Significance
Shift workers are affected by circadian misalignment and have an increased risk to develop metabolic diseases such as type 2 diabetes. Here, we show that during simulated short-term night shift work insulin sensitivity at the level of skeletal muscle is decreased in male volunteers, which could contribute to the development of type 2 diabetes in the long term. We also find that the muscle molecular clock does not align rapidly to the new behavioral cycle. Importantly, on the level of the transcriptome, circadian misalignment induced upregulation of fatty acid metabolism pathways, potentially resulting in substrate competition on the cellular level. These findings help to better understand the negative consequences during night shift work.
Keywords: circadian misalignment, shift work, diabetes, insulin sensitivity, skeletal muscle
Abstract
Circadian misalignment, such as in shift work, has been associated with obesity and type 2 diabetes. However, direct effects of circadian misalignment on skeletal muscle insulin sensitivity and the muscle molecular circadian clock have never been studied in humans. Here, we investigated insulin sensitivity and muscle metabolism in 14 healthy young lean men [age 22.4 ± 2.8 years; body mass index (BMI) 22.3 ± 2.1 kg/m2 (mean ± SD)] after a 3-d control protocol and a 3.5-d misalignment protocol induced by a 12-h rapid shift of the behavioral cycle. We show that short-term circadian misalignment results in a significant decrease in muscle insulin sensitivity due to a reduced skeletal muscle nonoxidative glucose disposal (rate of disappearance: 23.7 ± 2.4 vs. 18.4 ± 1.4 mg/kg per minute; control vs. misalignment; P = 0.024). Fasting glucose and free fatty acid levels as well as sleeping metabolic rate were higher during circadian misalignment. Molecular analysis of skeletal muscle biopsies revealed that the molecular circadian clock was not aligned to the inverted behavioral cycle, and transcriptome analysis revealed the human PPAR pathway as a key player in the disturbed energy metabolism upon circadian misalignment. Our findings may provide a mechanism underlying the increased risk of type 2 diabetes among shift workers.
The dramatic increase in the prevalence of obesity and type 2 diabetes mellitus (T2DM) is an important global health issue, as it comes with high morbidity and mortality and poses a major burden on health care costs. Obesity and T2DM are both strongly associated with a westernized lifestyle of low physical activity levels and high caloric intake. However, recently it has been recognized that also our 24-h culture, characterized by working and eating late, reduced sleep (quantity and quality), and excessive light exposure at night, should be considered as lifestyle factors that may negatively impact metabolic health. Indeed, epidemiologic studies show that night work, characterized by chronic circadian misalignment, is associated with adverse metabolic consequences and increased risk to develop T2DM (1, 2). Moreover, controlled circadian misalignment studies have demonstrated detrimental effects on (postprandial) glucose and insulin levels, which may indicate decreased insulin sensitivity (3, 4).
Circadian rhythmicity of metabolism is regulated by the core molecular clock, which consists of a transcriptional-translational feedback loop, in which the transcriptional activators BMAL1 and CLOCK induce expression of their own repressors CRY and PER, generating ∼24-h oscillations which affect up to 40% of the genome transcripts (5). Importantly, circadian oscillators are present in virtually every cell of the body, including skeletal muscle (6, 7), which is a major organ involved in the regulation of glucose homeostasis. The circadian clock is increasingly recognized as a key regulator of many metabolic processes and serves to anticipate metabolic challenges and demands across a normal day (8). Importantly, systemic or localized disruption of core clock genes in animal models has been associated with impaired glucose metabolism and the development of mitochondrial dysfunction. Thus, ablation of BMAL1 in mice resulted in decreased fatty acid oxidation and mitochondrial oxygen consumption rates (9, 10). Moreover, in rodent models, insulin resistance can be induced by liver or skeletal muscle-specific ablation of BMAL1 (11, 12) as well as circadian misalignment protocols (13). So far, evidence that circadian misalignment can also affect skeletal muscle metabolism in humans is lacking. We have previously shown that in humans, skeletal muscle is characterized by a day-night rhythm in clock gene expression and mitochondrial respiratory capacity (7). A reduction in mitochondrial respiratory capacity is commonly associated with compromised insulin sensitivity (14, 15). So far it is unknown if circadian misalignment in humans also leads to disturbed skeletal muscle metabolism and/or blunted insulin sensitivity.
To this end, we performed an in-depth human intervention study in male volunteers in which insulin sensitivity—assessed by the hyperinsulinemic euglycemic clamp—and molecular analysis of muscle biopsies were performed after a 3-d control protocol (circadian alignment) and after a 3.5-d protocol in which the behavioral cycle was abruptly shifted by 12 h, while male participants stayed in dim light conditions to minimize shifts of the central brain clock (circadian misalignment).
Materials and Methods
Participants.
Fourteen healthy lean young men [age: 22.4 ± 2.8 y; body mass index (BMI): 22.3 ± 2.1 kg/m2; mean ± SD] participated in the study. Participants were nonsmokers, had no active diseases, used no medication, and did not engage in exercise for more than 3 h per week, as verified by questionnaires. In addition, participants reported regular bedtimes (11 PM ± 2 h), regular sleep duration of 7–9 h, did not perform shift work or travel across more than one time zone in the last 3 mo, and were not definite morning larks or night owls, assessed with a Morningness-Eveningness Questionnaire Self Assessment (MEQ-SA: 54 ± 7, mean ± SD). The study was conducted in accordance with the principles of the declaration of Helsinki and approved by the Ethics Committee of the Maastricht University Medical Center. All participants provided written informed consent. The study was registered at https://clinicaltrials.gov with identifier NCT02580513.
Study Conditions.
Seven days before the study periods, participants were instructed to maintain a standardized normal lifestyle, including (trying to) sleep and consuming meals at regular times, consistent with the times during the study. In addition, participants were asked to abstain from consumption of caffeine and alcohol during those 7 d. Each participant underwent one control protocol and one circadian misalignment protocol, in a randomized, crossover fashion, with a wash-out of 4–10 wk (mean 6.5 vs. 6.8 wk, for those starting with control vs. misalignment). Participants resided in a respiration chamber: a small room with a bed, toilet, TV, and computer. Light exposure was 4 lx (horizontal angle of gaze) during wake episodes and darkness during sleep opportunities. Participants did not have access to devices that can display time (e.g., mobile phone), Internet, live television, or radio.
Study Design.
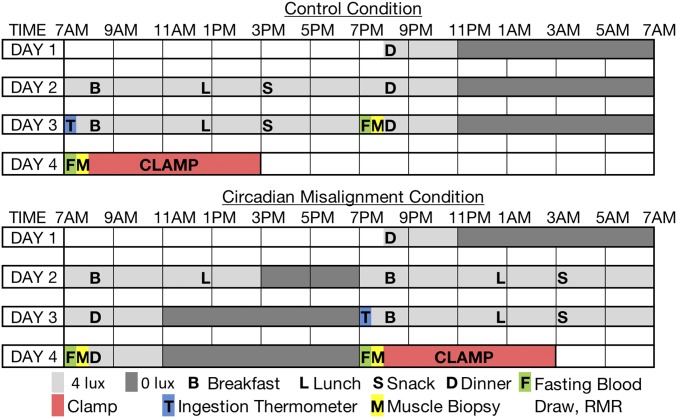
The study design is graphically depicted in Fig. 1. For the control period, participants were admitted to the research unit at 7:45 PM on day 1 and stayed until 5 PM on day 4. Sleep opportunities were from 11 PM until 7 AM, during which participants were required to switch off the lights and try to sleep. Participants were not allowed to switch on the lights or get out of bed during sleep opportunities, except for toilet breaks, and were not allowed to sleep during wake periods. Adherence to the protocol was checked by visual inspection at least every 15–20 min at all times. If participants had difficulties to stay awake during the biological night in the misaligned condition, researchers initiated conversations to promote wakefulness and supervision was intensified. During the test days (except for the last test day), participants received breakfast, lunch, a snack, and dinner (see below) at fixed times (8 AM, 12:30 PM, 3 PM, and 8 PM, respectively). To prevent sedentary behavior in our small-sized room calorimeters, participants were instructed to perform a low-intensity physical activity, consisting of 3 × 5 min of stepping exercises and 3 × 5 min of standing in an alternating pattern, 1 h after each meal. In the misaligned period, participants were admitted at 7:45 PM on day 1 and had a normal sleeping opportunity from 11 PM until 7 AM. On day 2, circadian misalignment was imposed by shifting the behavioral cycle by 12 h. Participants were instructed to switch off the lights, remain lying in bed and try to sleep from 3 PM until 7 PM. At 7 PM participants started a new behavioral morning with all study procedures equal to the control period but shifted by 12 h.
Fig. 1.
Study design. Participants underwent a control and a misalignment condition in a randomized crossover design. B, breakfast; Clamp, hyperinsulinemic euglycemic clamp; D, dinner; F, measurement of fasting blood substrates and energy metabolism; L, lunch; M, muscle biopsy; RMR, resting metabolic rate; S, snack.
Study Meals.
Two days before and during the study, participants were provided with standardized meals. Caloric requirements for the study days were estimated by multiplying sleeping metabolic rate as measured in the first night of each condition with an activity factor of 1.5. Caloric intake was spread over three daily meals and a snack. Breakfast accounted for ∼20 energy%, lunch for ∼25 energy%, a snack for ∼10 energy%, and dinner for ∼45 energy%. Daily macronutrient distribution was ∼52 energy% as carbohydrates, ∼31 energy% as fat (∼9% saturated), and ∼14 energy% as protein. Breakfast and lunch were bread meals, while dinner was a hot meal, resulting in a relatively higher fat content for dinner, as is common in The Netherlands. No additional snacks or drinks other than water were provided.
Indirect Calorimetry.
Whole-body energy expenditure and substrate metabolism were measured with an automated respiratory gas analyzer using a ventilated hood system and whole-room calorimeter (Omnical; Maastricht Instruments). Sleeping metabolic rate was assed as the lowest 3-h mean energy expenditure of the sleep period and extrapolated to 24-h energy expenditure (7).
Skeletal Muscle Biopsies.
During each study period, two skeletal muscle biopsies were obtained, at 8 AM and 8 PM from the m. vastus lateralis under local anesthesia (1% lidocaine, without epinephrine). Successive biopsies were taken moving from distal to proximal. The leg of the first biopsy during the first study condition was randomized, and the next biopsy was taken from the other leg, alternating between left and right leg (7).
Gene Transcript Quantification and Microarray Processing.
RNA was isolated from 50 mg of muscle material as described previously (7). Total RNA (100 ng) was labeled with the Whole-Transcript Sense Target Assay (P/N 900652; Affymetrix-ThermoFisher Scientific) and hybridized to whole-genome Affymetrix Human Gene 2.1 ST arrays. Detailed information can be found in the SI Appendix.
Two-Step Hyperinsulinemic Euglycemic Clamp.
To determine insulin sensitivity, a two-step hyperinsulinemic euglycemic clamp with infusion of d-[6,6-2H2]glucose tracer was performed. Insulin was infused at 10 mU/m2 per min for 3 h to assess hepatic insulin sensitivity [suppression of endogenous glucose production (EGP) by insulin] and subsequently at 40 mU/m2 per min for 2 h to determine rate of disappearance of glucose, mainly reflecting muscle insulin sensitivity (glucose Rd). Detailed information can be found in the SI Appendix.
Statistics.
Data are presented as mean ± SEM unless indicated otherwise. Statistical analyses were performed with the use of IBM Statistical Package for Social Sciences for MAC, version 23 (SPSS, Inc.). The effect of circadian misalignment on outcome variables was assessed by paired samples t test. Statistical significance was defined as a P < 0.05.
Results
12-H Rapid Shift in Behavior Induces Circadian Misalignment.
In the current study, we used a rapid 12-h shift protocol to induce circadian misalignment modeled after ref. 3. To confirm that also in the current study the volunteers were in circadian misalignment, we recorded core body temperature (CBT). As can be seen in SI Appendix, Fig. S1, CBT follows a classical 24-h rhythm during the control protocol, with a decrease in the late evening. During circadian misalignment, CBT decreased in the late biological evening despite subjects remaining awake, indicating that the shifted behavioral cycle was misaligned relative to the central circadian clock (SI Appendix, Fig. S1B).
Circadian Misalignment Increases Sleeping Metabolic Rate.
To examine if circadian misalignment of body temperature was also reflected in alterations in energy expenditure, we determined whole-body sleeping metabolic rate (SMR). Consistent with higher body temperature during sleep, SMR was elevated during circadian misalignment compared with the control condition (4.96 ± 0.12 vs. 5.16 ± 0.15 kJ/min, control vs. misalignment P = 0.014, SI Appendix, Fig. S2 A and C). Substrate oxidation during sleep was not affected by circadian alignment (RER: 0.83 ± 0.01 vs. 0.84 ± 0.01, control vs. misalignment, P = 0.687, SI Appendix, Fig. S2B). Radar counts from the respiration chamber during the sleeping period were not different between control and misaligned condition [average (±SEM) for control: 26.3 ± 1.2 vs. misaligned 28.1 ± 3.1 counts, P = 0.470]. Body weight, which is a determinant of SMR, was not different between control and misalignment (74 ± 12 vs. 74 ± 12 kg, P = 0.915).
Circadian Misalignment Alters Fasting Plasma Metabolites.
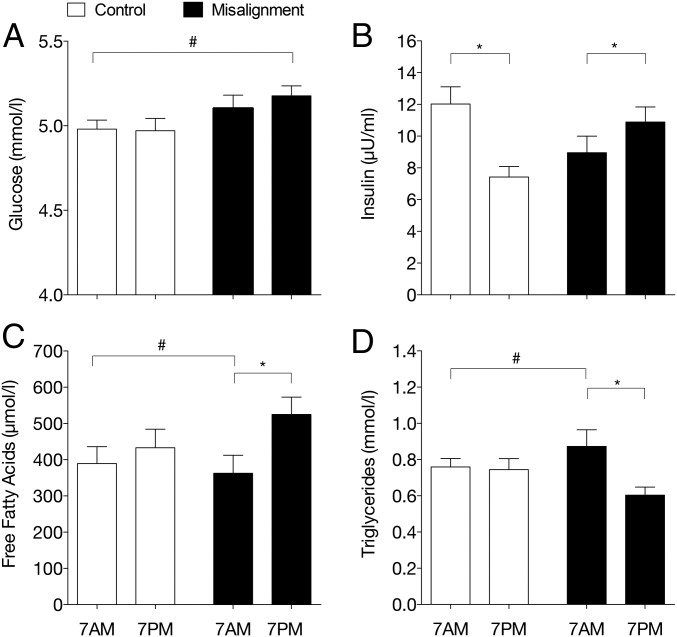
We next investigated the effect of circadian misalignment on plasma substrate values taken after an “overnight” fast and predinner, at 7 AM and 7 PM in the control and misaligned condition (Fig. 2). Plasma glucose levels were increased during circadian misalignment in the biological evening, i.e., at 7 PM (control: 5.0 ± 0.1 and 5.0 ± 0.1 mmol/L, 7 AM and 7 PM; misalignment: 5.1 ± 0.1 and 5.2 ± 0.1 mmol/L, 7 AM and 7 PM P = 0.015, Fig. 2A), while insulin levels were not statistically changed (control: 12.0 ± 1.1 and 7.4 ± 0.7 µU/mL, 7 AM and 7 PM; misalignment: 9.0 ± 1.0 and 10.9 ± 1.0 µU/mL, 7 AM and 7 PM P = 0.209, Fig. 2B). Plasma free fatty acid (FFA) levels were however markedly increased during circadian misalignment, with higher FFA levels in the biological evening after an 11-h overnight fast (7 PM in circadian misalignment) (control: 389 ± 47 and 433 ± 51 µmol/L, 7 AM and 7 PM; misalignment: 363 ± 50 and 525 ± 48 µmol/L, 7 AM and 7 PM P = 0.006, Fig. 2C). Also, plasma triglyceride levels showed a more pronounced evening-morning variation after circadian misalignment, with lower triglyceride (TG) levels in the biological evening after an overnight fast (control: 0.76 ± 0.05 and 0.74 ± 0.06 mmol/l, 7 AM and 7 PM; misaligned: 0.87 ± 0.09 and 0.60 ± 0.04 mmol/l, 7 AM and 7 PM P = 0.004, Fig. 2D).
Fig. 2.
Plasma metabolites are altered in circadian misalignment. Plasma glucose (A), insulin (B), free fatty acids (C), and triglycerides (D) were measured in the evening before dinner (nonfasted) and in the overnight fasted state 15 min after awaking, 7 AM and 7 PM in the control and misalignment condition, respectively. Data are mean ± SEM. *P < 0.05 biological morning and evening; #P < 0.05 behavioral morning and evening.
Circadian Misalignment Decreases Insulin Sensitivity.
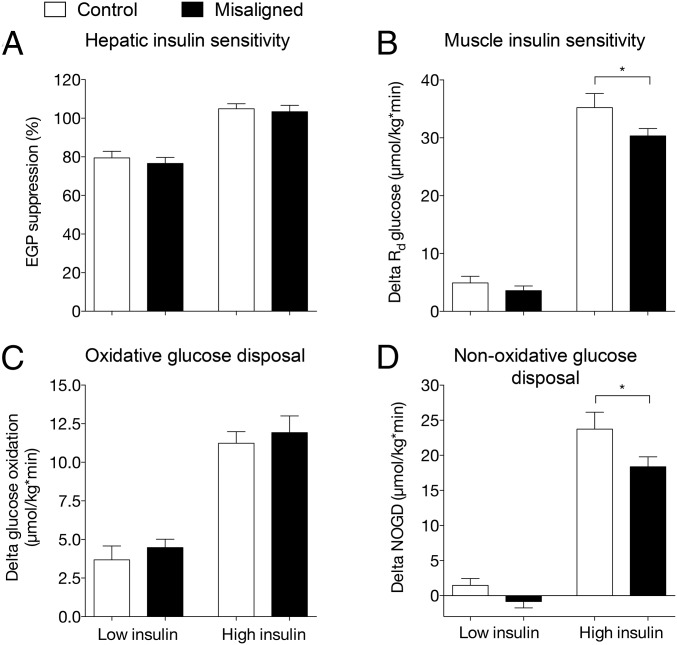
We next investigated if the changes in plasma metabolites upon circadian misalignment were accompanied by a reduction in insulin sensitivity. To this end, we performed a two-step hyperinsulinemic euglycemic clamp after an overnight fast, either at 8 AM or 8 PM in the control and misaligned condition, respectively. During low-dose insulin stimulation, EGP (2.2 ± 0.3 vs. 2.4 ± 0.2 µmol/kg per min, control vs. misalignment, P = 0.424) or the relative suppression of EGP (Fig. 3A, P = 0.309) was not affected by circadian misalignment. This suggests that circadian misalignment had no effect on hepatic insulin sensitivity. During high insulin infusion, EGP was fully suppressed in both study conditions, further supporting a lack of effect of circadian misalignment on hepatic insulin sensitivity. In contrast, circadian misalignment resulted in a ∼14% lower insulin-stimulated glucose disposal, mainly reflecting muscle insulin sensitivity, during high insulin infusion (delta Rd: 35.2 ± 2.4 vs. 30.3 ± 1.3 µmol/kg per min, control vs. misalignment P = 0.029, Fig. 3B). The decrease in insulin-stimulated glucose uptake was not accounted for by reduced glucose oxidation (delta glucose oxidation: 11.2 ± 0.8 vs. 11.9 ± 1.1 µmol/kg per min, control vs. misalignment, P = 0.583, Fig. 3C). However, nonoxidative glucose disposal was ∼23% lower upon circadian misalignment, suggesting decreased ability to form glycogen (delta NOGD: 23.7 ± 2.4 vs. 18.4 ± 1.4 µmol/kg per min, control vs. misalignment P = 0.024, Fig. 3D) (16). Free fatty acid levels decreased during the clamp from 386 ± 36 vs. 521 ± 43 µmol/L (control vs. misalignment P < 0.001) in the basal state to 74 ± 11 vs. 81 ± 12 µmol/L, (P = 0.422) upon low insulin and to 23 ± 2 vs. 26 ± 3 µmol/L (P = 0.456) upon high insulin infusion. We next performed analysis in skeletal muscle biopsies to investigate the mechanisms potentially underlying the reduction in skeletal muscle insulin sensitivity.
Fig. 3.
Insulin sensitivity is decreased in circadian misalignment. EGP suppression during low and high insulin infusion steady state is depicted in A. Insulin-stimulated glucose disposal is expressed as Rd low insulin − Rd basal (A) and Rd high insulin − Rd basal (B). Oxidative glucose disposal (C) and nonoxidative glucose disposal (NOGD) were corrected for basal values. All values were calculated for the last 30 min of the basal, low, and high insulin steady states. Data are mean ± SEM. Rd, rate of disappearance. *P < 0.05.
The Core Skeletal Muscle Molecular Clock Does Not Rapidly Align to the New Behavioral Rhythm.
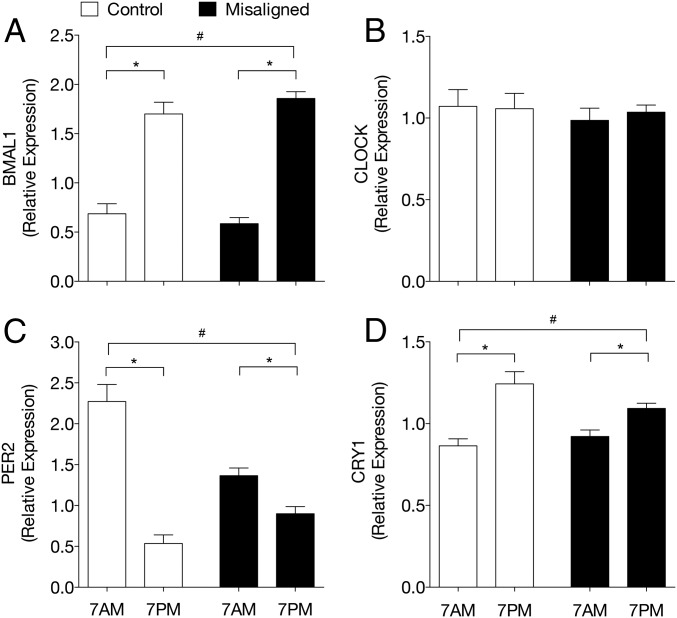
Given that circadian misalignment resulted in skeletal muscle insulin resistance, we first investigated whether circadian misalignment affected the skeletal muscle molecular clock. To this end, we performed quantitative PCR (qPCR) analysis on muscle biopsies that were obtained at 7 AM and 7 PM in both the control and circadian misalignment condition. In the control condition, the core clock genes BMAL1, CRY1, and PER2 displayed diurnal differences in mRNA expression levels, concordant with the results from our previous study (7) (Fig. 4). Intriguingly, upon circadian misalignment, mRNA expression levels of the core clock genes kept the same diurnal pattern as in the control condition, albeit blunted for PER and CRY, indicating that the core molecular clock in skeletal muscle indeed was misaligned relative to the inverted behavioral cycle.
Fig. 4.
Skeletal muscle core molecular clock genes are not aligned to behavioral rhythm upon circadian misalignment. mRNA expression levels of the core molecular clock genes BMAL1 (A), CLOCK (B), PER2 (C), and CRY1 (D) in skeletal muscle measured by RT-QPCR. Data are normalized to the geometric mean of three housekeeping genes and presented as mean ± SEM. *P < 0.05 comparing 7 AM and 7 PM in control and misalignment condition. #P < 0.05 comparing behavioral mornings in control vs. circadian misalignment condition (overnight fasted state before the clamp). Because only two time points per arm were investigated, the lack of difference in mRNA expression levels between 7 AM and 7 PM does not provide evidence that the measured parameter lacks 24 h rhythmicity.
Mitochondrial Oxidative Capacity Is Elevated upon Circadian Misalignment.
A reduced mitochondrial function has also been suggested as a determinant of skeletal muscle insulin resistance (15). Therefore, we measured ex vivo mitochondrial oxidative capacity using high-resolution respirometry in muscle fibers obtained at 7 AM and 7 PM in both the control and circadian misalignment condition. ADP-stimulated mitochondrial respiration (state 3) upon pyruvate as a substrate was not significantly different between evening and morning in the control condition (state 3 MP: 65.6 ± 4.4 and 71.0 ± 5.1 pmol/mg per s in control condition at 7 AM and 7 PM, P = 0.198, SI Appendix, Fig. S3A). However, upon circadian misalignment, state 3 respiration was higher at 7 PM (i.e., the behavioral morning) compared with 7 AM (state 3 MP: 69.3 ± 4.3 and 78.9 ± 5.8 pmol/mg per s, misaligned condition at 7 AM and 7 PM, P = 0.015, SI Appendix, Fig. S3A). Similar results were observed for pyruvate-fueled state U respiration (SI Appendix, Fig. S3A). As a result, state 3 and state U respiration was higher upon circadian misalignment when measured in the overnight fasted state (state 3 MP: P = 0.034). ADP-stimulated mitochondrial respiration (state 3) and uncoupled respiration (state U) upon octanoylcarnitine as a substrate did not show morning-evening variation, neither in the control nor in the misalignment condition (SI Appendix, Fig. S3B). Please note that oxidation of octanoylcarnitine is not dependent on CPT1 activity or proximal metabolism of fatty acids. Differences in mitochondrial oxidative capacity did not originate from mitochondrial content, as revealed by the absence of significant changes in mitochondrial protein content (4.95 ± 0.42 and 4.94 ± 0.34 AU, 7 AM and 7 PM vs. 5.03 ± 0.51 and 5.29 ± 0.49 AU, 7 AM and 7 PM, control vs. misalignment P ≥ 0.05). Muscle fiber type proved to be similar between control and misalignment condition.
Microarray Analysis Reveals Induction of PPAR-Mediated Oxidative Gene Expression upon Circadian Misalignment.
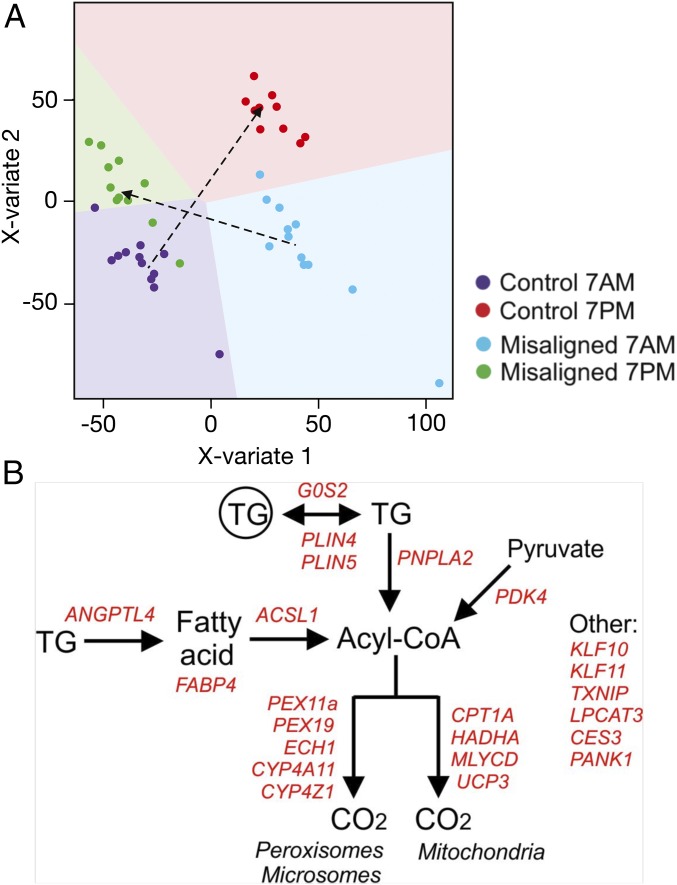
To further investigate the potential molecular mechanism underlying the difference in insulin sensitivity, we performed whole genome expression profiling on the muscle biopsies taken in the evening and morning of both conditions. Multilevel partial least squares-discriminant analysis (PLS-DA) showed that based on the global gene expression profiles, samples separated into four clusters that well represented the four conditions (i.e., 7 AM and 7 PM of the control condition and circadian misalignment) (Fig. 5A). Interestingly, the shift in the clustering of gene expression profiles from 7 AM to 7 PM (biological morning to biological evening) was clearly distinct in the control condition compared with the misaligned condition (Fig. 5A, arrows), which suggests (only) partial adaptation to the new (misaligned) behavioral cycle. A similar conclusion was reached when depicting the gene expression changes in a scatter plot (SI Appendix, Fig. S4). Correlation analysis indicated that on average the diurnal expression tended to have a reversed expression pattern in the misaligned condition compared with the control condition, suggesting partial rather than complete adaptation to the inverted behavioral cycle of the subjects. Clock-related genes that display a circadian rhythm tend to align into an opposite direction compared with the regression line, i.e., they have not aligned to the inverted behavioral cycle. These data thus confirm the data from the exploratory qPCR analysis, that several clock genes did not reverse their expression patterns during circadian misalignment [BMAL1 (ARNTL), PER1/2/3, and CIART]. This was a very consistent response in all of the subjects (SI Appendix, Fig. S5). At the level of individual genes, several ones were identified that exhibited diurnal differences between 7 AM and 7 PM in the control condition [296 genes, false discovery rate (FDR) < 0.05] and during circadian misalignment (238 genes, FDR < 0.05). To better characterize the potential functional implications of the differences in gene expression, we performed pathway analysis in the form of gene set enrichment analysis, comparing 7 AM with 7 PM in both the control and misaligned condition. Interestingly, many of the most highly enriched gene sets among the genes changed in expression from 7 AM to 7 PM in the misaligned condition were related to fatty acid metabolism and PPAR signaling. This observation indicates that fatty acid metabolism and PPAR signaling are most strongly increased from 7 AM to 7 PM in the misaligned condition (SI Appendix, Fig. S6A). Since insulin sensitivity was only determined once in each study condition, after the overnight fasts at 7 AM (control) and 7 PM (misaligned) respectively, we next compared the changed pathways between 7 AM in the control condition and 7 PM in the misaligned condition, representing the timepoints of the clamp. Again, fatty acid metabolism and PPAR signaling featured prominently among the most significantly changed pathways (SI Appendix, Fig. S6B). The functional role in lipid metabolism of the positively enriched genes in the geneset “human PPAR targets” is illustrated in Fig. 5B. Interestingly, pathways involved in glucose metabolism were not changed by circadian misalignment. These data suggest that upon circadian misalignment, lipid metabolism is induced in the behavioral morning compared with the control condition.
Fig. 5.
Global gene expression analysis indicates up-regulated PPAR target genes. (A) Multilevel PLS-DA of the transcriptome data indicates similar expression changes between participants. Sample prediction area plots from a PLS-DA model applied on the transcriptome data set with the expression levels of all 29,526 genes in 47 samples. Almost all samples could be correctly classified into four classes that represented the four conditions for each individual subject [control condition (7 AM and 7 PM) and misaligned condition (7 AM and 7 PM)]. Each point represents one array of a participant at one time point. Background color indicates the two-dimensional representation of the predicted classification space of the four classes (conditions), and show that only 3 of the 47 samples were misclassified. (B) Positively enriched genes in the geneset “human PPAR targets” that were significantly up-regulated at 7 PM in the misaligned condition compared with 7 AM in the control condition (representing the timepoints of the clamp) (P < 0.05) were positioned in a biochemical map of cellular lipid metabolism.
Discussion
A substantial part of the working population is engaged in shift work, which is associated with metabolic diseases. Here, we show in male volunteers that short-term circadian misalignment resulted in a significant decrease in insulin sensitivity that was mainly due to impairment in insulin-stimulated nonoxidative glucose disposal, but not hepatic insulin sensitivity. In addition, we show that the molecular biological clock in skeletal muscle was misaligned relative to the behavioral routine, suggesting that skeletal muscle does not adjust to a new day-night rhythm within 3 d. Interestingly, this was accompanied by an enriched molecular PPAR and fat (oxidative) metabolism signature, which may suggest a role for substrate competition at the level of the skeletal muscle in the induction of compromised skeletal muscle insulin sensitivity.
Controlled circadian misalignment decreased insulin sensitivity and was accompanied by an increase in fasting plasma glucose levels. This finding corroborates indirect observations from earlier studies, demonstrating higher plasma glucose levels after a meal, despite concomitantly increased levels of plasma insulin (3, 17), during circadian misalignment. Also, Sharma et al. (18) recently reported that postprandial glycemic excursion was higher after night shift in shift workers, due to impaired β-cell function. It has been suggested that eating at the wrong time is an important aspect in shift work conditions (19) and may be one explanation for our results. Our findings are also in line with the finding that insulin sensitivity is typically reduced in the evening (20). Interestingly, we here find that the decrease in insulin sensitivity under misaligned conditions was not due to a reduction in hepatic insulin sensitivity, as the suppression of hepatic glucose output upon physiological concentrations of insulin (10 mU/m2 per min infusion) was similar in both study conditions, indicating that hepatic insulin sensitivity is not affected during short-term circadian misalignment. In fact, the decrease in insulin sensitivity could be completely attributed to a 23% decrease in insulin-stimulated nonoxidative glucose disposal (NOGD).
Circadian misalignment also led to higher fasting FFA levels and lower triglyceride levels. Notably, elevated fasting glucose and FFA levels are important markers in the development of prediabetes and point toward a disturbed glucose homeostasis (21). The increase in fasting FFA with circadian misalignment is consistent with previous findings by Morris et al. (3) and suggests that basal rates of lipolysis display intrinsic circadian rhythmicity, as has also been shown in mice (22). Moreover, a recent human trial showed that the adipose tissue molecular clock responds to delayed meal timing with a shift in circadian phase (23). We also found altered triglyceride levels in circadian misalignment, which can be due to intrinsic circadian regulation and/or elevated clearance of very low density lipoprotein (VLDL)-TG by the skeletal muscle. In fasted rats, VLDL levels are lower during the active phase compared with the resting phase (24); however, human data in support of such regulation is lacking. Together, these results highlight the negative consequences of a short-term circadian misalignment.
We also found significantly higher SMR and a tendency for higher resting energy metabolism in the misaligned condition. These results are in line with a previous report of higher SMR after 3 d of circadian misalignment, induced by an artificial 27-h forced desynchrony protocol (25), but contrast with a recent report by McHill et al. (26). The reason for the different results are unclear. Importantly, we noted that energy expenditure was consistently higher (and very stable) throughout the entire sleeping period after circadian misalignment (SI Appendix, Fig. S1C). This fits well with the higher body temperature during the night upon misalignment. Although an elevation of energy metabolism may seem beneficial in the context of body weight regulation, improvements in metabolic health are usually characterized by reductions in energy metabolism, due to improved energy efficiency (27). Therefore, most likely the increase in sleeping metabolic rate is another indicator for an unfavorable metabolic profile during circadian misalignment.
Analysis of gene expression in skeletal muscle clearly showed that in this 3-d circadian misalignment protocol the molecular clock gene pathway had not adapted to the behavioral rhythm (i.e., the new day-night rhythm). We here demonstrate that in humans, upon short-term misalignment, the molecular clock in skeletal muscle does not realign to the new behavior. From preclinical studies, it is assumed that the skeletal molecular clock can be affected by behavioral factors such as food intake and exercise (28). Future studies are needed to investigate whether the muscle molecular clock can be realigned to the new behavioral rhythm via interventions with food intake or physical activity. A more global analysis of gene transcripts showed that the vast majority of gene transcripts with diurnal expression differences in the control condition show a tendency toward reversed expression in circadian misalignment and are thus not aligned to the new behavioral rhythm. Interestingly, pathway analysis revealed that particularly genes involved in lipid metabolism were affected by circadian misalignment. Analysis of the most significantly up-regulated genes upon circadian misalignment revealed a clear PPAR signature, accompanied by markers of elevated (fat) oxidative gene transcription. Consistent with a role for PPAR in the regulation of this gene program, genes involved in glucose oxidation were not affected. These findings are in line with a recent report showing that PPARδ overexpression in mice specifically affects cellular substrate choice by favoring fat oxidation over glucose and glycogen usage (29). The finding that specifically insulin-induced glycogen storage was reduced upon circadian misalignment fits with the concept that PPAR activation may have spared glycogen usage, similar as in mice overexpressing PPARδ (29). Subsequently, this would lead to a reduced urge to replenish glycogen stores under hyperinsulinemic euglycemic conditions, hence the observed reduced NOGD. Higher FFA levels measured after an overnight fast upon circadian misalignment fit with this concept of substrate competition and could then serve to fuel the muscle with fatty acid substrates. Follow-up studies are needed to unravel whether, indeed, intracellular substrate competition is responsible for the reduced insulin sensitivity upon circadian misalignment.
Limitations of the study are that we only included male volunteers and have only studied the effect of a short-term, acute day-night shift. Future studies are needed to investigate whether similar effects are seen in women and whether the observed effects also result in similar negative metabolic health effects under chronic shiftwork conditions, as suggested by a recent study (30). Another limitation is the lack of information on sleep quality, which can decrease insulin sensitivity (31), and therefore no conclusion can be made about the relative contribution of disturbed sleep versus circadian misalignment on insulin sensitivity. In a similar study protocol by Morris et al. (3), total sleep time, measured by polysomnography, was found to be reduced by ∼40 min. In daily practice, individuals in circadian misalignment are usually also affected by disturbed sleep, which thus can contribute to reduced insulin sensitivity. However, it has been shown that a decrease in insulin sensitivity, measured with the i.v. glucose tolerance test, during circadian misalignment can also occur independently of sleep loss (4).
In this study, we demonstrated that controlled short-term circadian misalignment reduced insulin sensitivity, which supports the role of circadian disruption in the development of insulin resistance and T2DM. Furthermore, we showed that the molecular clock in skeletal muscle has not aligned to the new day-night rhythm after 3 d of circadian misalignment, and that upon circadian misalignment, a human PPAR gene profile is enhanced that could favor intramuscular fatty acid metabolism over glucose metabolism. Future studies should assess whether similar results can be found in individuals at risk for the development of T2DM.
Supplementary Material
Acknowledgments
This work is partly financed by Netherlands Organization for Scientific Research Grant TOP 40-00812-98-14047 (to P.S. and A.K.); the Netherlands Cardiovascular Research Initiative: an initiative with support of the Dutch Heart Foundation Grant CVON2014-02 ENERGISE; J. Hansen is supported by the School of Nutrition and Translational Research in Metabolism (NUTRIM) Netherlands Organization for Scientific Research (NWO) Graduate Programme, which is financially supported by Netherlands Organization for Scientific Research Grant 022.003.011; J. Hoeks is supported by Vidi Grant 917.14.358 for innovative research from NWO and a Senior Fellowship from the Dutch Diabetes Research Foundation via Grant 2013.82.1639; F.A.J.L.S. has been funded in part by NIH Grants R01HL118601, R01DK099512, R01DK105072, R01DK102696, and R01HL140574; and B.S. was supported by the FP7 grant Eurhythdia and holds “ERC advanced Grant” 694717.
Footnotes
Conflict of interest statement: F.A.J.L.S. has received speaker fees from Bayer Healthcare, Sentara Healthcare, Kellogg, Philips, and Vanda Pharmaceuticals.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1722295115/-/DCSupplemental.
References
- 1.Buchvold HV, Pallesen S, Øyane NM, Bjorvatn B. Associations between night work and BMI, alcohol, smoking, caffeine and exercise–A cross-sectional study. BMC Public Health. 2015;15:1112. doi: 10.1186/s12889-015-2470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gan Y, et al. Shift work and diabetes mellitus: A meta-analysis of observational studies. Occup Environ Med. 2015;72:72–78. doi: 10.1136/oemed-2014-102150. [DOI] [PubMed] [Google Scholar]
- 3.Morris CJ, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci USA. 2015;112:E2225–E2234. doi: 10.1073/pnas.1418955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leproult R, Holmbäck U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860–1869. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc Natl Acad Sci USA. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckel-Mahan KL, et al. Reprogramming of the circadian clock by nutritional challenge. Cell. 2013;155:1464–1478. doi: 10.1016/j.cell.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Moorsel D, et al. Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. Mol Metab. 2016;5:635–645. doi: 10.1016/j.molmet.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian J, Scheer FAJL. Circadian system and glucose metabolism: Implications for physiology and disease. Trends Endocrinol Metab. 2016;27:282–293. doi: 10.1016/j.tem.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrews JL, et al. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc Natl Acad Sci USA. 2010;107:19090–19095. doi: 10.1073/pnas.1014523107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peek CB, et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342:1243417. doi: 10.1126/science.1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dyar KA, et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol Metab. 2013;3:29–41. doi: 10.1016/j.molmet.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobi D, et al. Hepatic Bmal1 regulates rhythmic mitochondrial dynamics and promotes metabolic fitness. Cell Metab. 2015;22:709–720. doi: 10.1016/j.cmet.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salgado-Delgado R, Angeles-Castellanos M, Saderi N, Buijs RM, Escobar C. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology. 2010;151:1019–1029. doi: 10.1210/en.2009-0864. [DOI] [PubMed] [Google Scholar]
- 14.Fabbri E, et al. Insulin resistance is associated with reduced mitochondrial oxidative capacity measured by 31P-magnetic resonance spectroscopy in participants without diabetes from the Baltimore longitudinal study of aging. Diabetes. 2017;66:170–176. doi: 10.2337/db16-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phielix E, et al. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes. 2008;57:2943–2949. doi: 10.2337/db08-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shulman GI, et al. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990;322:223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- 17.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma A, et al. Glucose metabolism during rotational shift-work in healthcare workers. Diabetologia. 2017;60:1483–1490. doi: 10.1007/s00125-017-4317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattson MP, et al. Meal frequency and timing in health and disease. Proc Natl Acad Sci USA. 2014;111:16647–16653. doi: 10.1073/pnas.1413965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Cauter E, Désir D, Decoster C, Féry F, Balasse EO. Nocturnal decrease in glucose tolerance during constant glucose infusion. J Clin Endocrinol Metab. 1989;69:604–611. doi: 10.1210/jcem-69-3-604. [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88:787–835. doi: 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Shostak A, Meyer-Kovac J, Oster H. Circadian regulation of lipid mobilization in white adipose tissues. Diabetes. 2013;62:2195–2203. doi: 10.2337/db12-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wehrens SMT, et al. Meal timing regulates the human circadian system. Curr Biol. 2017;27:1768–1775.e1763. doi: 10.1016/j.cub.2017.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mondola P, Gambardella P, Santangelo F, Santillo M, Greco AM. Circadian rhythms of lipid and apolipoprotein pattern in adult fasted rats. Physiol Behav. 1995;58:175–180. doi: 10.1016/0031-9384(95)00016-c. [DOI] [PubMed] [Google Scholar]
- 25.Gonnissen HK, et al. Effect of a phase advance and phase delay of the 24-h cycle on energy metabolism, appetite, and related hormones. Am J Clin Nutr. 2012;96:689–697. doi: 10.3945/ajcn.112.037192. [DOI] [PubMed] [Google Scholar]
- 26.McHill AW, et al. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc Natl Acad Sci USA. 2014;111:17302–17307. doi: 10.1073/pnas.1412021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Civitarese AE, et al. CALERIE Pennington Team Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Goede P, et al. Differential effects of diet composition and timing of feeding behavior on rat brown adipose tissue and skeletal muscle peripheral clocks. Neurobiol Sleep Circadian Rhythms. 2017;4:24–33. doi: 10.1016/j.nbscr.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan W, et al. PPARdelta promotes running endurance by preserving glucose. Cell Metab. 2017;25:1186–1193.e1184. doi: 10.1016/j.cmet.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris CJ, Purvis TE, Mistretta J, Scheer FA. Effects of the internal circadian system and circadian misalignment on glucose tolerance in chronic shift workers. J Clin Endocrinol Metab. 2016;101:1066–1074. doi: 10.1210/jc.2015-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao MN, et al. Subchronic sleep restriction causes tissue-specific insulin resistance. J Clin Endocrinol Metab. 2015;100:1664–1671. doi: 10.1210/jc.2014-3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.