Significance
Shift workers, whose schedules are misaligned relative to their suprachiasmatic nuclei (SCN) circadian pacemaker, are at elevated risk of metabolic disorders. In a study of simulated day- versus night-shift work followed by a constant routine, we separated plasma-circulating metabolites according to whether their 24-h rhythms aligned with the central SCN pacemaker or instead reflected externally imposed behavioral schedules. We found that rhythms in many metabolites implicated in food metabolism dissociated from the SCN pacemaker rhythm, with the vast majority aligning with the preceding sleep/wake and feeding/fasting cycles. Our metabolomics study yields insight into the link between prolonged exposure to shift work and the spectrum of associated metabolic disorders by providing a window into peripheral oscillators and the biobehavioral factors that orchestrate them.
Keywords: circadian misalignment, gastrointestinal tract, liver, metabolomics, shift work
Abstract
Misalignment between internal circadian rhythmicity and externally imposed behavioral schedules, such as occurs in shift workers, has been implicated in elevated risk of metabolic disorders. To determine underlying mechanisms, it is essential to assess whether and how peripheral clocks are disturbed during shift work and to what extent this is linked to the central suprachiasmatic nuclei (SCN) pacemaker and/or misaligned behavioral time cues. Investigating rhythms in circulating metabolites as biomarkers of peripheral clock disturbances may offer new insights. We evaluated the impact of misaligned sleep/wake and feeding/fasting cycles on circulating metabolites using a targeted metabolomics approach. Sequential plasma samples obtained during a 24-h constant routine that followed a 3-d simulated night-shift schedule, compared with a simulated day-shift schedule, were analyzed for 132 circulating metabolites. Nearly half of these metabolites showed a 24-h rhythmicity under constant routine following either or both simulated shift schedules. However, while traditional markers of the circadian clock in the SCN—melatonin, cortisol, and PER3 expression—maintained a stable phase alignment after both schedules, only a few metabolites did the same. Many showed reversed rhythms, lost their rhythms, or showed rhythmicity only under constant routine following the night-shift schedule. Here, 95% of the metabolites with a 24-h rhythmicity showed rhythms that were driven by behavioral time cues externally imposed during the preceding simulated shift schedule rather than being driven by the central SCN circadian clock. Characterization of these metabolite rhythms will provide insight into the underlying mechanisms linking shift work and metabolic disorders.
Endogenous circadian rhythms exist in nearly all physiological and behavioral processes, including metabolism. In normal physiology, the central circadian clock, located in the hypothalamic suprachiasmatic nuclei (SCN), synchronizes the timing of peripheral clocks in the liver, gut, pancreas, and adipose tissue via multiple neural and hormonal pathways (1). Desynchronization of this circadian timing system from externally imposed behavioral rhythms (light/dark exposure; sleep/wakefulness; rest/activity; feeding/fasting)—such as occurs in shift workers—over time results in a number of metabolic disorders including obesity, metabolic syndrome, and type 2 diabetes (2). To determine the underlying mechanisms linking shift work to metabolic disorders, it is essential to understand whether and how peripheral clocks are disturbed during shift work and to what extent these peripheral clocks are driven by the central SCN pacemaker versus misaligned behavioral time cues. Investigating rhythms in circulating metabolites as biomarkers of peripheral clock disturbances may enable this, thereby offering an important step forward.
Previous circadian and sleep metabolomics studies have shown time-of-day variation in plasma metabolites. As expected under fully entrained conditions with alignment of the central SCN pacemaker and behavioral cycles, a large number of metabolites are rhythmic (3, 4). However, under constant routine conditions in which exogenous factors are removed or fixed, only 10–20% of metabolites appear rhythmic (5–7). It remains unknown whether the oscillations seen in metabolites that remain rhythmic under constant routine conditions are driven by the central SCN pacemaker or whether they are reflections of peripheral oscillators that continue to cycle in the absence of externally imposed behavioral time cues that were initially driving them. Distinguishing these possibilities is critical for understanding how shift work may lead to peripheral rhythm disturbances that may be involved in the etiology of metabolic disorders.
The rhythmic production of the hormones melatonin and cortisol is driven directly by SCN timing; these hormones are therefore considered reliable markers of the phase of the central SCN clock (8). Rhythms that reflect the SCN pacemaker have also been observed in the expression of core clock genes, such as Period3 (PER3), that can be measured in blood-borne cells (9). Previous studies in both simulated (10, 11) and real-world (12, 13) shift work have shown that the rhythms of these circadian markers are resistant to phase shifting, indicating that the endogenous SCN rhythm is slow to adapt to shift work schedules. As such, exposure to a night-shift schedule produces misalignment between behavioral rhythms and the rhythm of the central SCN pacemaker.
In a between-groups, in-laboratory study, we assigned healthy volunteers (ages 22–34 y) to 3 d of either a simulated night-shift schedule (seven subjects) or a simulated day-shift (i.e., control) schedule (seven subjects). In each group, this was followed by a 24-h constant routine protocol, during which blood was drawn at 3-h intervals to measure plasma metabolite profiles using a targeted liquid chromatography/mass spectrometry (LC/MS) metabolomics approach (SI Appendix, Fig. S1). We thus characterized 24-h rhythms in metabolites, free of exogenous factors, after the central SCN clock and behaviorally induced rhythms were experimentally misaligned (night-shift condition) or aligned (day-shift condition). This allowed us to assess whether oscillations observed in metabolites are driven by the central SCN pacemaker—or whether they are the signature of shifted behavioral rhythms during the preceding days of shift work.
Results
During the constant routine after 3 d of simulated shift work, the dim light melatonin onset (DLMO) and the 24-h rhythm of plasma cortisol (traditional markers of the central SCN pacemaker) as well as the 24-h rhythm in clock gene PER3 expression all showed relatively little phase difference between the day-shift and night-shift conditions (SI Appendix, Figs. S2–S4). A delay of ∼2 h was observed after the night-shift condition, regardless of which rhythm marker was used, which is congruent with a free-running rhythm (circadian period τ > 24 h) (14). This indicates that the SCN pacemaker was resistant to phase shifting by the behaviorally imposed schedule, as expected, and that adaptation of the endogenous SCN timing system to the night-shift schedule was limited. Thus, the night-shift condition successfully produced misalignment between behavioral rhythms (light/dark exposure; sleep/wakefulness; rest/activity; feeding/fasting), which were shifted by 12 h under the simulated night-shift condition, and the relatively unyielding rhythm of the central SCN pacemaker.
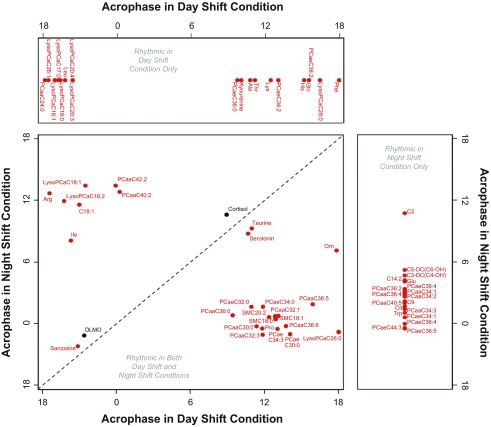
The blood samples taken during the 24-h constant routine were analyzed for 132 circulating metabolites from 5 metabolite classes using targeted LC/MS metabolomics. For each of these metabolites, the samples were analyzed with cosinor analysis to assess the presence and timing of 24-h rhythmicity (SI Appendix, Table S1). Nearly half of the metabolites analyzed (i.e., 65 of 132) showed significant 24-h rhythmicity during the constant routine under either or both conditions. Of these, a group of 27 metabolites showed rhythmicity following both the simulated day-shift and night-shift schedules. A group of 19 metabolites lost their rhythmicity under constant routine following the night-shift schedule. For another group of 19 metabolites, no significant rhythmicity was observed following the day-shift schedule, but rhythmicity emerged under constant routine following the night-shift schedule. For each of the metabolites that showed a significant 24-h rhythm, Fig. 1 shows the clock time at which the rhythm peaked (acrophase).
Fig. 1.
Timing of the peak of the 24-h rhythm (acrophase) during constant routine following the day-shift condition and following the night-shift condition in 27 metabolites with significant 24-h rhythmicity for both conditions (Bottom Left), in 19 metabolites with significant 24-h rhythmicity for the day-shift condition only (Top), and in 19 metabolites with significant 24-h rhythmicity for the night-shift condition only (Right). Time is indicated as clock time in hours (0 = midnight). Red: metabolites. Black: timing of melatonin onset (DLMO) and timing of cortisol peak (for reference). The dashed diagonal line indicates where metabolites would fall if the timing of their rhythmicity during constant routine was unperturbed by the night-shift condition relative to the day-shift condition.
Fig. 1, Bottom Left, shows the 27 metabolites with significant 24-h rhythmicity under both conditions. Only three of these metabolites maintained approximately the same peak time following both the simulated day-shift and night-shift schedules (as did the traditional circadian markers, DLMO and cortisol peak): serotonin, taurine, and sarcosine (Fig. 2, Left). By contrast, under constant routine following the simulated night-shift schedule, 24 metabolites significantly shifted—and in most cases essentially reversed (i.e., shifted by 12 h)—their rhythms. These metabolites comprised four amino acids (ornithine, arginine, isoleucine, and proline), octadecanoylcarnitine (AC-C18:1), and a collection of glycerophospholipids, lysophosphatidylcholines (lysoPCs), and sphingolipids (for an example, see Fig. 2, Right).
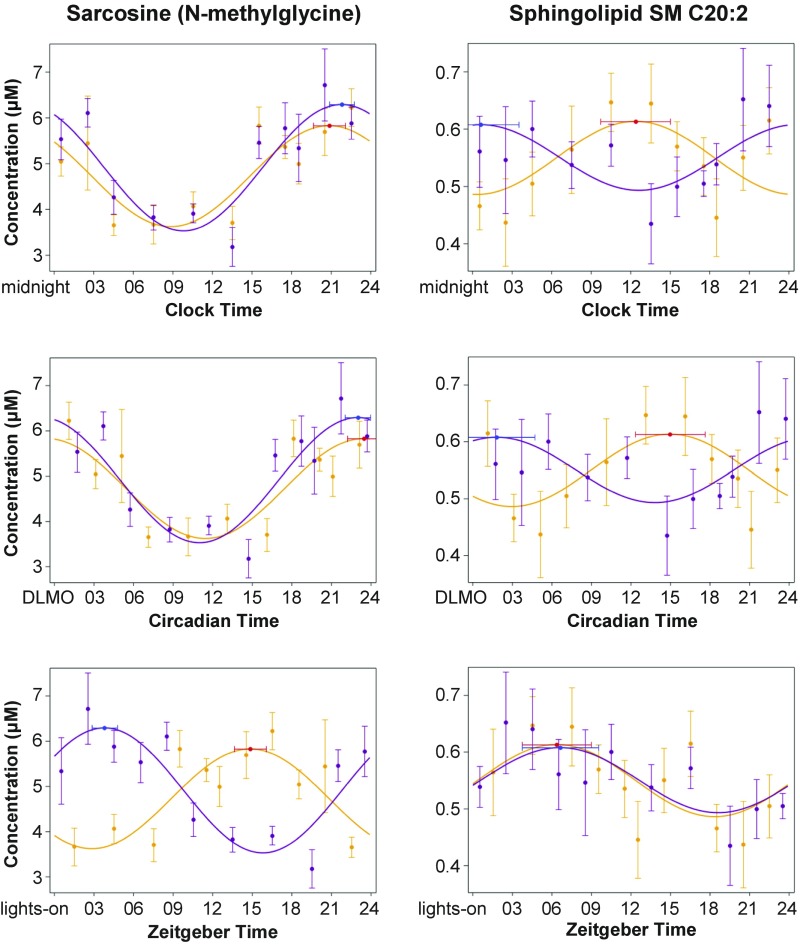
Fig. 2.
Three representations of the cosinor fits for two example metabolites: a biogenic amine, sarcosine (N-methylglycine, Left); and a sphingolipid, SM C20:2 (Right). (Top) By clock time (i.e., time of day, relative to midnight). (Middle) By circadian time (relative to the timing of melatonin onset, DLMO). (Bottom) By zeitgeber time (i.e., time awake, relative to lights on). Orange/red: constant routine after simulated day-shift condition. Purple/blue: constant routine after simulated night-shift condition. Circles: group means (±SE). Curves: cosinor fits. Red and blue markers: timing of acrophase (and 95% confidence interval).
Fig. 1, Top, shows the metabolites that exhibited significant 24-h rhythmicity following the day-shift condition, but lost rhythmicity following the night-shift schedule. These metabolites included half of the measured lysoPCs, and 7 of the 20 amino acids, all of which peaked during the daytime hours. Fig. 1, Right, on the other hand, shows the metabolites that did not exhibit significant 24-h rhythmicity following the day-shift condition, but exhibited rhythmicity following the night-shift condition. These metabolites comprised half of the measured acylcarnitines, glutamate, tryptophan, citrulline, and 11 phosphatidylcholines, all but 1 peaking during the nighttime hours.
Fig. 2, Top, illustrates the contrast between one of the three metabolites that maintained a stable peak time following both the simulated day-shift and night-shift schedules, namely the amino acid sarcosine (N-methylglycine, Left), and one of the 24 metabolites that reversed their rhythms under constant routine following the simulated night-shift schedule, namely the sphingolipid SM C20:2 (Right). As shown in Fig. 2 (Middle), when plotted against circadian time—i.e., relative to DLMO (which itself remained relatively stable between the two schedules)—the 24-h rhythm in sarcosine aligned nearly perfectly between the day- and night-shift schedules, indicating a strong influence of the central SCN pacemaker; but this was not the case for SM C20:2. By contrast, as shown in Fig. 2 (Bottom), when plotted against zeitgeber time—i.e., relative to the scheduled timing of the light/dark, sleep/wake, rest/activity, and feeding/fasting cycles in the preceding shift days—the 24-h rhythm in SM C20:2 lined up nearly perfectly between the day- and night-shift conditions. This was observed despite the fact that these behavioral cycles were no longer present under the constant routine conditions in which these metabolites were measured, providing substantial evidence that the observed rhythms in this metabolite (and others like it) were driven by behavioral rhythms in the preceding shift days and were subsequently retained.
The subdivision according to the observed rhythms following the day- and night-shift schedules as captured in Fig. 1 was corroborated by principal component analysis, which yielded three distinct clusters of metabolites (SI Appendix, Table S2). Cluster 1 represented the group of metabolites that showed 24-h rhythmicity under constant routine following both the day- and night-shift schedules, but reversed their rhythms following the night-shift schedule (SI Appendix, Fig. S5, Top). Heat maps revealed that this pattern was the primary response to the experiment in the overall data set (SI Appendix, Fig. S6). Cluster 1 was dominated by glycerophospholipids and sphingolipids. Cluster 2 represented the group of metabolites that showed significant 24-h rhythmicity under constant routine only after the day-shift schedule (SI Appendix, Fig. S5, Middle). This cluster was dominated by lysophosphatidylcholines and a variety of amino acids. Cluster 3 represented the group of metabolites that did not show significant 24-h rhythmicity under constant routine after the day-shift schedule, but was rhythmic after the night-shift schedule (SI Appendix, Fig. S5, Bottom). Similar to the other two clusters, cluster 3 was dominated by acylcarnitines, some amino acids, glycerophospholipids, and sphingolipids.
The 65 metabolites that showed significant rhythmicity during constant routine following the day-shift and/or night-shift schedules were entered into pathway analyses. Under the day-shift condition, nine pathways were significantly enriched (SI Appendix, Table S3), including aminoacyl-tRNA biosynthesis, nitrogen metabolism, arginine–proline metabolism, and d-glutamine–glutamate metabolism—as well as alanine–asparate–glutamate metabolism, cyanoamino acid metabolism, d-arginine and d-ornithine metabolism, glutathione metabolism, and glycine–serine–threonine metabolism, which were not significantly enriched under the night-shift condition. Under the night-shift condition, five pathways were significantly enriched (SI Appendix, Table S4), including aminoacyl-tRNA biosynthesis, nitrogen metabolism, arginine–proline metabolism, and d-arginine–ornithine metabolism—as well as valine–leucine–isoleucine biosynthesis, which was not significantly enriched under the day-shift condition. As such, the observed rhythms in circulating metabolites and the metabolic pathways affected appear to reflect metabolic processes in the liver, pancreas, and digestive tract.
Discussion
Night-shift workers are at elevated risk of a wide range of chronic medical conditions, including gastrointestinal disorders and debilitating metabolic disorders such as metabolic syndrome and type 2 diabetes mellitus (2, 15). It has been hypothesized that misalignment between internal circadian rhythmicity and externally imposed behavioral schedules associated with nighttime wakefulness underlies this elevated risk (15, 16). However, a reliable tool to separate circadian- and behavior-driven rhythms in metabolism to test this hypothesis has been lacking.
In the current laboratory-controlled, simulated shift work study, we investigated the metabolic consequences of misaligned sleep/wake and feeding/fasting cycles using a targeted metabolomics approach. We found that, whereas traditional markers of the circadian clock in the SCN (melatonin, cortisol, PER3 expression) remained relatively stable after 3 d of simulated shift work, many of the plasma metabolites (62 of 132) showed profound changes in the timing of their rhythms following simulated night work (Fig. 1; SI Appendix, Table S1). In the majority of cases, these changes involved nearly complete reversal of the 24-h rhythmicity in the metabolites (SI Appendix, Figs. S5 and S6)—typically peaking during the daytime after the day-shift condition and during the nighttime after the night-shift condition (e.g., Fig. 2, Right). In other instances, 24-h rhythms were seen only after the day-shift condition, peaking during the daytime, or only after the night-shift condition, peaking during the nighttime (SI Appendix, Fig. S5). Although with 14 subjects our sample was relatively small, these effects were robust and highly statistically significant. Importantly, the altered rhythms persisted under constant routine conditions in the absence of any externally imposed rhythms. This provides strong evidence that most of the metabolite rhythms observed were driven by behavioral time cues—from the light/dark exposure, sleep/wakefulness, rest/activity, and/or feeding/fasting cycles—during the preceding simulated shift schedules. Exogenous, behavioral time cues associated with externally imposed schedules thus have the capacity to drive endogenous rhythmicity in metabolism, likely by impacting on peripheral oscillators (17, 18) and dissociating them from the rhythm of the central SCN pacemaker.
The mechanisms linking shift work with an increased risk of type 2 diabetes are not well understood. Although metabolic profiling (metabolomics) has been used to investigate prediabetic/obese and type 2 diabetic phenotypes (19–21), this has been limited to single-time-point samples. To assess the interaction between circadian timing and metabolic physiology, we recently compared 24-h metabolite rhythms in overweight/obese individuals and in individuals with type 2 diabetes to age- and weight-matched controls (4). Several metabolites, including proline, sarcosine, and lysoPC a C18:1 and C18:2, exhibited robust daily rhythms in the majority of participants in all study groups and showed a progressive change in concentration from lean to overweight/obese to type 2 diabetes groups. Isoleucine and valine rhythms were lost in patients with type 2 diabetes compared with controls. These metabolites may play a role or act as biomarkers in the progression from a healthy weight to obesity with type 2 diabetes. In the current study, rhythms in proline, lysoPC a C18:1 and C18:2, leucine, and isoleucine were altered by externally imposed behavioral time cues (Fig. 1), and pathway analysis implicated proline metabolism and biosynthesis of branched-chain amino acids (leucine, isoleucine, valine) as impacted by simulated night-shift work (SI Appendix, Table S4). Whether these metabolite rhythms and pathways reflecting disrupted circadian rhythms in peripheral metabolism due to simulated night-shift work link to the metabolic phenotype of type 2 diabetes remains to be studied.
In addition to type 2 diabetes, shift work has been linked to a host of other chronic medical conditions, including renal dysfunction (22, 23) and various types of cancer (24–27). There is evidence that the kidneys have their own circadian oscillators (28), and it is possible that disruption of these peripheral oscillators leads to renal disease (29). Consistent with this possibility, we found that tryptophan and kynurenine, markers of renal function (30), were altered by prior exposure to simulated night-shift work. Peripheral clocks are also critically involved in the coordination of cellular processes such as cell cycles, DNA repair, and apoptosis (31). Animal models with externally disturbed circadian rhythms show dysregulation of these processes, promoting tumorigenesis and tumor growth (32–34). Our pathway analyses revealed that some of the major components of amino acid metabolism—including cyanoamino acid, glycine, serine, threonine, alanine, and glutathione pathways—were enriched under the day-shift condition but not under the night-shift condition (SI Appendix, Tables S3 and S4). These pathways have the capacity to influence cancer-cell gene-expression patterns, cell proliferation, and tumor microenvironments (35), and glutathione in particular plays a role in protection against oxidative stress, increased mitochondrial respiration, and protection from cell death (36). Our targeted metabolomics approach was not specifically focused on such effects, but the results suggest that altered metabolite rhythms after exposure to night-shift work could be involved in the development of a wide range of chronic diseases (37).
Of the 65 metabolites that showed 24-h rhythmicity under constant routine following either or both simulated shift schedules, only 3 maintained a rhythm after both schedules in which a stable phase alignment was kept relative to traditional markers of the central SCN clock (melatonin, cortisol, PER3 expression; SI Appendix, Figs. S2–S4). These metabolites—taurine, serotonin, and sarcosine (N-methylglycine; Fig. 2, Left)—thus appear to be resistant to externally imposed behavioral rhythms. Their temporal profiles may be controlled by the SCN clock (38), or they may be generally slower to adapt to shifted temporal cues. The observed 24-h rhythms in the other 62 metabolites, most of which are bioactive lipids, amino acids, acylcarnitines, and other compounds implicated in metabolic processes and energy metabolism, provide a window on peripheral clock function in the liver, pancreas, and digestive tract. Although our study involved acute exposure to simulated night-shift work in healthy non–shift-working volunteers inside the laboratory, and the results therefore do not readily generalize to real-world shift-work settings, our data nonetheless provide clues about which metabolic pathways to target for further research. Such research could, on the one hand, aim to identify early stage biomarkers of the pathophysiology of metabolic disorders associated with shift work and, on the other hand, aim to elucidate underlying mechanisms and enable the development of new therapeutic interventions to promote long-term shift worker health.
The 24-h duration of our constant routine protocol was relatively short, which is a limitation of the study. Even so, our results were robust to any transient effects from physiological adjustment to the constant routine procedures that may have occurred (SI Appendix, Materials and Methods). Also, the targeted metabolomics approach that we used (3, 4) did not cover the entire metabolome. As such, we do not know whether other classes of metabolites (e.g., polar metabolites) would show a similar predominance of synchronization to behavioral time cues rather than the central SCN pacemaker. While our targeted approach is restrictive in that regard, it is quantitative and reliable (39) and, compared with whole-metabolome analyses, can be more readily confirmed and validated in other laboratories. Through our targeted metabolomics approach, we were thus able to separate circadian- and behavior-driven rhythms in metabolism. The behavior-driven rhythms that we observed may have been influenced by the experimentally imposed time cues in light/dark exposure, sleep/wakefulness, rest/activity, and/or feeding/fasting; our experimental paradigm did not allow us to disentangle these external factors. However, night-shift workers typically experience changes in these behavioral time cues in tandem, as their work schedules force them to shift their wakefulness—and thereby also their activity and feeding patterns and exposure to light—into the night.
Time-restricted feeding has been proposed as a potential strategy to restore normal peripheral clock rhythmicity in metabolic pathways disturbed by circadian misalignment (40). In mouse models, feeding restriction during the inactive part of the day results in ∼12-h phase shifting in core clock genes in peripheral tissues such as the liver (17, 41) within a time span of just 2 d (42). Time-restricted feeding patterns also affect the mouse metabolome (43), but the phase shifting in peripheral oscillators appears to be independent of clock gene expression in the central SCN pacemaker (17, 42). Initial laboratory studies in humans suggest that time-restricted feeding may help to address abnormalities in glucose metabolism in night-shift workers (44). Our current finding of simulated night-shift work inducing changes in the timing of metabolite rhythms, indicative of disturbed peripheral clock function in the liver, pancreas, and digestive tract, provides a mechanistic foundation for this type of intervention and a quantitative tool to determine its effectiveness.
Materials and Methods
Clinical Study.
The study was approved by the Institutional Review Board of Washington State University. Participants gave written, informed consent. They had to meet defined inclusion criteria to be deemed eligible for the study (SI Appendix, Materials and Methods).
In-Laboratory Experiment.
The 7-d in-laboratory experiment (SI Appendix, Fig. S1) was conducted under controlled conditions (constant ambient temperature of 21 ± 1 °C, constant light level of less than 50 lx during scheduled wakefulness) in the Sleep and Performance Research Center at Washington State University Spokane. The study consisted of a baseline day and night followed by assignment to either a 3-d simulated day-shift schedule (sleep opportunity: 22:00–06:00) or, after a transition nap (sleep opportunity: 14:00–18:00), to a 3-d simulated night-shift schedule (sleep opportunity: 10:00–18:00). During the simulated shift days, breakfast, lunch, and dinner were provided at 1.5, 7, and 13.5 h of scheduled wakefulness, respectively. The 3-d simulated shift schedule was followed by a 24-h constant routine protocol, which involved controlled environmental conditions with fixed semirecumbent posture, hourly identical snacks, and sustained wakefulness to investigate rhythms free of exogenous factors (45). The study concluded with a recovery day.
During the 24-h constant routine, blood samples were taken through an i.v. catheter at intervals of 1–3 h (SI Appendix, Fig. S1). The samples were used for targeted LC/MS metabolomics and cortisol and PER3 assays. Additional i.v. blood samples were taken hourly during the baseline day (18:30–21:30) and during the constant routine (18:30–01:30) for melatonin assays (SI Appendix, Fig. S1).
Analyses.
See SI Appendix, Materials and Methods for details of the targeted LC/MS metabolomics analysis; melatonin, cortisol, and PER3 assays; and statistical analyses.
Supplementary Material
Acknowledgments
We thank the staff of the human sleep laboratory in the Sleep and Performance Research Center at Washington State University Spokane for their help conducting the clinical study; Dr. Matthew Layton for serving as physician of record for the clinical study; and the Metabolomics Core Facility at the University of Surrey. This work was supported by start-up funds from the College of Pharmacy and Pharmaceutical Sciences at Washington State University (to S.G.) and in part by Congressionally Directed Medical Research Program Award W81XWH-16-1-0319 (to H.P.A.V.D.); National Institutes of Health Grant R00ES022640 (to S.G.); UK Biotechnology and Biological Sciences Research Council Grant BB/I019405/1 (to D.J.S.); and European Union FP7-HEALTH-2011 EuRhythDia Grant 278397 (to D.J.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1801183115/-/DCSupplemental.
Change History
August 30, 2021: The SI Appendix has been updated to coincide with a formal Correction.
References
- 1.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 2.Kecklund G, Axelsson J. Health consequences of shift work and insufficient sleep. BMJ. 2016;355:i5210. doi: 10.1136/bmj.i5210. [DOI] [PubMed] [Google Scholar]
- 3.Davies SK, et al. Effect of sleep deprivation on the human metabolome. Proc Natl Acad Sci USA. 2014;111:10761–10766. doi: 10.1073/pnas.1402663111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isherwood CM, Van der Veen DR, Johnston JD, Skene DJ. Twenty-four-hour rhythmicity of circulating metabolites: Effect of body mass and type 2 diabetes. FASEB J. 2017;31:5557–5567. doi: 10.1096/fj.201700323R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dallmann R, Viola AU, Tarokh L, Cajochen C, Brown SA. The human circadian metabolome. Proc Natl Acad Sci USA. 2012;109:2625–2629. doi: 10.1073/pnas.1114410109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasukawa T, et al. Human blood metabolite timetable indicates internal body time. Proc Natl Acad Sci USA. 2012;109:15036–15041. doi: 10.1073/pnas.1207768109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chua EC, et al. Extensive diversity in circadian regulation of plasma lipids and evidence for different circadian metabolic phenotypes in humans. Proc Natl Acad Sci USA. 2013;110:14468–14473. doi: 10.1073/pnas.1222647110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev. 2005;9:25–39. doi: 10.1016/j.smrv.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Viola AU, et al. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol. 2007;17:613–618. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 10.Lee C, Smith MR, Eastman CI. A compromise phase position for permanent night shift workers: Circadian phase after two night shifts with scheduled sleep and light/dark exposure. Chronobiol Int. 2006;23:859–875. doi: 10.1080/07420520600827160. [DOI] [PubMed] [Google Scholar]
- 11.James FO, Cermakian N, Boivin DB. Circadian rhythms of melatonin, cortisol, and clock gene expression during simulated night shift work. Sleep. 2007;30:1427–1436. doi: 10.1093/sleep/30.11.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen JH, Geving IH, Reinertsen RE. Adaptation rate of 6-sulfatoxymelatonin and cognitive performance in offshore fleet shift workers: A field study. Int Arch Occup Environ Health. 2010;83:607–615. doi: 10.1007/s00420-010-0547-x. [DOI] [PubMed] [Google Scholar]
- 13.Boivin DB, Boudreau P, James FO, Kin NM. Photic resetting in night-shift work: Impact on nurses’ sleep. Chronobiol Int. 2012;29:619–628. doi: 10.3109/07420528.2012.675257. [DOI] [PubMed] [Google Scholar]
- 14.Wever RA. The Circadian System of Man. Springer; New York: 1979. [Google Scholar]
- 15.James SM, Honn KA, Gaddameedhi S, Van Dongen HPA. Shift work: Disrupted circadian rhythms and sleep—Implications for health and well-being. Curr Sleep Med Rep. 2017;3:104–112. doi: 10.1007/s40675-017-0071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leproult R, Holmbäck U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860–1869. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damiola F, et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wehrens SMT, et al. Meal timing regulates the human circadian system. Curr Biol. 2017;27:1768–1775.e3. doi: 10.1016/j.cub.2017.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newgard CB, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suhre K, et al. Metabolic footprint of diabetes: A multiplatform metabolomics study in an epidemiological setting. PLoS One. 2010;5:e13953. doi: 10.1371/journal.pone.0013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang-Sattler R, et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol Syst Biol. 2012;8:615. doi: 10.1038/msb.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charles LE, et al. Association between shiftwork and glomerular filtration rate in police officers. J Occup Environ Med. 2013;55:1323–1328. doi: 10.1097/JOM.0b013e3182a299c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasaki S, et al. Short sleep duration increases the risk of chronic kidney disease in shift workers. J Occup Environ Med. 2014;56:1243–1248. doi: 10.1097/JOM.0000000000000322. [DOI] [PubMed] [Google Scholar]
- 24.Parent MÉ, El-Zein M, Rousseau MC, Pintos J, Siemiatycki J. Night work and the risk of cancer among men. Am J Epidemiol. 2012;176:751–759. doi: 10.1093/aje/kws318. [DOI] [PubMed] [Google Scholar]
- 25.He C, Anand ST, Ebell MH, Vena JE, Robb SW. Circadian disrupting exposures and breast cancer risk: A meta-analysis. Int Arch Occup Environ Health. 2015;88:533–547. doi: 10.1007/s00420-014-0986-x. [DOI] [PubMed] [Google Scholar]
- 26.Lin X, et al. Night-shift work increases morbidity of breast cancer and all-cause mortality: A meta-analysis of 16 prospective cohort studies. Sleep Med. 2015;16:1381–1387. doi: 10.1016/j.sleep.2015.02.543. [DOI] [PubMed] [Google Scholar]
- 27.Rao D, Yu H, Bai Y, Zheng X, Xie L. Does night-shift work increase the risk of prostate cancer? A systematic review and meta-analysis. OncoTargets Ther. 2015;8:2817–2826. doi: 10.2147/OTT.S89769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stow LR, Gumz ML. The circadian clock in the kidney. J Am Soc Nephrol. 2011;22:598–604. doi: 10.1681/ASN.2010080803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martino TA, et al. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1675–R1683. doi: 10.1152/ajpregu.00829.2007. [DOI] [PubMed] [Google Scholar]
- 30.Pawlak D, Tankiewicz A, Mysliwiec P, Buczko W. Tryptophan metabolism via the kynurenine pathway in experimental chronic renal failure. Nephron. 2002;90:328–335. doi: 10.1159/000049069. [DOI] [PubMed] [Google Scholar]
- 31.Fu L, Kettner NM. The circadian clock in cancer development and therapy. Prog Mol Biol Transl Sci. 2013;119:221–282. doi: 10.1016/B978-0-12-396971-2.00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S, Donehower LA, Herron AJ, Moore DD, Fu L. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS One. 2010;5:e10995. doi: 10.1371/journal.pone.0010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Dycke KC, et al. Chronically alternating light cycles increase breast cancer risk in mice. Curr Biol. 2015;25:1932–1937. doi: 10.1016/j.cub.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Papagiannakopoulos T, et al. Circadian rhythm disruption promotes lung tumorigenesis. Cell Metab. 2016;24:324–331. doi: 10.1016/j.cmet.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Circu ML, Aw TY. Glutathione and modulation of cell apoptosis. Biochim Biophys Acta. 2012;1823:1767–1777. doi: 10.1016/j.bbamcr.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGinnis GR, Young ME. Circadian regulation of metabolic homeostasis: Causes and consequences. Nat Sci Sleep. 2016;8:163–180. doi: 10.2147/NSS.S78946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panda S, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 39.Siskos AP, et al. Interlaboratory reproducibility of a targeted metabolomics platform for analysis of human serum and plasma. Anal Chem. 2017;89:656–665. doi: 10.1021/acs.analchem.6b02930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panda S. Circadian physiology of metabolism. Science. 2016;354:1008–1015. doi: 10.1126/science.aah4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davidson AJ, Poole AS, Yamazaki S, Menaker M. Is the food-entrainable circadian oscillator in the digestive system? Genes Brain Behav. 2003;2:32–39. doi: 10.1034/j.1601-183x.2003.00005.x. [DOI] [PubMed] [Google Scholar]
- 42.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 43.Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20:991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grant CL, et al. Timing of food intake during simulated night shift impacts glucose metabolism: A controlled study. Chronobiol Int. 2017;34:1003–1013. doi: 10.1080/07420528.2017.1335318. [DOI] [PubMed] [Google Scholar]
- 45.Duffy JF, Dijk DJ. Getting through to circadian oscillators: Why use constant routines? J Biol Rhythms. 2002;17:4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.