Using intrinsic signal optical imaging, Chen et al. (1) show that disparity information in visual area V2 is decodable from correlated random dot stereograms (cRDSs), but not from anticorrelated RDSs (aRDSs). The authors conclude that “V2 is the initial locus of false matching elimination,” indicating that the correspondence problem is solved within or immediately after V2. We disagree with this conclusion based on previous single-unit studies. Two immediate downstream areas of V2, middle temporal area (MT) and V4, still encode disparities of aRDSs. Anticorrelation reduces the disparity selectivity by only ∼50% in MT (2, 3) and ∼60% in V4 (3, 4) (as evaluated by the mean amplitude ratio between the tuning curves for aRDSs and cRDSs). These findings suggest that the stereo correspondence problem is not yet fully solved within or immediately after V2. Although direct projections from V1 to MT might explain retained selectivity in MT (1), this same explanation is less plausible in V4. Relative disparity selectivity progressively develops from V1 through V2 to V4 (5–7), suggesting that V2 is an important area bridging V1 and V4 in disparity processing. Therefore, we suspect that artificial pooling inherent to intrinsic signal optical imaging may have attenuated false-match responses to a greater degree than biological pooling between V2 and V4.
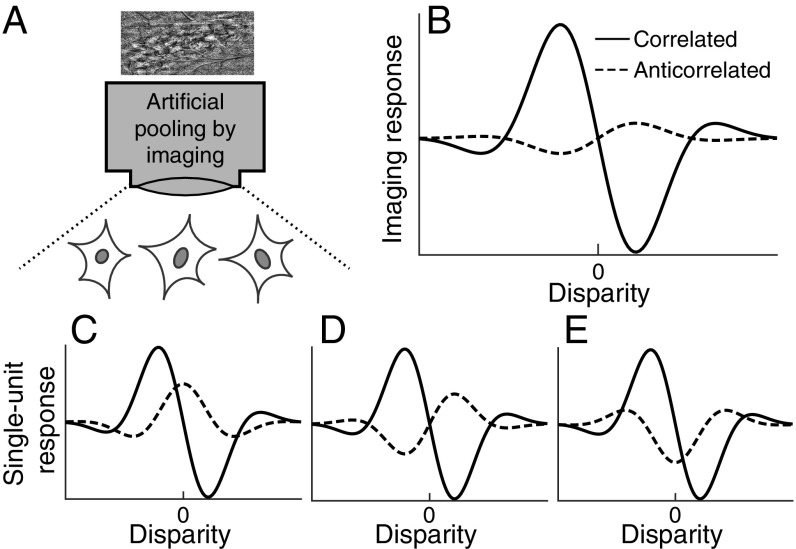
Chen et al. list several parameters over which pooling may reduce the responses to false matches. These parameters include preferred orientations, spatial scales, and receptive-field locations. We propose to add another important parameter to this list: the difference in tuning shape (phase) for cRDSs and aRDSs. Fig. 1 depicts a scenario that can explain observations made by Chen et al. without contradicting the aforementioned physiological findings. Neighboring neurons prefer similar disparities in response to cRDSs (Fig. 1 C–E). Anticorrelation partially attenuates their response magnitudes. Importantly, anticorrelation changes the tuning shape differently among different neurons. As their responses are averaged, the imaging response shows greater tuning attenuation for aRDSs than individual responses, while keeping the response to cRDSs intact (Fig. 1B).
Fig. 1.
A possible explanation for the selective response attention for anticorrelated RDSs with intrinsic signal optical imaging. (A) Schematic diagram showing that the responses of three neurons are averaged through the imaging. (B) Disparity tuning curve consistent with the optical imaging data reported in ref. 1. This could arise even when the neurons have substantial disparity tunings for anticorrelated RDSs as reported in single-unit studies (C–E). The key assumption of this scenario is that nearby single units share the disparity selectivity for correlated RDSs but not for anticorrelated RDSs.
Our proposed explanation lacks direct evidence but is biologically plausible. The disparity energy model predicts that anticorrelation inverts tuning shape (Fig. 1D). Even in V1, some neurons deviate from this prediction (Fig. 1 C and E) (8). These deviations are large enough that pooling individual V1 neurons attenuates the responses to aRDSs (3). In V4, the deviation is even larger, to the extent that pooling across a population of V4 neurons can eliminate the disparity selectivity for aRDSs (3). Such anticorrelation effects can be explained by combining several energy-model subunits with an output nonlinearity (3), a type of computation that presumably takes place in V2 (9). We suggest that the spatial pooling inherent to the intrinsic signal optical imaging might artificially eliminate false-match responses, possibly because of the incoherent tuning shapes to aRDSs among nearby neurons. In actual visual processing, this pooling is likely to complete only after V4.
Acknowledgments
We thank Seiji Tanabe and David White for valuable comments.
Footnotes
The authors declare no conflict of interest.
References
- 1.Chen G, Lu HD, Tanigawa H, Roe AW. Solving visual correspondence between the two eyes via domain-based population encoding in nonhuman primates. Proc Natl Acad Sci USA. 2017;114:13024–13029. doi: 10.1073/pnas.1614452114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krug K, Cumming BG, Parker AJ. Comparing perceptual signals of single V5/MT neurons in two binocular depth tasks. J Neurophysiol. 2004;92:1586–1596. doi: 10.1152/jn.00851.2003. [DOI] [PubMed] [Google Scholar]
- 3.Abdolrahmani M, Doi T, Shiozaki HM, Fujita I. Pooled, but not single-neuron, responses in macaque V4 represent a solution to the stereo correspondence problem. J Neurophysiol. 2016;115:1917–1931. doi: 10.1152/jn.00487.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanabe S, Umeda K, Fujita I. Rejection of false matches for binocular correspondence in macaque visual cortical area V4. J Neurosci. 2004;24:8170–8180. doi: 10.1523/JNEUROSCI.5292-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cumming BG, Parker AJ. Binocular neurons in V1 of awake monkeys are selective for absolute, not relative, disparity. J Neurosci. 1999;19:5602–5618. doi: 10.1523/JNEUROSCI.19-13-05602.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas OM, Cumming BG, Parker AJ. A specialization for relative disparity in V2. Nat Neurosci. 2002;5:472–478. doi: 10.1038/nn837. [DOI] [PubMed] [Google Scholar]
- 7.Umeda K, Tanabe S, Fujita I. Representation of stereoscopic depth based on relative disparity in macaque area V4. J Neurophysiol. 2007;98:241–252. doi: 10.1152/jn.01336.2006. [DOI] [PubMed] [Google Scholar]
- 8.Cumming BG, Parker AJ. Responses of primary visual cortical neurons to binocular disparity without depth perception. Nature. 1997;389:280–283. doi: 10.1038/38487. [DOI] [PubMed] [Google Scholar]
- 9.Tanabe S, Cumming BG. Mechanisms underlying the transformation of disparity signals from V1 to V2 in the macaque. J Neurosci. 2008;28:11304–11314. doi: 10.1523/JNEUROSCI.3477-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]