Significance
What determines the role of brain regions and their plasticity when typical inputs or experience is not provided? To what extent can extreme compensatory use affect brain organization? We tested the reorganization of primary and association sensorimotor cortex hand-selective areas in people born without hands, who use their feet for everyday tasks. We found that their primary sensorimotor hand area is preferentially activated for nearby body parts that cannot serve as effectors. In contrast, foot-selective compensatory plasticity was found in the association cortex, in an area typically involved in manual tool use. This shows limitations of compensatory plasticity and experience in modifying brain organization of early topographical cortex, as compared with association cortices where function-based organization is the driving factor.
Keywords: brain development, hands, motor cortex, plasticity, somatosensory cortex
Abstract
What forces direct brain organization and its plasticity? When brain regions are deprived of their input, which regions reorganize based on compensation for the disability and experience, and which regions show topographically constrained plasticity? People born without hands activate their primary sensorimotor hand region while moving body parts used to compensate for this disability (e.g., their feet). This was taken to suggest a neural organization based on functions, such as performing manual-like dexterous actions, rather than on body parts, in primary sensorimotor cortex. We tested the selectivity for the compensatory body parts in the primary and association sensorimotor cortex of people born without hands (dysplasic individuals). Despite clear compensatory foot use, the primary sensorimotor hand area in the dysplasic subjects showed preference for adjacent body parts that are not compensatorily used as effectors. This suggests that function-based organization, proposed for congenital blindness and deafness, does not apply to the primary sensorimotor cortex deprivation in dysplasia. These findings stress the roles of neuroanatomical constraints like topographical proximity and connectivity in determining the functional development of primary cortex even in extreme, congenital deprivation. In contrast, increased and selective foot movement preference was found in dysplasics’ association cortex in the inferior parietal lobule. This suggests that the typical motor selectivity of this region for manual actions may correspond to high-level action representations that are effector-invariant. These findings reveal limitations to compensatory plasticity and experience in modifying brain organization of early topographical cortex compared with association cortices driven by function-based organization.
What determines the role of brain regions and their plasticity when typical inputs or experience is not provided? To what extent can extreme compensatory use affect brain organization? Is brain organization limited in its plasticity due to strong neuroanatomical constraints? We investigated this question by studying the neural activity profile in primary and association sensorimotor cortices in people born without hands who use their feet to perform everyday manual tasks.
Both animal studies and human cases of deafferentation or amputation have found that the primary sensory and motor cortices reorganize to show activity expansion of adjacent body parts, and that this reorganization is greater when it occurs early in life (1–10).
However, several recent functional neuroimaging studies in people with congenitally missing hands (dysplasic individuals) have stressed the role of experience-based plasticity in the reorganization of the primary sensorimotor hand region. Specifically, it has been proposed that the hand area supports the use of other body parts in performing manual-type, everyday actions, and that this plasticity is not constrained by topographical factors. Results have been reported showing activation of the hand primary sensorimotor cortex for body parts used in place of congenitally missing hands (11, 12). The hand-responsive area in somatosensory cortex of individuals with partially missing hands shrinks depending on the size and use of the hand remains (13, 14). Importantly, in dysplasic individuals, somatosensory stimulation of the foot activated the lateral sensory cortex (15). Furthermore, in two individuals born without hands bilaterally, it has been shown that strong transcranial magnetic stimulation of the lateral motor cortex generated motor evoked potentials not only in the participants’ residual finger or shoulder but also in the foot. Such stimulation also interfered with performance of a foot motor task (11). These findings were interpreted as evidence for robust plasticity of the sensorimotor cortex and functional takeover of the hand areas by the foot. These studies were interpreted as raising the possibility that the primary sensorimotor cortex is functionally selective rather than selective for a specific body part. On this view, the hand area stands for any effector that functions as a hand in everyday tasks like grasping and manipulating objects (12).
However, the specificity of such supposed compensatory reorganization (for the body part used as hands) has not been thoroughly tested in people born without hands. None of the studies with dysplasic individuals tested whether the hand area is activated more for compensatorily used body parts than for other proximal, but noncompensatorily used organs. Is the hand sensorimotor cortex indeed selective for compensatorily used body parts that serve as effectors? Alternatively, plasticity due to compensatory effector experience may be limited by neuroanatomical constraints such as topographical proximity and connectivity of this brain system, enabling only a takeover by closer cortical territories or contralateral intact body parts, akin to what is found in late-onset amputation or deafferentation.
A related question concerns the effects of missing limbs on the organization of the association sensorimotor action system. Parts of the posterior parietal cortex show preference for specific body parts, specifically the eyes and hands, when participating in goal-directed visuomotor action (16–20). These preferences in the fronto-parietal network also depend on the functional use of body parts such as in the execution of manual tasks like reaching, object manipulation, and grasping (21–25). Given that the foot rather than the hand is the major effector in dysplasics, we additionally tested whether typically hand-selective regions in the parietal and frontal cortex now respond preferentially to the foot, reflecting the effects of effector use-based compensatory plasticity.
We investigated these questions by mapping sensorimotor responses to movement of various body parts in five individuals born without hands who use their feet for everyday functions. The results are discussed in the context of the broader issue of the roles of neural proximity and functional equivalence in shaping the reorganization of primary and association cortices in the absence of relevant sensorimotor experience.
Results
To test the specificity of plasticity in the sensorimotor hand areas in people born without arms or hands (SI Appendix, Table S1), we used an active motor paradigm, designed to activate both primary somatosensory and primary motor cortices, similar to previous studies of reorganization (refs. 12 and 26–29; for supporting findings in a passive somatosensory stimulation design, see below). We scanned five dysplasic subjects, as well as a control group, as they performed simple flexing movements of different body parts. The body parts chosen for this experiment included the hands (in the controls, used as a localizer) and the feet, which the dysplasic subjects use to overcome their disability. Our study participants, according to self-report, rely largely on their dexterous feet (dominantly their right foot; all were right-footed) to perform daily typical manual activities. Their feet are extraordinarily dexterous, allowing them to use cell phones, utensils and nearly all other everyday tools (see list at SI Appendix, Table S2). In a questionnaire of tool use, all of the dysplasic subjects reported using their lower limbs for most of the tools they have used (SI Appendix, Fig. S1). Foot tool use accounted for a minimum of 92% of the used tools, although few tools were jointly manipulated by the lower face or remaining upper limbs in specific individuals (SI Appendix, Fig. S1 and Table S1). Additionally, we inspected movement of the shoulder and lips, expected to activate neighboring cortical regions on both sides of the missing hand territory, as well as a control body part, which is not an immediate cortical neighbor of the hand and is not used compensatorily to replace hand function as a dexterous effector: the abdomen. While abdominal core muscles may be used to stabilize the body and may thus be used in excess by the dysplasics while using their feet, they cannot be used to replace hand function.
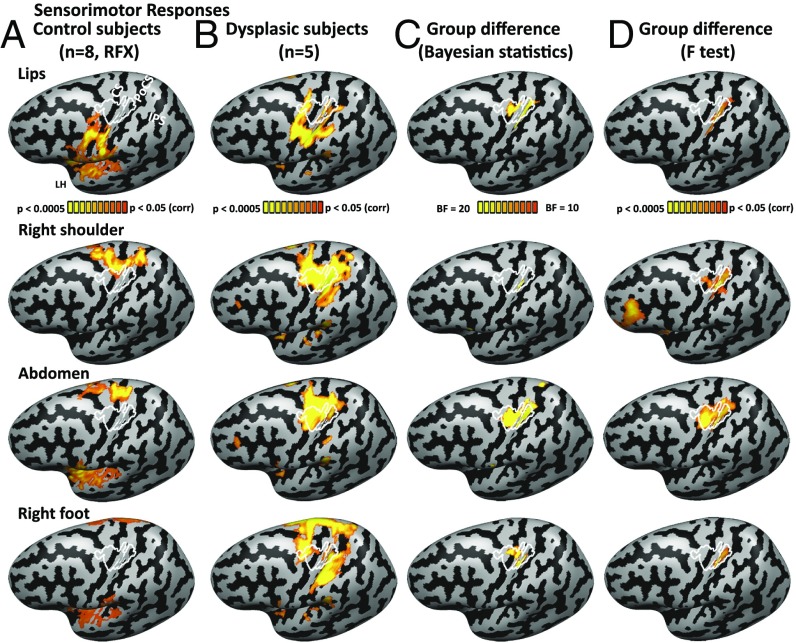
In the control group, this protocol resulted in a typical somatotopic activation pattern with sensorimotor responses along a superior-inferior axis for the foot, abdomen, shoulder, hand, and lips movements (Fig. 1A; hand peak is delineated in white), replicating the known Penfield homunculus (30). Topographic responses were found along the central sulcus, postcentral gyrus, and postcentral sulcus, covering Brodmann areas 3a, 3b, and 1 (31), and extending anteriorly from the central sulcus also to the precentral gyrus, covering areas 4a and 4p (32) and potentially parts of the premotor cortex. In the dysplasic subjects, although the peak responses remained topographic along the primary sensorimotor cortex, movement in every tested body part extended toward the deprived area and generated some activation in the hand region (Fig. 1B). This also included increased activation of the primary hand area while they moved their right foot, used by these subjects to perform typically manual actions, as reported before (11, 12). Indeed, plotting the group differences showed that the sensorimotor hand area had stronger activation in the dysplasic subjects than in the controls for moving the various body parts, including the foot (Fig. 1 C and D).
Fig. 1.
Activation for multiple body parts in the sensorimotor hand area in dysplasic subjects (born without hands). (A) The activation for body part movement (contraction of the lips, right shoulder, abdomen, and right foot) in the typically developed control subject group (random effect GLM analysis; P < 0.05 corrected for multiple comparisons) is shown on the left cortical hemisphere, following the standard Penfield homunculus. The sensorimotor hand area, delineated in white, represents the core area activated by right hand movement in all of the control participants (each at P < 0.05 corrected). This area encompasses both primary somatosensory and motor areas and extends beyond them to the postcentral sulcus posteriorly and precentral gyrus anteriorly. CS, central sulcus; IPS, intraparietal sulcus; PoCS, postcentral sulcus. (B) Activation for body part movement is shown for the dysplasic individuals, born without hands (fixed effect GLM analysis, P < 0.05 corrected; see SI Appendix, Table S1 for subject upper limb structure). Movement of each of the tested body parts elicited activation in the hand-selective area to some extent, including, as previously reported, movement of the right foot. (C and D) Group difference in activation of body part movement is shown in both Bayesian analysis (C; BF10 > 10 represents strong evidence for the existence of a group difference) and frequentist statistics (D; mixed effects F test). The sensorimotor hand area is activated more by the dysplasic group for multiple body parts. Group differences extend somewhat more inferiorly in the posterior somatosensory cortex (postcentral gyrus) compared with the motor cortex, in accordance with a larger cortical representation to the upper limbs in this area (82).
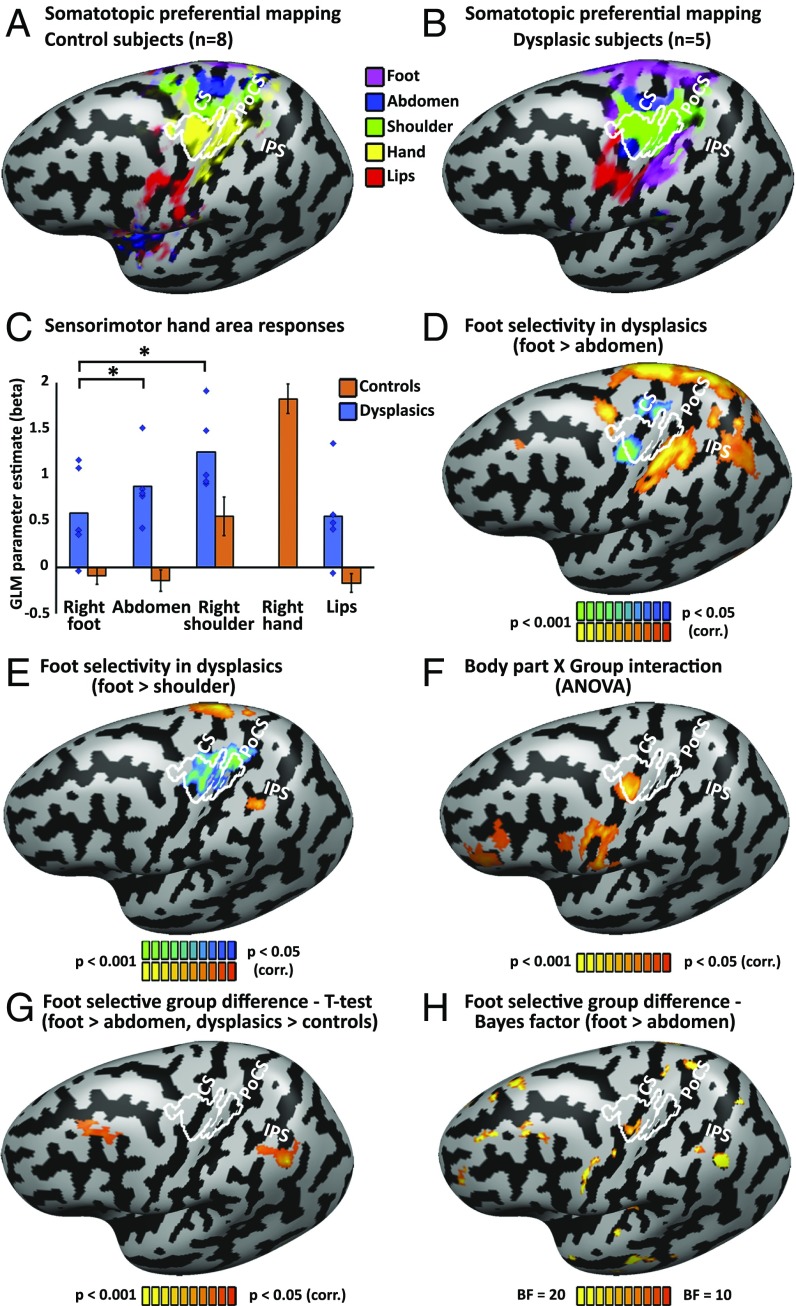
When exploring selectivity of sensorimotor responses, plotting the preferential activation per cortical vertex (in a winner-takes-all approach; Fig. 2 A and B), the dysplasic subjects show a preference for shoulder and abdomen movements in the typical hand area (Fig. 2B; hand area delineated white), which seem to have been displaced and expanded toward the hand area compared with the activation pattern in the controls. This reorganization for the shoulder appears to be similar across the sensory and motor cortices, although a preference for the abdomen is found more anteriorly, in the central sulcus itself, likely originating from the motor cortex. These patterns were consistent across the individual subjects (SI Appendix, Fig. S2) and found also in the right hemisphere for movement of the contralateral body parts (see SI Appendix, Fig. S3 for comparable analyses of the right hemisphere). We further sampled the response pattern within the hand region of interest (ROI). This ROI was defined as significant overlapping activation for hand movement, compared with baseline, in all control subjects, covering both primary sensory and motor cortex. The activation in the dysplasics’ primary hand region is strongest for the movement of the shoulder, which is proximal to the missing hand (and therefore also activates the hand region in controls; see also Figs. 1A and 2C), but is also significant for the abdomen. Crucially, moving the foot does not generate strong significant activation in the hand area at large (Fig. 2C). Despite the preferential compensatory use of the right foot in these subjects, movements of the foot do not seem to favorably overtake this region. In fact, movement of the shoulder and abdomen activated the hand region in the dysplasics significantly more than foot movement (P < 0.005 for both). Similarly, in a whole brain analysis, when contrasting moving the foot compared with the noncompensatory but more proximal abdomen, the dysplasic subjects do not show significant activation in their primary sensorimotor hand area, and activity was found for the reverse contrast (Fig. 2D; see SI Appendix, Fig. S5 A and C for unsmoothed individual subjects data and overlap analysis).
Fig. 2.
Selectivity for the compensatorily used foot in the dysplasics is found in associative somatosensory cortex, but not primary sensorimotor hand area. (A) Preferred body part responses for contraction movements (winner-takes-all approach) for the control subjects follows the standard Penfield homunculus. The sensorimotor hand area is delineated in white, representing the core area activated by right hand movement in all of the control participants (each at P < 0.05 corrected), as in Fig. 1. For a presentation on grooved (uninflated) cortical reconstruction, see SI Appendix, Fig. S6B, and for an overlay of probabilistic cytoarchitectonic parcellation, see SI Appendix, Fig. S7B. CS, central sulcus; IPS, intraparietal sulcus; PoCS, postcentral sulcus. (B) Preferred body part responses for contraction movements for the dysplasic group shows a preference for shoulder (and to some extent abdomen, in the motor cortex; see below for details) movements in the hand area, despite the extensive use of the feet to perform typically manual fine-motor tasks. Preferential activation for abdomen movement was found also on the anterior inferior border of the hand region, in the central sulcus, in agreement with evidence of a potential discontinuity in the motor cortex surrounding the hand area (83, 84). This abdomen preference is not found in passive tactile stimulation of the body; compare with SI Appendix, Fig. S4. Extensive preference for the foot is found outside the primary sensorimotor hand area, in the depth of the postcentral sulcus (potentially area BA 2) and extending to the aIPS. (C) Sensorimotor responses were sampled from the hand area, showing that this region in the dysplasics is more activated by proximal body parts (shoulder and abdomen/trunk) than by foot movements (P < 0.005 for both comparisons). Error bars for the control group (orange bars) represent SEM. Individual data points (blue diamonds) are presented for the five dysplasic individuals in addition to the group average. (D) Foot movement selectivity (over abdomen movement, representing a control body part that does not serve compensatorily as an effector) in the dysplasics can be found in the postcentral sulcus, intraparietal sulcus, superior parietal lobule, and premotor cortex (PMd), but not in the hand primary sensorimotor cortex, which shows the reverse preference. (E) Movement selectivity comparing the shoulder and foot in the dysplasics shows a robust preference to shoulder movement (a proximal, noncompensatory body part), rather than to foot movement, in the hand area. A preference for the foot is found in the aIPS. (F) Overall body part selectivity (comparing movement of all shared body parts; e.g., lips, shoulder, abdomen, and foot) differs between the dysplasics and controls (ANOVA body part x group interaction) in the inferior frontal lobe (including PMv) and in the sensorimotor hand area. (G) A direct comparison of the selectivity to right foot movement (vs. abdomen movement) between the dysplasics and control subjects shows potential for plasticity specific to the compensatorily used foot in the association cortices, in the inferior parietal lobule, in aIPS extending to the angular/supramarginal gyri, as well as the middle frontal gyrus, but not in the primary sensorimotor cortex. (H) Bayes factor (BF10) for difference between the groups in their differential activation to right foot movement (vs. abdomen movement) is shown. The dysplasics show different selectivity level for right foot movement compared with the controls in various cortical loci, including the sensorimotor hand area. However, the group difference found in the primary sensorimotor hand area in this analysis reflects a preference in the dysplasics group toward the abdomen movement (compare with D).
Even more robustly, whole brain analysis contrasting foot movement to shoulder movement shows extensive significant selectivity for shoulder movement in the primary hand area of the dysplasics (Fig. 2E and SI Appendix, Fig. S5 B and D for individual subjects’ data and overlap analysis). Preference for the shoulder in the hand area was also found in the somatosensory cortex in a passive somatosensory stimulation experiment (see SI Appendix, Fig. S4 for right and left hemispheres; preference for the abdomen in the anterior motor hand area was not found for passive stimulation; compare with Fig. 2B).
This does not mean that no foot selectivity exists in these subjects: When directly contrasting foot movement to movement of the abdomen in the dysplasic subjects (Fig. 2D), activation is found not only in the primary sensorimotor cortex foot region, which extends laterally from that of the controls, but also in the superior and inferior parietal lobule and posterior superior frontal sulcus. In the more stringent contrast (foot vs. shoulder), a significant cluster is found in the anterior intraparietal sulcus (aIPS), on its border with the postcentral sulcus. Overlaying our findings on a cytoarchitectonic parcellation probabilistic atlas (33–35) places this cluster on the aIPS at the border of Brodmann area 2 and area PFt in the inferior parietal lobule (see SI Appendix, Fig. S6 for findings overlaid on uninflated cortical reconstructions and SI Appendix, Fig. S7 for an overlay on the cytoarchitectonic parcellation probabilistic atlas).
Body part selectivity differs between the dysplasics and controls also in the frontal lobe (including PMv) and in the sensorimotor hand area itself (ANOVA Body part X Group interaction; Fig. 2F). Both frequentist (Fig. 2G) and Bayesian statistics (Fig. 2H) of foot-selectivity group difference shows additional regions that may be related to compensatory use in the frontal (middle frontal gyrus) and parietal lobes. The inferior parietal cluster extends along the intraparietal sulcus (SI Appendix, Fig. S6), over the angular/supramarginal gyri and overlaps several cytoarchitectonic areas, including hIP1 and hIP2 (SI Appendix, Fig. S7). The more sensitive Bayesian analysis hints at group effects also in additional foci and in the temporal lobe, but these may require verification from additional studies, perhaps with increased group size. Therefore, plasticity related to extensive foot use in the dysplasics is found in the association parietal cortex, in the inferior parietal lobule (aIPS), and not in the primary sensorimotor hand area.
We also inspected whether functional connectivity (FC) may reflect use-based plasticity within the primary sensorimotor cortex and in the association cortices. The group differences in FC from the hand area across the entire cortex showed FC differences only in the association cortex, in the middle intraparietal sulcus (mIPS; SI Appendix, Fig. S8).
Discussion
We tested people born without hands who use their feet to perform everyday manual tasks. We found that their primary sensorimotor hand area is activated by foot movements more than in typically developed control subjects (Fig. 1), replicating previous findings of plasticity in congenital limb absence (11, 12, 15). However, in contrast to previous research, we additionally tested for the selectivity of the compensatorily used effector and found that the hand area shows no foot selectivity. Instead, the hand area is significantly more activated by proximal but noncompensatory body parts, such as the shoulder and abdomen, which are not used as dexterous effectors (Fig. 2). In the association sensorimotor cortex, we found evidence for compensatory plasticity specific to the foot. Foot selectivity in the aIPS and inferior parietal lobule (Fig. 2 D, E, and G) was found exclusively in the dysplasic participants.
Past findings of primary sensorimotor plasticity in people born without hands were thought to reflect use-dependent, compensatory plasticity for sensorimotor loss (11, 12, 15). Those results were interpreted as evidence that the primary sensorimotor cortex shows functional selectivity for performance of tasks typically conducted with the hands, and not necessarily for the specific body part (12). This interpretation builds on a model based on the organization of association sensory cortex in congenital blindness and deafness. In these cases, the role of association sensory cortices appears to be defined not strictly by their typical primary sensory input (e.g., vision) but by their function. This was demonstrated for high-level visual cortex in the blind where domain selectivity was found for distinct object domains, including artifacts, body parts, and scenes (36–42) with nonvisual stimuli. Similar results were obtained for nonvisual spatial localization (42) and motion perception (43). Analogous results of retained functional specialization independent of modality of input have been found for the deaf (44–46). These findings encouraged the view that the observation of seemingly compensatory reorganization in people born with partial dysplasia is driven by similarity of function as opposed to a topographically driven specialization in early sensorimotor cortex (12). Our findings of significant plasticity showing preference for body parts not used as effectors instead of compensatory foot selectivity, even in the complete absence of hands and in individuals who show strong compensatory strategies with foot use, point to important limitations of the function-specific model of primary sensorimotor cortex.
The case of primary sensorimotor cortex in dysplasics differs in several respects from the findings in the blind and deaf. Beyond the different senses, these cases represent different stations of the cortical processing hierarchy. Specifically, in the blind and deaf, claims of functionally selective organization are limited to the associative sensory cortices, whereas plasticity in people born without hands was tested for the primary sensory-motor cortex. Therefore, while it may be that functionally driven plasticity principles could govern the reorganization of higher sensorimotor cortices, they do not seem to apply to the first cortical stations involved in processing touch and movement, which is governed by topographic mapping of the body. Consistent with these contrasting effects, we found foot selectivity in the dysplasics in the association parietal cortex, which may be indicative of compensatory plasticity and effector-function organization.
An additional difference between these cases is the extent of the deprivation of the sensory-motor modality. In complete blindness or deafness, there are no competing inputs within the sensory modality in the early sensory cortices. Therefore, while the functional role of these regions is still debated (47–49), the topographic organization in the early stations of the hierarchy is retained (50–52). In higher stations of cortical processing, the visual and auditory cortices also receive inputs from other modalities and from downstream cortical stations via feedback connectivity, which become more dominant in the complete absence of visual input. These may then drive cortex organization toward similar functions and domains even in the absence of the typical visual/auditory features driving these regions. However, the case of absence of one body part leaves intact inputs from proximal body parts in the topographic organization, generating within-modality competition and overtake. In the somatosensory cortex, topographic overtake is mediated through horizontal, direct, and indirect (transsynaptic) connections (53) as well as from close body parts in subcortical nuclei. Moreover, in both motor and somatosensory cortex, there is overlap of the fields for different, close-by body parts (1, 53). This overlap is evident in our data: The hand area of control subjects is significantly activated as they move their shoulder (Fig. 2C), causing a limited activation difference between the groups for shoulder movement (Fig. 1C).
The ability to evoke plasticity and takeover by nearby body parts has been confirmed in multiple studies of the effects of adult-stage and even early-life amputation or deafferentation (1–10, 54). Related findings of lesion-induced map plasticity and takeover in early sensory cortical areas following sensory loss in adulthood were also found for audition following cochlear lesions (resulting in tinnitus; ref. 55) and for vision following retinal lesions (56). Additionally, use-dependent plasticity in adults learning a new motor skill also allows for the expansion of relevant body parts (57, 58). It follows naturally that the same level of plasticity is possible in earlier development, and in the case of congenital limb absence, even without being strongly driven by extreme compensatory use. Importantly, topographical intramodal connectivity appears to more strongly drive the primary sensorimotor cortex than inputs from other functionally significant downstream stations or topographically remote body parts such as the foot, which could direct it toward compensatory roles. While some connectivity can be modulated to permit innervation from remote body parts in cases of deafferentation (6), our data suggest it is not sufficient, even in congenital bilateral upper limb dysplasia, to evoke preferential takeover. Thus, the current study reveals clear limitations for brain compensatory plasticity and for the role of experience in modifying brain organization, and highlights the topographical nature of the early sensory and motor cortices.
In contrast to the results for primary sensorimotor cortex, we found increased selectivity for the foot in dysplasics’ association cortex. Specifically, we found selectivity for foot movement compared with movement of the abdomen and shoulder in parts of the inferior parietal lobule, mainly in the anterior IPS area, bordering the postcentral sulcus and, to some extent, in the angular/supramarginal gyri (Fig. 2 B, D, and E). We also found increased FC from the primary sensorimotor hand area to IPS, albeit in a more posterior region, in mIPS (SI Appendix, Fig. S8B). The strongest result was found in the aIPS and, inferiorly to it, in the inferior parietal lobule, falling with high probability within several cytoarchitectonic areas, from BA2 to hIP2 and PFt. Electrophysiological data in nonhuman primates and fMRI data in humans have shown that these regions support various types of manual behaviors. These include visually guided object manipulation, tool use and grasping movements (23, 25, 59–62), action observation and imitation (63), and additional more abstract action representations of function and goal (64, 65). Therefore, this region’s motor selectivity for the foot may be functional and related to compensatory use, linking it to a role in representing effector-invariant/effector-independent action; i.e., related to any body part used as an effector.
Whereas several studies have shown effector-invariant action representations in the parietal lobe with regard to hand laterality and correspondence between hand and eye (66–69), generally in mIPS and pIPS, aIPS shows effector selectivity for the hand, compared with foot or eye movements (70). Importantly, aIPS appears to support grasping actions not only with the hands but also with tools (71–74). Our findings would therefore suggest that this region’s role may have to do with the use of the hands or tools as effectors, enabling it to also extend its selectivity to the foot, when it is used as an effector throughout life.
Thus, the current study shows a clear division in organization principles between the primary, topographic cortices, and the association cortex. We find that these regions differ in their compensatory plasticity and in the manner in which experience can affect their organization. In the primary sensorimotor cortex, we find that similarly to the lesion-induced map plasticity in late-onset animal and human studies, even congenital deprivation and compensatory use cannot overcome neuroanatomical constraints such as topographical proximity and connectivity, which determine the functional development of these brain regions. In contrast, functional specialization occurs at relatively high levels of representation in the parietal lobe of the dysplasics. Analogously to the case of the association cortices of the blind and deaf, the aIPS in dysplasics appears to show effector-invariant plasticity and selectivity—to the foot in this case. This suggests its role extends beyond the hand to support object manipulation with other bodily effectors.
Methods
Participants.
Five individuals born with severely shortened or completely absent upper limbs (individuals with upper limb dysplasia; dysplasics 1–5), and eight typically developed control subjects, matched for age (no group difference; P < 0.25) participated in the experiment. The causes of dysplasia were genetic, teratogenic medications (thalidomide), or unknown. See SI Appendix, Table S1 and Supplemental Methods for the summary of the characteristics of the dysplasics, as well as images of their residual limbs. None of the dysplasics had a history of phantom limb sensations or movements, and all were adept at performing everyday actions and tool use with their feet (see SI Appendix, Table S2). All of the dysplasic participants used their right foot dominantly. None of participants had a history of psychiatric or neurological disorder. They gave written informed consent in accordance with the institutional review board of Harvard University, which approved all of the experiments.
Experimental Design.
The motor experiment was carried out in a block design fMRI experiment. Mouth, abdomen and either side hands (for the control subjects), shoulders, and feet were moved (simple flexing/contraction movement) in separate blocks (6 s movement and 6 s rest) in randomized order according to an auditory cue (metronome). Four flex and relax movements were performed in each block at a frequency of 0.66 Hz. Due to our focus on the compensatory use of the feet, and as all of the dysplasic participants were dominantly right-footed, we used the movements of the right hand and foot for further examination and provide evidence for similar organization of the right hemisphere in response to left hand and foot movement in SI Appendix, Fig. S3. A supplementary somatosensory experiment with four of the five dysplasic subjects was carried out in a block design fMRI experiment (see acquisition detail and detail of the paradigm in SI Appendix, Supplemental Methods).
Functional Imaging.
The blood oxygen level-dependent (BOLD) fMRI measurements were obtained in a Siemens Trio 3-T scanner at the Center for Brain Science at Harvard University. For acquisition detail, see SI Appendix, Supplemental Methods. The main experiment had three runs of 186 whole-brain images each collected in one functional scan. Separate 3D recordings were used for coregistration and surface reconstruction.
Data analysis was performed using the Brain Voyager QX 2.8 software package (Brain Innovation) using standard preprocessing procedures (SI Appendix, Supplemental Methods). Functional and anatomical datasets for each subject were aligned and fit to standardized Talairach space (75). Anatomical cortical reconstruction procedures included the segmentation of the white matter using a grow-region function embedded in Brain Voyager. The Talairach normalized cortical surface was then used for surface-based alignment conducted across the subjects according to their cortical curvature (sulci and gyri) patterns. All further analyses were conducted in cortical space. Single-subject data were spatially smoothed with a 2D 4 vertex full-width at half-maximum Gaussian to reduce intersubject anatomical variability for group analysis. Unsmoothed data are presented at the single-subjects cortical level (SI Appendix, Figs. S2 and S5). Due to the small sample size of the unique dysplasic group, analyses were based on converging evidence from the group analyses (general linear model; GLM; Figs. 1 A and B and 2 A, B, D, and E and SI Appendix, Fig. S3 A, B, D, and E), single-subject level (SI Appendix, Figs. S2 and S5), mixed effects ANOVA (Figs. 1D and 2 F and G and SI Appendix, Fig. S3 F and H), and Bayesian analysis (Figs. 1C and 2H and SI Appendix, Fig. S3G) to enable an assessment of the consistency of the findings. The minimum significance level of the results presented in this study (both individual and group level analyses) was set at P < 0.05, corrected for multiple comparisons, using the spatial extent method, a set-level statistical inference correction (76).
The hand sensorimotor cortex (delineated in white in Figs. 1 and 2) was defined according to a full overlap (100%) of activation to right hand flexing across all control subjects (each at P < 0.05 corrected) to overcome potential individual biases. This region was further used to sample movement responses in each group (Fig. 2C) and as a seed to compute FC (SI Appendix, Fig. S8). Somatotopic preferential mapping was computed at a surface level for each dysplasic subject (SI Appendix, Fig. S2). Each cortical vertex is colored based on the body part whose movement elicited the highest activation (GLM estimate, beta value).
Group analyses in the control group were conducted in a hierarchical random effects analysis (RFX GLM; ref. 77) and, in the dysplasics, a fixed-effect GLM was implemented, due to the group size.
For group level somatotopic preferential mapping, each cortical vertex is colored based on the body part whose movement elicited the highest average activation across the group (Fig. 2 A and B). The same analyses were performed for the somatosensory supplemental experiment (SI Appendix, Fig. S4). Group analyses are presented on the Colin27 brain inflated (or folded; SI Appendix, Fig. S6) cortices, to which individual surface (cortical) data were aligned based on the curvature patterns. To link our findings to the anatomic characterization of these regions, activation for some of the contrasts was overlaid on the probabilistic cytoarchitectonic atlas (33–35, 78) (SI Appendix, Figs. S6 and S7). All areas defined by these maps are shown at 40% probability of belonging to this cytoarchitectonic region.
Group comparisons were conducted using both frequentist (t test and mixed effects ANOVA; with group and body part factors; Figs. 1D and 2F and SI Appendix, Fig. S3F) and sensitive Bayesian analyses (refs. 79 and 80, Figs. 1C and 2H and SI Appendix, Figs. S3G and S8B), appropriate for testing small samples of unique populations and patients. See detail of the Bayesian analyses in SI Appendix, Supplemental Methods. Bayes factor (BF) of over 3 is considered substantial evidence and BF over 10 is considered strong evidence against the null hypothesis (79), in our case suggesting a group difference. The data generated and analyzed for the current study are available from the corresponding author upon request.
FC.
A dataset of spontaneous BOLD fluctuations for the investigation of intrinsic (rest state; ref. 81) FC was collected while the subjects lay in the scanner with no external stimulation or task. For details, see SI Appendix, Supplemental Methods.
Supplementary Material
Acknowledgments
We thank the dysplasic subjects who participated in our experiments, Himanshu Bhat and Thomas Benner of Siemens Healthcare for the simultaneous multislice echo-planar imaging (SMS-EPI) sequence, Steven Cauley of Massachusetts General Hospital for modifications that enabled implementation of our protocols in a routine session, and Tamar Makin for her helpful comments on an earlier version of the manuscript. This work was supported by Società Scienze Mente Cervello–Fondazione Cassa di Risparmio di Trento e Rovereto, a grant from the Provincia Autonoma di Trento, and a Harvard Provostial postdoctoral fund (to A.C.), and by the European Union’s Horizon 2020 Research and Innovation Programme under Marie Sklodowska-Curie Grant Agreement 654837 and the Israel National Postdoctoral Award Program for Advancing Women in Science (to E.S.-A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1803926115/-/DCSupplemental.
References
- 1.Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- 2.Hall EJ, Flament D, Fraser C, Lemon RN. Non-invasive brain stimulation reveals reorganized cortical outputs in amputees. Neurosci Lett. 1990;116:379–386. doi: 10.1016/0304-3940(90)90105-i. [DOI] [PubMed] [Google Scholar]
- 3.Pons TP, et al. Massive cortical reorganization after sensory deafferentation in adult macaques. Science. 1991;252:1857–1860. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- 4.Merzenich MM, et al. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983;8:33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- 5.Kaas JH, Merzenich MM, Killackey HP. The reorganization of somatosensory cortex following peripheral nerve damage in adult and developing mammals. Annu Rev Neurosci. 1983;6:325–356. doi: 10.1146/annurev.ne.06.030183.001545. [DOI] [PubMed] [Google Scholar]
- 6.Jain N, Qi H-X, Collins CE, Kaas JH. Large-scale reorganization in the somatosensory cortex and thalamus after sensory loss in macaque monkeys. J Neurosci. 2008;28:11042–11060. doi: 10.1523/JNEUROSCI.2334-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schieber MH, Deuel RK. Primary motor cortex reorganization in a long-term monkey amputee. Somatosens Mot Res. 1997;14:157–167. doi: 10.1080/08990229771024. [DOI] [PubMed] [Google Scholar]
- 8.Flor H, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375:482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- 9.Philip BA, Frey SH. Compensatory changes accompanying chronic forced use of the nondominant hand by unilateral amputees. J Neurosci. 2014;34:3622–3631. doi: 10.1523/JNEUROSCI.3770-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makin TR, et al. Deprivation-related and use-dependent plasticity go hand in hand. eLife. 2013;2:e01273. doi: 10.7554/eLife.01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoeckel MC, Seitz RJ, Buetefisch CM. Congenitally altered motor experience alters somatotopic organization of human primary motor cortex. Proc Natl Acad Sci USA. 2009;106:2395–2400. doi: 10.1073/pnas.0803733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahamy A, et al. Representation of multiple body parts in the missing hand territory of congenital onehanders. Curr Biol. 2017;27:1350–1355. doi: 10.1016/j.cub.2017.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoeckel MC, Jörgens S, Witte OW, Seitz RJ. Reduced somatosensory hand representation in thalidomide-induced dysmelia as revealed by fMRI. Eur J Neurosci. 2005;21:556–562. doi: 10.1111/j.1460-9568.2005.03866.x. [DOI] [PubMed] [Google Scholar]
- 14.Stoeckel MC, Pollok B, Witte OW, Seitz RJ, Schnitzler A. Shrinkage of somatosensory hand area in subjects with upper extremity dysmelia revealed by magnetoencephalography. J Neurophysiol. 2005;93:813–818. doi: 10.1152/jn.00749.2004. [DOI] [PubMed] [Google Scholar]
- 15.Stoeckel MC, Pollok B, Schnitzler A, Witte OW, Seitz RJ. Use-dependent cortical plasticity in thalidomide-induced upper extremity dysplasia: Evidence from somaesthesia and neuroimaging. Exp Brain Res. 2004;156:333–341. doi: 10.1007/s00221-003-1794-9. [DOI] [PubMed] [Google Scholar]
- 16.Crawford JD, Henriques DY, Medendorp WP. Three-dimensional transformations for goal-directed action. Annu Rev Neurosci. 2011;34:309–331. doi: 10.1146/annurev-neuro-061010-113749. [DOI] [PubMed] [Google Scholar]
- 17.Fattori P, Gamberini M, Kutz DF, Galletti C. ‘Arm-reaching’ neurons in the parietal area V6A of the macaque monkey. Eur J Neurosci. 2001;13:2309–2313. doi: 10.1046/j.0953-816x.2001.01618.x. [DOI] [PubMed] [Google Scholar]
- 18.Ferraina S, et al. Combination of hand and gaze signals during reaching: Activity in parietal area 7 m of the monkey. J Neurophysiol. 1997;77:1034–1038. doi: 10.1152/jn.1997.77.2.1034. [DOI] [PubMed] [Google Scholar]
- 19.Dickinson AR, Calton JL, Snyder LH. Nonspatial saccade-specific activation in area LIP of monkey parietal cortex. J Neurophysiol. 2003;90:2460–2464. doi: 10.1152/jn.00788.2002. [DOI] [PubMed] [Google Scholar]
- 20.Calton JL, Dickinson AR, Snyder LH. Non-spatial, motor-specific activation in posterior parietal cortex. Nat Neurosci. 2002;5:580–588. doi: 10.1038/nn0602-862. [DOI] [PubMed] [Google Scholar]
- 21.Kaas JH, Stepniewska I, Gharbawie O. Cortical networks subserving upper limb movements in primates. Eur J Phys Rehabil Med. 2012;48:299–306. [PMC free article] [PubMed] [Google Scholar]
- 22.Fogassi L, Luppino G. Motor functions of the parietal lobe. Curr Opin Neurobiol. 2005;15:626–631. doi: 10.1016/j.conb.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Grefkes C, Fink GR. The functional organization of the intraparietal sulcus in humans and monkeys. J Anat. 2005;207:3–17. doi: 10.1111/j.1469-7580.2005.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaas JH, Gharbawie OA, Stepniewska I. The organization and evolution of dorsal stream multisensory motor pathways in primates. Front Neuroanat. 2011;5:34. doi: 10.3389/fnana.2011.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vingerhoets G. Contribution of the posterior parietal cortex in reaching, grasping, and using objects and tools. Front Psychol. 2014;5:151. doi: 10.3389/fpsyg.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makin TR, Scholz J, Henderson Slater D, Johansen-Berg H, Tracey I. Reassessing cortical reorganization in the primary sensorimotor cortex following arm amputation. Brain. 2015;138:2140–2146. doi: 10.1093/brain/awv161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lotze M, Flor H, Grodd W, Larbig W, Birbaumer N. Phantom movements and pain. An fMRI study in upper limb amputees. Brain. 2001;124:2268–2277. doi: 10.1093/brain/124.11.2268. [DOI] [PubMed] [Google Scholar]
- 28.MacIver K, Lloyd DM, Kelly S, Roberts N, Nurmikko T. Phantom limb pain, cortical reorganization and the therapeutic effect of mental imagery. Brain. 2008;131:2181–2191. doi: 10.1093/brain/awn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foell J, Bekrater-Bodmann R, Diers M, Flor H. Mirror therapy for phantom limb pain: Brain changes and the role of body representation. Eur J Pain. 2014;18:729–739. doi: 10.1002/j.1532-2149.2013.00433.x. [DOI] [PubMed] [Google Scholar]
- 30.Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- 31.Geyer S, Schleicher A, Zilles K. Microstructural Organization and Interindividual Variability Areas 3a, 3b, and 1 of human primary somatosensory cortex. Neuroimage. 1999;10:63–83. doi: 10.1006/nimg.1999.0440. [DOI] [PubMed] [Google Scholar]
- 32.Geyer S, et al. Two different areas within the primary motor cortex of man. Nature. 1996;382:805–807. doi: 10.1038/382805a0. [DOI] [PubMed] [Google Scholar]
- 33.Zilles K, Amunts K. Centenary of Brodmann’s map–Conception and fate. Nat Rev Neurosci. 2010;11:139–145. doi: 10.1038/nrn2776. [DOI] [PubMed] [Google Scholar]
- 34.Amunts K, Schleicher A, Zilles K. Cytoarchitecture of the cerebral cortex–More than localization. Neuroimage. 2007;37:1061–1065. doi: 10.1016/j.neuroimage.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 35.Zilles K, Schleicher A, Palomero-Gallagher N, Amunts K. Brain Mapping: The Methods. 2nd Ed. Elsevier; San Diego: 2002. Quantitative analysis of cyto-and receptor architecture of the human brain; pp. 573–602. [Google Scholar]
- 36.Mahon BZ, Caramazza A. What drives the organization of object knowledge in the brain? Trends Cogn Sci. 2011;15:97–103. doi: 10.1016/j.tics.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heimler B, Striem-Amit E, Amedi A. Origins of task-specific sensory-independent organization in the visual and auditory brain: Neuroscience evidence, open questions and clinical implications. Curr Opin Neurobiol. 2015;35:169–177. doi: 10.1016/j.conb.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Thaler L, Goodale MA. Echolocation in humans: An overview. Wiley Interdiscip Rev Cogn Sci. 2016;7:382–393. doi: 10.1002/wcs.1408. [DOI] [PubMed] [Google Scholar]
- 39.Bi Y, Wang X, Caramazza A. Object domain and modality in the ventral visual pathway. Trends Cogn Sci. 2016;20:282–290. doi: 10.1016/j.tics.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Ricciardi E, Handjaras G, Pietrini P. The blind brain: How (lack of) vision shapes the morphological and functional architecture of the human brain. Exp Biol Med (Maywood) 2014;239:1414–1420. doi: 10.1177/1535370214538740. [DOI] [PubMed] [Google Scholar]
- 41.Bock AS, Fine I. Anatomical and functional plasticity in early blind individuals and the mixture of experts architecture. Front Hum Neurosci. 2014;8:971. doi: 10.3389/fnhum.2014.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Renier L, De Volder AG, Rauschecker JP. Cortical plasticity and preserved function in early blindness. Neurosci Biobehav Rev. 2014;41:53–63. doi: 10.1016/j.neubiorev.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ptito M, Matteau I, Gjedde A, Kupers R. Recruitment of the middle temporal area by tactile motion in congenital blindness. Neuroreport. 2009;20:543–547. doi: 10.1097/WNR.0b013e3283279909. [DOI] [PubMed] [Google Scholar]
- 44.Lomber SG. What is the function of auditory cortex when it develops in the absence of acoustic input? Cogn Dev. 2017;42:49–61. [Google Scholar]
- 45.Bola Ł, et al. Task-specific reorganization of the auditory cortex in deaf humans. Proc Natl Acad Sci USA. 2017;114:E600–E609. doi: 10.1073/pnas.1609000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Striem-Amit E, Vannuscorps G, Caramazza A. Sensorimotor-independent development of hands and tools selectivity in the visual cortex. Proc Natl Acad Sci USA. 2017;114:4787–4792. doi: 10.1073/pnas.1620289114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bedny M. Evidence from blindness for a cognitively pluripotent cortex. Trends Cogn Sci. 2017;21:637–648. doi: 10.1016/j.tics.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Amedi A, Hofstetter S, Maidenbaum S, Heimler B. Task selectivity as a comprehensive principle for brain organization. Trends Cogn Sci. 2017;21:307–310. doi: 10.1016/j.tics.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 49.Campus C, Sandini G, Concetta Morrone M, Gori M. Spatial localization of sound elicits early responses from occipital visual cortex in humans. Sci Rep. 2017;7:10415. doi: 10.1038/s41598-017-09142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Striem-Amit E, et al. Topographical functional connectivity patterns exist in the congenitally, prelingually deaf. Sci Rep. 2016;6:29375. doi: 10.1038/srep29375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Striem-Amit E, et al. Functional connectivity of visual cortex in the blind follows retinotopic organization principles. Brain. 2015;138:1679–1695. doi: 10.1093/brain/awv083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bock AS, et al. Resting-state retinotopic organization in the absence of retinal input and visual experience. J Neurosci. 2015;35:12366–12382. doi: 10.1523/JNEUROSCI.4715-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schieber MH. Constraints on somatotopic organization in the primary motor cortex. J Neurophysiol. 2001;86:2125–2143. doi: 10.1152/jn.2001.86.5.2125. [DOI] [PubMed] [Google Scholar]
- 54.Ramachandran VS, Rogers-Ramachandran D, Stewart M. Perceptual correlates of massive cortical reorganization. Science. 1992;258:1159–1160. doi: 10.1126/science.1439826. [DOI] [PubMed] [Google Scholar]
- 55.Rauschecker JP. Auditory cortical plasticity: A comparison with other sensory systems. Trends Neurosci. 1999;22:74–80. doi: 10.1016/s0166-2236(98)01303-4. [DOI] [PubMed] [Google Scholar]
- 56.Kaas JH, et al. Reorganization of retinotopic cortical maps in adult mammals after lesions of the retina. Science. 1990;248:229–231. doi: 10.1126/science.2326637. [DOI] [PubMed] [Google Scholar]
- 57.Karni A, et al. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- 58.Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Janssen P, Scherberger H. Visual guidance in control of grasping. Annu Rev Neurosci. 2015;38:69–86. doi: 10.1146/annurev-neuro-071714-034028. [DOI] [PubMed] [Google Scholar]
- 60.Martel M, Cardinali L, Roy AC, Farnè A. Tool-use: An open window into body representation and its plasticity. Cogn Neuropsychol. 2016;33:82–101. doi: 10.1080/02643294.2016.1167678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goldenberg G, Spatt J. The neural basis of tool use. Brain. 2009;132:1645–1655. doi: 10.1093/brain/awp080. [DOI] [PubMed] [Google Scholar]
- 62.Tarhan LY, Watson CE, Buxbaum LJ. Shared and distinct neuroanatomic regions critical for tool-related action production and recognition: Evidence from 131 left-hemisphere stroke patients. J Cogn Neurosci. 2015;27:2491–2511. doi: 10.1162/jocn_a_00876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50:1148–1167. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tunik E, Rice NJ, Hamilton A, Grafton ST. Beyond grasping: Representation of action in human anterior intraparietal sulcus. Neuroimage. 2007;36:T77–T86. doi: 10.1016/j.neuroimage.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leshinskaya A, Caramazza A. Abstract categories of functions in anterior parietal lobe. Neuropsychologia. 2015;76:27–40. doi: 10.1016/j.neuropsychologia.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 66.Gallivan JP, McLean DA, Flanagan JR, Culham JC. Where one hand meets the other: Limb-specific and action-dependent movement plans decoded from preparatory signals in single human frontoparietal brain areas. J Neurosci. 2013;33:1991–2008. doi: 10.1523/JNEUROSCI.0541-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gallivan JP, McLean DA, Smith FW, Culham JC. Decoding effector-dependent and effector-independent movement intentions from human parieto-frontal brain activity. J Neurosci. 2011;31:17149–17168. doi: 10.1523/JNEUROSCI.1058-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haar S, Dinstein I, Shelef I, Donchin O. Effector-invariant movement encoding in the human motor system. J Neurosci. 2017;37:9054–9063. doi: 10.1523/JNEUROSCI.1663-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ogawa K, Imai F. Hand-independent representation of tool-use pantomimes in the left anterior intraparietal cortex. Exp Brain Res. 2016;234:3677–3687. doi: 10.1007/s00221-016-4765-7. [DOI] [PubMed] [Google Scholar]
- 70.Heed T, Leone FTM, Toni I, Medendorp WP. Functional versus effector-specific organization of the human posterior parietal cortex: Revisited. J Neurophysiol. 2016;116:1885–1899. doi: 10.1152/jn.00312.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacobs S, Danielmeier C, Frey SH. Human anterior intraparietal and ventral premotor cortices support representations of grasping with the hand or a novel tool. J Cogn Neurosci. 2010;22:2594–2608. doi: 10.1162/jocn.2009.21372. [DOI] [PubMed] [Google Scholar]
- 72.Lewis JW. Cortical networks related to human use of tools. Neuroscientist. 2006;12:211–231. doi: 10.1177/1073858406288327. [DOI] [PubMed] [Google Scholar]
- 73.Gallivan JP, Cavina-Pratesi C, Culham JC. Is that within reach? fMRI reveals that the human superior parieto-occipital cortex encodes objects reachable by the hand. J Neurosci. 2009;29:4381–4391. doi: 10.1523/JNEUROSCI.0377-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peeters R, et al. The representation of tool use in humans and monkeys: Common and uniquely human features. J Neurosci. 2009;29:11523–11539. doi: 10.1523/JNEUROSCI.2040-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme; New York: 1988. [Google Scholar]
- 76.Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- 77.Friston KJ, Holmes AP, Worsley KJ. How many subjects constitute a study? Neuroimage. 1999;10:1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- 78.Eickhoff SB, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 79.Jeffreys H. The Theory of Probability. Oxford Univ Press; Oxford: 1998. [Google Scholar]
- 80.Rouder JN, Speckman PL, Sun D, Morey RD, Iverson G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychon Bull Rev. 2009;16:225–237. doi: 10.3758/PBR.16.2.225. [DOI] [PubMed] [Google Scholar]
- 81.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 82.Glasser MF, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536:171–178. doi: 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meier JD, Aflalo TN, Kastner S, Graziano MS. Complex organization of human primary motor cortex: A high-resolution fMRI study. J Neurophysiol. 2008;100:1800–1812. doi: 10.1152/jn.90531.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Strother L, Medendorp WP, Coros AM, Vilis T. Double representation of the wrist and elbow in human motor cortex. Eur J Neurosci. 2012;36:3291–3298. doi: 10.1111/j.1460-9568.2012.08241.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.