Abstract
Background Appropriate patient selection and optimal timing of dialysis initiation among older adults with advanced CKD are uncertain. We determined the association between dialysis versus medical management and survival at different ages and levels of kidney function.
Methods We assembled a nationally representative 20% sample of United States veterans with eGFR<30 ml/min per 1.73 m2 between 2005 and 2010 (n=73,349), with follow-up through 2012. We used an extended Cox model to determine associations among the time-varying exposures, age (<65, 65–74, 75–84, and ≥85 years), eGFR (<6, 6–<9, 9–<12, 12–<15, and 15–<29 ml/min per 1.73 m2), and provision of dialysis, and survival.
Result Over the mean±SEM follow-up of 3.4±2.2 years, 15% of patients started dialysis and 52% died. The eGFR at which dialysis, compared with medical management, associated with lower mortality varied by age (P<0.001). For patients aged <65, 65–74, 75–84, and ≥85 years, dialysis associated with lower mortality for those with eGFR not exceeding 6–<9, <6, 9–<12, and 9–<12 ml/min per 1.73 m2, respectively. Dialysis initiation at eGFR<6 ml/min per 1.73 m2 associated with a higher median life expectancy of 26, 25, and 19 months for patients aged 65, 75, and 85 years, respectively. When dialysis was initiated at eGFR 9–<12 ml/min per 1.73 m2, the estimated difference in median life expectancy was <1 year for these patients.
Conclusions Provision of dialysis at higher levels of kidney function may extend survival for some older patients.
Keywords: dialysis, end-stage renal disease, geriatric nephrology
Each year, >115,000 patients start maintenance dialysis therapy for ESRD in the United States, including >25,000 patients over the age of 75.1 Although maintenance dialysis is intended to be life-sustaining, appropriate patient selection and the optimal timing of dialysis initiation among older adults are uncertain. The 2-year mortality rate for patients who initiate dialysis over the age of 75 exceeds 50%,1 and observational studies suggest that dialysis may not increase survival for older patients with high comorbidity or low functional status.2–9 Compared with younger patients, older adults are more likely to start dialysis with a higher eGFR, reflecting dialysis initiation earlier in the course of kidney disease, reduced muscle mass with lower creatinine generation, or both. This practice is controversial; the Initiating Dialysis Early and Late (IDEAL) trial found no benefit when dialysis was initiated with a higher (10–14 ml/min per 1.73 m2) versus lower eGFR.10 Because older adults with frailty or serious comorbidities are underrepresented in clinical trials, it is unclear whether IDEAL can be extrapolated to this population.
Previous studies evaluating the comparative effectiveness of dialysis in older adults were conducted in health care systems outside of the United States, which may differ in their patient selection practices.3–8 Importantly, prior studies were subject to length-time and lead-time biases, because they did not account for differences in the rate of kidney disease progression preceding dialysis initiation or the level of kidney function at the start of dialysis.
We aimed to evaluate the association between dialysis versus medical (nondialytic) management on survival at different ages and levels of kidney function in a large, contemporary, national cohort of United States veterans with advanced CKD, a population which accounts for almost one fifth of all older adults who start maintenance dialysis in the United States. Our goal was to determine whether the association between dialysis initiation and survival was modified by age or eGFR, and to estimate median increase in survival with dialysis initiation compared with medical management at different ages and levels of kidney function.
Methods
Cohort
We conducted a cohort study of United States veterans using laboratory and administrative data from the Department of Veterans Affairs (VA), Medicare claims, and the United States Renal Data System (USRDS), a national registry of patients receiving dialysis therapy for ESRD. We used laboratory data from the VA Decision Support System Laboratory Results file to identify all veterans with an outpatient measurement of serum creatinine between January 1, 2005 and December 31, 2010. We identified 367,188 individuals with the first (index) outpatient eGFR measurement <30 ml/min per 1.73 m2 who had not received dialysis or a kidney transplant on or before the index date. We randomly selected 20%, resulting in an analytic cohort of 73,349 patients. The Institutional Review Board at Stanford University and the Research and Development Committee of VA Palo Alto Health Care System approved conduct of the study.
Exposures
The primary exposure for this study was the time-varying status of dialysis initiation. We ascertained dialysis initiation using the first service date in the USRDS and with dialysis International Classification of Diseases, Ninth Revision codes (3995, 5498, V56, V56.8, V45.1) and Current Procedural Terminology codes (90935, 90937, 90945, 90947) in VA and Medicare claims. The date of dialysis initiation was defined as the first occurrence of dialysis in any of the three data sources. In total, 8369 patients (77.9%) had a record in USRDS.
Two other key exposure variables were age and eGFR. We abstracted age from VA administrative files, categorized as <65, 65–74, 75–84, and ≥85 years. We ascertained all inpatient and outpatient serum creatinine measurements after the index date from VA laboratory files. We used the Chronic Kidney Disease Epidemiology Collaboration equation to estimate GFR from serum creatinine measurements and demographic characteristics. We recorded eGFR during follow-up including at dialysis initiation, categorized according to CKD stage for eGFR≥15, and in 3 ml/min increments for eGFR<15: <6, 6–<9, 9–<12, 12–<15, 15–29, and >29 ml/min per 1.73 m2. If the serum creatinine at dialysis initiation was not available from VA laboratory files (i.e., the patient started dialysis outside of the VA), we used the serum creatinine reported on the USRDS Medical Evidence form to estimate GFR. When neither were available, we used a last observation carried forward (LOCF) method to update eGFR values, described further below.
Outcome
The primary outcome for this study was mortality, ascertained from VA Vital Status Files through September 30, 2012. Each patient’s observation period began at the time that their index eGFR measurement <30 ml/min per 1.73 m2 was recorded. Patients who did not die during their observation period were censored at the first occurrence of either kidney transplantation (n=32) or end of study follow-up.
Covariates
We ascertained sex, race, marital status, VA facility complexity, ZIP code median income, and residential location (urban, city, rural) using VA administrative data, Medicare files, and census data. We recorded driving distance to the nearest VA facility and VA copayment as surrogates for access to care. We recorded chronic conditions from VA and Medicare files using International Classification of Diseases, Ninth Revision and Current Procedural Terminology codes. We also recorded the change in eGFR over the preceding interval (eGFR slope) and cumulative hospital and nursing facility days from the index date up to dialysis initiation as another metric of comorbidity.
Statistical Analyses
For each patient, we created time intervals corresponding to each new eGFR measurement and/or dialysis initiation. The number and length of time intervals, therefore, varied across patients according to the frequency and timing of eGFR measurements, and whether dialysis was initiated and/or the patient died during the observation period. The time-varying variables age, eGFR, eGFR slope, provision of dialysis, and cumulative hospital and nursing facility days were recorded at the start of each time interval.
We evaluated baseline patient characteristics and the frequency of dialysis initiation according to index age category. We used a Cox model extended to accommodate time-varying covariates to evaluate the association between dialysis initiation and mortality. Patients contributed observation time to the medical management group until dialysis initiation. We modeled the hazard of mortality as a function of dialysis initiation, age, eGFR, their pairwise interactions, and a three-way interaction term between age, eGFR, and dialysis initiation. The models included the covariates listed above, as well as calendar year. We used robust sandwich estimation to account for correlation of patients within facilities. The results are presented as the hazard ratio (HR) of death for levels of time-varying eGFR and age. The HR for time-varying eGFR is interpreted as the instantaneous risk of death associated with starting dialysis with the current eGFR, compared with not starting dialysis at that eGFR. A similar interpretation applies to time-varying age.
We used this model to depict the predicted 2-year survival by age, eGFR, and dialysis initiation status for various “typical” patients to illustrate variation in survival by these factors. We also constructed predicted survival curves to estimate median life expectancy for typical patients who initiate dialysis and typical patients who receive medical management for age and eGFR strata in which dialysis was associated with a benefit.
Missing Data and Sensitivity Analyses
Among patients who initiated dialysis, 26% of patients lacked an eGFR within 30 days of dialysis initiation. To address this, we carried forward the most recent observation as we did for eGFR observations during noninitiation periods, referred to as LOCF.11 To address missing values for other baseline covariates, we used multiple imputation techniques on the basis of the joint modeling approach employed in SAS’s PROC MI and summarized findings across models for each imputed data set in R using Rubin’s rules to yield finalized point estimates for describing associations and corresponding SEM estimates under the Cox model.11 Variables in the imputation model included all those in the scientific model as well as albumin and BUN. We used a two-sided Wald test to assess whether variation in the magnitude of the association between dialysis initiation and mortality by eGFR differed by age group at the 0.05 level of significance.
We conducted three sensitivity analyses to determine if our results were robust to missing data assumptions and dialysis definition. First, we used multiple imputation for all missing eGFR values at dialysis initiation. Second, we employed a modification of LOCF, in which we used serum creatinine values obtained shortly after dialysis initiation (available in 64% of patients with missing eGFRs at dialysis initiation) to singly impute eGFR at dialysis initiation before applying LOCF in the whole sample. Thus, LOCF was applied in fewer cases than in the primary analysis. Our primary analysis was conducted using intention-to-treat principles; that is, we assumed that once dialysis was initiated, the patient remained on dialysis. In a third sensitivity analysis, we excluded patients who may have initiated dialysis for AKI, defined as patients who were absent from the USRDS registry and whose first and last dialysis procedure codes were <30 days apart (n=2139).
Comparison to IDEAL Trial
Finally, to assess the comparability of our cohort to the IDEAL population, we estimated the percentage of patients who met the eligibility criteria for IDEAL: (1) 10<eGFR<15 ml/min per 1.73 m2 between the index date and the start of dialysis and (2) no history of dementia, metastatic cancer, hematologic malignancy, or paralysis.10 We then determined the association between eGFR at dialysis initiation and survival among these patients.
Results
Patient Characteristics
Patient characteristics stratified by age group are presented in Table 1. Mean index age was 74±12 years and mean index eGFR was 24±6 ml/min per 1.73 m2. The majority of patients were male (97%), white (73%), and lived in urban areas (81%). The prevalence of most chronic conditions was higher at older ages.
Table 1.
Patient characteristics, stratified by index age
| Patient Characteristics | Age Group, yr | |||
|---|---|---|---|---|
| <65 (n=19,149) | 65–74 (n=14,839) | 75–84 (n=27,012) | ≥85 (n=12,349) | |
| Age, yr | 57±7 | 71±3 | 80±3 | 88±3 |
| Male, % | 94 | 98 | 98 | 97 |
| Race, % | ||||
| Black | 25 | 14 | 9 | 9 |
| White | 62 | 74 | 78 | 78 |
| Other | 10 | 8 | 7 | 7 |
| Married, % | 45 | 59 | 63 | 56 |
| Distance to closest VA facility (miles) | 43±45 | 48±46 | 48±45 | 46±46 |
| High complexity VA, % | 79 | 75 | 74 | 74 |
| Copayment for VA services, % | 69 | 84 | 87 | 83 |
| ZIP code median income, % | ||||
| ≤$35,000 | 21 | 18 | 17 | 16 |
| $35,001–$42,000 | 20 | 20 | 20 | 19 |
| $42,001–$50,000 | 20 | 22 | 22 | 22 |
| $50,001–$62,000 | 19 | 20 | 20 | 19 |
| ≥$62,001 | 17 | 18 | 20 | 22 |
| Residence, % | ||||
| Urban | 84 | 81 | 80 | 82 |
| Large city | 6 | 7 | 8 | 8 |
| Rural | 6 | 8 | 9 | 8 |
| Comorbidities, % | ||||
| Diabetes mellitus | 46 | 56 | 46 | 34 |
| Ischemic heart disease | 24 | 46 | 53 | 51 |
| Heart failure | 15 | 27 | 32 | 33 |
| Peripheral arterial disease | 9 | 19 | 22 | 21 |
| Cerebrovascular disease | 7 | 14 | 17 | 17 |
| Paralysis | 1 | 1 | 1 | 1 |
| Chronic pulmonary disease | 16 | 27 | 28 | 27 |
| Chronic liver disease | 9 | 3 | 2 | 2 |
| Connective tissue disease | 2 | 2 | 2 | 3 |
| Peptic ulcer disease | 1 | 2 | 3 | 3 |
| Malignancy | 11 | 19 | 23 | 25 |
| Metastatic solid tumor | 1 | 2 | 2 | 1 |
| AIDS | 2 | 0.4 | 0.1 | 0.1 |
| Dementia | 1 | 3 | 7 | 12 |
| Depression | 30 | 15 | 13 | 15 |
| Index eGFR, ml/min per 1.73 m2 | 23±6 | 24±6 | 25±5 | 25±5 |
| Year of index date, % | ||||
| 2005–2007 | 48 | 59 | 66 | 54 |
| 2008–2010 | 52 | 42 | 34 | 46 |
4% of patients were missing information on race, 0.7% were missing facility complexity, 0.4% were missing copayment, 4% were missing residence, and 2% were missing ZIP code median income. Data are presented as mean ± SEM or as percentages.
Mean follow-up time was 3.4±2.2 years. The median number of follow-up eGFR measurements per patient was 10 (25th, 75th percentile 4, 23) for patients who received medical management, and for patients who started dialysis, the median number of follow-up eGFR measurements before dialysis initiation was 9 (25th, 75th percentile 4, 21). During the follow-up period, 15% of patients started dialysis and 52% died (Supplemental Table 1). The cumulative incidence of dialysis initiation was lower at older ages, occurring in 21% of patients<65 years, 18% of patients 65–74 years, 12% of patients 75–84 years, and 5% of patients aged ≥85 years at the end of follow-up (Figure 1). Among patients who started dialysis, 97% received hemodialysis as the initial dialysis modality. Older patients were more likely to have a higher eGFR or a missing eGFR at dialysis initiation compared with younger patients (Supplemental Figure 1, Supplemental Table 2).
Figure 1.
Cumulative incidence of dialysis initiation, demonstrating lower frequency of dialysis initiation at older ages.
Dialysis Mortality Risk by Age and eGFR
The association between dialysis and mortality was modified by eGFR, and this association was further modified by age (P value for three-way interaction <0.001, Table 2). For example, among patients≥75 years, dialysis was associated with a lower risk for death compared with medical management at eGFR 9–<12 ml/min per 1.73 m2, with larger magnitudes of the association at progressively lower levels of kidney function, and equivocal or higher risk for death above this level (Table 2). Among patients 65–74 years, dialysis was associated with a lower risk for death compared with medical management at eGFR<6 ml/min per 1.73 m2, and equivocal or higher risk for death above this level. Among patients<65 years, dialysis was associated with a lower risk for death compared with medical management at levels of eGFR 6–<9 ml/min per 1.73 m2 and lower, and equivocal or progressively higher risk for death above this level.
Table 2.
Adjusted mortality HRs (95% CIs) for patients who initiated dialysis compared with patients who received medical management, stratified by time-varying age and eGFR
| Time-Varying eGFR, ml/min per 1.73 m2 | Time-Varying Age | |||
|---|---|---|---|---|
| <65 | 65–74 | 75–84 | >85 | |
| <6 | 0.28 (0.16 to 0.45) | 0.37 (0.22 to 0.62) | 0.34 (0.20 to 0.57) | 0.36 (0.21 to 0.63) |
| 6 to <9 | 0.41 (0.32 to 0.52) | 0.87 (0.64 to 1.18) | 0.37 (0.30 to 0.45) | 0.39 (0.30 to 0.50) |
| 9 to <12 | 0.83 (0.67 to 1.02) | 0.86 (0.68 to 1.09) | 0.74 (0.63 to 0.88) | 0.61 (0.49 to 0.75) |
| 12 to <15 | 0.88 (0.70 to 1.11) | 1.49 (1.18 to 1.87) | 1.20 (1.05 to 1.37) | 1.08 (0.86 to 1.36) |
| 15–29 | 1.50 (1.32 to 1.71) | 1.76 (1.55 to 1.99) | 1.78 (1.63 to 1.95) | 2.04 (1.74 to 2.40) |
| >29 | 3.70 (2.99 to 4.58) | 3.76 (2.87 to 4.92) | 3.16 (2.59 to 3.87) | 3.37 (2.40 to 4.73) |
Model includes main effects for age, eGFR, dialysis, and interactions between age×eGFR, age×dialysis, and eGFR×dialysis, in addition to index eGFR, eGFR slope, sex, race, marital status, ZIP code median income, distance to VA facility, VA facility complexity, VA copayment, residence, chronic conditions (diabetes, ischemic heart disease, heart failure, peripheral arterial disease, cerebrovascular disease, paralysis, chronic pulmonary disease, chronic liver disease, connective tissue disease, peptic ulcer disease, malignancy, metastatic solid tumor, AIDS, dementia, and depression), cumulative hospital and nursing facility days, calendar year, and correlation of patients within facilities.
Predicted 2-Year and Overall Survival
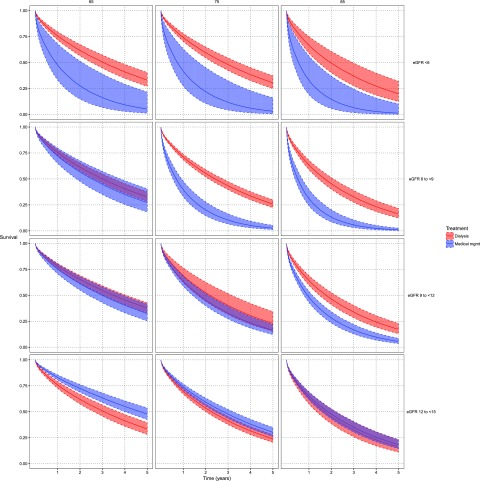
Predicted 2-year survival for a typical patient by age, eGFR, and dialysis initiation status is depicted in Figure 2. Among patients receiving medical management, predicted survival was markedly lower with progressively lower eGFRs in each age strata. Conversely, among patients initiating dialysis, predicted survival was comparatively less heterogeneous across eGFR strata. Survival curves for dialysis and medical management diverged at higher levels of eGFR for older patients (Figure 3).
Figure 2.
Predicted two-year survival by age, eGFR and dialysis initiation status. In the medical management group, predicted survival is lower at lower levels of eGFR. In the dialysis group, predicted survival is less heterogeneous across eGFR levels.
Figure 3.
Adjusted predicted survival curves for typical cohort members according to age, eGFR and dialysis, demonstrating that dialysis is associated with survival benefit at higher levels of eGFR for older patients. The figure depicts predicted survival curves with 95% confidence intervals for a typical patient who initiates dialysis (in red) and a typical patient who receives medical management (in blue), at ages 60, 65, 75 and 85 years, and at eGFR<6 ml/min per 1.73 m2, 6 to <9 ml/min per 1.73 m2, 9 to <12 ml/min per 1.73 m2, and 12 to <15 ml/min per 1.73 m2. eGFR values >15 ml/min per 1.73m2 are not depicted because these were not associated with a survival benefit for dialysis in any age group. mgmt, management.
Median Life Expectancy
Estimated median life expectancies for typical patients who initiate dialysis and others who receive medical management are presented in Table 3 by age group and eGFR. Dialysis initiation at eGFR<6 ml/min per 1.73 m2 was associated with a higher median life expectancy of 54, 26, 25, and 17 months for patients 60, 65, 75, and 85 years, respectively. A smaller absolute difference in median life expectancy was observed in all age groups when dialysis was initiated at higher eGFR. For example, when dialysis was initiated at eGFR 9–<12 ml/min per 1.73 m2, the median difference in life expectancy was <1 year.
Table 3.
Estimated survival for patients who initiate dialysis compared with patients who receive medical management, by age group and eGFR
| Age, yr | eGFR (ml/min per 1.73 m2) | Median Survival in Months (25th, 75th percentile) | Difference in Median Survival (mo) | |
|---|---|---|---|---|
| Dialysis | Medical Management | |||
| 60 | <6 | 71 (28, NE) | 17 (5, 39) | 54 |
| 6–<9 | 67 (26, NE) | 45 (16, 90) | 22 | |
| 9–<12 | 54 (20, NE) | 52 (19, NE) | 2 | |
| 65 | <6 | 37 (13, 75) | 11 (3, 26) | 26 |
| 6–<9 | 36 (12, 74) | 30 (10, 64) | 6 | |
| 9–<12 | 42 (15, 85) | 36 (12, 76) | 6 | |
| 75 | <6 | 34 (11, 70) | 9 (3, 22) | 25 |
| 6–<9 | 29 (10, 62) | 8 (2, 20) | 21 | |
| 9–<12 | 26 (9, 57) | 21 (7, 46) | 5 | |
| 85 | <6 | 24 (8, 52) | 7 (2, 16) | 17 |
| 6–<9 | 21 (7, 46) | 6 (2, 15) | 15 | |
| 9–<12 | 21 (7, 47) | 11 (3, 27) | 10 | |
NE values correspond to times which extend beyond the maximum follow-up time. eGFR values >12 ml/min per 1.73 m2 are not presented because they were not associated with higher dialysis survival in any age group.
Sensitivity Analyses
The findings were similar using multiple imputation or the modified LOCF approach for missing eGFR values at dialysis initiation (Supplemental Table 3). In a separate sensitivity analysis excluding possible cases of dialysis initiation for AKI, the association between dialysis and an increased risk for death at higher eGFR levels was attenuated (Supplemental Table 3).
For example, the HRs for death associated with dialysis initiation for probable AKI at eGFR 15–<29 ml/min per 1.73 m2 were 1.05 (95% confidence interval [95% CI], 0.91 to 1.21), 1.17 (95% CI, 1.04 to 1.33), 1.12 (95% CI, 1.10 to 1.25), and 1.39 (95% CI, 1.19 to 1.64), at ages <65, 65–74, 75–84, and ≥85, respectively.
Comparison to IDEAL Trial
Overall, 8817 patients (12%) met the inclusion criteria for IDEAL, ranging from 17% of patients age<65 years, to 7% of patients age≥85 years. Among the 4719 IDEAL-eligible patients who started dialysis during follow-up, we evaluated the association between eGFR at the start of dialysis and mortality. Compared with IDEAL-eligible patients who started dialysis at eGFR 10–<14 ml/min per 1.73 m2, patients who started dialysis at eGFR 6–<10 ml/min per 1.73 m2 had a similar risk for death (HR, 1.04; 95% CI, 0.95 to 1.15), and those who started dialysis at eGFR<6 ml/min per 1.73 m2 had a lower risk for death (HR, 0.79; 95% CI, 0.68 to 0.91).
Discussion
This study, in a contemporary cohort of United States veterans with advanced CKD, found that the eGFR at which survival with dialysis exceeded survival with medical management varied by age; an apparent benefit of dialysis was evident at higher eGFRs for older patients. At the same time, the potential gain in life expectancy associated with dialysis was diminished for older patients and at higher eGFRs.
These results address a key knowledge gap regarding the effectiveness of dialysis in older adults with advanced CKD. Although older adults have similar or larger absolute and relative mortality risk reductions with dialysis, the corresponding increase in median life expectancy is modest, ranging between 5 and 25 months for patients≥75 years. These estimates, generated from a national cohort with almost 250,000 person-years of follow up and robust to several analytic assumptions, may facilitate shared decision making with older patients.
Estimates of life expectancy, considered in conjunction with quality of life and patient preferences, can be useful to patients and families weighing the potential burdens and complications of maintenance dialysis therapy against its benefits. Patients with serious illness vary widely in their willingness to accept a high-burden life-sustaining treatment.12,13 In a discrete choice experiment, patients with CKD were willing to forgo 7–15 months of life expectancy in order to reduce hospital visits and increase their independence, estimates that are similar to the gain in life expectancy we observed.14 The extent to which dialysis alleviates distressing symptoms or improves quality of life compared with medical management could not be determined from our study. Prior studies have reported mixed results, illustrating the heterogeneity of patient responses to dialysis. Although older patients receiving dialysis generally report similar quality of life compared with younger patients,15 older patients also have higher rates of dialysis discontinuation,16 and frequently experience loss of independence.17,18 Limited observational data suggest that peritoneal dialysis is associated with similar quality of life and physical function compared with hemodialysis, although it is infrequently utilized among older veterans.19
The IDEAL trial found no difference in survival among patients randomized to earlier initiation of dialysis (eGFR 10–14 ml/min per 1.73 m2) compared with patients randomized to later initiation, calling the practice of dialysis initiation at higher eGFRs into question.10 This practice is widespread among older adults, although somewhat less pronounced in the VA,20 reflecting uncertainty about the generalizability of IDEAL to older adults. A related question arising from IDEAL is whether it is safe to defer dialysis for patients who have symptoms of uremia but lack urgent clinical manifestations. O’Hare et al.21 reviewed the clinical context for dialysis initiation among 1691 veterans who started dialysis between 2000 and 2009 and found that the trend toward initiation of dialysis with higher eGFRs in more recent years has occurred among these patients—those who were neither acutely ill nor asymptomatic.
Our results complement IDEAL and the study by O’Hare et al. by providing information about the potential benefits of dialysis at different eGFR levels in a “real-world” population of older adults with advanced CKD, many of whom would not meet IDEAL’s eligibility criteria. Compared with the IDEAL cohort, the frequencies of comorbidities such as heart failure and malignancy were higher in the veteran population, whereas the use of peritoneal dialysis was lower. Within a cohort of IDEAL-eligible patients who started dialysis, and among patients<75 years, our study mirrors the IDEAL findings. We also demonstrate that dialysis is associated with a modest survival benefit compared with medical management between eGFR 9 and <12 ml/min per 1.73 m2 for older patients. Although the latter finding may seem to contradict IDEAL, it should be noted that IDEAL was not powered to identify differences in survival of <10% between treatment strategies. It may also reflect the selection of healthier patients in IDEAL. On the other hand, we cannot eliminate the possibility that the survival differences observed in this study are attributable to unmeasured confounding due to the nonrandomized design.
Similar to most prior observational studies, we found no evidence of a benefit from dialysis initiation at eGFR>15 ml/min per 1.73 m2. The apparent harm associated with dialysis initiation in this eGFR range should be interpreted cautiously. In sensitivity analyses excluding AKI, this effect was diminished. Furthermore, patients who initiate dialysis at very high (or very low) levels of eGFR are likely to have other coexisting conditions and/or socioeconomic disadvantages that confound the association between eGFR, dialysis initiation, and mortality. Taken together, the results reinforce the idea that the conventional definition of ESRD, an eGFR<15 ml/min per 1.73 m2, is not synonymous with the appropriate timing of dialysis initiation.
Patient surveys suggest the quality of dialysis decision making is often poor and may not meet the standard for informed consent in some circumstances.22,23 Few patients receive prognostic information or have their preferences elicited, and many are not presented with the option to forgo dialysis.24,25 Decision aids that incorporate prognostic information along with other expected outcomes could improve the quality of dialysis decision making and reduce variation in dialysis initiation practices.26 Such a tool could help patients understand trade-offs involved in deferring or forgoing dialysis initiation and promote conversations about patient goals and preferences before the clinical circumstances become urgent.
Our study has several strengths. We used data from the Veterans Health Administration, the largest healthcare system providing care to patients with CKD in the United States. We used a novel study design which included patients with advanced CKD who did not receive dialysis; this approach allowed us to account for patient selection and timing of dialysis initiation with time-varying measurements of eGFR. Our study also has several limitations. eGFR at dialysis initiation was imputed for 26% of patients; however, the results were robust to missing data assumptions and sensitivity analyses excluding AKI. We lacked information about the clinical context at dialysis initiation, and we could not determine the effect of dialysis initiation on quality of life, symptom burden, or functional status. The results may not be generalizable to women, nonveterans, or patients who initiate peritoneal dialysis. Finally, although we accounted for a variety of health characteristics in our analysis, there may be unmeasured confounding. If unmeasured confounding is present, our study may have overestimated the benefit of dialysis over medical management.
In summary, these results illustrate potential gains in life expectancy from dialysis initiation at different levels of kidney function for older adults. Although decisions regarding whether and when to initiate dialysis should continue to be made on a patient-to-patient basis, these data can be used to inform and improve shared decision making.
Disclosures
None.
Supplementary Material
Acknowledgments
This study is supported by HX001262 from the Department of Veterans Affairs. G.M.C. is supported by K24 DK085446.
Views expressed are those of the authors and not necessarily those of the Department of Veterans Affairs.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Now or Later? Understanding Timing of Dialysis Initiation beyond IDEAL,” on pages 2033–2035.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017121273/-/DCSupplemental.
References
- 1.Saran R, Li Y, Robinson B, Abbott KC, Agodoa LY, Ayanian J, et al. : US renal data system 2015 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 67[3 Suppl 1]: Svii, S1–305, 2016 [DOI] [PMC free article] [PubMed]
- 2.Murtagh FE, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE: Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant 22: 1955–1962, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Hussain JA, Mooney A, Russon L: Comparison of survival analysis and palliative care involvement in patients aged over 70 years choosing conservative management or renal replacement therapy in advanced chronic kidney disease. Palliat Med 27: 829–839, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Carson RC, Juszczak M, Davenport A, Burns A: Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol 4: 1611–1619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandna SM, Da Silva-Gane M, Marshall C, Warwicker P, Greenwood RN, Farrington K: Survival of elderly patients with stage 5 CKD: Comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant 26: 1608–1614, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joly D, Anglicheau D, Alberti C, Nguyen AT, Touam M, Grünfeld JP, et al. : Octogenarians reaching end-stage renal disease: Cohort study of decision-making and clinical outcomes. J Am Soc Nephrol 14: 1012–1021, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Morton RL, Webster AC, McGeechan K, Howard K, Murtagh FE, Gray NA, et al. : Conservative management and end-of-life care in an Australian cohort with ESRD. Clin J Am Soc Nephrol 11: 2195–2203, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verberne WR, Geers AB, Jellema WT, Vincent HH, van Delden JJ, Bos WJ: Comparative survival among older adults with advanced kidney disease managed conservatively versus with dialysis. Clin J Am Soc Nephrol 11: 633–640, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacilio M, Minutolo R, Garofalo C, Liberti ME, Conte G, De Nicola L: Stage 5-CKD under nephrology care: To dialyze or not to dialyze, that is the question. J Nephrol 29: 153–161, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, et al. : IDEAL Study: A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 363: 609–619, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Little RJA, Rubin DB: Statistical Analysis with Missing Data, New York, John Wiley & Sons, 2014 [Google Scholar]
- 12.Fried TR, Bradley EH, Towle VR, Allore H: Understanding the treatment preferences of seriously ill patients. N Engl J Med 346: 1061–1066, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Fried TR, Tinetti ME, Iannone L, O’Leary JR, Towle V, Van Ness PH: Health outcome prioritization as a tool for decision making among older persons with multiple chronic conditions. Arch Intern Med 171: 1854–1856, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morton RL, Snelling P, Webster AC, Rose J, Masterson R, Johnson DW, et al. : Factors influencing patient choice of dialysis versus conservative care to treat end-stage kidney disease. CMAJ 184: E277–E283, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unruh ML, Newman AB, Larive B, Dew MA, Miskulin DC, Greene T, et al. ; Hemodialysis Study Group : The influence of age on changes in health-related quality of life over three years in a cohort undergoing hemodialysis. J Am Geriatr Soc 56: 1608–1617, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellwood AD, Jassal SV, Suri RS, Clark WF, Na Y, Moist LM: Early dialysis initiation and rates and timing of withdrawal from dialysis in Canada. Clin J Am Soc Nephrol 8: 265–270, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE: Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 361: 1539–1547, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jassal SV, Chiu E, Hladunewich M: Loss of independence in patients starting dialysis at 80 years of age or older. N Engl J Med 361: 1612–1613, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Iyasere OU, Brown EA, Johansson L, Huson L, Smee J, Maxwell AP, et al. : Quality of life and physical function in older patients on dialysis: A comparison of assisted peritoneal dialysis with hemodialysis. Clin J Am Soc Nephrol 11: 423–430, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu MK, O’Hare AM, Batten A, Sulc CA, Neely EL, Liu CF, et al. : Trends in timing of dialysis initiation within versus outside the department of veterans affairs. Clin J Am Soc Nephrol 10: 1418–1427, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Hare AM, Wong SP, Yu MK, Wynar B, Perkins M, Liu CF, et al. : Trends in the timing and clinical context of maintenance dialysis initiation. J Am Soc Nephrol 26: 1975–1981, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davison SN: End-of-life care preferences and needs: Perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 195–204, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brennan F, Stewart C, Burgess H, Davison SN, Moss AH, Murtagh FEM, et al. : Time to improve informed consent for dialysis: An international perspective. Clin J Am Soc Nephrol 12: 1001–1009, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song MK, Lin FC, Gilet CA, Arnold RM, Bridgman JC, Ward SE: Patient perspectives on informed decision-making surrounding dialysis initiation. Nephrol Dial Transplant 28: 2815–2823, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ladin K, Lin N, Hahn E, Zhang G, Koch-Weser S, Weiner DE: Engagement in decision-making and patient satisfaction: A qualitative study of older patients’ perceptions of dialysis initiation and modality decisions. Nephrol Dial Transplant 32: 1394–1401, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, et al. : Shared decision making: A model for clinical practice. J Gen Intern Med 27: 1361–1367, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.