Abstract
Background
The hallmark of podocytopathies, such as FSGS, is podocyte injury resulting in proteinuria. Transient receptor potential channel C6 (TRPC6) is a calcium-conducting ion channel expressed at the slit diaphragm. TRPC6 gain-of-function mutations and glomerular TRPC6 overexpression are associated with proteinuria. However, the pathways linking TRPC6 to podocyte injury, which is characterized by loss of the slit diaphragm protein nephrin, activation of several intracellular pathways (including calcineurin-NFAT signaling), and cytoskeletal rearrangement, remain elusive.
Methods
We tested whether the calcium-dependent protease calpain-1 mediates TRPC6-dependent podocyte injury in human and experimental FSGS and cultured podocytes.
Results
Compared with kidneys of healthy controls, kidneys of patients with FSGS had increased TRPC6 expression, increased calpain and calcineurin activity, and reduced expression of the calpain target Talin-1, which links the actin cytoskeleton to integrins and is critical for podocyte cytoskeletal stability. In a rat model of human FSGS, increased glomerular and urinary calpain activity associated with reduced Talin-1 abundance, enhanced calcineurin activity, and increased proteinuria. Treatment with the calpain inhibitor calpeptin prevented these effects. In cultured podocytes, pharmacologic stimulation of TRPC6-dependent calcium influx increased calpain-1 and calcineurin activity and reduced Talin-1 expression, and knockdown of TRPC6 or calpain-1 prevented these effects.
Conclusions
We elucidated a novel mechanism that links TRPC6 activity to calpain-1 activation and through Talin-1 loss and possibly, calcineurin activation, the podocyte injury characterizing FSGS. Therefore, calpain-1 and/or TRPC6 inhibition could be future therapeutic options to treat patients with FSGS or other podocytopathies.
Keywords: podocyte, intracellular signal, proteinuria, glomerular disease, cytoskeleton, ion channel
Podocytes are terminally differentiated epithelial cells in the glomerular filtration barrier with interdigitating foot processes that are interconnected by the slit diaphragm protein complex, and they play a vital role in the blood filtration process in the kidneys. Structural podocyte injury is central in the pathogenesis of most inherited and acquired glomerular diseases, which are all associated with decreased expression of slit diaphragm proteins, such as nephrin, but also, podocyte cytoskeletal rearrangement.1–4
Transient Receptor Potential channel C6 (TRPC6) is a nonspecific calcium (Ca2+)-conducting ion channel that is expressed at or near the slit diaphragm of the podocyte.5 TRPC6 gain-of-function mutations lead to hereditary FSGS, whereas acquired glomerular proteinuric diseases are associated with TRPC6 overexpression.5–9 Therefore, it is suggested that TRPC6 plays an important role during the initiation of podocyte injury, and TRPC6 activity was shown to activate several intracellular signaling pathways. One of these signaling cascades downstream of TRPC6 involves the Ca2+-dependent phosphatase calcineurin, which when activated, subsequently activates NF of activated T cells (NFAT).8 An important transcriptional target of NFAT is TRPC6 itself, thereby creating a deleterious feed forward loop, which we previously elucidated.8 Direct activation of calcineurin by TRPC6-medicated Ca2+ influx was, however, never proven in the podocyte. Although TRPC6 expression and activity are known to be deleterious, the exact molecular pathways linking TRPC6 to structural podocyte injury remain incompletely explained.
Calpains are a family of nonlysosomal cysteine proteases that are activated by Ca2+ and cleave proteins with a specific tertiary structure.10 In the kidney, calpain activity was associated with kidney injury, in particular renal ischemia-reperfusion and antiglomerular basement membrane injury, but not in a salt-sensitive hypertension model.11–14 Moreover, calpain was shown to be able to cleave the slit diaphragm protein nephrin and the podocyte cytoskeleton–associated anchoring protein Talin-1.13 Recently, it was reported that loss of Talin-1 is a key mechanism for the initiation and progression of podocyte injury, suggesting that calpain-dependent cleavage of (structural) podocyte proteins could disturb podocyte integrity and induce severe proteinuria.15 However, the mechanism by which calpains would be activated in the slit diaphragm microdomain of the podocyte remains elusive.
We hypothesized that one or more calpains are activated by TRPC6-mediated Ca2+ influx, which would ultimately lead to degradation of Talin-1 and other structural proteins in the podocyte as well as activation of the calcineurin/NFAT pathway. This study aims to determine whether calpain activation plays a role in patients with FSGS, elucidate the mechanism of calpain activation in the podocyte, and evaluate the potential renoprotective effect of calpain inhibition during experimental FSGS.
Methods
FSGS Patient Material
Snap-frozen kidneys of three patients with FSGS after nephrectomy and five healthy controls were used. Calpain activity was determined in urine of seven patients with FSGS and five healthy controls. The Medical Ethics Committee of the Radboud University Medical Center in Nijmegen, The Netherlands previously approved collection of patient material (reference no. 09/073). None of the subjects objected to the collection and storage of their material and use of their clinical data where applicable.
Adriamycin Nephropathy Rat Model
The adriamycin nephropathy model for human FSGS was induced as described previously.16 Male Wistar rats (200 g and approximately 8 weeks old; Charles River Laboratories, Wilmington, MA) were treated three times a week with intraperitoneal injections of 1 mg/kg body wt calpeptin (Sigma-Aldrich, Zwijndrecht, The Netherlands) or vehicle for 6 weeks. Hereafter, rats were housed in metabolism cages to collect 24-hour urine samples, after which kidneys and blood were collected.
All procedures involving animals were approved by the Animal Ethics Committee of the Radboud University in Nijmegen, The Netherlands in accordance with the guidelines of the Dutch Council for Animal Care and the European Communities Council Directive (86/609/EEC).
Isolation of Glomeruli
Glomeruli from rat kidneys were isolated by a sieving technique as previously described.17 Glomeruli were collected through sieves (150 and 75 μm) with cold Tris-buffered saline. The isolated glomeruli were examined under a microscope to verify their purity (consistently 90%–95%). Glomeruli were lysed and centrifuged, and the supernatant was used for Western blotting and the calpain activity assay as mentioned below.
Cell Culture
Cultured conditionally immortalized mouse podocytes (MPC-5) were transiently transfected with calpain-1 small interfering RNA (siRNA) (cpn-1KD) or nontargeting siRNA (cpn-1scr). MPC-5 untransfected or stably transfected with tetracycline-inducible TRPC6 knockdown (TRPC6KD) or scrambled siRNA constructs were cultured as described previously.7,8 Podocytes were injured using adriamycin (0.25 μg/ml) for 24 hours, or TRPC channels were activated using 1-oleoyl-2-acetyl-glycerol (OAG; 100 μM) for 20 seconds before a calpain activity assay for 4 hours when assessing expressional changes. Cells were simultaneously treated with calpeptin (1 μM), 2-aminoethoxydiphenyl borate (2-APB; 100 μM), or vehicle. Intracellular Ca2+ was visualized using the fluorescent Ca2+ indicator FURA-2. Data are presented as 340-to-380-nm ratios.
Real-Time Quantitative RT-PCR Analysis
Total RNA was isolated and reverse transcribed (transcriptor kit; Roche Diagnostics GmbH, Mannheim, Germany). Quantitative RT-PCR was performed using SYBR Green SuperMix (Roche Diagnostics), and glyceraldehyde-3-phosphate dehydrogenase was used as the housekeeping gene.
Immunofluorescence Analyses
Immunofluorescence staining for TRPC6 (Abcam, Cambridge, United Kingdom and Alomone, Jerusalem, Israel), Talin-1 (Acris Antibodies, Herford, Germany), nephrin (R&D, Minneapolis, MN), synaptopodin (Santa Cruz), calpastatin (Santa Cruz), and desmin (Santa Cruz) was performed on 2-μm cryosections of rat and human kidney cortex. Mean fluorescent intensity in 30–40 glomeruli per slide was determined.
Western Blot Analyses
Talin-1, calpain, and TRPC6 protein expression were assessed by Western blot analysis. Blots were incubated with mouse monoclonal anti-human Talin-1 antibody (Novusbio), rabbit polyclonal anti-rat calpain-1 (Abcam), rabbit polyclonal anti-rat TRPC6, and mouse anti–β-actin antibody (AC-15; Sigma-Aldrich). Proteins were visualized using chemiluminescence.
Calpain and Calcineurin Activity Analyses
Calpain activity was determined by a calpain activity assay according to the manufacturer’s protocol (Abcam). In situ zymography was performed by incubating the substrate of the calpain activity assay diluted in the appropriate buffer as described in the manufacturer’s protocol on 10-μm cryocut kidney sections for 24 hours at 37°C. The slides were then subsequently stained for Talin-1 or synaptopodin and examined using a fluorescent microscope. The mean fluorescent intensity in 30–40 glomeruli per slide was determined. The supernatant of the sections was taken, and calpain activity was determined and normalized for surface area of the section.
Calcineurin activity was determined by a calcineurin activity assay according to the manufacturer’s protocol (Merck).
Analytic Procedures
Urinary albumin and creatinine levels were determined by radial immunodiffusion and the Architect C16000 (Abbott Diagnostics, Chicago, IL), respectively. Urinary IgG levels were measured using ELISA according to the manufacturer’s protocol (Antibodies-Online, Aachen, Germany).
Statistical Analyses
All results are depicted as mean±SEM; t test or ANOVA was used to test for significance using SPSS software (IBM, New York, NY) followed by a Bonferroni post hoc test. P values <0.05 were considered significant.
Results
Calpains Are Expressed in the Kidney and Podocytes
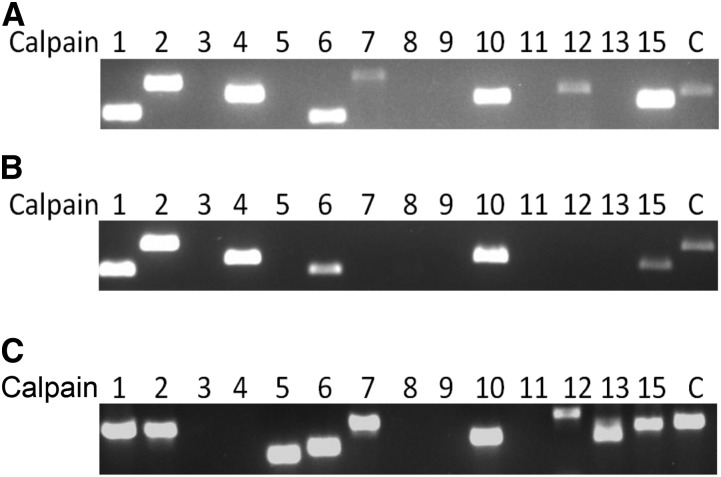
Currently, it is unknown which calpains are expressed in kidney cortex and podocytes. We could show that calpains 1, 2, 4, 6, 7, 10, 12, and 15 and the endogenous calpain inhibitor calpastatin are expressed in mouse kidney cortex (Figure 1A). Except for calpains 7 and 12, conditionally immortalized mouse podocytes also expressed these calpains as well as calpastatin (Figure 1B). In both control and adriamycin-treated rats, as a model for human FSGS, calpains 1, 2, 5, 6, 7, 10, 12, 13, and 15 and calpastatin were expressed (Figure 1C, Supplemental Figure 1).
Figure 1.
Calpains are expressed in the kidney and in podocytes. PCR was used to determine which calpains are (A) expressed in mouse renal cortex and (B) cultured conditionally immortalized mouse podocytes and (C) rat renal cortex. Numbers correspond to the calpain family member. Notably, there is no calpain-4 ortholog present in the rat genome and no calpain-14 ortholog present in the mouse and rat genome. C, calpastatin.
Renal Calpain Activity Is Increased in Patients with FSGS
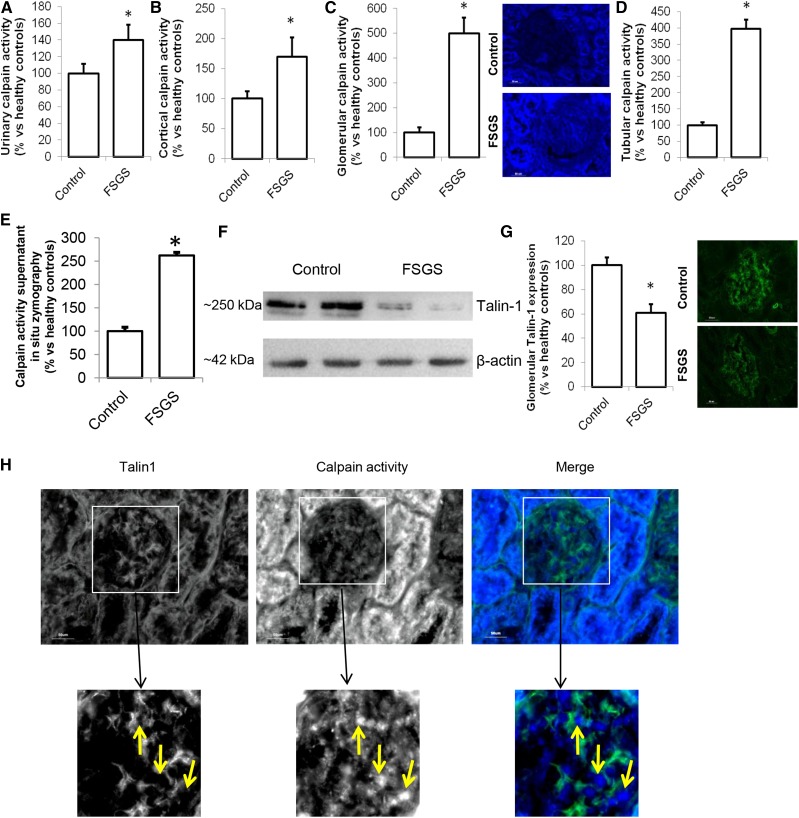
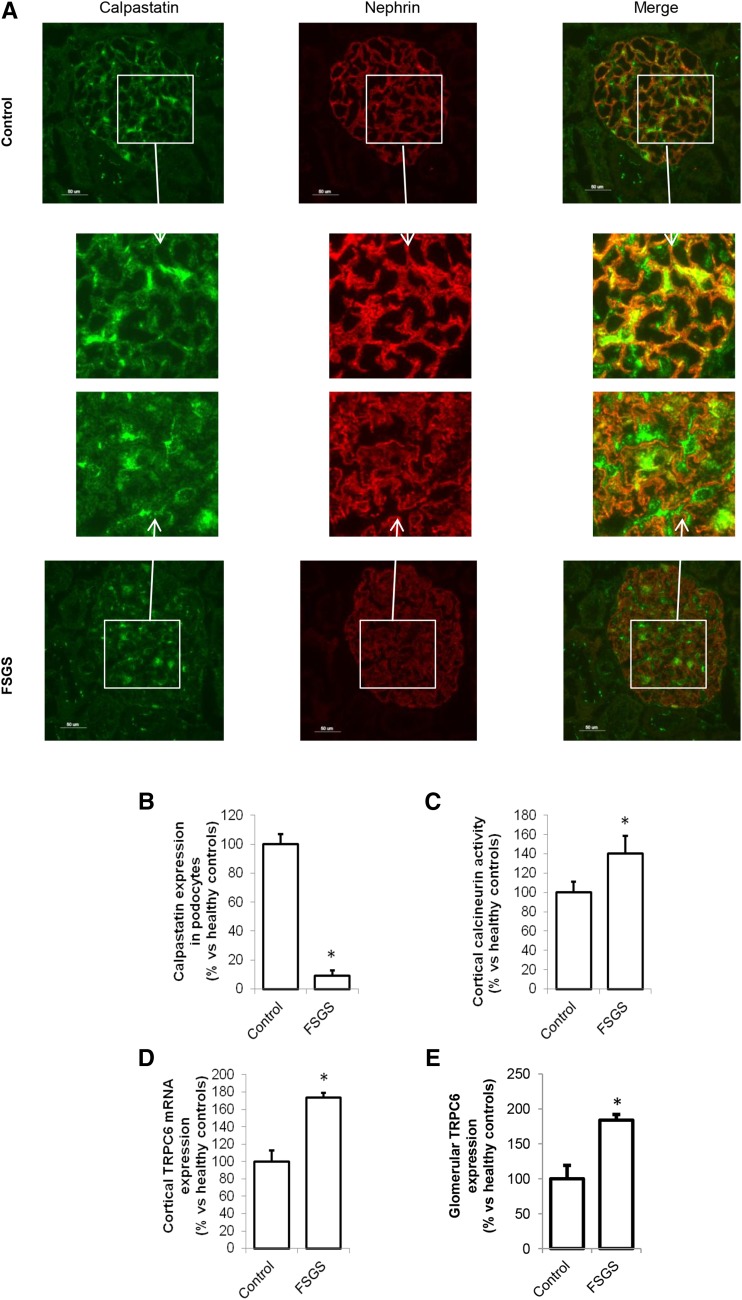
It was previously suggested that proteinuria involves breakdown of Talin-1 in the podocyte, possibly facilitated by calpain-induced Talin-1 cleavage, and increased calpain activity in urine of patients with FSGS was shown.15 The origin of and pathway inducing calpain activation was not determined. We confirmed the increased calpain activity in urine of patients with FSGS (Figure 2A). In addition, we showed increased calpain activity in renal cortical protein extracts from patients with FSGS compared with healthy controls (Figure 2B). We developed a fluorescent in situ zymography and found that glomeruli (Figure 2C) and tubuli (Figure 2D) of patients with FSGS showed more calpain activity compared with those in healthy controls (representative staining is shown in Figure 2C). Similar results were obtained by measuring the calpain activity in the supernatant of the in situ zymography (Figure 2E). This was accompanied by a decreased cortical Talin-1 expression (Figure 2F) and glomerular Talin-1 expression (Figure 2G). Notably, Talin-1 expression was low where calpain activity was high (Figure 2H, Supplemental Figure 2). An explanation of the increased calpain activity could be downregulation of the endogenous calpain inhibitor calpastatin in podocytes of patients with FSGS. Indeed, calpastatin expression was reduced in podocytes and appeared to be localized primarily outside of the podocyte in patients with FSGS, whereas calpastatin is highly expressed in podocytes in healthy controls (Figure 3A; calpastatin expression is quantified in Figure 3B). Calcineurin is a known calpain substrate that is irreversibly activated by calpain.18 Calcineurin activity was increased in the renal cortex of patients with FSGS compared with healthy persons (Figure 3C).
Figure 2.
Calpain activation occurs in glomeruli of patients with FSGS. (A) Calpain activity was determined in the urine of patients with FSGS (n=7) or healthy controls (control; n=5). (B) Protein was extracted from renal cortex samples, and a calpain activity assay was performed. Sections of the cortex were cut, and using an in situ zymography assay, (C) glomerular and (D) tubular calpain activity was determined. (E) Calpain activity in the section supernatant was measured and normalized to surface area of the section. (F) Western blot analysis was performed to determine the cortical expression of the calpain cleavage target Talin-1 (Talin-1–to–β-actin ratio: 1.22±0.17 in control versus 0.25±0.20 in FSGS samples). (G) Glomerular Talin-1 was costained with synaptopodin, and its expression was quantified. (H) Talin-1 was stained in combination with an in situ zymography for calpain activity in sections from patients with FSGS. (B–H) FSGS, n=3; control, n=5. *P<0.05 compared to healthy controls.
Figure 3.
Reduced calpastatin, increased Transient Receptor Potential channel C6 (TRPC6) expression, and enhanced calcineurin activation in patients with FSGS. (A) Representative pictures of costaining of the endogenous calpain inhibitor calpastatin and nephrin in healthy controls and patients with FSGS. (B) Calpastatin expression in podocytes was determined by quantifying the costaining of calpastatin and nephrin. (C) Cortical calcineurin activity, as a downstream calpain target, was determined as well as (D) cortical TRPC6 mRNA and (E) glomerular TRPC6 protein expression as quantified by immunofluorescence costained with synaptopodin. FSGS, n=3; control, n=5. *P<0.05 compared with healthy controls.
We now hypothesized that TRPC6-dependent Ca2+ influx leads to activation of calpain. Correspondingly, cortical TRPC6 mRNA (Figure 3D) and glomerular TRPC6 protein expression (Figure 3E, Supplemental Figure 3A) were increased in patients with FSGS. Taken together, we showed that TRPC6 expression is increased in patients with FSGS along with increased calpain and calcineurin activity and decreased Talin-1 expression.
Inhibition of Calpain in a Model for Human FSGS Reduces Breakdown/Activation of Calpain Targets Talin-1 and Calcineurin and Ameliorates Proteinuria
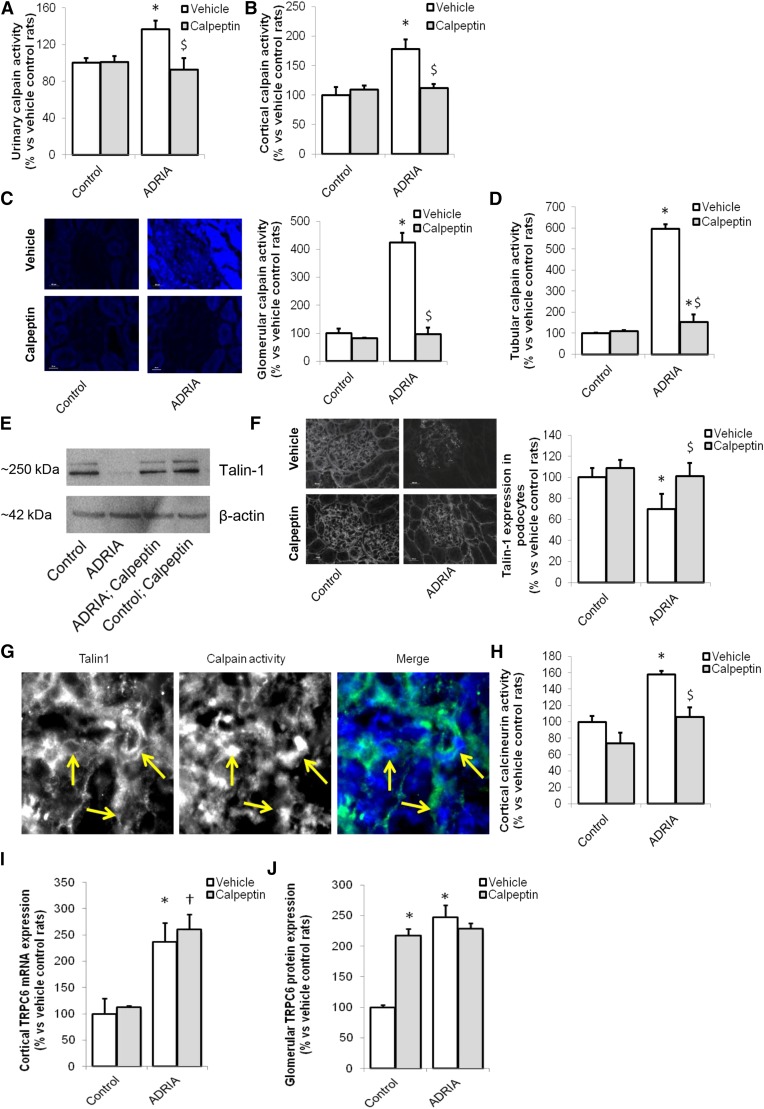
To study the role of calpain activation in podocyte injury and test the potential beneficial effect of calpain inhibition in an experimental model for human FSGS, we studied adriamycin-induced nephropathy rats and treated them with the calpain inhibitor calpeptin. Adriamycin treatment resulted in proteinuria (Supplemental Figure 4A) as described previously.16 Importantly, calpeptin treatment reduced urinary albumin (224±46 versus 1029±368 μg/mM creatinine) (Supplemental Figure 4A) and IgG excretion (6.8±2.7 versus 19.1±0.9 μg/ml) (Supplemental Figure 4B) in adriamycin-treated animals. Urinary calpain activity was increased in proteinuric rats, and it was normalized by calpeptin treatment (Figure 4A). Serum calpain activity showed no difference between groups, suggesting that enhanced renal calpain activity caused the increase in urinary calpain activity (Supplemental Figure 4C).
Figure 4.
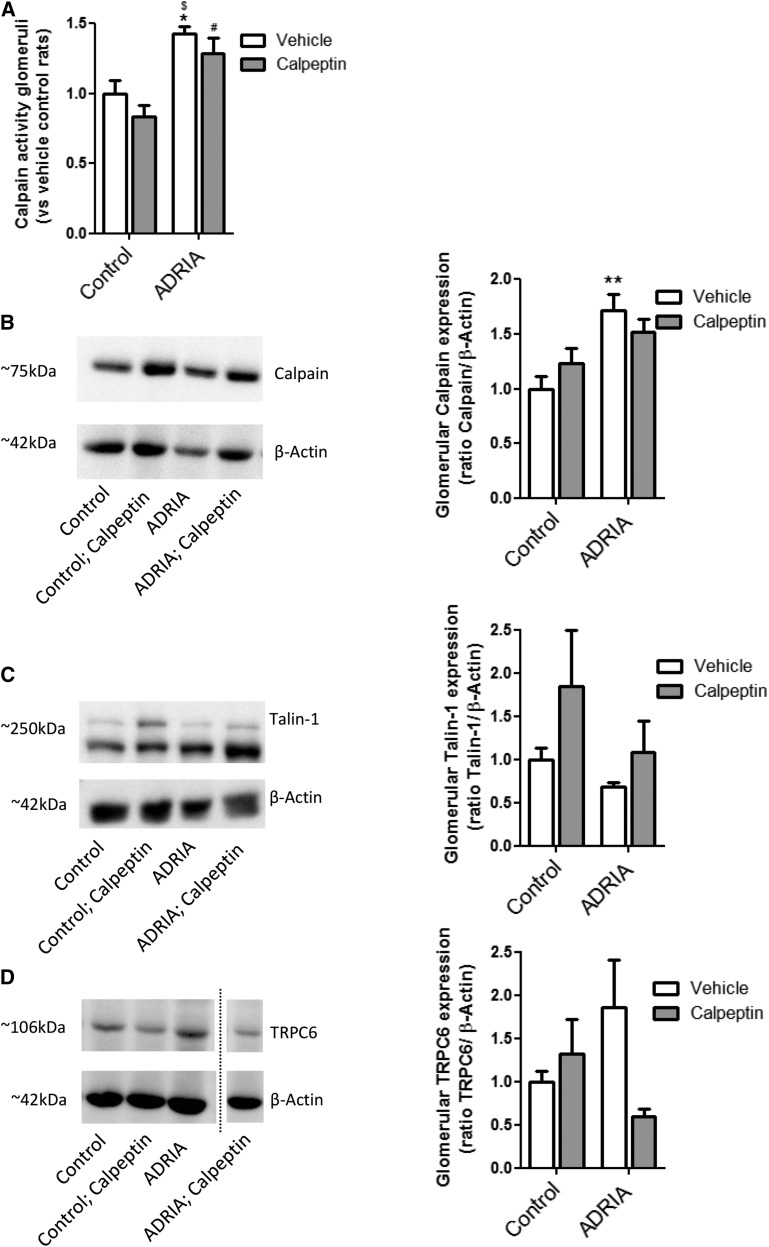
Inhibition of the enhanced calpain activity in an animal model for human FSGS improves renal outcome. Wistar rats were injected with vehicle (control) or adriamycin (ADRIA) to induce ADRIA nephropathy, a model for human FSGS. Subsequently, animals were treated with vehicle or calpeptin for 6 weeks, after which they were euthanized. (A) Urinary calpain activity was determined. (B) Protein was isolated from renal cortex, and a calpain activity assay was performed. Sections of the cortex were cut, an in situ zymography assay was performed, and (C) glomerular and (D) tubular calpain activity was determined. (E) Western blot analysis was performed to determine the expression of the calpain cleavage target Talin-1 in cortex extracts of snap-frozen kidneys (Talin-1–to–β-actin ratios: control, 0.20; ADRIA, 0.01; ADRIA + calpeptin, 0.25; control + calpeptin, 0.35). (F) Glomerular Talin-1 was costained with synaptopodin, and its expression was quantified. (G) Talin-1 was stained in combination with an in situ zymography for calpain activity. (H) Cortical calcineurin activity, as a downstream calpain target, was determined. (I) Cortical expression of Transient Receptor Potential channel C6 (TRPC6) mRNA was determined as well as (J) glomerular TRPC6 protein expression costained with synaptopodin measured by immunofluorescence. *P<0.05 compared with vehicle-treated control rats (n=8 rats per treatment group); †P<0.05 compared with calpeptin-treated vehicle rats (n=8 rats per treatment group); $P<0.05 compared with vehicle-treated ADRIA rats (n=8 rats per treatment group).
In line with the observations in human FSGS, calpain activity was increased in renal cortical protein extracts of adriamycin-induced nephropathy rats compared with controls, which was prevented by calpeptin treatment (Figure 4B). In situ zymography showed that glomerular and tubular calpain activity was greatly enhanced in adriamycin nephropathy, which was normalized by calpeptin treatment (Figure 4, C and D). Similar results were obtained by measuring the calpain activity in the supernatants of the in situ zymography (Supplemental Figure 4D). To confirm that aforementioned effects occur in glomeruli, calpain activity was determined in isolated glomeruli. Calpain activity was significantly increased in adriamycin-treated rats compared with control rats (Figure 5A). In addition, calpain expression was significantly increased in isolated glomeruli of adriamycin-treated rats compared with the control rats (Figure 5B). Moreover, cortical Talin-1 expression (Figure 4E) and glomerular Talin-1 expression (Figures 4F and 5C) were less abundant in adriamycin-treated animals, and they were completely restored by calpeptin treatment (Figures 4, E and F and 5C). At glomerular sites of high calpain activity, Talin-1 expression was low (Figure 4G). In addition, cortical calcineurin activity was increased in adriamycin-treated rats compared with healthy and calpeptin-treated animals (Figure 4H). Expression of the podocyte injury marker desmin was upregulated during adriamycin nephropathy, whereas this was normalized by calpeptin (Supplemental Figure 4E). The increased TRPC6 mRNA (Figure 4I) and protein (Figure 4J, Supplemental Figure 3B) expressions in renal cortex and podocytes of adriamycin-treated rats were not affected by treatment with calpeptin. In isolated glomeruli, the enhanced glomerular TRPC6 expression was confirmed as well as the effect of treatment with calpeptin in adriamycin-treated rats (Figure 5D). Calpeptin treatment increased glomerular TRPC6 protein abundance but not cortical TRPC6 mRNA expression. Taken together, TRPC6 expression is increased and calcineurin and calpain activated in experimental FSGS, leading to loss of the calpain target Talin-1 and proteinuria, which can be prevented by calpain inhibitor treatment.
Figure 5.
Increased calpain and Transient Receptor Potential channel C6 (TRPC6) in isolated glomeruli in an animal model for human FSGS. Wistar rats were injected with vehicle (control) or adriamycin (ADRIA) to induce ADRIA nephropathy, a model for human FSGS. Subsequently, animals were treated with vehicle or calpeptin for 6 weeks, after which they were euthanized. (A) Glomeruli were isolated, and calpain activity was determined. Western blot analysis was performed to determine the glomerular expression of (B) calpain, (C) Talin-1, and (D) TRPC6 in isolated glomeruli. Calpain activity is representative of two experiments, in which there were n=4 samples of isolated glomeruli per treatment group (material from two rats pooled per sample). Representative Western blots are shown, and graphs show combined data of two separate experiments using n=4 samples of isolated glomeruli per treatment group (material from two rats pooled per sample). *P<0.05 compared with vehicle-treated control rats; **P<0.01 compared with vehicle-treated control rats; #P<0.05 compared with calpeptin-treated control rats; $P<0.001 compared with calpeptin-treated control rats.
TRPC6-Dependent Ca2+ Influx Activates Calpain-1
In neurons, it has been shown that calpain-1 is the predominantly expressed calpain in the cell body and dendrites. Several papers showed clear similarities between neurons and podocytes, and dendrites share many features with podocyte foot processes. Therefore, we hypothesized that TRPC6-dependent Ca2+ influx would be responsible for the activation of calpain-1, which degrades Talin-1 and causes podocyte injury.
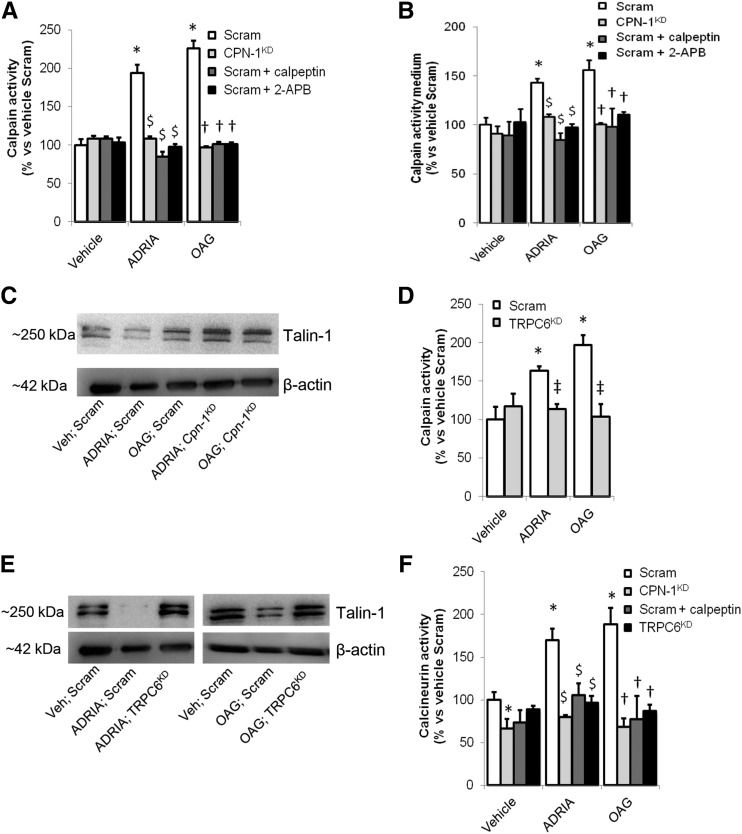
To determine whether TRPC6-dependent Ca2+ influx mediates calpain(-1) activation in the podocyte, cultured podocytes were transfected with calpain-1 siRNA (cpn-1KD), resulting in 72%±3% knockdown as quantified by quantitative PCR. Cpn-1KD or podocytes transfected with nontargeting scrambled siRNA (scram) were injured using adriamycin, or Ca2+ influx was induced using the TRPC channel activator OAG. Subsequently, the calpain inhibitor calpeptin, the TRP-channel blocker 2-APB, or vehicle was applied. Adriamycin and OAG increased cellular calpain activity (Figure 6A) and excretion of active calpain into the culture medium (Figure 6B). Importantly, in podocytes treated with calpeptin or 2-APB as well as in cpn-1KD cells, adriamycin- and OAG-induced calpain activation did not occur. Thus, calpain-1 is activated during adriamycin-induced podocyte injury and by activation of an OAG-sensitive, Ca2+-conducting TRP channel. Calpain-1 mRNA levels did not differ between adriamycin-treated and control scrambled podocytes (Supplemental Figure 5A), showing that the increased calpain activity is not caused by transcriptional regulation. Importantly, calpain-1 knockdown restored Talin-1 expression in adriamycin- and OAG-treated podocytes (Figure 6C).
Figure 6.
Calpain-1 is activated by Transient Receptor Potential channel C6 (TRPC6)–dependent Ca2+ influx into the podocyte. Differentiated immortalized cultured podocytes were transfected with nontargeting scrambled (Scram) or calpain-1 (CPN-1KD) siRNA. (A) Subsequently, they were treated with vehicle, adriamycin (ADRIA), or 1-oleoyl-2-acetyl-glycerol (OAG), and a calpain activity assay was performed. (B) In addition, medium was collected, and a calpain activity assay was performed to determine excretion of active calpain by podocytes in the culture medium. (C) Western blot analysis was performed to detect the downstream calpain target Talin-1 (Talin-1–to–β-actin ratio: 0.21 in ADRIA-treated cells versus 0.38 in ADRIA-treated Cpn-1KD cells; 0.35 in OAG-treated cells versus 0.51 in OAG-treated Cpn-1KD cells). To confirm that TRPC6 activates calpain-1, we used podocytes with an inducible stably transfected TRPC6 (TRPC6KD) or scrambled siRNA construct (scram). (D) After activation of the constructs, we treated the cells with vehicle, ADRIA, or OAG and measured calpain activity. (E) Western blotting for Talin-1 was performed using TRPC6KD or Scram cells treated with ADRIA or OAG (Talin-1–to–β-actin ratios: Veh-Scram, 0.20; ADRIA-scram, 0.02; ADRIA-TRPC6KD, 0.24; OAG-scram, 0.18; OAG-TRPC6KD, 0.23). (F) Subsequently, a calcineurin activity assay was performed. Results are representative of two experiments, with n=4 per group. 2-APB, 2-aminoethoxydiphenyl borate; siRNA, small interfering RNA. *P<0.05 compared with vehicle-treated scrambled cells; $P<0.05 compared with nontargeted transfected ADRIA-treated podocytes; †P<0.05 compared with scrambled siRNA transfected OAG-treated podocytes; ‡P<0.05 compared with scrambled siRNA transfected vehicle-treated podocytes.
Previously, we have shown that TRPC6 expression was upregulated during adriamycin-induced podocyte injury.7,16 To test the hypothesis that TRPC6-mediated Ca2+ influx activates calpain-1, we used podocytes stably transfected with TRPC6 (TRPC6KD) or scrambled siRNA (scram). We showed that the increased Ca2+ influx after adriamycin pretreatment in podocytes is TRPC6 dependent (Supplemental Figure 5B) as we did previously.8 Importantly, adriamycin- and OAG-induced calpain activation (Figure 6D) and loss of Talin-1 (Figure 6E) were prevented in TRPC6KD podocytes. TRPC6 was overexpressed after adriamycin application, irrespective of calpain-1 knockdown (Supplemental Figures 5C), suggesting that calpain-1 is downstream of TRPC6. Of note, calcineurin, which was hypothesized to be activated by TRPC6-dependent Ca2+ influx and/or calpain cleavage, is not activated in TRPC6KD and cpn-1KD cells (Figure 6F). Taken together, these data are in line with TRPC6-mediated Ca2+ influx, through activation of calpain-1, inducing subsequent loss of the cytoskeletal protein Talin-1 and activation of calcineurin.
Discussion
In this study, we elucidated a novel mechanism that links TRPC6 to activity of the nonlysosomal cysteine protease calpain-1, which is known to induce Talin-1 loss and calcineurin activation, thereby causing podocyte injury characteristic of FSGS and other podocytopathies. Podocyte injury–induced and Ca2+ influx–mediated calpain activity, Talin-1 loss, and calcineurin activation were inhibited by silencing TRPC6 or calpain-1 in cultured podocytes. These data support the hypothesis that TRPC6-mediated Ca2+ influx activates cytoplasmic calpain-1. In accordance, we showed similar findings in experimental and human FSGS.
Increased calpain activity in the kidney has been previously shown in renal ischemia-reperfusion and antiglomerular basement membrane injury, which could be prevented by blocking calpain activation.11–14 Recently, the large cytoskeletal-associated anchoring protein Talin-1 was shown to be critical for glomerular filtration barrier maintenance.15 Loss of Talin-1 seemed to be a key mechanism for the initiation and progression of podocyte injury associated with reduced β1-integrin activation and podocyte cell adhesion, thereby inducing profound alterations of the podocyte actin cytoskeleton.15 Importantly, in addition to Talin-1, calpains can also cleave the slit diaphragm protein nephrin.13 These findings concomitantly suggested that calpain-dependent cleavage of (structural) podocyte proteins, like nephrin and Talin-1, could disturb podocyte integrity, thereby inducing severe proteinuria.15
However, the underlying mechanism that leads to activation of calpains in the slit diaphragm microdomain of the podocyte remained elusive. TRPC6 was previously identified as a slit diaphragm–associated, Ca2+-conducting ion channel involved in podocyte injury, and it could, therefore, be responsible for the Ca2+-dependent activation of cytoplasmic calpains in the podocyte. Our data indeed showed that TRPC6-dependent Ca2+ influx into the podocyte activates the calpain family member calpain-1 and leads to loss of Talin-1. Calpain-1 knockdown as well as inhibition with calpeptin prevented the calpain activity resulting from both adriamycin-induced podocyte injury and Ca2+ influx through TRPC channels. Importantly, TRPC6 knockdown prevented both the calpain-1 activation as well as the loss of Talin-1 in these situations. In line with these results from cultured podocytes, patients with FSGS as well as the rat adriamycin nephropathy animal model for FSGS showed increased glomerular TRPC6 expression, enhanced glomerular and urinary calpain activity, and reduced glomerular Talin-1 expression. Accordingly, glomerular sites of high calpain activity colocalized with low Talin-1 expression in both human and rat FSGS. Importantly, the proteinuria and loss of Talin-1 in adriamycin nephropathy were ameliorated by calpeptin treatment. Whereas calpeptin could have affected other podocyte signaling pathways beyond calpain as well, our data are all in line with the initial hypothesis that TRPC6-mediated Ca2+ influx into the podocyte induces podocyte injury by activating calpain and subsequent loss of Talin-1, which would induce podocyte cytoskeletal changes.15
We and others previously showed that Ca2+ influx through TRPC6 activates calcineurin, leading to an angiotensin II–induced feed forward mechanism enhancing TRPC6 expression.8 Direct activation of the Ca2+-dependent phosphatase calcineurin by TRPC6-medicated Ca2+ influx was, however, never proven in the podocyte. Because calcineurin is a target of calpain and calpain has been shown to irreversibly activate calcineurin, calpain could be held responsible for the TRPC6-dependent activation of calcineurin.18 Notably, angiotensin II activates calpain in cardiomyocytes, which leads to calcineurin activation.19 Our current data showed that calcineurin activation coincided with TRPC6 overexpression and calpain activation in human and rat FSGS. Calcineurin activation in cultured podocytes was stimulated by OAG-induced Ca2+ influx, and it was absent when TRPC6 was knocked down, suggesting that calcineurin activation occurs downstream of TRPC6 as previously suggested. Importantly, calpain-1 knockdown completely prevented adriamycin- and OAG-induced calcineurin activation and even significantly reduced calcineurin activity in vehicle-treated control cells. These results suggest that calpain-1 is an important factor governing calcineurin activation in the podocyte and that at least the podocyte injury–induced and OAG-stimulated Ca2+ influx through TRPC6 may not directly activate calcineurin but necessitates the presence of calpain-1. Recently, such a calpain-mediated calcineurin cleavage mechanism was also suggested by data from Ding et al.20 in puromycin aminonucleoside–treated podocytes, where calcineurin was restored after calpain-1 knockdown.
Calpain-1 knockdown and calpain inhibition by calpeptin did not alter TRPC6 mRNA expression in cultured podocytes and animal studies. However, calpeptin significantly increased TRPC6 protein levels in the absence of primary podocyte injury. Interestingly, it was previously shown that calpain is able to cleave the TRPC6 protein in neurons and that inhibition of calpain will increase neuronal TRPC6 protein levels.21 Thus, the combined observations of increased glomerular TRPC6 protein expression, but not TRPC6 mRNA expression, in calpeptin-treated control animals could be due to inhibition of TRPC6 protein cleavage by calpain. Therefore, calpain-mediated TRPC6 cleavage could represent a negative feedback loop to control potentially unrestrained TRPC6 expression and thereby, activity in podocyte injury (a schematic summary is provided in Supplemental Figure 7). This also raises the possibility of different intracellular versus extracellular action of calpain(s), which opens interesting new perspectives regarding TRPC6-triggered enzymatic actions in kidney epithelial cells, including podocytes.
In conclusion, we have elucidated a novel mechanism that links deleterious Ca2+ influx through the slit diaphragm–associated ion channel TRPC6, via calpain-1 activation, to important events that lead to podocyte injury, such as loss of Talin-1 and activation of calcineurin. Identification of other possible downstream targets of calpain-1 in the podocyte, thereby initiating or propagating podocyte injury and/or apoptosis (whereas morphologically, we could not observe signs of apoptosis), awaits further investigation as does a possible effect of calpain on TRPC6 itself. Our data also reveal that calpain activity is induced in the tubular compartment in FSGS and adriamycin-induced nephropathy, where Talin-1 expression seems to be reduced as well. The significance of these findings for tubular function and possibly, tubular injury in this primary glomerular disease context remains to be established in future research. Importantly, our data highlight that TRPC6-dependent activation of calpain-1 might be a key mechanism in the initiation and progression of podocyte injury in glomerular diseases. This identifies both TRPC6 and calpain-1 as future therapeutic targets in the treatment of proteinuria and prevention of glomerular injury, which we substantiated by showing the effect of calpeptin administration in experimental FSGS.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Prof. Dr. Jochen Reiser (Rush University Medical Center, Chicago, IL) for providing mouse podocyte cell lines.
This work was financially supported by Kolff Senior Postdoc Career Stimulation Grant 13OKS023 (to T.N.) from the Dutch Kidney Foundation.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016111248/-/DCSupplemental.
References
- 1.Durvasula RV, Petermann AT, Hiromura K, Blonski M, Pippin J, Mundel P, et al.: Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int 65: 30–39, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Endlich N, Kress KR, Reiser J, Uttenweiler D, Kriz W, Mundel P, et al.: Podocytes respond to mechanical stress in vitro. J Am Soc Nephrol 12: 413–422, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, et al.: Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24: 251–256, 2000 [DOI] [PubMed] [Google Scholar]
- 4.White KE, Bilous RW; Diabiopsies Study Group : Structural alterations to the podocyte are related to proteinuria in type 2 diabetic patients. Nephrol Dial Transplant 19: 1437–1440, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Reiser J, Polu KR, Möller CC, Kenlan P, Altintas MM, Wei C, et al.: TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet 37: 739–744, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, et al.: A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308: 1801–1804, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Sonneveld R, van der Vlag J, Baltissen MP, Verkaart SA, Wetzels JF, Berden JH, et al.: Glucose specifically regulates TRPC6 expression in the podocyte in an AngII-dependent manner. Am J Pathol 184: 1715–1726, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Nijenhuis T, Sloan AJ, Hoenderop JG, Flesche J, van Goor H, Kistler AD, et al.: Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am J Pathol 179: 1719–1732, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Möller CC, Wei C, Altintas MM, Li J, Greka A, Ohse T, et al.: Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol 18: 29–36, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Goll DE, Thompson VF, Li H, Wei W, Cong J: The calpain system. Physiol Rev 83: 731–801, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee PK, Brown PA, Cuzzocrea S, Zacharowski K, Stewart KN, Mota-Filipe H, et al.: Calpain inhibitor-1 reduces renal ischemia/reperfusion injury in the rat. Kidney Int 59: 2073–2083, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee PK, Todorovic Z, Sivarajah A, Mota-Filipe H, Brown PA, Stewart KN, et al.: Inhibitors of calpain activation (PD150606 and E-64) and renal ischemia-reperfusion injury. Biochem Pharmacol 69: 1121–1131, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Peltier J, Bellocq A, Perez J, Doublier S, Dubois YC, Haymann JP, et al.: Calpain activation and secretion promote glomerular injury in experimental glomerulonephritis: Evidence from calpastatin-transgenic mice. J Am Soc Nephrol 17: 3415–3423, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Blass G, Levchenko V, Ilatovskaya DV, Staruschenko A: Chronic cathepsin inhibition by E-64 in Dahl salt-sensitive rats. Physiol Rep 4: e12950, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian X, Kim JJ, Monkley SM, Gotoh N, Nandez R, Soda K, et al.: Podocyte-associated talin1 is critical for glomerular filtration barrier maintenance. J Clin Invest 124: 1098–1113, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonneveld R, Ferrè S, Hoenderop JG, Dijkman HB, Berden JH, Bindels RJ, et al.: Vitamin D down-regulates TRPC6 expression in podocyte injury and proteinuric glomerular disease. Am J Pathol 182: 1196–1204, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Nakatani S, Kakehashi A, Ishimura E, Yamano S, Mori K, Wei M, et al.: Targeted proteomics of isolated glomeruli from the kidneys of diabetic rats: Sorbin and SH3 domain containing 2 is a novel protein associated with diabetic nephropathy. Exp Diabetes Res 2011: 979354, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SH, Choi J, Kim H, Lee DH, Roh GS, Kim HJ, et al.: FK506 reduces calpain-regulated calcineurin activity in both the cytoplasm and the nucleus. Anat Cell Biol 47: 91–100, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu HY, Tomizawa K, Matsui H: Calpain-calcineurin signaling in the pathogenesis of calcium-dependent disorder. Acta Med Okayama 61: 123–137, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Ding F, Li X, Li B, Guo J, Zhang Y, Ding J: Calpain-mediated cleavage of calcineurin in puromycin aminonucleoside-induced podocyte injury. PLoS One 11: e0155504, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du W, Huang J, Yao H, Zhou K, Duan B, Wang Y: Inhibition of TRPC6 degradation suppresses ischemic brain damage in rats. J Clin Invest 120: 3480–3492, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.