Abstract
Background Although pharmacotherapeutic proteinuria lowering was found to be nephroprotective in adults, the predictive value of early drug-induced proteinuria reduction for long-term renal survival in pediatric CKD is unknown. We analyzed data from the ESCAPE Trial for a potential association between initial antiproteinuric effect of standardized angiotensin-converting enzyme (ACE) inhibition and renal disease progression in children with CKD.
Methods In total, 280 eligible children with CKD stages 2–4 (mean age 11.7 years old, median eGFR 46 ml/min per 1.73 m2, 71% congenital renal malformations) received a fixed dose of ramipril (6 mg/m2 per day) and were subsequently randomized to conventional or intensified BP control. We assessed initial proteinuria reduction from baseline to first measurement on ramipril (at 2.5±1.3 months). We used multivariable Cox modeling to estimate the association between initial proteinuria reduction and the risk of reaching a renal end point (50% eGFR decline or ESRD), which occurred in 80 patients during 5 years of observation.
Results Ramipril therapy lowered proteinuria by a mean of 43.5% (95% confidence interval, 36.3% to 49.9%). Relative to proteinuria reduction <30%, 30%–60% and >60% reduction resulted in hazard ratios (95% confidence intervals) of 0.70 (0.40 to 1.22) and 0.42 (0.22 to 0.79), respectively. This association was independent of age, sex, CKD diagnosis, baseline eGFR, baseline proteinuria, initial BP, and concomitant BP reduction.
Conclusions The early antiproteinuric effect of ACE inhibition is associated with long-term preservation of renal function in children with CKD. Proteinuria lowering should be considered an important target in the management of pediatric CKD.
Keywords: proteinuria, chronic kidney disease, children, ACE inhibition
In the general adult population, increased levels of urinary albumin are present in approximately 7% of individuals, and they are associated with a higher risk of renal and cardiovascular disease.1–3 We recently showed that, in the general toddler population, increased albuminuria is present in approximately 7% of the children, in analogy to that in the adult population.4 Also, in children with CKD, higher proteinuria levels are associated with an increased risk of cardiovascular and renal disease progression.5–7
Antagonists of the renin-angiotensin system (RAS) efficiently lower proteinuria. Studies with angiotensin-converting enzyme (ACE) inhibitors and angiotensin II type I receptor blockers in adults with CKD due to glomerular disorders have shown that the larger the reduction in albuminuria induced by these agents during the first months of treatment, the larger the reduction in renal and cardiovascular risk during subsequent follow-up.8–10 Also in children, in whom CKD is most often caused by a congenital nephron deficit due to kidney maldevelopment, ACE inhibitors and angiotensin II type I receptor blockers have been shown to reduce albuminuria.11,12 However, the effect of proteinuria lowering on long-term renal survival has not been established in the pediatric CKD population.
Here, we made use of the largest pharmacologic nephroprotection trial performed to date in children to investigate a possible quantitative association between the initial antiproteinuric effect of ACE inhibition and subsequent CKD progression. We also determined whether residual proteinuria (i.e., the proteinuria level during ACE inhibition) is associated with a higher renal risk, arguing that positive findings for these two associations would strengthen the hypothesis that albuminuria is also an important modifiable determinant of renal disease progression in pediatric CKD.
Methods
Study Design and Patients
For this study, we used data from the Effect of Strict Blood Pressure Control and ACE inhibition on the Progression of CRF in Pediatric Patients (ESCAPE) Trial. Rationale, study design, and results of this study have been published elsewhere.11 In short, the ESCAPE Trial was an investigator-initiated, randomized, controlled trial investigating whether intensified BP control (<50th percentile for age) would delay the progression of renal disease in children with CKD who were receiving a fixed dose of ACE inhibition. In this study, 385 children with CKD (ages 3–18 years old, eGFR of 15–80 ml/min per 1.73 m2 body surface area), whose 24-hour mean arterial pressure (MAP) was either elevated (>95th percentile) or controlled with antihypertensive medication, were included. Exclusion criteria were renal artery stenosis, a history of kidney transplantation, an unstable clinical condition, treatment with immunosuppressive agents (including glucocorticoids), and major primary cardiac, hepatic, or gastrointestinal disorders. The study protocol was approved by the central ethics committee of the medical faculty of the University of Heidelberg and the local institutional review board of each site. Parents of all children provided written informed consent.
At screening, eligible patients underwent an ambulatory BP monitoring. Eligible patients started a run-in period of 6 months, during which they attended clinic visits every 2 months. At least 2 months before the end of the run-in period, any treatment with an inhibitor of the RAS was discontinued. After the run-in period, all children received the same dose of the ACE inhibitor ramipril (6 mg/m2 body surface area per day, equivalent to the maximum approved dose of 10 mg/d in adults). The dosage was gradually uptitrated over the course of 2 months. Subsequently, patients were stratified to either a conventional BP target (50th–95th percentile of 24-hour MAP) or an intensified BP target (<50th percentile of 24-hour MAP). To reach the BP target, any antihypertensive agent could be prescribed, except for other inhibitors of the RAS.
During the 5-year study period, BP, proteinuria, and eGFR were assessed every 2 months, and 24-hour ambulatory BP measurements were performed every 6 months. eGFR was assessed by means of the Schwartz formula with the use of measurements of serum creatinine and height and a k constant of 0.55.13 ACE I/D genotype was measured in blood collected during the run-in period using the method that has been extensively described previously.14
Proteinuria Measurements
Urine collections (where possible, a 24-hour urine collection) were performed every 2 months. If collection of a 24-hour urine sample was not possible due to young age or enuresis, a random urine sample was collected during the clinical visit. At the baseline visit, a random sample instead of a 24-hour urine sample was collected in 43 individuals. Total protein and creatinine concentrations were measured in the samples with the use of Coomassie blue staining and modified Jaffe reaction, respectively. Proteinuria was measured as the urinary protein-to-creatinine ratio in milligrams per milligram. Throughout the article, we use the term proteinuria.
The initial proteinuria reduction was defined as the natural logarithm of the reduction in proteinuria from baseline measurement to the first proteinuria measurement after attainment of the full dose of ramipril. It was calculated as follows: ln(first measurement after full dose of ramipril/baseline measurement). We defined residual proteinuria as the level of proteinuria present during treatment with 6 mg/m2 ramipril. Proteinuria exposure over time was calculated as the area under the curve (AUC) of all proteinuria measurements from baseline until reaching either the end point or the end of the study. The median number of proteinuria measurements to calculate the AUC was 15 (25th–75th percentile: 8–24 measurements).
In this study, we included 280 children of the initial ESCAPE Trial population. Children with a missing proteinuria level at baseline (n=43), with no follow-up measurement within 6 months after starting ramipril (n=29), with a missing ambulatory BP measurement at baseline (n=3) or after 6 months (n=21), or who were lost to follow-up (n=9) were excluded.
Outcomes
The prespecified primary efficacy measure of the ESCAPE Trial was time from attainment of the full dose of ramipril until the first event of the composite end point, which was defined as a sustained 50% reduction of eGFR or progression to ESRD (eGFR<10 ml/min per 1.73 m2 or start of RRT). In this study, the same composite end point was used.
Statistical Analyses
The cumulative event rate for the composite renal end point in subgroups of proteinuria reduction was assessed using the Kaplan–Meier procedure. Proteinuria reduction was stratified into three subgroups: >60% reduction, 30%–60% reduction, and <30% reduction. These groups were chosen post hoc to provide easily understandable thresholds, whereas the number of patients within the groups remains similar. Differences in population characteristics between the three groups of proteinuria reduction were tested with chi-squared test, ANOVA, or Kruskal–Wallis test where appropriate. A Cox proportional hazards model was used to estimate the renal risk difference among the three subgroups of proteinuria reduction. The Cox model was adjusted for the following covariates: age, sex, CKD diagnosis (glomerulopathy, congenital anomalies of the kidney and urinary tract, or other diagnosis), baseline eGFR, baseline proteinuria, baseline BP, and BP reduction from baseline to month 6. Multivariable linear regression was performed to assess which baseline variables were associated with proteinuria reduction. To ascertain that the effect of proteinuria reduction on the composite renal end point was not modified by either baseline proteinuria or BP reduction, we performed two separate multivariable Cox models, in which an interaction term between either baseline proteinuria and proteinuria reduction or BP reduction and proteinuria reduction was included.
The association between residual proteinuria and the renal end point was estimated using the Kaplan–Meier procedure, with residual proteinuria categorized into three subgroups: <0.2, 0.2–1.0, and >1.0 mg/mg. To assess the effect of residual proteinuria on the renal end point, a multivariable Cox proportional hazards model with the three groups of residual proteinuria was performed, and it was adjusted for the covariates mentioned earlier. Because proteinuria may change over time during prolonged exposure to Renin-Angiotensin-Aldosterone system blockade, we also assessed the association between subgroups of exposure to proteinuria over time and renal risk using both the Kaplan–Meier analysis and a multivariable Cox proportional hazards model adjusted for the covariates mentioned above.15 Exposure to proteinuria was stratified into tertiles.
To assess whether the findings of the association between proteinuria reduction and the composite renal end point were robust and were not dependent on the selection of the thresholds, we analyzed the association between proteinuria reduction as a continuous measure and the composite renal end point in a sensitivity analysis. Sensitivity of the proteinuria assay in the low-normal proteinuria range is low, and measurements below the limit of detection may introduce misclassifications in proteinuria reduction. Therefore, a sensitivity analysis was performed, in which children with a baseline proteinuria <0.1 mg/mg were excluded. In this sensitivity analysis, we used a Cox proportional hazards model with initial proteinuria reduction and the same covariates as described earlier. To ascertain that the results are not driven by the individuals with the highest baseline proteinuria, we performed a sensitivity analysis, in which patients with a baseline proteinuria >90th percentile were excluded. A final sensitivity analysis was performed, in which children who started additional antihypertensive medication to reach the BP target were excluded to avoid interference of other antihypertensive therapy on the association between proteinuria change and renal outcome. In this analysis, proteinuria reduction was analyzed as a continuous measure, because we did not have enough power to use three proteinuria reduction groups.
Data are expressed as either mean and SD or median and interquartile range for continuous variables and percentage and count for categorical variables. All analyses were performed using STATA version 14 (Statacorp LP).
Results
Mean patient age was 11.7 years old (SD=3.9), and 60% were boys. The mean time interval between baseline proteinuria measurement and first measurement on full-dose ramipril was 2.5 months (SD=1.3 months). The characteristics of the population included in this study were similar to those of the overall ESCAPE Trial population (Table 1).
Table 1.
Population characteristics (on the basis of proteinuria reduction divided into three categories)
| Variable | Total ESCAPE Trial Population, n=385 | Population Selected for This Analysis, n=280 | P Valuea | |||
|---|---|---|---|---|---|---|
| Total Study Population, n=280 | Proteinuria Reduction >60%, n=92 | Proteinuria Reduction 30%–60%, n=76 | Proteinuria Reduction <30%, n=112 | |||
| Boys, n | 230 (60) | 167 (60) | 54 (59) | 49 (64) | 64 (57) | 0.59 |
| Age, yr | 11.6 (4.0) | 11.7 (3.9) | 10.9 (3.8) | 12.4 (3.9) | 11.5 (3.8) | 0.04 |
| Conventional treatment arm, n | 195 (51) | 140 (50) | 41 (45) | 40 (53) | 59 (53) | 0.45 |
| Renal diagnosis, n | 0.44 | |||||
| Glomerulopathies | 51 (13) | 36 (13) | 14 (15) | 10 (13) | 12 (11) | |
| CAKUT | 267 (70) | 199 (71) | 60 (65) | 58 (76) | 81 (72) | |
| Other | 66 (17) | 45 (16) | 18 (20) | 8 (11) | 19 (17) | |
| ACE polymorphism, n | 0.29 | |||||
| II genotype | 70 (20) | 52 (19) | 23 (25) | 12 (16) | 17 (16) | |
| ID genotype | 184 (59) | 139 (52) | 44 (48) | 43 (58) | 52 (55) | |
| DD genotype | 103 (29) | 79 (29) | 25 (27) | 19 (26) | 35 (34) | |
| Reached end point, n | 133 (36) | 80 (29) | 17 (18) | 22 (29) | 41 (37) | 0.27 |
| Baseline eGFR, ml/min per 1.73 m2 | 44 (29–59) | 46 (33–59) | 50 (37–61) | 43 (28–55) | 45 (31–60) | 0.06 |
| Baseline urinary protein-to-creatinine ratio, mg/mg | 0.9 (0.3–2.0) | 0.8 (0.3–1.8) | 1.0 (0.4–2.3) | 1.0 (0.4–1.8) | 0.6 (0.2–1.5) | 0.001 |
| Baseline serum urea, mmol/L | 13.1 (6.5) | 12.8 (6.1) | 12.1 (5.7) | 13.5 (6.1) | 13.0 (6.5) | 0.44 |
| Baseline ambulatory MAP, SDS | 1.2 (0.2–2.3) | 1.2 (0.2–2.3) | 1.3 (0.4–2.1) | 1.0 (0.2–2.2) | 1.1 (0.2–2.4) | 0.97 |
| Ambulatory MAP reduction, SDS | 1.2 (0.3–2.1) | 1.2 (0.4–2.1) | 1.4 (0.5–2.2) | 1.2 (0.6–2.0) | 1.1 (0.1–2.1) | 0.12 |
Values for continuous variables are described as mean±SD or median (25th–75th percentile); values for categorical variables as number (percentage). ESCAPE, Effect of Strict Blood Pressure Control and ACE inhibition on the Progression of CRF in Pediatric Patients; CAKUT, congenital anomalies of the kidney and urinary tract; ACE, angiotensin-converting enzyme; MAP, mean arterial pressure; SDS, standard deviation score.
P value for difference among the three strata of proteinuria reduction.
Initial Proteinuria Reduction within the Study Population
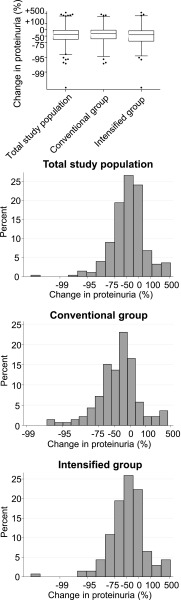
The mean initial proteinuria change was −40.2% (95% confidence interval [95% CI], −49.1 to −29.6%) in the conventional BP control group and −46.7% (95% CI, −55.5% to −36.2%) in the intensified BP control group (P=0.35 for the between-group difference) (Figure 1). Because of the similar initial proteinuria change in the two study arms, the groups were combined for further analysis. Initial proteinuria change was similar between the different renal diagnoses, with a mean initial proteinuria change of −48.7% (95% CI, −61.1% to −32.1%) in the children with glomerulopathies, −39.9% (95% CI, −47.7% to −30.9%) in the children with congenital anomalies of the kidney and urinary tract, and −53.6% (95% CI, −68.3% to −32.2%) in the children with other diagnoses (P=0.26). In the total study population, initial proteinuria was reduced by a mean of 43.5% (95% CI, 36.3% to 49.9%), with a large interindividual variation (range, −99.8%–547%) (Figure 1). An initial proteinuria reduction by >60% was observed in 33% of the patients, an initial proteinuria reduction by 30%–60% was observed in 27% of the patients, and an initial proteinuria reduction by <30% was observed in 40% of the patients. Baseline proteinuria was lower in the group with the least proteinuria reduction, and age was different between the three groups of proteinuria reduction. Other population characteristics were similar among the groups (Table 1). By multivariable linear regression analysis, higher proteinuria (β=0.14 per 1 mg/mg; P<0.001) was independently associated with a larger proteinuria reduction, whereas sex, age, treatment assignment, primary renal diagnosis, baseline eGFR, baseline BP, baseline serum urea, and ACE I/D genotype were not independently associated with proteinuria lowering (Supplemental Table 1).
Figure 1.
Initial proteinuria change has a large interindividual variation. Distribution of initial proteinuria change in the total study population. (Upper panel) Box and whisker plots of proteinuria change. Whiskers represent 2.5th to 97.5th percentiles. (Lower panel) Distribution of intraindividual proteinuria change in the total study population, the conventional BP control group, and the intensified BP control group.
Initial Proteinuria Reduction as a Predictor of Renal Outcomes
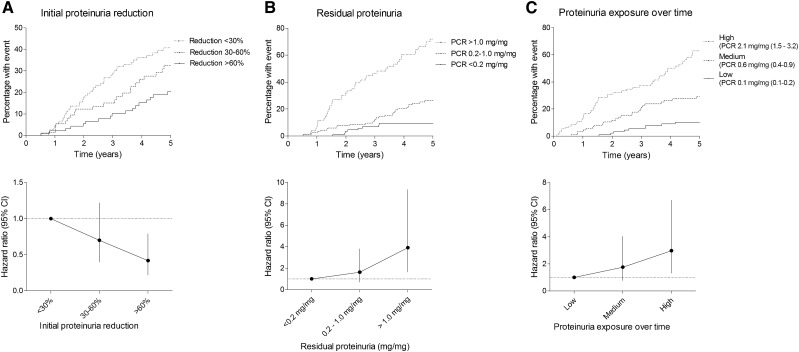
Larger initial proteinuria reduction was associated with a larger risk reduction for the primary renal end point (Figure 2A). Taking the differences in baseline characteristics into account in a multivariable Cox model, the subgroup with the largest initial proteinuria reduction (>60%) remained at lowest risk (hazard ratio, 0.42; 95% CI, 0.22 to 0.79) relative to the patients with <30% reduction. The intermediate proteinuria responders (30%–60% reduction) showed an insignificant relative risk reduction (hazard ratio, 0.70; 95% CI, 0.40 to 1.22) (Figure 2A, Table 2). The effect of proteinuria reduction was not modified by either baseline proteinuria or BP reduction as evidenced by the insignificant interaction terms represented in Supplemental Tables 2 and 3. Results were similar when proteinuria reduction was analyzed as a continuous variable (Supplemental Table 4).
Figure 2.
More proteinuria reduction, lower residual proteinuria, and lower proteinuria exposure over time are associated with lower renal risk. Risk on the composite renal end point with Kaplan–Meier analysis and hazard ratios calculated with the Cox proportional hazard model1 for (A) initial proteinuria reduction, (B) residual proteinuria, and (C) exposure to proteinuria over time.1 Initial proteinuria reduction was adjusted for age, sex, CKD diagnosis, baseline ambulatory mean arterial pressure (MAP), baseline eGFR, baseline proteinuria, and change in ambulatory MAP. Residual proteinuria was adjusted for age, sex, CKD diagnosis, baseline ambulatory MAP, baseline eGFR, and change in ambulatory MAP. Long-term exposure to proteinuria was adjusted for age, sex, CKD diagnosis, baseline ambulatory MAP, baseline eGFR, and change in ambulatory MAP. PCR, protein-to-creatinine ratio.
Table 2.
Adjusted Cox proportional hazards model with association between initial proteinuria reduction and composite renal end point
| Variable | Hazard Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Proteinuria reduction, % | |||
| <30 | 1.00 (reference group) | 0.40 to 1.22 | 0.21 |
| 30–60 | 0.70 | 0.22 to 0.79 | <0.01 |
| >60 | 0.42 | ||
| Boys | 0.77 | 0.47 to 1.25 | 0.29 |
| Age, yr | 1.08 | 1.02 to 1.15 | 0.01 |
| Baseline ambulatory MAP, SDS | 1.18 | 1.00 to 1.38 | 0.05 |
| Diagnosis group | |||
| Glomerulopathies | 1.00 (reference group) | 0.31 to 1.05 | 0.07 |
| CAKUT | 0.57 | 0.38 to 1.89 | 0.69 |
| Other | 0.85 | ||
| Baseline eGFR, ml/min per 1.73 m2 | 0.93 | 0.91 to 0.94 | <0.001 |
| Baseline urinary protein-to-creatinine ratio, mg/mg | 1.21 | 1.09 to 1.34 | <0.001 |
| Ambulatory MAP reduction, SDS | 0.92 | 0.77 to 1.10 | 0.39 |
No interaction was detected between proteinuria reduction and baseline proteinuria when added to the model (P=0.12). All model parameters are shown in Supplemental Table 2. No interaction was detected between proteinuria reduction and BP reduction when added to the model (P=0.10). All model parameters are shown in Supplemental Table 3. MAP, mean arterial pressure; SDS, standard deviation score; CAKUT, congenital anomalies of the kidney and urinary tract.
A sensitivity analysis that excluded 26 individuals with baseline proteinuria below 0.1 mg/mg showed a similar pattern in that patients with a larger initial proteinuria reduction had a lower risk of the composite end point, with hazard ratios of 0.43 (95% CI, 0.23 to 0.83) for the >60% reduction group and 0.75 (95% CI, 0.43 to 1.33) for the 30%–60% reduction group compared with the <30% reduction group (Supplemental Table 5). The same results were seen when patients with a baseline proteinuria >90th percentile were excluded (Supplemental Table 6). Results were also confirmed when 138 patients with additional antihypertensive medication during the study were excluded, with larger initial proteinuria reduction remaining associated with a lower renal risk (hazard ratio, 0.41; 95% CI, 0.22 to 0.77) (Supplemental Table 7).
Residual Proteinuria as a Predictor of Renal Outcomes
After attaining the full dose of ramipril, the median residual proteinuria was 0.44 mg/mg (25th–75th percentile, 0.14–1.04 mg/mg). Residual proteinuria was <0.2 mg/mg in 32%, between 0.2 and 1.0 mg/mg in 42%, and >1.0 mg/mg in 26% of patients. Kaplan–Meier analysis and Cox modeling revealed that children with a higher residual proteinuria level carry a higher risk of reaching the composite renal end point (Figure 2B), with hazard ratios calculated with the multivariable Cox model of 3.91 (95% CI, 1.64 to 9.33) for the residual proteinuria >2.0 mg/mg group and 1.63 (95% CI, 0.70 to 3.81) for the residual proteinuria 0.2–2.0 mg/mg group compared with the residual proteinuria <0.2 mg/mg group.
The median exposures to proteinuria over time during the entire study period as calculated with the AUC of all proteinuria measurements from baseline until either reaching the end point or the end of the study were 0.1 (25th–75th percentile, 0.1–0.2) mg/mg in the group with the lowest exposure, 0.6 (25th–75th percentile, 0.4–0.9) mg/mg in the medium proteinuria exposure group, and 2.1 (25th–75th percentile, 1.5–3.2) mg/mg in the highest exposure group. Higher proteinuria exposure over time was an independent predictor for reaching the renal end point (Figure 2C, Supplemental Table 8).
Discussion
In this post hoc analysis of a large interventional trial, we studied the effect of proteinuria lowering with standardized ACE inhibition on renal survival in children with CKD. A higher degree of proteinuria lowering during the first months of treatment was independently associated with a lower risk of CKD progression. In addition, both a higher residual proteinuria level after attaining the full dose of ramipril and the total exposure to proteinuria during follow-up accounted for a higher risk of CKD progression. These findings were independent of the underlying disease, baseline proteinuria, and BP control. Collectively, these data extend previous studies in adult populations and highlight the importance of proteinuria, next to BP, as a risk factor for renal disease progression in children with CKD.
Whereas the degree of proteinuria has been described as a risk factor for progression of renal disease in children, the long-term effect of drug-induced proteinuria lowering on renal survival has not been well established in children. In adults, several post hoc trial analyses have shown a positive association between initial reduction of proteinuria or albuminuria and renal disease progression. These studies have been performed in different patient populations with heterogeneous disorders (e.g., diabetic nephropathies, nondiabetic hypertensive kidney disease, and proteinuric chronic nephropathy), and they have used drugs intervening in the RAS.8–10,16,17 One observational study in 20 children with chronic nephropathies investigated the effect of combined treatment of ramipril and losartan on eGFR slopes. Children who achieved remission of proteinuria (defined as >50% reduction in proteinuria to <200 mg/d) had improved eGFR slopes compared with children who did not achieve remission.12 However, that study did not assess the effect of proteinuria reduction during RAS blockade on clinical outcomes. Here, we show in a large, prospectively followed pediatric cohort that more efficient proteinuria lowering during the first few months of ACE inhibitor therapy is associated with a reduced risk of renal failure progression. In keeping with previous findings in adults, our results emphasize the potential importance of proteinuria as a therapeutic target in children with CKD.
The initial proteinuria-lowering effect varied widely between individuals, in keeping with observations in adults.18,19 We found no clear indicators that could explain the large variation of proteinuria response to a defined ACE inhibitor dose. In adults, determinants of the individual response to an ACE inhibitor include dietary consumption of salt and proteins and the ACE I/D polymorphism.20–23 In this study, the ACE I/D genotype did not explain the variability of the proteinuria response to ramipril.
To further investigate the role of proteinuria as a risk predictor of renal outcomes during continued ACE inhibition, we also assessed the association between residual proteinuria and renal outcome. Several adult studies have shown that exposure to proteinuria over time is a very important, if not the most important, predictor of renal outcomes in adults.24–26 To our knowledge, no study in children has investigated the predictive value of long-term exposure to proteinuria. Both higher residual proteinuria and higher proteinuria exposure over time accounted for a higher renal risk in this study population, further suggesting that proteinuria might be a very important parameter in renal disease progression in children with CKD. It should be emphasized, however, that the early proteinuria reduction predicted renal survival as well as the long-term evolution of proteinuria, highlighting the true predictive value of this early effect.
The observation that patients with the least proteinuria reduction were at highest risk of CKD progression warrants additional strategies to further lower proteinuria in these patients. These include the addition of hydrochlorothiazide to the treatment protocols; dietary interventions, such as a moderation of dietary sodium or protein intake; or possibly, novel proteinuria lowering therapies other than RAS inhibition (e.g., SGLT-2 inhibitors).6,27–31 Finally, monitoring BP and treating hypertension are also important in these patients, because BP control is the only other intervention that has been proven to be nephroprotective in children with CKD.11 Hence, ACE inhibition aimed at improving renal outcomes in children with CKD may require a dual strategy that optimizes both BP and proteinuria control.
We recognize several limitations to our study. It should be noted that this study is a post hoc analysis of a clinical trial that was not designed to investigate the association between ACE inhibition–induced proteinuria lowering and renal end points and that results must be interpreted accordingly. A prospective study aimed at investigating the effect of proteinuria lowering on renal survival in children with CKD would be required to unequivocally confirm the findings described in this paper. Furthermore, additional antihypertensive medication initiated during the trial in approximately one half of the study population to reach the BP target could have interfered with CKD progression. A sensitivity analysis that excluded patients who received additional antihypertensive medication showed similar results as our main analyses, suggesting minimal effect of additional antihypertensive medication use on our findings. A final caveat relates to the difficulty of separating out the relative benefits of proteinuria and BP reduction. However, because BP lowering was taken into account as a covariate in the multivariable Cox models, it is suggested that the effect of proteinuria reduction was largely independent of the effect of BP lowering. Moreover, the statistically nonsignificant interaction between BP reduction and proteinuria reduction suggests that the effect of proteinuria reduction is not modified by BP reduction.
In conclusion, this post hoc analysis shows that the early antiproteinuric effect of ACE inhibition independently predicts long-term preservation of renal function in children with CKD. Moreover, higher residual proteinuria and higher long-term proteinuria exposure account for worse renal survival. These findings indicate that proteinuria lowering is an important target in the management of children with CKD.
Disclosures
F.S. reports serving as a paid advisor for Abbvie, Astellas, and Bayer. No other potential conflicts of interest relevant to this article were reported
Supplementary Material
Acknowledgments
The study protocol was developed exclusively by the participants. Onsite monitoring of study data collection was performed by an independent clinical research organization (Omnicare Clinical Research).
The Effect of Strict Blood Pressure Control and ACE inhibition on the Progression of CRF in Pediatric Patients (ESCAPE) Trial was supported by grants from the Boehringer Ingelheim Stiftung, the European Commission (Fifth Framework Programme QLRT-2001-00908), the Kuratorium für Dialyse und Nierentransplantation e.V., Neu-Isenburg, and the Baxter Extramural Grant Program. Aventis Pharmaceuticals supplied ramipril and financed the Good Clinical Practice audit.
The following are members of the ESCAPE Study Group – Local investigators (in alphabetical order of center):
A. Anarat (Cukurova University School of Medicine, Balcali, Adana, Turkey); A. Bakkaloglu and F. Ozaltin (Hacettepe University Faculty of Medicine, Sihhiye, Ankara, Turkey); A. Peco-Antic (University Children’s Hospital, Belgrade, Serbia); U. Querfeld and J. Gellermann (Charité Children’s Hospital, Berlin, Germany); P. Sallay (Semmelweis University Budapest, First Department of Pediatrics, Budapest, Hungary); D. Drozdz (Polish-American Children’s Hospital, Jagiellonian University Collegium Medicum, Krakow, Poland); K.-E. Bonzel and A.-M. Wingen (University Hospital Essen, Essen, Germany); A. Zurowska and I. Balasz (Department of Pediatric and Adolescent Nephrology and Hypertension, Medical University of Gdansk, Gdansk, Poland); A. Trivelli and F. Perfumo (G. Gaslini Institute, Genova, Italy); D.E. Müller-Wiefel and K. Möller (University Children’s Hospital Hamburg-Eppendorf, Hamburg, Germany); G. Offner and B. Enke (Hannover Medical School, Children’s Hospital, Hannover, Germany); E. Wühl, C. Gimpel, O.Mehls, and F. Schaefer (Center for Pediatric and Adolescent Medicine, Heidelberg, Germany); S. Emre (University of Istanbul, Istanbul Medical Faculty, Capa, Istanbul, Turkey); S. Caliskan (Istanbul University, Cerrahpasa Medical Faculty, Istanbul, Turkey); S. Mir (Ege University Medical Faculty, Department of Pediatrics Bornova, Izmir, Turkey); S. Wygoda (Urban Hospital St. Georg, Leipzig, Germany); K. Hohbach-Hohenfellner (University Children’s Hospital, Mainz, Germany); N. Jeck and G. Klaus (Department of Pediatrics, Philipps University Marburg, Marburg, Germany); G. Ardissino and S. Testa (IRCCS Ospedale Maggiore, Policlinico-Mangiagalli, Milano, Italy); G. Montini (Azienda Ospedaliera-Università di Padova, Padova, Italy); M. Charbit and P. Niaudet (Hospital Necker, Paris, France); A. Caldas-Afonso and A. Fernandes-Teixeira (Hospital S. Joao, Porto, Portugal); J. Dušek (Department of Pediatrics, University Hospital Motol, Prague, Czech Republic); M.C. Matteucci, S. Picca, and A. Mastrostefano (Ospedale Pediatrico Bambino Gesù, Rome, Italy); M. Wigger (University Children’s Hospital, Rostock, Germany); U.B. Berg and G. Celsi (Karolinska Institute, Huddinge University Hospital, Stockholm, Sweden); M. Fischbach and J. Terzic (Hopitaux Universitaires de Strasbourg, Pediatrie 1, Strasbourg, France); J. Fydryk and T. Urasinski (Pomeranian Academy of Medicine, Szczecin, Poland); R. Coppo and Licia Peruzzi (Ospedale Infantile Regina Margherita, Torino, Italy); K. Arbeiter (University Children’s Hospital, Vienna, Austria); A. Jankauskiené (Vilnius University Pediatric Center, Vilnius, Lithuania); R. Grenda, M. Litwin, and R. Janas (Children’s Memorial Health Hospital, Warsaw, Poland); G. Laube and T.J. Neuhaus (University Children’s Hospital, Zürich, Switzerland).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018010036/-/DCSupplemental.
Contributor Information
Collaborators: A. Anarat, A. Bakkaloglu, F. Ozaltin, A. Peco-Antic, U. Querfeld, J. Gellermann, P. Sallay, D. Drozdz, K.-E. Bonzel, A.-M. Wingen, A. Zurowska, I. Balasz, A. Trivelli, F. Perfumo, D.E. Müller-Wiefel, K. Möller, G. Offner, B. Enke, E. Wühl, C. Gimpel, O. Mehls, F. Schaefer, S. Emre, S. Caliskan, S. Mir, S. Wygoda, K. Hohbach-Hohenfellner, N. Jeck, G. Klaus, G. Ardissino, S. Testa, G. Montini, M. Charbit, P. Niaudet, A. Caldas-Afonso, A. Fernandes-Teixeira, J. Dušek, M.C. Matteucci, S. Picca, A. Mastrostefano, M. Wigger, U.B. Berg, G. Celsi, M. Fischbach, J. Terzic, J. Fydryk, T. Urasinski, R. Coppo, Licia Peruzzi, K. Arbeiter, A. Jankauskiené, R. Grenda, M. Litwin, R. Janas, G. Laube, and T.J. Neuhaus
References
- 1.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, et al.; Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group : Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 106: 1777–1782, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, et al.; CKD Prognosis Consortium : Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: A collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 3: 514–525, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Velde M, Halbesma N, de Charro FT, Bakker SJ, de Zeeuw D, de Jong PE, et al.: Screening for albuminuria identifies individuals at increased renal risk. J Am Soc Nephrol 20: 852–862, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gracchi V, van den Belt SM, Kupers LK, Corpeleijn E, de Zeeuw D, Heerspink HJ: Prevalence and distribution of (micro)albuminuria in toddlers. Nephrol Dial Transplant 31: 1686–1692, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, et al.; American Heart Association Expert Panel on Population and Prevention Science; American Heart Association Council on Cardiovascular Disease in the Young; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Nutrition, Physical Activity and Metabolism; American Heart Association Council on High Blood Pressure Research; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on the Kidney in Heart Disease; Interdisciplinary Working Group on Quality of Care and Outcomes Research : Cardiovascular risk reduction in high-risk pediatric patients: A scientific statement from the American Heart Association expert panel on population and prevention science; the councils on cardiovascular disease in the young, epidemiology and prevention, nutrition, physical activity and metabolism, high blood pressure research, cardiovascular nursing, and the kidney in heart disease; and the interdisciplinary working group on quality of care and outcomes research: Endorsed by the American Academy of Pediatrics. Circulation 114: 2710–2738, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Wingen AM, Fabian-Bach C, Schaefer F, Mehls O; European Study Group of Nutritional Treatment of Chronic Renal Failure in Childhood : Randomised multicentre study of a low-protein diet on the progression of chronic renal failure in children. Lancet 349: 1117–1123, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Warady BA, Abraham AG, Schwartz GJ, Wong CS, Muñoz A, Betoko A, et al.: Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: The chronic kidney disease in children (CKiD) cohort. Am J Kidney Dis 65: 878–888, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, et al.: Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 110: 921–927, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Lea J, Greene T, Hebert L, Lipkowitz M, Massry S, Middleton J, et al.: The relationship between magnitude of proteinuria reduction and risk of end-stage renal disease: Results of the African American study of kidney disease and hypertension. Arch Intern Med 165: 947–953, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Eijkelkamp WB, Zhang Z, Remuzzi G, Parving HH, Cooper ME, Keane WF, et al.: Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: Post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. J Am Soc Nephrol 18: 1540–1546, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, et al.; ESCAPE Trial Group : Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361: 1639–1650, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Ruggenenti P, Cravedi P, Chianca A, Caruso M, Remuzzi G: Achieving remission of proteinuria in childhood CKD. Pediatr Nephrol 32: 321–330, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz GJ, Brion LP, Spitzer A: The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 34: 571–590, 1987 [DOI] [PubMed] [Google Scholar]
- 14.Hohenfellner K, Wingen AM, Nauroth O, Wühl E, Mehls O, Schaefer F: Impact of ACE I/D gene polymorphism on congenital renal malformations. Pediatr Nephrol 16: 356–361, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Felix Kröpelin T, de Zeeuw D, Holtkamp FA, Packham DK, L Heerspink HJ: Individual long-term albuminuria exposure during angiotensin receptor blocker therapy is the optimal predictor for renal outcome. Nephrol Dial Transplant 31: 1471–1477, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Ruggenenti P, Perna A, Remuzzi G; GISEN Group Investigators : Retarding progression of chronic renal disease: The neglected issue of residual proteinuria. Kidney Int 63: 2254–2261, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Hellemons ME, Persson F, Bakker SJ, Rossing P, Parving HH, De Zeeuw D, et al.: Initial angiotensin receptor blockade-induced decrease in albuminuria is associated with long-term renal outcome in type 2 diabetic patients with microalbuminuria: A post hoc analysis of the IRMA-2 trial. Diabetes Care 34: 2078–2083, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, et al.: Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int 65: 2309–2320, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Schievink B, de Zeeuw D, Parving HH, Rossing P, Lambers Heerspink HJ: The renal protective effect of angiotensin receptor blockers depends on intra-individual response variation in multiple risk markers. Br J Clin Pharmacol 80: 678–686, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parving HH, de Zeeuw D, Cooper ME, Remuzzi G, Liu N, Lunceford J, et al.: ACE gene polymorphism and losartan treatment in type 2 diabetic patients with nephropathy. J Am Soc Nephrol 19: 771–779, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perna A, Ruggenenti P, Testa A, Spoto B, Benini R, Misefari V, et al.: ACE genotype and ACE inhibitors induced renoprotection in chronic proteinuric nephropathies1. Kidney Int 57: 274–281, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Lambers Heerspink HJ, Holtkamp FA, Parving HH, Navis GJ, Lewis JB, Ritz E, et al.: Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int 82: 330–337, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Vegter S, Perna A, Postma MJ, Navis G, Remuzzi G, Ruggenenti P: Sodium intake, ACE inhibition, and progression to ESRD. J Am Soc Nephrol 23: 165–173, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li PK, Ho KK, Szeto CC, Yu L, Lai FM: Prognostic indicators of IgA nephropathy in the Chinese--clinical and pathological perspectives. Nephrol Dial Transplant 17: 64–69, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Reich HN, Troyanov S, Scholey JW, Cattran DC; Toronto Glomerulonephritis Registry : Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol 18: 3177–3183, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Bello A, Thompson S, Lloyd A, Hemmelgarn B, Klarenbach S, Manns B, et al.; Alberta Kidney Disease Network : Multiple versus single and other estimates of baseline proteinuria status as predictors of adverse outcomes in the general population. Am J Kidney Dis 59: 364–371, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Heeg JE, de Jong PE, van der Hem GK, de Zeeuw D: Efficacy and variability of the antiproteinuric effect of ACE inhibition by lisinopril. Kidney Int 36: 272–279, 1989 [DOI] [PubMed] [Google Scholar]
- 28.Abe M, Okada K, Maruyama T, Matsumoto K: Antiproteinuric and blood pressure-lowering effects of a fixed-dose combination of losartan and hydrochlorothiazide in hypertensive patients with stage 3 chronic kidney disease. Pharmacotherapy 29: 1061–1072, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Halbesma N, Bakker SJ, Jansen DF, Stolk RP, De Zeeuw D, De Jong PE, et al.; PREVEND Study Group : High protein intake associates with cardiovascular events but not with loss of renal function. J Am Soc Nephrol 20: 1797–1804, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G: Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 19: 999–1007, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chin HJ, Kim DK, Park JH, Shin SJ, Lee SH, Choi BS, et al.: Effect of urine urea nitrogen and protein intake adjusted by using the estimated urine creatinine excretion rate on the antiproteinuric effect of angiotensin II type I receptor blockers. Nutrition 31: 1333–1338, 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.