Abstract
Nephrotoxicity from cancer therapies is common and increasingly encountered in clinical practice, such that the subfield of “onco-nephrology” has emerged. Conventional chemotherapeutic drugs and novel agents targeting specific genes/proteins are effective cancer therapies but suffer from a number of adverse kidney effects. An effective avenue of cancer treatment is immunotherapy, which uses drugs that augment immune system–mediated recognition and targeting of tumor cells. As such, leveraging the immune system to target malignant cells represents an important modality in eradicating cancer. IFN and high-dose IL-2 are older immunotherapies used in clinical practice to treat various malignancies, whereas new cancer immunotherapies have emerged over the past decade that offer even more effective treatment options. The immune checkpoint inhibitors are an exciting addition to the cancer immunotherapy armamentarium. Chimeric antigen receptor T cells are also a new immunotherapy used to treat various hematologic malignancies. However, as with the conventional and targeted cancer agents, the immunotherapies are also associated with immune-related adverse effects, which includes nephrotoxicity.
Keywords: immunotherapies, interferon, interleukin-2, immune checkpoint inhibitors, chimeric antigen receptor T-cells, acute kidney injury
Conventional chemotherapeutic drugs are first-line agents to treat several malignancies but cause kidney injury through nontargeted cell killing.1 Drugs that target specific cancer genes or proteins are extremely effective but also suffer from nephrotoxicity.1,2 Manipulating the immune system represents an attractive therapy as our understanding of the concept of cancer immunoediting has evolved.3 Immunoediting characterizes the interaction of tumor cells with the immune system and consists of three phases. These include elimination of clinically silent cancer cells by innate and adaptive immune responses; equilibrium between tumors and the immune system whereby surviving cancer cells, which have developed a nonimmunogenic phenotype selected for growth, are counterbalanced by negative pressure applied by immune cells; and escape from immune destruction by surviving tumor cells, which have altered their immune recognition and/or created a resistant tumor microenvironment, permitting continued cancer growth/metastasis.3 Developing immunotherapies that can successfully eradicate cancers that have exploited immunoediting to avert immune killing is the ultimate goal of modern cancer treatment.4–6
Oncologists have used immunotherapies to eradicate cancers since the 1980s and 1990s. The first immunotherapies used were exogenous cytokines, such as IFN-α,7,8 which is synthesized mainly by nonlymphocyte leukocytes, and the T cell cytokine IL-2.9–12 Immunotherapy with IFN and high-dose IL-2 has been effectively used for several decades to treat a number of malignancies. However, these agents are complicated by systemic toxicity and may lose efficacy over time as cancers develop treatment resistance.
Over the past decade, cancer therapeutics have advanced further by intensifying the immune response with a more targeted approach against tumor cells.4–6 The fine balance between cancer surveillance and preserving self-tolerance is accomplished in part by immune checkpoints. These checkpoints regulate T cell activation to either allow anticancer responses or maintain T cell quiescence.1,4–6 Some immune checkpoints also regulate activated T cells at later stages by either allowing continued cancer killing or deactivating T cells to avoid autoimmunity. Many cancers are able to co-opt these checkpoints by attenuating activated cancer-specific T cells, which allows unchecked tumor cell proliferation and metastasis—a process known as “immune-evasion.”4–6 To overcome this, immune checkpoint inhibitors (CPIs) activate quiescent T cells and impair cancer’s ability to turn off activated T cells in the tumor microenvironment.4–6 Another effective immunotherapy uses genetically engineered host T cells known as chimeric antigen receptor (CAR) T cells, which directly bind/destroy cancer cells, thereby overcoming immune roadblocks exploited by cancer cells.4,5,13
Unfortunately, immunotherapies also suffer from nephrotoxicity. IFN-α and high-dose IL-2 have well known adverse kidney effects, which will be briefly reviewed. Immune-mediated nephrotoxicity is also an important complication of the immune CPIs and CAR T cells, which will be the focus of this paper.
IFN-α Therapy
IFN is a critical component of the innate immune system to protect against viral infection and participate in cancer killing.7 IFN-α was Food and Drug Administration (FDA) approved to treat CML in 1981 followed by hairy cell leukemia, AIDS-related Kaposi sarcoma, metastatic melanoma, and follicular non-Hodgkin lymphoma. IFN-α activates the immune system by promoting effector T cell–mediated responses, such as IL-12 secretion, via several signaling events.8 As seen in Table 1, IFN-α is complicated by kidney injury.14–20 Minimal change disease (MCD) and FSGS are manifestations of podocyte injury,15–18 whereas thrombotic microangiopathy (TMA) reflects vascular endothelial damage.14,19,20 FSGS and MCD present with AKI and high-grade proteinuria after 3 months to 6 years of therapy.15–18 FSGS generally responds poorly to drug cessation and corticosteroids, whereas MCD is more responsive. TMA occurs after approximately 35 months of IFN-α therapy and is associated with severe AKI and significant mortality.14,19,20 Prompt drug discontinuation is critical.
Table 1.
IFN therapy–associated kidney lesions from selected series
| Reference and Patients (n) | Timing of AKI Onset with IFN Therapy | Kidney Syndrome | Kidney Biopsy | Treatment Data | Outcome Data |
|---|---|---|---|---|---|
| Zuber et al.14 | |||||
| 29 | 32.1±7.9 mo (eight patients), 34.0±7.1 mo (21 patients) | AKI, hypertension, proteinuria | TMA | Drug discontinuation ± corticosteroids, FFP, and plasma exchange | Kidney function: seven CR or PR, nine required chronic dialysis, 13 died |
| Markowitz et al.15 | |||||
| 11 | 4 mo (median), 12.6 mo (mean) | AKI, nephrotic proteinuria | cFSGS | Drug discontinuation ± corticosteroids | Kidney function: four CR, five PR |
| Proteinuria: one CR, two PR | |||||
| Markowitz et al.15 (literature review) | |||||
| 21 | 5 d to 22 mo (range), 4.6 mo (mean) | AKI, nephrotic-range proteinuria | Eight MCD, ten FSGS, three cFSGS | Drug discontinuation ± corticosteroids | Kidney function: CR or PR in all MCD, improved in all FSGS but <50% CR or PR |
| Kayar et al.17 | |||||
| 1 | 3 mo | AKI, nephrotic proteinuria | FSGS | Drug discontinuation, corticosteroids | Kidney function: CR |
| Proteinuria: CR | |||||
| Ozturk et al.18 | |||||
| 1 | 6 yr | Proteinuria | FSGS | Drug discontinuation | Proteinuria: CR |
| Kundra and Wang20 | |||||
| 68 | 35 mo (IFN-α for CML), 12 mo (IFN-α for HCV), 68.6 mo (IFN-β for MS) | AKI | TMA | Plasma exchange ± corticosteroids, FFP ± corticosteroids or rituximab or drug dose reduction or discontinuation | Kidney function: 27 CR, 28 CKD, 12 died |
TMA, thrombotic microangiopathy; FFP, fresh frozen plasma; CR, complete remission; PR, partial remission; cFSGS, collapsing FSGS; MCD, minimal change disease; CML, chronic myelogenous leukemia; HCV, hepatitis C virus; MS, multiple sclerosis.
IL-2 THERAPY
IL-2 is a cytokine with both activating and regulatory immune functions that mediate its effects by binding to receptors found on activated T cells, regulatory T cells, memory T cells, and natural killer cells.9 High-dose IL-2 has been used to treat patients with metastatic renal cell carcinoma since 1991 after seven phase 2 trials showed disease response.9–12 In these trials,11 hypotension occurred in 96% of patients. High-dose IL-2 also gained FDA approval for metastatic melanoma after eight clinical trials revealed clinical efficacy,12 with hypotension in 64% of patients. High-dose IL-2 causes cytokine-driven capillary leak syndrome, which can lead to intravascular volume depletion, third spacing of fluids, and nephrotoxicity (Table 2).21–25 AKI occurs due to reversible prerenal azotemia from capillary leak23; however, ischemic acute tubular injury (ATI) can also develop with severe hypotension.21,22 Cytokine-mediated inflammatory kidney injury may also occur.26 As such, patients receiving high-dose IL-2 should have regular monitoring of kidney function and urine output. Although AKI resolves with post-treatment discontinuation in most, recovery may be delayed if intrinsic injury occurs.
Table 2.
High-dose IL-2–associated nephrotoxicity from selected series
| Reference and Patients (n) | Systemic Manifestations | Kidney Syndrome | Outcome Data |
|---|---|---|---|
| Shalmi et al.22 | |||
| 10 | Capillary leak syndrome, hypotension, weight gain, edema, ascites, pleural effusions | Nine of 10 developed increase in sCr (mean 1.9 mg/dl); nine of 10 developed trace/1+proteinuria | Measured GFR decreased in nine of 10; of these, five had <30% decrease in ERPF, whereas four had increase in ERPF (suggesting intrinsic kidney injury) |
| Guleria et al.23 | |||
| 199 | Capillary leak syndrome, hypotension, edema, weight gain | Oliguria, AKI (13%), proteinuria (11%), hematuria, pyuria, granular casts (30%) | More severe AKI with NSAID coadministration, discontinued IL-2 for AKI, partial or complete kidney recovery after discontinued IL-2 |
| Belldegrun et al.24 | |||
| 99; IL-2 (n=23); IL-2 + LAK cells (n=76) | Capillary leak syndrome, hypotension, abdominal distension, weight gain | 90% Developed increased sCr (mean 3.44 mg/dl), oliguria (77.5%), mean FeNa =0.07% | Complete recovery of kidney function: 84% at 2 wk, 95% at 1 mo; faster kidney recovery in patients with baseline sCr <1.5 mg/dl |
| Memoli et al.25 | |||
| Nine: all received concomitant NSAID | Not mentioned | Increased sCr, decreased urine output and FeNa over 5 d of IL-2 without renal dose dopamine | Complete recovery of kidney function after discontinued IL-2; renal dose dopamine on day 3 of IL-2 prevented increase in sCr and decrease in urine output and FeNa |
sCr, serum creatinine; ERPF, effective renal plasma flow; NSAID, nonsteroidal anti-inflammatory drug; LAK, lymphokine-activated killer cell; FeNa, fractional excretion of sodium.
Immune Checkpoint Inhibitors
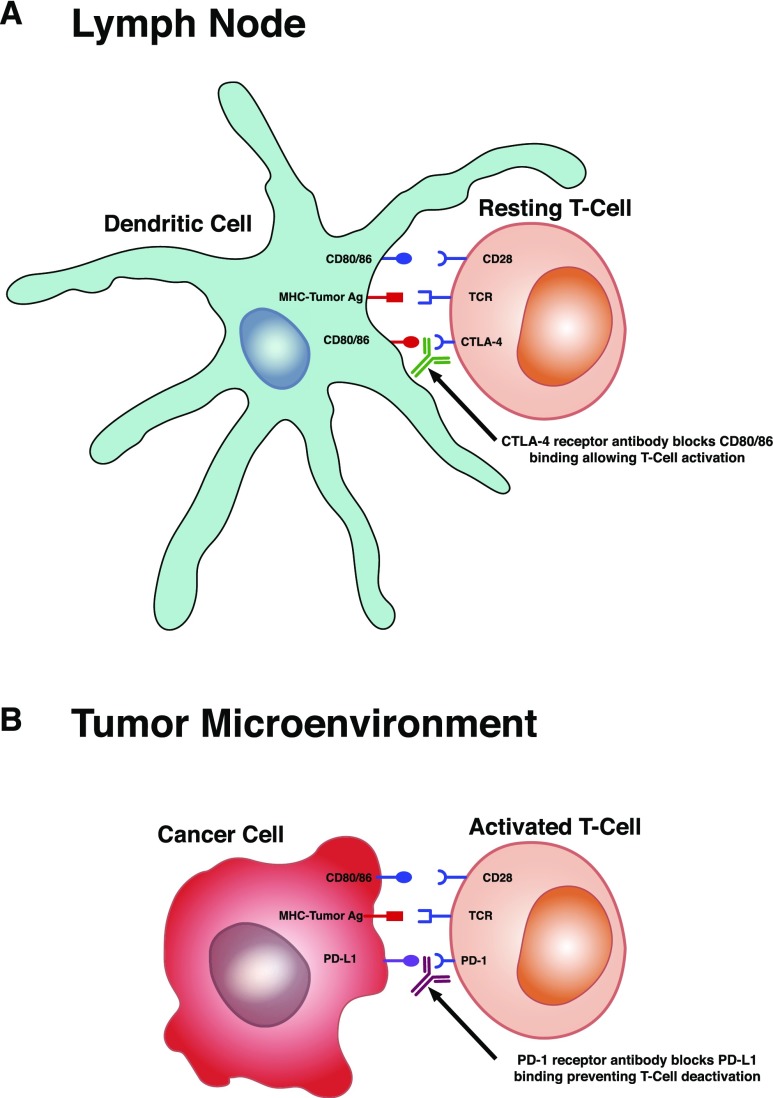
Enhancing or suppressing T cell activation via costimulatory or coinhibitory molecules modifies effector T cell response and provides “checkpoints” for immune regulation. The constitutively expressed T cell membrane receptor, CD28, is stimulated and interacts with the CD80/CD86 receptors on antigen-presenting cells when the T cell receptor engages with MHC/antigen complex. This costimulatory signal promotes activation of intracellular signaling proteins that activate T cells and allow differentiation into effector T cells,27–30 a process that can be inhibited at various steps or checkpoints (Figure 1). Cytotoxic lymphocyte–associated antigen-4 (CTLA-4) and programmed cell death protein-1 (PD-1) are two receptors that play an important role in negatively regulating T cell activation/function via these intracellular signaling pathways.31–33
Figure 1.
Immune checkpoint inhibitor target sites. (A) At the level of the lymph node, T cells express cytotoxic lymphocyte–associated antigen-4 (CTLA-4) receptor, which competes with CD28 receptor binding to CD80/86 on dendritic cells. This competitive binding reduces the magnitude of the CD28 costimulatory response, thus inhibiting T cell activation. This maintains balance to prevent T cell activation against self-antigen, thereby avoiding autoimmunity. Blocking CD80/86 binding to the CTLA-4 receptor with the mAb ipilimumab allows CD28 receptor binding to fully activate the T cells. This is appropriate for activation against presented tumor antigen (Ag). (B) In the tumor microenvironment, programmed death-1 (PD-1) receptor is a protein expressed on activated T cells. Certain tumors express ligands (programmed death ligand-1 [PD-L1] and programmed death ligand-2 [PD-L2]) that bind T cell PD-1 receptors and blunt T cell function. Blocking tumor cell PD-L1 or PD-L2 binding to T cell PD-1 receptor with the mAbs nivolumab and pembrolizumab leads to further T cell activation (or blocks deactivation of T cells) and promotes tumor cell killing. TCR, T cell receptor.
CTLA-4 receptor is expressed on the T cell surface upon significant T cell receptor stimulation and competes with CD28 to bind CD80/CD86, thereby reducing the magnitude of the CD28 costimulatory response.34,35 CTLA-4 binding can also inhibit T cell activation in the absence of CD28 by directly antagonizing T cell receptor–driven signaling pathways.36 Ultimately, CTLA-4 engagement disrupts effective T cell signal transmission, proliferation, and cell cycle progression,37,38 rendering the newly antigen-specific T cell anergic. This pathway also suppresses effector T cells and promotes the development of immunosuppressive Tregs.39–42 PD-1 receptor is a transmembrane protein expressed on effector T cells, Tregs, and B cells that binds the PD ligands (programmed death ligand-1 [PD-L1] and PD-L2) that are expressed on tumor cells, antigen-presenting cells, and other cells.31 On ligand binding, the PD-1 cytoplasmic domain is phosphorylated and recruits two tyrosine phosphatases,32,33 which dephosphorylate signaling intermediates and reduce downstream effects, thereby blunting T cell function.
Ligand binding to CTLA-4 and PD-1 receptors modifies the immune system response to antigens by inhibiting T cell activation, allows immunologic self-tolerance, and prevents autoimmunity. PD-1 blockade in both wild-type and CD-28–deficient mice accelerates a severe form of autoimmune encephalomyelitis characterized by increased antigen-specific T cell expansion and cytokine production.43 PD-1–deficient mice also have increased B cell proliferation and reduced expression of CD5 compared with control animals.44 CTLA-4 blockade in mice enhances antitumor immunity but also elicits autoimmune responses,45,46 an effect noted in six of 14 metastatic melanoma patients.47
Immune CPIs are mAbs aimed at targeting specific checkpoints via their ligand-receptor interactions48–51 (Figure 1). Activating T cells with ipilimumab, the CTLA-4 receptor antagonist (Figure 1A), is currently approved for patients with advanced melanoma.52 Allowing reactivation of quiescent T cells with pembrolizumab, a PD-1 receptor antagonist (Figure 1B), is currently approved for use in subsets of patients with advanced nonsmall cell lung cancer, advanced melanoma, advanced urothelial bladder cancer, advanced head and neck squamous cell cancer, and Hodgkin lymphoma.53,54 Nivolumab, another PD-1 receptor antagonist, is approved for a subset of patients with advanced nonsmall cell lung cancer, advanced melanoma, advanced urothelial bladder cancer, advanced head and neck squamous cell cancer, and Hodgkin lymphoma but can also be used in select patients with hepatocellular carcinoma, advanced renal cell cancer, and metastatic colorectal cancer.55
AKI and Electrolyte Disorders
The robust immune response generated by CPIs is complicated by a number of adverse autoimmune effects, including dermatitis, colitis, hypophysitis, pneumonitis, and various endocrinopathies.56 The incidence of these immune-related adverse events (IRAEs) range from 15% to 90%, with severe IRAEs ranging from 0.5% to 13%.56 Immune-related toxicities also involve the kidneys. Incidence of CPI-related nephrotoxicity was noted in phase 2/3 clinical trials data. From a total of 3695 patients, overall incidence of AKI was 2.2%. The incidence of grade 3/4 AKI was 0.6%. When stratified, AKI was more common with ipilimumab/nivolumab combination therapy (4.9%) than with monotherapy with ipilimumab (2%), nivolumab (1.9%), or pembrolizumab (1.4%).57 A meta-analysis of 48 clinical trials of PD-1 inhibitor therapy showed an overall AKI incidence rate of 2.1% and abnormal electrolyte incidence rates of 0.6%–1.3%.58 Thirteen studies had a pooled relative risk of AKI of 1.86 (95% confidence interval [95% CI], 0.95 to 3.64; P=0.07) that increased to 4.9 (95% CI, 1.57 to 11.18; P<0.001) compared with non-nephrotoxic controls. Although FAERS describes various electrolyte disorders (hyponatremia, hypokalemia, etc.) with CPI therapy, five studies noted an overall pooled relative risk of 1.67 (95% CI, 0.89 to 3.12; P=0.11) for electrolyte abnormalities, with only hypocalcemia significant. These data suggest that, although the incidence rates of CPI-induced AKI and electrolyte disorders are generally low, AKI risk is increased with combination therapy. Acute interstitial nephritis (AIN) is the most common kidney lesion (Table 3); however, several others have also been described.55,57–64 On the basis of abstracts and unpublished data, however, the incidence of nephrotoxicity may be rising (9.9%–29%).65
Table 3.
Immune checkpoint inhibitor therapy–associated kidney lesions
| Reference and CPI Therapy | AKI after CPI Initiation | Kidney Syndrome | Urinalysis/Urine Sediment | Kidney Biopsy | Treatment Data | Outcome Data |
|---|---|---|---|---|---|---|
| Cortazar et al.57 | ||||||
| Ipilimumab | 54 d | AKI | 5–10 WBCs, 2 RBCs | G-AIN | Drug DC, pred | PR |
| Ipilimumab + nivolumab | 91 d | AKI | 2–3 WBCs, 3–5 RBCs | G-AIN | Drug DC, pred | CR |
| Ipilimumab + nivolumab | 69 d | AKI | 5–10 WBCs, 0 RBCs | G-AIN | Drug DC, pred | PR |
| Ipilimumab | 70 d | AKI | 0–2 WBC casts, 16–34 WBCs | AIN | Drug DC only | NR |
| Ipilimumab + nivolumab | 245 d | AKI | 5 WBCs, 1 RBC | AIN | Drug DC, MP/pred | PR |
| Ipilimumab | 183 d | AKI | 0 WBC, 0 RBC | AIN | Drug DC, MP/pred | NR |
| Nivolumab | 224 d | AKI | 0 WBC, 0 RBC | AIN | Drug DC, pred | PR |
| Ipilimumab | 154 d | AKI | 6–9 WBCs, 0–3 RBCs | TMA | Drug DC, pred | NR |
| Ipilimumab + nivolumab | 42 d | AKI | 9 WBCs, 8 RBCs | AIN | Drug DC, MP/pred, MMF | PR |
| Ipilimumab | 120 d | AKI | 3 WBCs/HPF, WBC casts | AIN and ICD | Drug continued | NR |
| Ipilimumab | 60 d | AKI | 50–100 WBCs, 0–2 RBCs | AIN | Drug DC, pred | PR |
| Pembrolizumab | 21 d | AKI | 20–50 WBCs, 0–2 RBCs | AIN | Drug DC, MP/pred | PR |
| Pembrolizumab | 231 d | AKI | 11–20 WBCs, 0 RBCs | AIN | Drug DC, MP/pred | CR |
| Shirali et al.60 | ||||||
| Nivolumab | 11 mo | AKI | Bland | AIN | Drug continued initially, then held | CR 6 mo after drug DC |
| Nivolumab | 16 mo | AKI | 2 WBC casts/LPF | AIN | Drug DC, pred | CR |
| Nivolumab + bevacizumab | 10 mo | AKI | 2–5 WBCs/LPF | AIN | Drug continued, MP/pred | CR |
| Pembrolizumab | 3 mo | AKI | Numerous WBCs/HPF | AIN | Drug DC, pred ×2 courses | CR after second steroid course |
| Nivolumab + ipilimumab | 8 mo | AKI | 1 WBC cast/LPF | AIN | Drug DC, recurrent AKI with retrial treated with steroids | CR, recurrent AKI with retrial, PR after steroids |
| Pembrolizumab | 365 d | AKI | 15–30 WBCs/HPF | AIN | Drug DC, pred | CR |
| Izzedine et al.66 | ||||||
| Ipilimumab | 42 d | AKI | 35 WBCs and 70 RBCs/HPF | G-AIN | Drug DC, pred | CR |
| Ipilimumab | 42 d | AKI | N/A | G-AIN | Drug DC, pred | CR |
| Belliere et al.71 | ||||||
| Nivolumab | 48 d | AKI | Bland | G-AIN | Drug DC, steroids | PR |
| Pembrolizumab | 137 d | AKI | Bland | AIN | Drug DC, steroids | PR |
| Ipilimumab | 130 d | AKI | Bland | AIN | Drug DC, steroids | CR |
| Murakami et al.31 | ||||||
| Nivolumab + ipilimumab | 21 d | AKI | 9 WBCs and 8 RBCs/HPF, WBC and granular casts | AIN | Drug DC, MP/pred, MMF after failure | NR, fatal septic shock |
| Kitchlu et al.62 | ||||||
| Pembrolizumab | 1 mo | NS, AKI | N/A | MCD, ATI | Drug DC, steroids | PR |
| Ipilimumab | 18 mo | NS | N/A | MCD | Drug DC, pred | CR, NS relapse with retrial, CR with DC |
| Fadel et al.69 | ||||||
| Ipilimumab | 21 d | NS | 25 RBCs per 1 cubic mm | Lupus GN | Drug DC, pred | CR |
| Jung et al.70 | ||||||
| Nivolumab | 10 mo | AKI | Numerous RBCs, 3–5 WBCs, 1–3 granular casts | IgA dominant GN | Drug DC, MP followed by pred | HD dependent for 6 mo, CR off HD |
| Daanen et al.63 | ||||||
| Nivolumab | 1.5 mo | NS, AKI | Many hyaline casts, few WBCs/RBCs | FSGS | Drug DC, MP/pred | PR |
| Kidd and Gizaw55 | ||||||
| Ipilimumab | N/A | NS, AKI | N/A | MCD | Drug DC, pred | CR (NS), PR (AKI) |
CPI, immune checkpoint inhibitor; WBC, white blood cell; RBC, red blood cell; G-AIN, granulomatous acute interstitial nephritis; DC, discontinued; pred, prednisone; PR, partial remission; CR, complete remission; AIN, acute interstitial nephritis; NR, no response; MP, methylprednisolone; TMA, thrombotic microangiopathy; MMF, mycophenolate mofetil; HPF, high power field; ICD, immune complex deposit; LPF, low power field; N/A, not available; NS, nephrotic syndrome; MCD, minimal change disease; ATI, acute tubular injury; HD, hemodialysis; ; cFSGS, collapsing FSGS.
Two patients with granulomatous AIN complicating ipilimumab therapy were reported in 2014.66 Literature review revealed several patients with AIN and one patients with an immune complex GN from this CTLA-4 antagonist.65 Biopsy-proven AIN was described with the combination of ipilimumab/nivolumab after the second treatment cycle.66 Glucocorticoid therapy was initially associated with improved kidney function; however, AKI redeveloped a week later.
We observed six cases of biopsy-proven AIN in patients with nonsmall cell lung cancer during treatment with nivolumab and pembrolizumab.60 AKI developed after 3–16 months of drug exposure, with median time of 10.5 months. Serum creatinine ranged from 1.8 to 10.6 mg/dl, with mean serum creatinine of 4.1 mg/dl. Interestingly, all patients were on either PPIs or nonsteroidal anti-inflammatory drug for months to years before development of CPI-associated AIN. None had clinical evidence of an allergic drug reaction, except for two patients with mild serum eosinophilia. Urine studies suggested an inflammatory kidney lesion in four of six patients with sterile pyuria (n=3) or white blood cell (WBC) casts (n=2). After CPI discontinuation and corticosteroid therapy in five of six patients (intravenous methylprednisolone in one), five patients had complete recovery and one had partial recovery of kidney function. Drug reinitiation was associated with AKI. No patient required temporary or permanent dialysis.60
Thirteen patients from seven academic medical centers developed AKI after CPI exposure.57 Of these, six were on ipilimumab monotherapy, one was on nivolumab monotherapy, one was on pembrolizumab monotherapy, and four were on combination therapy with ipilimumab/nivolumab. Eight patients had an extrarenal IRAE. AKI developed at 7–63 days after the last CPI dose. Eight patients had pyuria, three had hematuria, and one had eosinophilia. Mean peak serum creatinine was 4.5 mg/dl, and the majority of patients had subnephrotic proteinuria. Biopsy-proven AIN was observed in 12 of 13 patients, and one patient had acute TMA. Three of 12 AIN biopsies had granulomas. CPI therapy was discontinued in 12 of 13, and all but two patients with AIN were treated with glucocorticoids (intravenous methylprednisolone in five). In ten patients with AIN administered glucocorticoids, seven had partial recovery, and two had complete recovery of kidney function. The patient with TMA remained dialysis dependent. Two patients with AIN who were not treated with glucocorticoids did not recover kidney function, and one became dialysis dependent. Two patients who recovered after glucocorticoid treatment were rechallenged with CPI therapy, and neither developed AKI.57
The literature supports a clear link between the CPIs and AIN, which lacks consistent allergic symptoms and signs. Eight of 13 patients compiled by Cortazar et al.57 developed extrarenal IRAEs, whereas two of six patients reported by Shirali et al.60 had eosinophilia. Sterile pyuria and WBC casts were present in some but not all patients with AIN, making urine microscopy somewhat insensitive. The timeline for CPI-mediated AIN was more variable than traditional drug-induced AIN, often developing long after the first/last dose of drug. This also contrasts other end organ IRAEs. For example, dermatologic effects occur within the first few weeks, endocrinopathies and hepatic involvement occur after approximately 6 weeks, and gastrointestinal effects tend to occur at 5–10 weeks.67
Complete or partial kidney function recovery may be achieved with some combination of drug discontinuation and glucocorticoid treatment.57,60 However, a small number may require long-term dialysis. Permanent drug cessation is recommended if grade 3/4 nephrotoxicity develops.68 Retrialing of CPI therapy remains controversial. Two patients with AIN who were rechallenged after recovery from glucocorticoid therapy did not develop recurrent AKI,57 whereas two developed recurrent AKI with re-exposure.57,60 Finally, although AIN is by far the most common nephrotoxicity of CPI therapy, patients with immune complex GN, MCD, FSGS, and TMA have also been reported (Table 3).55,57,62–64,69–71
Kidney Transplantation Rejection
CPI therapy in patients with kidney transplants and cancer raises concern for development of rejection. Current literature notes kidney rejection in one of three patients exposed to ipilimumab alone and eight of 14 rejection episodes with PD-1 inhibitor therapy.72–78 Three PD-1 inhibitor-associated rejections were previously exposed to ipilimumab without suffering a rejection episode. Rejection also occurred in one patient with combination therapy.79 Time to rejection ranged from 1 to 8 weeks, and most patients suffered graft loss, because they did not respond to pulse corticosteroids and drug discontinuation. The potential mechanism of acute cellular rejection is reactivation of T cells that are specific for graft antigen. Although data are limited, more rejection with PD-1 blockade suggests a role for PD-1 in establishing and maintaining tolerance in peripheral tissues compared with CTLA-4, which is primarily involved in initial T cell activation in lymph nodes.
Mechanism of Action
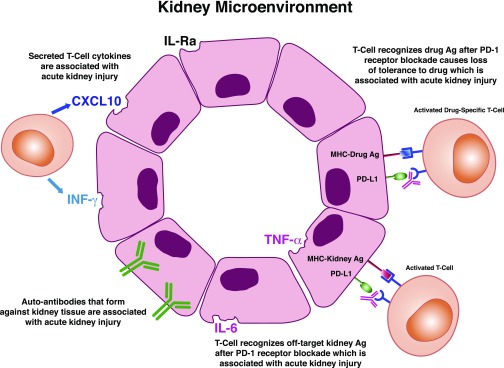
The mechanism underlying CPI-induced kidney injury is unknown, but there are some potential hypothesis-driven explanations primarily on the basis of nonrenal IRAEs and less so on renal IRAE and mouse models (Figure 2). First, CPIs may favor development of autoantibodies that are pathogenic to the kidney. This was seen in a case where ipilumumab was associated with a lupus-like glomerulopathy.73 Serum testing revealed the presence of antidouble-stranded DNA and antinuclear antigen antibodies, which disappeared after stopping ipilumumab and starting glucocorticoids. Second, certain tissues may normally express checkpoint receptors that then bind to anti-CPI antibodies, triggering an immune reaction against that tissue. An autopsy case described severe hypophysitis after therapy with anti–CTLA-4 antibody, tremilumumab.80 In addition to histologic evidence of necrotizing hypophysitis, immunohistochemistry showed significant CTLA-4 expression on pituitary endocrine cells. Control patients with less severe hypophysitis or pituitary adenomas also displayed variable CTLA-4 expression, suggesting that this was not a treatment-related phenomenon. Pituitary cells also showed deposition of both anti-IgG2 antibody and complement 4D. Taken together, this study illustrates that anti–CTLA-4 antibodies may bind to CTLA-4 at sites other than T cells, leading to off-target effects. To date, CTLA-4 expression has not been described in kidneys, but PD-L1 is expressed on renal tubular epithelial cells.81 Studies are required to determine if these checkpoint receptors are targets for anti-CPI antibody binding. Another mechanistic explanation may be the formation of new or reactivated T cells against tumor antigens that crossreact with off-target kidney tissues. Robust data for this come from autopsy cases of CPI-induced myocarditis, in which histologic analysis showed diffuse CD4+ and CD8+ T cell infiltration in the myocardium as well as skeletal muscle and tumor.82 Moreover, T cell receptor sequencing of these cells showed a high degree of clonality among different affected tissues, potentially suggesting a shared epitope among these tissues. Alternately, T cells may recognize a distinct tumor antigen, which is different but homologous to antigens in cardiac and skeletal tissues. T cell receptor clonality in tumor and kidney has not yet been studied in CPI-induced kidney disease to see if a similar pathway exists. Reactivation of drug-specific T cells through CPI-induced loss of tolerance may be another mechanism of CPI-associated AIN.60 Many of the patients in two published series57,60 were on drugs known to cause AIN (nonsteroidal anti-inflammatory drug, PPIs, etc.). Given the causal link between drug-specific T cells and AIN, it is plausible that CPIs induce reactivation of latent drug-specific T cells. Positive drug-specific lymphocyte testing to lansoprazole was reported in a patient who developed AIN after treatment with nivolumab while on lansoprazole.83 Lastly, increased proinflammatory cytokines and chemokines may also mediate the inflammatory kidney injury from CPIs. A patient with AIN that developed during ipilimumab/nivolumab treatment had increased serum levels of proinflammatory cytokines/chemokines (IL-1Ra, C-X-C motif chemokine ligand 10, TNF-α).31 Whether these are the cause or result of kidney injury is unclear; however, data from nonrenal IRAEs suggest that the former is plausible. For example, increased IL-17 levels correlated with severity of diarrhea in patients with CTLA-4–induced colitis.84
Figure 2.
Potential mechanisms underlying immune checkpoint inhibitor–induced kidney injury. Formation of new or reactivated T cells against tumor antigens (Ags) that crossreact with off-target kidney tissues, loss of tolerance with reactivation of drug-specific T cells induced by the immune checkpoint inhibitors, increase in proinflammatory cytokines/chemokines in kidney tissue, and generation of autoantibodies (antikidney tissue antibodies) are potential mechanisms of immune checkpoint–induced kidney injury. CXCL10, C-X-C motif chemokine ligand 10; PD-1, programmed death-1; PD-L1, programmed death ligand-1.
On the basis of published data showing that AKI tends to develop at 3–12 months after CPI exposure, surveillance for kidney injury measuring serum creatinine and urinalysis at baseline, 3 and 12 months, and then yearly may be worthwhile. A reasonable approach to patients developing CPI-related AKI should take in account severity of kidney dysfunction and tumor response. Patients with Kidney Disease Improving Global Outcomes stage 1 AKI can be monitored and evaluated for reversible causes, such as volume depletion, urinary obstruction, and medication-related AKI. Nephrology consultation is indicated to evaluate for the cause of AKI and possible kidney biopsy in those with stage 2/3 AKI and/or proteinuria >1 g/d. Workup should include kidney ultrasonography, urinalysis/urine microscopy, and spot urine protein/creatinine. Urine microscopy revealing sterile pyuria and WBC casts is suggestive of an intrinsic inflammatory kidney lesion. However, because serum and urine tests lack both sensitivity and specificity for AIN, kidney biopsy to define the underlying lesion is recommended when the cause of AKI is unclear. The drug should be held with stage 2/3 AKI until the biopsy results are available. Biopsy will help guide therapy, because CPI-induced AIN is generally responsive to drug discontinuation and glucocorticoids, whereas other AKI lesions, such as ATI, TMA, and FSGS, are typically not. This would limit unnecessary and potentially harmful steroid exposure (hypertension, edema, hyperglycemia, etc.) and perhaps allow continued CPI therapy if AKI is unrelated to immunotherapy. Also, if AIN is observed, the patient’s medication list should be reviewed, and drugs known to cause AIN should be discontinued. Reinitiation of CPI therapy may be reasonable if AKI was not severe (stage 3/requiring dialysis) or if AKI recovered fully after glucocorticoids. However, close monitoring is warranted, because a retrospective study of anti–PD-1 re-exposure in patients with lung cancer was complicated by similar (24%) or new (26%) nonrenal IRAEs.85 Switch between CPI classes has been rarely undertaken without recurrence of AKI. However, we do not recommend this approach unless immunotherapy is considered life saving. Oncology/nephrology team discussion with patients who developed nephrotoxicity about the risks/benefits of restarting immunotherapy is required. CPI therapy in patients with kidney transplants and cancer (in particular, the PD-1 antagonists) may be complicated by rejection. Ipilimumab may be safer, but data are currently limited. If CPIs are used, patients should be monitored closely.
CAR T Cells
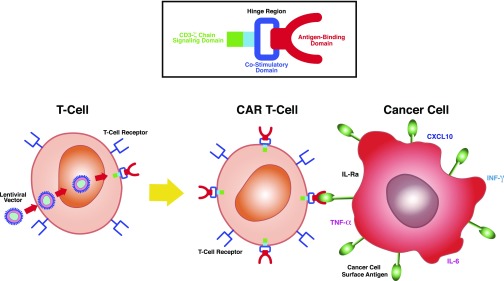
CAR T cell therapy represents a novel use of immunotherapy to treat various cancers. Autologous or allogeneic donor T cells are obtained, and they are modified using a single-chain variable fragment from an antibody along with a costimulatory/signaling domains to target specific extracellular antigens.86 Engineered CAR T cells are grown in the laboratory and then infused into patients, where they multiply and kill cancer cells that harbor the surface antigen (Figure 3).
Figure 3.
Chimeric antigen receptor (CAR) T cells. T cells harvested from patients are genetically modified using lentiviral vector to place an antigen binding domain (recognizes tumor antigen), which is linked to an intracellular costimulatory domain (CD28 or 4–1BB) and CD3-ζ signaling domain to amplify the immune response against tumor cells. CAR T cells engage tumor antigen by using extracellular antigen receptors, which are linked to intracellular costimulatory and signaling domains to amplify the immune response against tumor cells. Proinflammatory cytokines and chemokines are produced, which participate in eradication of cancer cells. CXCL10, C-X-C motif chemokine ligand 10.
A proof-of-concept clinical trial of autologous modified CD20-specific CAR T cells showed efficacy in eight patients with relapsed/refractory B cell NHL or mantle cell lymphoma.87 Trials have used CD19-specific CAR T cells to target follicular lymphoma88 as well as CLL.89 CD19-specific CAR T cells were also used to treat refractory/relapsed pre–B cell ALL.90 In a follow-up trial, this therapy successfully treated ALL, with patients achieving sustained remission for up to 24 months.91 In 2017, tisagenlecleucel was approved to treat pediatric patients with nonresponding/relapsing B cell ALL.92 Subsequently, axicabtagene ciloleucel was approved to treat adult patients with nonresponding/relapsing large B cell lymphomas.93 At present, clinical trials are examining CAR T cell therapy in glioblastoma multiforme, ovarian cancer, pancreatic cancer, and mesothelioma.86,94
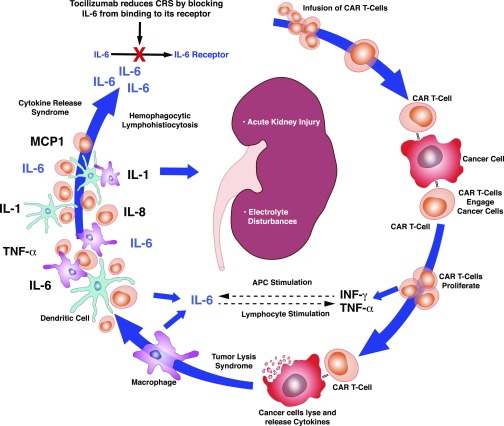
Effective targeted cancer killing by CAR T cells carries risk for systemic toxicity, which includes potential nephrotoxicity (Figure 4). Cytokine release syndrome (CRS) is a systemic inflammatory response that occurs on activation of CAR T cells and destruction of tumor cells.95–98 In this setting, IL-6, IL-10, and IFN-γ as well as inflammatory markers C-reactive protein and ferritin are produced. This syndrome manifests with high fever, myalgias, and tachycardia within 1–14 days of CAR T cell infusion95 and can progress to vasodilatory shock and capillary leak with multiorgan failure. Rhabdomyolysis may develop, whereas rapid, effective cancer killing may also cause tumor lysis syndrome (TLS). Adverse kidney effects include AKI and various electrolyte abnormalities.96
Figure 4.
Chimeric antigen receptor (CAR) T cell infusion–mediated toxicity. CAR T cells are infused and then interact with cancer cells, where they expand further and release IFN-γ and TNF-α. These stimulate macrophage activation. CAR T cells also cause lysis of cancer cells, which leads to release of cytokines and activation of macrophages and dendritic cells. IL-1, IL-6, and IL-8 are released as well as TNF-α and monocyte chemo-attractant 1 (MCP1). IL-6 is the most significant cytokine in the cytokine release syndrome. AKI and electrolyte abnormalities may occur in this setting. Tocilizumab blocks IL-6 from binding to its receptor, reducing the effects of cytokine release syndrome (CRS). APC, antigen-presenting cell.
TLS has been described in patients with ALL, CLL, and other hematologic malignancies after CAR T cell therapy.99,100 Increased lactate dehydrogenase and hyperuricemia were observed after approximately 22 days in initial clinical trials.99,100 A subset of patients with CRS also developed hepatosplenomegaly and liver dysfunction, increased ferritin levels, and decreased fibrinogen levels with coagulopathy.99 These clinical findings coupled with elevations of IL-6, IL-10, and IFN-γ suggest a secondary form of hemophagocytic lymphohistiocytosis (HLH). In fact, 12 of 39 patients with ALL who developed CRS manifested a clinical picture similar to HLH.96
Systemic toxicities associated with CAR T cells can potentially lead to AKI (Table 4).86,96 CRS and HLH can trigger cytokine-mediated vasodilation and capillary leak, with significant third spacing of fluids that causes prerenal physiology. In addition, high fever and nausea/vomiting associated with CRS induce intravascular volume depletion. Acute cardiomyopathy from CRS can promote hypotension and further exacerbate kidney hypoperfusion akin to acute cardiorenal syndrome.86,96 These effects reduce kidney perfusion, which can lead to prerenal AKI or progress to ischemic ATI when severe.
Table 4.
Chimeric antigen receptor T cell–associated nephrotoxicity
| Potential Nephrotoxicity |
|---|
| AKI |
| Prerenal AKI/acute tubular injury |
| Cytokine release syndrome with capillary leak and hypotension |
| Hemophagocytic lymphohistiocytosis with inflammation |
| Acute cardiac dysfunction with reduced cardiac output and hypotension |
| Intravascular volume depletion from fever, N/V, and diarrhea |
| Tumor lysis syndrome |
| Electrolyte disorders |
| Hypokalemia, hypophosphatemia, and hyponatremia |
| Prevention/treatment of toxicity |
| Chemotherapy to reduce tumor burden |
| Corticosteroids to reduce inflammatory response |
| Supportive care for hypotension with vasopressors, iv fluids, and oxygen |
| IL-6 blockade with tocilizumab for cytokine release syndrome and hemophagocytic lymphohistiocytosis |
N/V, nausea and vomiting.
In patients with large tumor burdens treated with CAR T cells, TLS constitutes another potential mechanism for AKI. The cytokine storm occurring with TLS can promote AKI and increase risk for further kidney injury from intratubular uric acid and calcium-phosphate crystal precipitation. Another potential mechanism of AKI is HLH-related kidney injury, which can cause ATI, AIN, or acute GN.100 Although these kidney lesions are described with HLH from other causes, they have not been described with CAR T cells and remain speculative. Hypokalemia (47%), hypophosphatemia (37%), and hyponatremia (5%) can also develop with CAR T cell therapy.101
Risk for CAR T cell complications can be anticipated on the basis of tumor burden and predicted tumor response. Prevention includes pretreatment with chemotherapy to decrease the tumor burden and steroids to dampen inflammation (Table 4). When indicated, intravenous fluid resuscitation and vasopressors to maintain systemic hemodynamics and renal perfusion are helpful. Tocilizumab, a monoclonal anti–IL-6 receptor antibody, is indicated for severe grade 3/4 CRS and catecholamine-dependent vasodilatory shock to improve BP and prevent multiorgan failure.94,95 Additionally, corticosteroids can be used in patients with only partial response to tocilizumab or who develop recurrent symptoms.
In conclusion, novel immunotherapies offer effective cancer treatment but are associated with kidney disease. IFN and high-dose IL-2 are older immunotherapies with well known nephrotoxicities. Immune CPIs are associated primarily with AIN, whereas CAR T cell therapy may be complicated by AKI and electrolyte disturbances.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Rosner MH, Perazella MA: Acute kidney injury in patients with cancer. N Engl J Med 376: 1770–1781, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Porta C, Cosmai L, Gallieni M, Pedrazzoli P, Malberti F: Renal effects of targeted anticancer therapies. Nat Rev Nephrol 11: 354–370, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Schreiber RD, Old LJ, Smyth MJ: Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 331: 1565–1570, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Khalil DN, Smith EL, Brentjens RJ, Wolchok JD: The future of cancer treatment: Immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol 13: 273–290, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA: Hallmarks of cancer: The next generation. Cell 144: 646–674, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Weber J: Immune checkpoint proteins: A new therapeutic paradigm for cancer--preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol 37: 430–439, 2010 [DOI] [PubMed] [Google Scholar]
- 7.FDA : Product information intron A. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/103132s5190lbl.pdf. Accessed May 6, 2018 [Google Scholar]
- 8.Lesinski GB, Anghelina M, Zimmerer J, Bakalakos T, Badgwell B, Parihar R, et al.: The antitumor effects of IFN-alpha are abrogated in a STAT1-deficient mouse. J Clin Invest 112: 170–180, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krieg C, Létourneau S, Pantaleo G, Boyman O: Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc Natl Acad Sci U S A 107: 11906–11911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutcher JP: Current status of interleukin-2 therapy for metastatic renal cell carcinoma and metastatic melanoma. Oncology (Williston Park) 16[Suppl 13]: 4–10, 2002 [PubMed] [Google Scholar]
- 11.Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC: Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol 13: 688–696, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al.: High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 17: 2105–2116, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Lee DW, Barrett DM, Mackall C, Orentas R, Grupp SA: The future is now: Chimeric antigen receptors as new targeted therapies for childhood cancer. Clin Cancer Res 18: 2780–2790, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuber J, Martinez F, Droz D, Oksenhendler E, Legendre C; Groupe D’stude Des Nephrologues D’ile-de-France (GENIF) : Alpha-interferon-associated thrombotic microangiopathy: A clinicopathologic study of 8 patients and review of the literature. Medicine (Baltimore) 81: 321–331, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Markowitz GS, Nasr SH, Stokes MB, D’Agati VD: Treatment with IFN-alpha, -beta, or -gamma is associated with collapsing focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 5: 607–615, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markowitz GS, Bomback AS, Perazella MA: Drug-induced glomerular disease: Direct cellular injury. Clin J Am Soc Nephrol 10: 1291–1299, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayar Y, Bayram Kayar N, Alpay N, Hamdard J, Ekinci I, Emegil S, et al.: Interferon induced focal segmental glomerulosclerosis. Case Rep Nephrol 2016: 6967378, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozturk M, Basoglu F, Yilmaz M, Ozagari AA, Baybas S: Interferon β associated nephropathy in a Multiple Sclerosis patient: A case and review. Mult Scler Relat Disord 9: 50–53, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Kavanagh D, McGlasson S, Jury A, Williams J, Scolding N, Bellamy C, et al.: Type I interferon causes thrombotic microangiopathy by a dose-dependent toxic effect on the microvasculature. Blood 128: 2824–2833, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kundra A, Wang JC: Interferon induced thrombotic microangiopathy (TMA): Analysis and concise review. Crit Rev Oncol Hematol 112: 103–112, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Webb DE, Austin HA 3rd ., Belldegrun A, Vaughan E, Linehan WM, Rosenberg SA: Metabolic and renal effects of interleukin-2 immunotherapy for metastatic cancer. Clin Nephrol 30: 141–145, 1988 [PubMed] [Google Scholar]
- 22.Shalmi CL, Dutcher JP, Feinfeld DA, Chun KJ, Saleemi KR, Freeman LM, et al.: Acute renal dysfunction during interleukin-2 treatment: Suggestion of an intrinsic renal lesion. J Clin Oncol 8: 1839–1846, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Guleria AS, Yang JC, Topalian SL, Weber JS, Parkinson DR, MacFarlane MP, et al.: Renal dysfunction associated with the administration of high-dose interleukin-2 in 199 consecutive patients with metastatic melanoma or renal carcinoma. J Clin Oncol 12: 2714–2722, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Belldegrun A, Webb DE, Austin HA 3rd ., Steinberg SM, White DE, Linehan WM, et al.: Effects of interleukin-2 on renal function in patients receiving immunotherapy for advanced cancer. Ann Intern Med 106: 817–822, 1987 [DOI] [PubMed] [Google Scholar]
- 25.Memoli B, De Nicola L, Libetta C, Scialò A, Pacchiano G, Romano P, et al.: Interleukin-2-induced renal dysfunction in cancer patients is reversed by low-dose dopamine infusion. Am J Kidney Dis 26: 27–33, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Marabondo S, Kaufman HL: High-dose interleukin-2 (IL-2) for the treatment of melanoma: Safety considerations and future directions. Expert Opin Drug Saf 16: 1347–1357, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Appleman LJ, van Puijenbroek AA, Shu KM, Nadler LM, Boussiotis VA: CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J Immunol 168: 2729–2736, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, et al.: CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-xL. Immunity. 1995. 3: 87-98. J Immunol 185: 3788–3799, 2010 [PubMed] [Google Scholar]
- 29.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, et al.: CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 25: 9543–9553, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward SG, Westwick J, Hall ND, Sansom DM: Ligation of CD28 receptor by B7 induces formation of D-3 phosphoinositides in T lymphocytes independently of T cell receptor/CD3 activation. Eur J Immunol 23: 2572–2577, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Murakami N, Motwani S, Riella LV: Renal complications of immune checkpoint blockade. Curr Probl Cancer 41: 100–110, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T: PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A 98: 13866–13871, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J, et al.: PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett 574: 37–41, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Linsley PS, Bradshaw J, Greene J, Peach R, Bennett KL, Mittler RS: Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity 4: 535–543, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Vandenborre K, Van Gool SW, Kasran A, Ceuppens JL, Boogaerts MA, Vandenberghe P: Interaction of CTLA-4 (CD152) with CD80 or CD86 inhibits human T-cell activation. Immunology 98: 413–421, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fallarino F, Fields PE, Gajewski TF: B7-1 engagement of cytotoxic T lymphocyte antigen 4 inhibits T cell activation in the absence of CD28. J Exp Med 188: 205–210, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider H, Smith X, Liu H, Bismuth G, Rudd CE: CTLA-4 disrupts ZAP70 microcluster formation with reduced T cell/APC dwell times and calcium mobilization. Eur J Immunol 38: 40–47, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krummel MF, Allison JP: CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med 183: 2533–2540, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudd CE, Taylor A, Schneider H: CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev 229: 12–26, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al.: CTLA-4 control over Foxp3+ regulatory T cell function. Science 322: 271–275, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK: Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity 6: 411–417, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi T, Kishi A, Osaki M, Morikawa H, Prieto-Martin P, Wing K, et al.: Construction of self-recognizing regulatory T cells from conventional T cells by controlling CTLA-4 and IL-2 expression. Proc Natl Acad Sci U S A 110: E2116–E2125, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salama AD, Chitnis T, Imitola J, Ansari MJ, Akiba H, Tushima F, et al.: Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med 198: 71–78, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishimura H, Minato N, Nakano T, Honjo T: Immunological studies on PD-1 deficient mice: Implication of PD-1 as a negative regulator for B cell responses. Int Immunol 10: 1563–1572, 1998 [DOI] [PubMed] [Google Scholar]
- 45.van Elsas A, Hurwitz AA, Allison JP: Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med 190: 355–366, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, et al.: Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res 60: 2444–2448, 2000 [PubMed] [Google Scholar]
- 47.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, et al.: Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A 100: 8372–8377, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keir ME, Butte MJ, Freeman GJ, Sharpe AH: PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26: 677–704, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al.; KEYNOTE-001 Investigators : Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372: 2018–2028, 2015 [DOI] [PubMed] [Google Scholar]
- 50.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al.: Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med 8: 793–800, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Pardoll DM: The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12: 252–264, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.FDA : YERVOY (ipilimumab) injection, 2011. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/125377Orig1s000TOC.cfm. Accessed May 6, 2018
- 53.FDA : Pembrolizumab (KEYTRUDA), checkpoint inhibitor, 2016. Available at: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm526430.htm. Accessed May 7, 2018 [Google Scholar]
- 54.FDA : FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication, 2017. Available at: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm560040.htm. Accessed May 7, 2018
- 55.Kidd JM, Gizaw AB: Ipilimumab-associated minimal-change disease. Kidney Int 89: 720, 2016 [DOI] [PubMed] [Google Scholar]
- 56.Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, Carbonnel F, et al.: Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Ann Oncol 27: 559–574, 2016 [DOI] [PubMed] [Google Scholar]
- 57.Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, et al.: Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 90: 638–647, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manohar S, Kompotiatis P, Thongprayoon C, Cheungpasitporn W, Herrmann J, Herrmann SM: Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: Meta-analysis [published online ahead of print May 11, 2018]. Nephrol Dial Transplant 10.1093/ndt/gfy105 [DOI] [PubMed] [Google Scholar]
- 59.Perazella MA: Checkmate: Kidney injury associated with targeted cancer immunotherapy. Kidney Int 90: 474–476, 2016 [DOI] [PubMed] [Google Scholar]
- 60.Shirali AC, Perazella MA, Gettinger S: Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis 68: 287–291, 2016 [DOI] [PubMed] [Google Scholar]
- 61.Izzedine H, Mateus C, Boutros C, Robert C, Rouvier P, Amoura Z, et al.: Renal effects of immune checkpoint inhibitors. Nephrol Dial Transplant 32: 936–942, 2017 [DOI] [PubMed] [Google Scholar]
- 62.Kitchlu A, Fingrut W, Avila-Casado C, Chan CT, Crump M, Hogg D, et al.: Nephrotic syndrome with cancer immunotherapies: A report of 2 cases. Am J Kidney Dis 70: 581–585, 2017 [DOI] [PubMed] [Google Scholar]
- 63.Daanen RA, Maas RJH, Koornstra RHT, Steenbergen EJ, van Herpen CML, Willemsen AECAB: Nivolumab-associated nephrotic syndrome in a patient with renal cell carcinoma: A case report. J Immunother 40: 345–348, 2017 [DOI] [PubMed] [Google Scholar]
- 64.Ray A, Ghosh S, Ghosh M, Yarlagadda S: Nivolumab induced renal failure with collapsing focal segmental glomerulosclerosis (FSGS). J Am Soc Nephrol 27: 102A, 2016 [Google Scholar]
- 65.Wanchoo R, Karam S, Uppal NN, Barta VS, Deray G, Devoe C, et al.; Cancer and Kidney International Network Workgroup on Immune Checkpoint Inhibitors : Adverse renal effects of immune checkpoint inhibitors: A narrative review. Am J Nephrol 45: 160–169, 2017 [DOI] [PubMed] [Google Scholar]
- 66.Izzedine H, Gueutin V, Gharbi C, Mateus C, Robert C, Routier E, et al.: Kidney injuries related to ipilimumab. Invest New Drugs 32: 769–773, 2014 [DOI] [PubMed] [Google Scholar]
- 67.Weber JS, Dummer R, de Pril V, Lebbé C, Hodi FS; MDX010-20 Investigators : Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: Detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer 119: 1675–1682, 2013 [DOI] [PubMed] [Google Scholar]
- 68.Spain L, Diem S, Larkin J: Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 44: 51–60, 2016 [DOI] [PubMed] [Google Scholar]
- 69.Fadel F, El Karoui K, Knebelmann B: Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med 361: 211–212, 2009 [DOI] [PubMed] [Google Scholar]
- 70.Jung K, Zeng X, Bilusic M: Nivolumab-associated acute glomerulonephritis: A case report and literature review. BMC Nephrol 17: 188, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Belliere J, Meyer N, Mazieres J, Ollier S, Boulinguez S, Delas A, et al.: Acute interstitial nephritis related to immune checkpoint inhibitors. Br J Cancer 115: 1457–1461, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maggiore U, Pascual J: The bad and the good news on cancer immunotherapy: Implications for organ transplant recipients. Adv Chronic Kidney Dis 23: 312–316, 2016 [DOI] [PubMed] [Google Scholar]
- 73.Chae YK, Galvez C, Anker JF, Iams WT, Bhave M: Cancer immunotherapy in a neglected population: The current use and future of T-cell-mediated checkpoint inhibitors in organ transplant patients. Cancer Treat Rev 63: 116–121, 2018 [DOI] [PubMed] [Google Scholar]
- 74.Barnett R, Barta VS, Jhaveri KD: Preserved renal-allograft function and the PD-1 pathway inhibitor nivolumab. N Engl J Med 376: 191–192, 2017 [DOI] [PubMed] [Google Scholar]
- 75.Winkler JK, Gutzmer R, Bender C, Lang N, Zeier M, Enk AH, et al.: Safe administration of an anti-PD-1 antibody to kidney-transplant patients: 2 Clinical cases and review of the literature. J Immunother 40: 341–344, 2017 [DOI] [PubMed] [Google Scholar]
- 76.Wu CK, Juang GD, Lai HC: Tumor regression and preservation of graft function after combination with anti-PD-1 immunotherapy without immunosuppressant titration. Ann Oncol 28: 2895–2896, 2017 [DOI] [PubMed] [Google Scholar]
- 77.Sadaat M, Jang S: Complete tumor response to pembrolizumab and allograft preservation in renal allograft recipient on immunosuppressive therapy. J Oncol Pract 14: 198–199, 2018 [DOI] [PubMed] [Google Scholar]
- 78.Deltombe C, Garandeau C, Renaudin K, Hourmant M: Severe allograft rejection and autoimmune hemolytic anemia after anti-PD1 therapy in a kidney transplanted patient. Transplantation 101: e291, 2017 [DOI] [PubMed] [Google Scholar]
- 79.Miller DM, Faulkner-Jones BE, Stone JR, Drews RE: Complete pathologic response of metastatic cutaneous squamous cell carcinoma and allograft rejection after treatment with combination immune checkpoint blockade. JAAD Case Rep 3: 412–415, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caturegli P, Di Dalmazi G, Lombardi M, Grosso F, Larman HB, Larman T, et al.: Hypophysitis secondary to cytotoxic T-lymphocyte-associated protein 4 blockade: Insights into pathogenesis from an autopsy series. Am J Pathol 186: 3225–3235, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ding H, Wu X, Gao W: PD-L1 is expressed by human renal tubular epithelial cells and suppresses T cell cytokine synthesis. Clin Immunol 115: 184–191, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al.: Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 375: 1749–1755, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koda R, Watanabe H, Tsuchida M, Iino N, Suzuki K, Hasegawa G, et al.: Immune checkpoint inhibitor (nivolumab)-associated kidney injury and the importance of recognizing concomitant medications known to cause acute tubulointerstitial nephritis: A case report. BMC Nephrol 19: 48, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tarhini AA, Zahoor H, Lin Y, Malhotra U, Sander C, Butterfield LH, et al.: Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer 3: 39–44, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Postow MA, Sidlow R, Hellmann MD: Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378: 158–168, 2018 [DOI] [PubMed] [Google Scholar]
- 86.Gill S, Maus MV, Porter DL: Chimeric antigen receptor T cell therapy: 25 Years in the making. Blood Rev 30: 157–167, 2016 [DOI] [PubMed] [Google Scholar]
- 87.Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, et al.: Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood 112: 2261–2271, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, et al.: Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116: 4099–4102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Porter DL, Levine BL, Kalos M, Bagg A, June CH: Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365: 725–733, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al.: Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368: 1509–1518, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al.: Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371: 1507–1517, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.US FDA : FDA approval brings first gene therapy to the United States, 2017. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm574058.htm. Accessed May 8, 2018
- 93.US FDA : FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma, 2017. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm581216.htm. Accessed May 10, 2018
- 94.Brudno JN, Kochenderfer JN: Toxicities of chimeric antigen receptor T cells: Recognition and management. Blood 127: 3321–3330, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frey NV, Porter DL: Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Hematology (Am Soc Hematol Educ Program) 2016: 567–572, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fitzgerald JC, Weiss SL, Maude SL, Barrett DM, Lacey SF, Melenhorst JJ, et al.: Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med 45: e124–e131, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ: Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics 3: 16011, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al.: Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124: 188–195, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Namuduri M, Brentjens RJ: Medical management of side effects related to CAR T cell therapy in hematologic malignancies. Expert Rev Hematol 9: 511–513, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Santoriello D, Hogan J, D’Agati VD: Hemophagocytic syndrome with histiocytic glomerulopathy and intraglomerular hemophagocytosis. Am J Kidney Dis 67: 978–983, 2016 [DOI] [PubMed] [Google Scholar]
- 101.Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al.: Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol 15: 47–62, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]