Abstract
Background C3 glomerulopathy (C3G) is a life-threatening kidney disease caused by dysregulation of the alternative pathway of complement (AP) activation. No approved specific therapy is available for C3G, although an anti-C5 mAb has been used off-label in some patients with C3G, with mixed results. Thus, there is an unmet medical need to develop other inhibitors of complement for C3G.
Methods We used a murine model of lethal C3G to test the potential efficacy of an Fc fusion protein of complement receptor of the Ig superfamily (CRIg-Fc) in the treatment of C3G. CRIg-Fc binds C3b and inhibits C3 and C5 convertases of the AP. Mice with mutations in the factor H and properdin genes (FHm/mP−/−) develop early-onset C3G, with AP consumption, high proteinuria, and lethal crescentic GN.
Results Treatment of FHm/mP−/− mice with CRIg-Fc, but not a control IgG, inhibited AP activation and diminished the consumption of plasma C3, factor B, and C5. CRIg-Fc–treated FHm/mP−/− mice also had significantly improved survival and reduced proteinuria, hematuria, BUN, glomerular C3 fragment, C9 and fibrin deposition, and GN pathology scores.
Conclusions Therapeutics developed on the basis of the mechanism of action of soluble CRIg may be effective for the treatment of C3G and should be explored clinically.
Keywords: complement, glomerulopathy, membranoproliferative glomerulonephritis (MPGN)
C3 glomerulopathy (C3G) is a rare kidney disease that is defined mechanistically by dysregulation of the alternative pathway of complement (AP) activation. C3G can be caused by defects in fluid phase or cell surface complement regulators.1–5 In addition, autoimmune forms of C3G attributable to autoantibodies against complement regulators or C3 convertase are also well documented.6 Pathologically, C3G is characterized by plasma C3 consumption and prominent C3 fragment depositions in the mesangium and capillary walls, with typically no or trace Ig presence in the glomeruli. Two subcategories of C3G are distinguished histologically by electron microscopy: dense deposit disease (DDD) and C3 glomerulonephritis (C3GN).7 A pathognomonic feature of DDD is dense deposit in the lamina densa of the glomerular basement membrane (GBM), whereas in C3GN, the pattern of electron-dense deposit is more variable and can present as mesangial, subendothelial, intramembranous, or subepithelial.8,9 The complement effectors responsible for C3G pathogenesis may not be identical across the clinical spectrum of the disease, but both human and mouse model studies have clearly shown the importance of C5-mediated terminal complement pathway in C3G pathogenesis,10–17 and the blood level of soluble C5b-9 (sC5b-9) has been used as a biomarker for disease activity and predicted response of anti-C5 therapy.11,14,16,18,19
Prognosis of C3G, particularly DDD, is poor and approximately 50% patients progress to end stage renal failure within 10 years of diagnosis.1,20 Currently there is no specific therapy for C3G and the disease is managed primarily by empirical and supportive treatments, including plasma infusion/exchange, immunosuppression, and BP control.21,22 These conventional treatments lack consistent efficacy and do not usually achieve long-term satisfactory clinical outcome. Renal transplantation in C3G is also not a reliable option because disease recurrence in the transplanted kidneys is common.1 More recently, eculizumab, an anticomplement C5 mAb, has been tested for C3G in isolated cases and in a small, open-label, clinical trial. Although some patients responded positively to anti-C5 treatment, others showed no improvement.12,14,16,23 Blocking C5 will inhibit terminal complement activation but is not expected to have an effect on plasma C3 activation and glomerular C3 fragment deposition. Whether and to what degree these processes contribute to C3G pathogenesis is not yet fully understood, but given the underlying mechanism of C3G being dysregulation of the AP, there is a strong rationale and need to explore other complement inhibitors targeting the proximal steps of AP that can block both C3 and C5 activation in C3G.
In this study, we have tested a soluble Fc fusion protein of the extracellular domain of complement receptor of the Ig superfamily (CRIg-Fc) in a murine model of lethal C3G. CRIg-Fc was previously shown to be a potent inhibitor of AP activation.24–26 It works by binding to. and interfering with, the substrate binding function of C3b, thereby inhibiting the formation of both C3 and C5 convertases.27,28 CRIg-Fc is a specific AP inhibitor because it does not interfere with C5 binding to the classic pathway C5 convertase C3bC4bC2a.27 We previously created a factor H (FH) and properdin (P) gene double-mutant mouse (FHm/mP−/−) that developed lethal C3G with features of human disease. FHm/mP−/− mice displayed dense deposits in the GBM, had low plasma C3, factor B (FB), and C5 levels, and developed early-onset proteinuria.5 All FHm/mP−/− mice died from rapidly progressive GN by 8–12 weeks.5 We show here that treatment of FHm/mP−/− mice with CRIg-Fc, but not an isotype control mAb, markedly ameliorated C3G when assessed by multiple disease parameters including survival, renal pathology, and renal function. Our results suggest that soluble CRIg may inform a promising therapeutic strategy for human C3G and should be explored clinically.
Methods
In Vitro Complement Activation Assays
Mouse CRIg-Fc (murine IgG1 Fc) and an isotype control murine α-HIV gp120-IgG1 antibody were produced as described previously.25 LPS-dependent AP activity assay of wild-type (WT) mouse sera with or without CRIg-Fc was performed as described.29 Classical pathway complement activity, in the presence or absence of CRIg, was measured using an ovalbumin (OVA)/anti–OVA-based ELISA method and FB knockout mouse serum as described.29
In Vivo Kinetics of CRIg-Fc Activity
WT C57BL/6 mice were purchased from The Jackson Laboratory. FHm/m mice were produced as described.5 Mice were intraperitoneally injected with 50 mg/kg or 1 mg per mouse (corresponding to 40–50 mg/kg) of CRIg-Fc, as specified. Blood was collected before and at various time points post-treatment for LPS-dependent AP activity assay or plasma C3 detection.5,29
Treatment of FHm/mP−/− Mice
FHm/mP−/− mice were generated as described.5 Beginning at 4 weeks of age, they were treated every 4 days (50 mg/kg, administered intraperitoneally) with either CRIg-Fc or the control IgG (n=10 per group). Treatment lasted for 10 weeks unless the mice became moribund and had to be euthanized. Only male mice were used in the treatment experiment.
Survival Curve
Survival curve was analyzed using the GraphPad Prism program (La Jolla, CA) as described previously.30
Measurement of Renal Function
Blood and urine samples were collected every 2 weeks during treatment. Urine samples were collected using metabolic cages for 16 hours and volumes recorded. blood urea nitrogen (BUN), serum creatinine, proteinuria, leukocyturia, and hematuria were assessed as described.5,10,31
Immunofluorescence Staining and Histology
Immunofluorescence staining of C3, C9, and fibrin in the mouse kidney, preparation of paraffin sections and hematoxylin and eosin and periodic acid–Schiff staining of kidney sections were performed as described previously.5,10,32 In some experiments, glomerular C3 deposition was also stained with a FITC-conjugated mouse anti-mouse C3b/iC3b/C3d mAb (used at 1 μg/ml; Cederlane Labs) or a biotinylated polyclonal goat anti-mouse C3d antibody (catalog #BAF2655, used at 1 mg/ml; R&D Systems) with streptavidin-FITC (catalog #554060, used at 1:100 dilution; BD Pharmingen) as a detecting reagent. Renal pathology was graded in a blind fashion. The following characteristics were recorded: glomerular hypercellularity and glomerular crescent formation. The severity of each change in a given mouse was graded as a percentage of glomeruli showing the respective changes (0–100%). Approximately 50 glomeruli were examined for each sample. Electron microscopy analysis of kidney sections was performed at the Electron Microscopy Resource Laboratory of University of Pennsylvania, as described previously.5
Western Blotting of C3, FB, and C5
Western blotting of mouse plasma C3, FB, and C5 was performed as described.5,10
Detection of Anti CRIg-Fc Antibody Response
To detect antibody response against CRIg-Fc, ELISA plates were coated with 2 μg/ml purified CRIg-Fc in PBS for 1 hour at 37°C. Plates were then blocked with 1% BSA-PBS for 1 hour at room temperature and washed three times with PBS-Tween 20, followed by addition of serially diluted mouse serum in 1% BSA-PBS, starting at 1:100. After washing, plates were incubated with a light-chain-specific, horseradish peroxidase-conjugated goat anti-mouse IgG (catalog #115–005–174, used at 2 μg/ml; Jackson Immunoresearch) for 1 hour at room temperature. After washing, plates were developed using Ultra TMB ELISA substrate (Thermo Fisher Scientific).
Statistical Analyses
Statistical comparisons were performed using GraphPad Prism 4.0 software. Data are presented as mean±SD. The difference between two groups was calculated using the two-tailed t test for normally distributed data. For multiple group comparison, one-way ANOVA with a Tukey test was used. P value <0.05 was considered statistically significant.
Result and Discussion
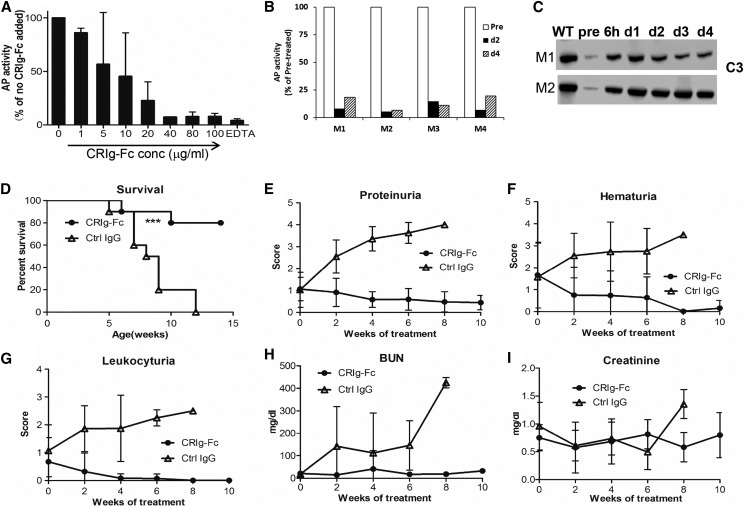
To confirm the activity of CRIg-Fc as an AP-specific inhibitor, we performed LPS-dependent AP and OVA/anti-OVA immune complex-mediated classical pathway complement activation assays using ELISA. Figure 1A shows that at 40 μg/ml CRIg-Fc completely suppressed AP activity in 50% mouse serum diluted in EGTA-containing gelatin-veronal buffered saline. Using OVA/anti-OVA immune complex and FB knockout mouse serum, we confirmed previous findings25 that CRIg does not inhibit classical pathway complement (Supplemental Figure 1).When administered to WT mice at 50 mg/kg, it also produced sustained inhibition of AP activity (>80% inhibition) for up to 4 days (Figure 1B). We next tested CRIg-Fc in FHm/m mice that carried a C-terminal truncation mutation in FH and expressed only a small amount of the mutant FH.5 Because of AP dysregulation, FHm/m mice had profound systemic complement activation and consumption with very low plasma C3 levels (Figure 1C, lane “pre”). After a single CRIg-Fc injection (1 mg per mouse), plasma C3 was markedly elevated when examined between 6 hours and 4 days postinjection (Figure 1C). These data suggested that CRIg-Fc can effectively inhibit AP activity both in normal mice and mice suffering from AP dysregulation.
Figure 1.
CRIg-Fc inhibits AP activity in normal and mutant mice and prevents fatal C3G disease. (A) At 40 μg/ml or higher, CRIg-Fc effectively inhibited AP activity in 50% WT mouse serum. Data from two independent experiments (one with duplicate and the other triplicate assays) were pooled and plotted together. Results are presented as mean (SD). (B) CRIg-Fc inhibited AP activity in vivo in WT mice. Data from four WT mice (M01, M02, M03, and M04) were plotted individually. Mice were injected with 50 mg/kg CRIg-Fc and LPS-dependent AP activity was measured using 10% serum, in duplicate assays (average is shown). AP activity at day 2 and day 4 was normalized to that of the pretreatment sample (pre). (C) Western blotting shows that CRIg-Fc treatment inhibited C3 consumption and markedly elevated plasma C3 levels in two FHm/m mice (M1 and M2). Western blot band shown denotes intact C3 α-chain. A single dose of 1 mg CRIg-Fc was administered to each mouse. Blood samples were collected immediately before and at 6 hours and 1, 2, 3, and 4 days after CRIg-Fc injection. (D–I). Treatment of FHm/mP−/− mice with control IgG had no effect on survival (100% mortality) and C3G disease development as assessed by proteinuria, hematuria, leukocyturia, BUN, and serum creatinine. In contrast, treatment with CRIg-Fc significantly improved survival (80%) and multiple disease parameters. FHm/mP−/− mice received treatments (n=10 mice per group) starting at 4 weeks of age and lasting for 10 weeks (50 mg/kg, administered intraperitoneally). Data shown in (E–I) represent mean (SD). ***P<0.001, log-rank test. ctrl, control; conc, concentration.
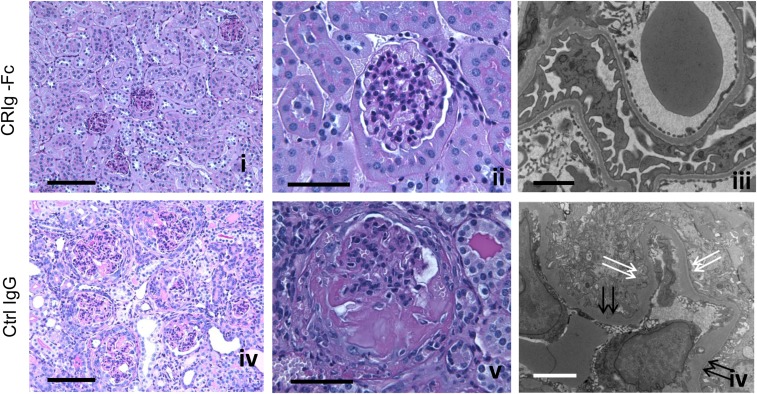
To test the therapeutic potential of CRIg-Fc, we next tested CRIg-Fc in FHm/mP−/− mice, who develop a more aggressive and lethal form of C3G.5 FHm/mP−/− mice were randomized into either CRIg-Fc or isotype control IgG treatment group (50 mg/kg, intraperitoneal injection every fourth day, n=10 mice per group). Treatment was initiated at 4 weeks of age and lasted for 10 weeks. Figure 1D shows that FHm/mP−/− mice treated with control IgG all died or became moribund within 8 weeks of initiating treatment, whereas eight out of ten (80%) CRIg-Fc–treated mice survived the entire 10-week treatment period. Furthermore, CRIg-Fc but not control IgG treatment reversed and/or maintained at baseline levels several C3G disease markers, including proteinuria, hematuria, leukocyturia, and BUN (Figure 1, D–I). Renal pathology was performed on eight out ten and seven out of ten mice in the CRIg-Fc and control IgG group, respectively. These mice represented all surviving mice in the CRIg-Fc group at week 10 of treatment and those that became moribund in the control IgG group that had to be terminated before week 10. We were not able to collect tissues from the remainder of experimental mice (two in the CRIg-Fc group and three in the control IgG group) as they died suddenly. As shown in Figure 2 and Supplemental Table 1, moribund mice in the control treatment group developed glomerular hypercellularity, diffuse cellular and fibrocellular glomerular crescents, and segmental fibrinoid necrosis. Interstitial fibrosis, tubular protein, and erythrocyte casts were also present. In contrast, CRIg-Fc–treated mice showed essentially normal kidney histology, with no glomerular crescent seen and hypercellularity in only 2–5% of the glomeruli (Figure 2, Supplemental Table 1). On electron microscopy, glomeruli of control IgG–treated mice had extensive foot process effacement, irregular GBM thickening, and intramembranous dense deposit, whereas CRIg-Fc–treated mice had normal GBM architecture with intact podocyte foot processes (Figure 2).
Figure 2.
FHm/mP−/− mice treated with CRIg-Fc but not isotype control IgG mAb had normal kidney histology. Period Periodic acid–Schiff staining shows that a representative control IgG–treated mouse developed fatal kidney damage with numerous crescents, significant glomerular enlargement, inflammation, and tubulointerstitial injury (D and E). In contrast, in a representative mouse treated with CRIg-Fc, only mild hypercellularity was observed in some glomeruli and no signs of significant crescents or tissue damage were evident (A and B). By electron microscopy, in a representative CRIg-Fc–treated mouse, normal GBM and intact podocyte foot processes (C) were seen. Signs of podocyte injury, irregular GBM thickening (F, double black arrows) and foot process effacement (F, double white arrows) were clearly present in a representative control IgG–treated FHm/mP−/− mouse. Bars, 100 μm in (A and D), 50 μm in (B and E), 2 μm in (C), and 4 μm in (F).
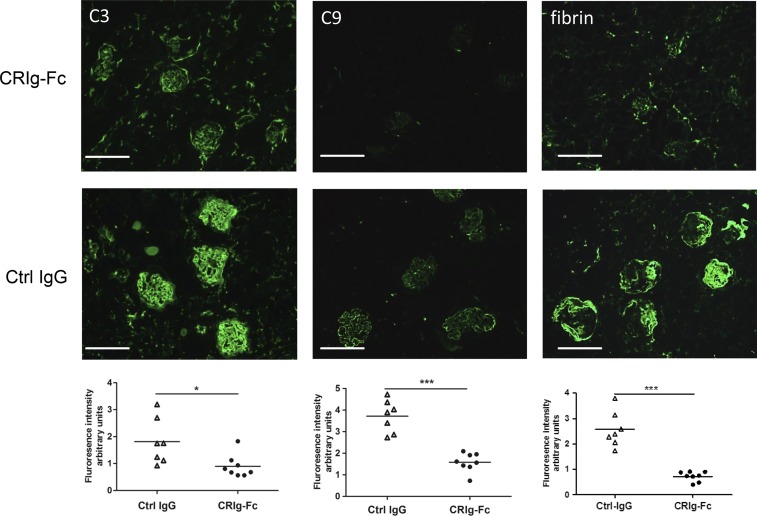
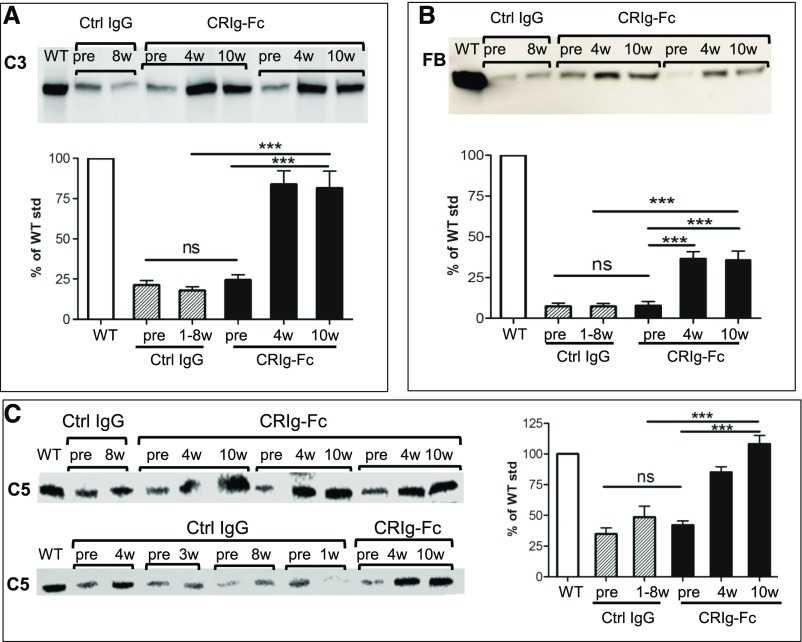
Results of immunofluorescence staining of C3, C9, and fibrin in the glomeruli were consistent with the histology data, with CRIg-Fc–treated mice showing significantly reduced staining of all three markers (Figure 3). In particular, C9 and fibrin staining were almost negative in CRIg-Fc–treated mice, whereas capillary C9 staining and fibrin staining in necrotic and crescentic glomeruli were prominent in control IgG–treated mice (Figure 3). The exact nature of activated C3 fragments deposited in the FHm/mP−/− mouse glomeruli remains to be established, but glomerular staining could also be detected using two commercial antibodies against C3b/iC3b/C3d or C3d (Supplemental Figure 2). Because C3, FB, and to a lesser extent, C5 were excessively consumed in FHm/mP−/− mice,5,10 we next examined if treatment with CRIg-Fc led to recovery of these complement components in the plasma. Figure 4 shows that control IgG treatment had no effect on plasma C3, FB, and C5 levels, but CRIg-Fc treatment resulted in significant elevation of plasma C3, C5, and FB proteins. These data further confirmed the efficacy of CRIg-Fc as a C3 and C5 convertase inhibitor of the AP. It is notable that CRIg-Fc had a more significant effect on plasma C5 and C3 than on FB. This differential effect is likely related to the mechanism of action of CRIg-Fc, which inhibits C3 and C5 cleavage by the respective C3 and C5 convertases but does not directly inhibit the cleavage of FB by factor D once C3bB is formed.33 The moderate increase in plasma FB likely reflected a secondary effect of C3 inhibition, leading to less C3b and C3bB production. It is also interesting that CRIg-Fc treatment produced a more dramatic inhibitory effect on glomerular C9 deposition than C3 deposition (Figure 3). This observation is consistent with the known property of CRIg-Fc, which has a higher affinity for, and is a better inhibitor of, the C5 convertase than the C3 convertase.25
Figure 3.
Reduction of immunofluorescence staining of C3, C9, and fibrin/fibrinogen in kidney sections of CRIg-Fc-treated mice compared with that of control IgG-treated mice. Compared with control IgG–treated group, CRIg-Fc treatment significantly decreased glomerular C3 and fibrin/fibrinogen deposition in FHm/mP−/− mice. Representative fluorescence images from a control IgG– and a CRIg-Fc–treated mouse are shown in the top panels and quantitative analysis of C3, C9 and fibrin/fibrinogen staining is provided in the scatter plots underneath the immunofluorescence images. Each dot in the scatter plot represents a single mouse. Fluorescence intensity of C3, C9, and fibrin/fibrinogen was calculated using ImageJ and expressed in arbitrary units. Arbitrary unit was calibrated separately for each antibody staining group. We analyzed and scored 10–15 glomeruli in three different viewing fields of each kidney section. Horizontal bars through scatter plots represent average values (n=7 mice in control IgG group and n=8 mice in CRIg-Fc group). Bars, 100 μm. * P<0.05; *** P<0.001 (t test).
Figure 4.
CRIg-Fc treatment significantly elevated plasma C3, FB, and C5 levels in FHm/mP−/− mice. Western blot analysis of intact plasma C3 (A), FB (B), and C5 (C) in WT, control IgG–treated FHm/mP−/− mice, and CRIg-Fc–treated FHm/mP−/− mice. Bands shown in (A–C) denote intact C3 α-chain, intact FB, and intact C5 (nonreducing gel), respectively. Blot images show only representative mice in each group and the bar graphs show densitometry scanning of all mouse sera available for analysis (n=6 for the control IgG group and n=7 for CRIg-Fc group). Plasma samples in the control IgG group were from pretreatment or 1–8 weeks after treatment, depending on when the mice became moribund. Plasma samples from the CRIg-Fc treatment group were more reliably collected because of better mouse survival and those from pretreatment (pre) and 4 and 10 weeks after treatment were used for analysis. *** P<0.001 (one-way ANOVA with Tukey test). ctrl, control; std, standard.
C3G is a serious kidney disease in need of a specific and effective treatment. Several proximal complement inhibitors are currently under development for C3G, but their efficacy in vivo remains to be tested.34 Our data here using the robust FHm/mP−/− mouse model have provided strong proof of concept that therapeutics on the basis of the mechanism of action of CRIg-Fc could represent a new and effective therapy for C3G. The treatment regimen of CRIg-Fc used in this study (50 mg/kg, every 4 days) is similar to what is required to inhibit mouse C5 by an mAb.10 If translatable to humans and by analogy of anti-C5 mAb therapy in humans,11–17 the pharmacology of CRIg-Fc may be superior to inhibitors targeting C335 or factor D,36 considering the abundance or fast turnover rate of the latter two complement proteins. Of note, by ELISA assay we detected low titers of antibody response to CRIg-Fc in the treated mice (Supplemental Figure 3). However, the observed efficacy of CRIg-Fc therapy in C3G mice suggested that such immune response likely had a limited effect on the pharmacology of CRIg-Fc. On the other hand, as a macrophage phagocytic receptor, CRIg plays a critical role in removing complement-opsonized bacteria in circulation,28 and whether the use of CRIg-Fc as a therapeutic drug creates an unacceptable or manageable infection risk needs to be carefully considered. In this context, complement inhibitors that share a similar mechanism of action to CRIg-Fc but do not block cellular functions of CRIg, e.g., C3b-specific mAbs as reported in the literature,37,38 may be another class of promising drugs that could be explored clinically for the treatment of C3G.
Disclosures
W.-C.S. has received a research grant from Genentech, Inc. All other author(s) have no competing financial interests to declare.
Supplementary Material
Acknowledgments
We thank the Electron Microscopy Core of the Perelman School of Medicine, University of Pennsylvania, for services provided.
W.-C.S., X.W., T.M., D.G., and G.X. designed experiments and interpreted data. X.W., T.M., D.G., Y.U., and Y.W. performed experiments. M.P. performed pathological analysis and interpreted data. M.V.L.C. and K.J.K. provided testing reagents. X.W., T.M., D.G., and W.-C.S. prepared figures. W.-C.S. and X.W. wrote the manuscript and all coauthors critically commented and/or edited the manuscript.
This work is supported by National Institutes of Health grants AI117410, AI44970, and AI085596, and a research grant from Genentech, Inc to W.-C.S.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Novel Approaches to Control of the Alternative Complement Pathway for the Treatment of C3 Glomerulopathies,” on pages 2032–2033.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018030270/-/DCSupplemental.
References
- 1.Servais A, Noël LH, Roumenina LT, Le Quintrec M, Ngo S, Dragon-Durey MA, et al.: Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int 82: 454–464, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Jansen JH, Høgåsen K, Mollnes TE: Extensive complement activation in hereditary porcine membranoproliferative glomerulonephritis type II (porcine dense deposit disease). Am J Pathol 143: 1356–1365, 1993 [PMC free article] [PubMed] [Google Scholar]
- 3.Jansen JH: Porcine membranoproliferative glomerulonephritis with intramembranous dense deposits (porcine dense deposit disease). APMIS 101: 281–289, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Pickering MC, Cook HT, Warren J, Bygrave AE, Moss J, Walport MJ, et al.: Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet 31: 424–428, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Lesher AM, Zhou L, Kimura Y, Sato S, Gullipalli D, Herbert AP, et al.: Combination of factor H mutation and properdin deficiency causes severe C3 glomerulonephritis. J Am Soc Nephrol 24: 53–65, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Józsi M, Reuter S, Nozal P, López-Trascasa M, Sánchez-Corral P, Prohászka Z, et al.: Autoantibodies to complement components in C3 glomerulopathy and atypical hemolytic uremic syndrome. Immunol Lett 160: 163–171, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Pickering MC, D’Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, et al.: C3 glomerulopathy: Consensus report. Kidney Int 84: 1079–1089, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao X, Pickering MC, Smith RJH: C3 glomerulopathy: The genetic and clinical findings in dense deposit disease and C3 glomerulonephritis. Semin Thromb Hemost 40: 465–471, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Barbour TD, Pickering MC, Terence Cook H: Dense deposit disease and C3 glomerulopathy. Semin Nephrol 33: 493–507, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams AL, Gullipalli D, Ueda Y, Sato S, Zhou L, Miwa T, et al.: C5 inhibition prevents renal failure in a mouse model of lethal C3 glomerulopathy. Kidney Int 91: 1386–1397, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuber J, Fakhouri F, Roumenina LT, Loirat C, Frémeaux-Bacchi V; French Study Group for aHUS/C3G : Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol 8: 643–657, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Bomback AS, Smith RJ, Barile GR, Zhang Y, Heher EC, Herlitz L, et al.: Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin J Am Soc Nephrol 7: 748–756, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herlitz LC, Bomback AS, Markowitz GS, Stokes MB, Smith RN, Colvin RB, et al.: Pathology after eculizumab in dense deposit disease and C3 GN. J Am Soc Nephrol 23: 1229–1237, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gurkan S, Fyfe B, Weiss L, Xiao X, Zhang Y, Smith RJ: Eculizumab and recurrent C3 glomerulonephritis. Pediatr Nephrol 28: 1975–1981, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daina E, Noris M, Remuzzi G: Eculizumab in a patient with dense-deposit disease. N Engl J Med 366: 1161–1163, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Le Quintrec M, Lionet A, Kandel C, Bourdon F, Gnemmi V, Colombat M, et al.: Eculizumab for treatment of rapidly progressive C3 glomerulopathy. Am J Kidney Dis 65: 484–489, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Radhakrishnan S, Lunn A, Kirschfink M, Thorner P, Hebert D, Langlois V, et al.: Eculizumab and refractory membranoproliferative glomerulonephritis. N Engl J Med 366: 1165–1166, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Wehling C, Amon O, Bommer M, Hoppe B, Kentouche K, Schalk G, et al.: Monitoring of complement activation biomarkers and eculizumab in complement-mediated renal disorders. Clin Exp Immunol 187: 304–315, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Nester CM, Martin B, Skjoedt MO, Meyer NC, Shao D, et al.: Defining the complement biomarker profile of C3 glomerulopathy. Clin J Am Soc Nephrol 9: 1876–1882, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith RJ, Harris CL, Pickering MC: Dense deposit disease. Mol Immunol 48: 1604–1610, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbour TD, Pickering MC, Cook HT: Recent insights into C3 glomerulopathy. Nephrol Dial Transplant 28: 1685–1693, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith RJ, Alexander J, Barlow PN, Botto M, Cassavant TL, Cook HT, et al.; Dense Deposit Disease Focus Group : New approaches to the treatment of dense deposit disease. J Am Soc Nephrol 18: 2447–2456, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lebreton C, Bacchetta J, Dijoud F, Bessenay L, Fremeaux-Bacchi V, Sellier-Leclerc AL: C3 glomerulopathy and eculizumab: A report on four paediatric cases. Pediatr Nephrol 32: 1023–1028, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Chen M, Muckersie E, Luo C, Forrester JV, Xu H: Inhibition of the alternative pathway of complement activation reduces inflammation in experimental autoimmune uveoretinitis. Eur J Immunol 40: 2870–2881, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Katschke KJ Jr, Helmy KY, Steffek M, Xi H, Yin J, Lee WP, et al.: A novel inhibitor of the alternative pathway of complement reverses inflammation and bone destruction in experimental arthritis. J Exp Med 204: 1319–1325, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieberman LA, Mizui M, Nalbandian A, Bossé R, Crispín JC, Tsokos GC: Complement receptor of the immunoglobulin superfamily reduces murine lupus nephritis and cutaneous disease. Clin Immunol 160: 286–291, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Wiesmann C, Katschke KJ, Yin J, Helmy KY, Steffek M, Fairbrother WJ, et al.: Structure of C3b in complex with CRIg gives insights into regulation of complement activation. Nature 444: 217–220, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Helmy KY, Katschke KJ Jr, Gorgani NN, Kljavin NM, Elliott JM, Diehl L, et al.: CRIg: A macrophage complement receptor required for phagocytosis of circulating pathogens. Cell 124: 915–927, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Kimura Y, Miwa T, Zhou L, Song WC: Activator-specific requirement of properdin in the initiation and amplification of the alternative pathway complement. Blood 111: 732–740, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altman DG: Practical Statistics for Medical Research, Boca Raton, FL, CRC press, 1990 [Google Scholar]
- 31.Yamada K, Miwa T, Liu J, Nangaku M, Song W-C: Critical protection from renal ischemia reperfusion injury by CD55 and CD59. J Immunol 172: 3869–3875, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Ueda Y, Mohammed I, Song D, Gullipalli D, Zhou L, Sato S, et al.: Murine systemic thrombophilia and hemolytic uremic syndrome from a factor H point mutation. Blood 129: 1184–1196, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bexborn F, Andersson PO, Chen H, Nilsson B, Ekdahl KN: The tick-over theory revisited: Formation and regulation of the soluble alternative complement C3 convertase (C3(H2O)Bb). Mol Immunol 45: 2370–2379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan BP, Harris CL: Complement, a target for therapy in inflammatory and degenerative diseases. Nat Rev Drug Discov 14: 857–877, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mastellos DC, Yancopoulou D, Kokkinos P, Huber-Lang M, Hajishengallis G, Biglarnia AR, et al.: Compstatin: A C3-targeted complement inhibitor reaching its prime for bedside intervention. Eur J Clin Invest 45: 423–440, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan X, Gavriilaki E, Thanassi JA, Yang G, Baines AC, Podos SD, et al.: Small-molecule factor D inhibitors selectively block the alternative pathway of complement in paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. Haematologica 102: 466–475, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katschke KJ Jr, Stawicki S, Yin J, Steffek M, Xi H, Sturgeon L, et al.: Structural and functional analysis of a C3b-specific antibody that selectively inhibits the alternative pathway of complement. J Biol Chem 284: 10473–10479, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DiLillo DJ, Pawluczkowycz AW, Peng W, Kennedy AD, Beum PV, Lindorfer MA, et al.: Selective and efficient inhibition of the alternative pathway of complement by a mAb that recognizes C3b/iC3b. Mol Immunol 43: 1010–1019, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.