Abstract
Background Despite epidemiologic evidence for increased cardiovascular morbidity and mortality associated with both high dietary and serum phosphate in humans with normal renal function, no controlled phosphate intervention studies of systemic hemodynamics have been reported. Higher serum 25(OH) vitamin D levels are associated with better cardiovascular outcomes, but vitamin D increases intestinal phosphate absorption.
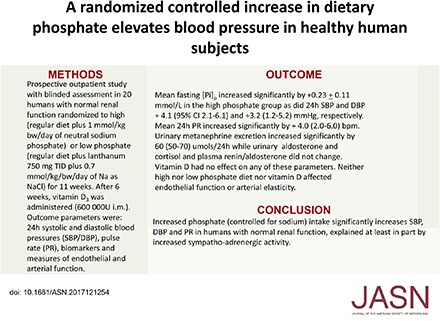
Methods We conducted a prospective outpatient study with blinded assessment in 20 young adults with normal renal function randomized to high phosphate (regular diet plus 1 mmol/kg body wt per day of Na as neutral sodium phosphate) or low phosphate (regular diet plus lanthanum, 750 mg thrice/day, plus 0.7 mmol/kg body wt per day of Na as NaCl) for 11 weeks. After 6 weeks, all subjects received vitamin D3 (600,000 U) by intramuscular injection. Outcome parameters were 24-hour ambulatory systolic and diastolic BP (SBP and DBP), pulse rate (PR), biomarkers, and measures of endothelial and arterial function.
Results Compared with the low-phosphate diet group, the high-phosphate diet group had a significant increase in mean±SEM fasting plasma phosphate concentration (0.23±0.11 mmol/L); 24-hour SBP and DBP (+4.1; 95% confidence interval [95% CI], 2.1 to 6.1; and +3.2; 95% CI, 1.2 to 5.2 mm Hg, respectively); mean 24-hour PR (+4.0; 95% CI, 2.0 to 6.0 beats/min); and urinary metanephrine and normetanephrine excretion (54; 95% CI, 50 to 70; and 122; 95% CI, 85 to 159 µg/24 hr, respectively). Vitamin D had no effect on any of these parameters. Neither high- nor low-phosphate diet nor vitamin D affected endothelial function or arterial elasticity.
Conclusions Increased phosphate intake (controlled for sodium) significantly increases SBP, DBP, and PR in humans with normal renal function, in part, by increasing sympathoadrenergic activity.
Keywords: phosphate, hypertension, hyperphosphatemia, FGF-23, Vitamin D
Vascular calcification is a cardiovascular risk factor leading to increased mortality in both CKD and general populations.1 Both traditional (hypertension, diabetes, dyslipidemia) and nontraditional risk factors (inflammation, oxidative stress, advanced glycation end products, hyperphosphatemia, elevated FGF-23 levels)2 are important in inducing vascular calcification. Elevated extracellular phosphate concentration has been found in observational epidemiologic assessments to play a highly suggestive role in initiating and exacerbating vascular disease and cardiovascular mortality in patients with CKD.3,4
The negative effect on cardiovascular morbidity/mortality of high dietary phosphate load/hyperphosphatemia may, however, also affect the general population5,6:
The age-associated decrease in GFR could contribute to a slowly progressive increase in net phosphate load per nephron and associated incremental hyperphosphatemia, but such experiments would require accurate measured GFR and control of diurnal phosphate sampling time effects.
Mostly because of its use in processed “fast foods,” phosphate intake has risen in recent decades and is now far above the current recommended daily allowance for inorganic phosphate as established by the National Academy of Medicine (700 mg).
Independent associations of fasting plasma phosphate concentration and dietary phosphate loads with cardiac calcification, left ventricular hypertrophy, cardiovascular events, and death are evident in the general population, even in younger adults, and cardiovascular event risk accelerates with phosphate levels >1.15–1.47 mmol/L.7–11
1,25(OH)2D stimulates intestinal phosphate absorption, thereby increasing the systemic phosphate load.12 Lower 25(OH) vitamin D levels or decreased supply of dietary vitamin D are associated with increased cardiovascular morbidity and cardiovascular as well as all-cause mortality.13–16 Therefore, it is unclear whether the routinely recommended vitamin D supplements might protect against cardiovascular phosphate toxicity or aggravate it via increasing phosphate absorption.
We examined in young healthy subjects with normal renal function, by randomized single-blind design, the effects of low-phosphate (LP) and high-phosphate (HP) intake for 6 weeks on a variety of cardiovascular hemodynamic effects (i.e., 24-hour BP and pulse profiles and measures of endothelial and arterial function) and cardiovascular risk and aging markers, as well as phosphate homeostasis. In addition, we evaluated the effect of supplemental vitamin D3 on these parameters with continued LP and HP intake for another 5 weeks.
Methods
Study Protocol
We evaluated the chronic effects of decreasing and increasing the dietary phosphate load without and with vitamin D3 supplementation on cardiovascular outcome parameters, i.e., 24-hour systolic and diastolic BP (SBP and DBP) and mean pulse rate, reactive hyperemia index as a measure of endothelial function, and aortic pulse wave velocity (PWV) and the augmentation index (AI) as a measure of arterial elasticity.
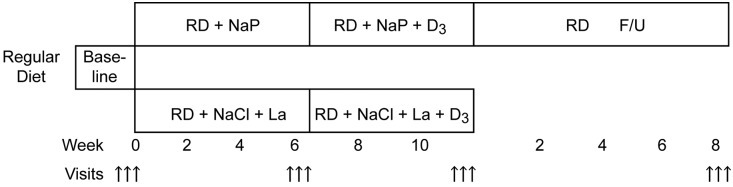
In a prospective, exploratory, single-blind outpatient study design (see Figure 1), 20 young, healthy human subjects of both sexes were studied. Exclusion criteria were: any evidence for kidney disease; electrolyte or acid-base disturbance; office BP≥125/80 mm Hg; ingestion of any drug (prescribed, over the counter, or illicit) during the 3 months before and during the study; smoking; excessive alcohol intake (more than 1 drink/d); any evidence for adrenal (24-hour urinary cortisol and aldosterone excretion), thyroid, or parathyroid dysfunction; or 25(OH)D serum concentrations <50 or >100 nmol/L.
Figure 1.
Flowchart of study protocol. Each arrow denotes daily complete assessments (three consecutive days/evaluations per study period). F/U, follow-up; La, lanthanum; NaCl, sodium chloride; NaP, neutral sodium phosphate; RD, regular diet.
The subjects were randomized by a research administrator, unblocked, and unstratified 1:1 to either the HP group (n=10), ingesting regular diet plus 1 mmol sodium per kg body weight per os as neutral sodium phosphate (yielding 0.55 mmol P per mmol Na) in three divided doses per day with meals, or to the LP group (n=10), ingesting a regular diet and the intestinal phosphate binder lanthanum (Fosrenol, 750 mg; Shire Pharmaceuticals, Lexington, MA, in three per os divided doses with meals). The LP group was supplemented with 0.7 mmol/kg body wt per day NaCl commensurate with the excess sodium intake of the HP group assuming an intestinal absorption of sodium phosphate of 70%.17 After 6 weeks of LP or HP loading, all subjects received a single intramuscular injection of vitamin D3 (600,000 U) and were studied for another 5 weeks. There were three visits each on three consecutive days at baseline, at the end of week 6, and at the end of week 11 (5 weeks after vitamin D administration). There were additional visits every 2 weeks to assess tolerance, safety, and compliance and a close-out visit at the end of week 12. After study unblinding, the HP group was re-examined (24-hour ambulatory BP measurement [ABPM] and tests of endothelial and arterial function only) in a recovery visit on regular diet 2 months after the close-out visit.
Measurements
Twenty-four-hour ABPMs were made at the nondominant arm using the commercial Schiller BR-102 device with vendor software. BP measurements were made every 15 minutes (06:00–22:00 hours) and every 30 minutes during the nighttime (22:00–06:00 hours), consistent with ABPM method guidance.18
Arterial pressure waveforms were recorded and analyzed using established techniques using vendor software (Sphygmocor; AtCor Medical, Australia). In brief, the aortic PWV (m/s) is estimated by calculating the time between the foot of the pressure wave and the inflection point. Augmentation represents the difference between the second reflected systolic peak and first systolic peak of the central pressure waveform, and AI is defined as the augmentation increment expressed as a percentage of the pulse pressure and is a measure of systemic arterial stiffness.19
Endothelial function was analyzed by determining the reactive hyperemia index, measured as flow-mediated vasodilation after 5 minutes of tourniquet-induced hypoxia using the Endopat (Itamar, Israel) pulse arterial volume change as assessed with a fingertip sensor and vendor software. The tourniquet was applied to the dominant arm and finger probes to both hands, the nondominant side serving as control.
Before these measurements, the fasting subjects were lying comfortably for 30 minutes in a noise-shielded, dimly lit room, at a constant room temperature of 22°C–24°C and constant humidity of 58%–62%. Measurements were always performed between 08:30 am and 11:00 am. In our study lab, the interassay coefficient for PWV measurements was 5.9%, and 8.7% for reactive hypoxia index.
Biochemical Analysis
Fasting samples for plasma electrolyte and blood acid-base measurements and for serum endocrine and aging markers were drawn after preheating the forearm to 43°C without use of tourniquets between 07:00 am and 08:00 am. Twenty-four-hour urines (three consecutive days of each study period) were collected under paraffin oil with thymol as a preservative. Plasma and urine electrolytes were measured in the hospital routine lab.
1,25(OH)2D was measured by ELISA (Immundiagnostik AG, Bensheim, Germany), 25(OH) vitamin D by the liquid chromatography–mass spectrometry method (MassChrom), and intact PTH and insulin by the Unicel Dxl 800 immunoassay system (Beckman Coulter).
Human FGF-23 (second generation, dual antibody method to detect both C-terminal and intact FGF-23) was measured using an ELISA kit (#60–6100; Immunotopics, San Clemente, CA). Serum αKlotho protein was precipitated from the serum using the sb-106 Fab, reported to recognize both vector HEK cell–expressed human full-length membrane Klotho (Isoform 1; Uniprot Consortium, Switzerland) and Klotho extracellular domain (ECD) (α cut) as nominal 130-kD bands. After gel electrophoresis, the immune-precipitated αKlotho protein was further detected on immunoblot using the clone KM2076 antibody (Transgenic Inc., Kobe, Japan). This antibody was generated from KL1 domain immunization in rats and was reported to detect (western blot) an approximately 130-kD human renal membrane-associated klotho and thus may detect human full-length membrane klotho (Isoform 1), proteolytic ECD (α cut) near 130 kD, or the secreted alternatively spliced isoform at a lower molecular mass (Isoform 2; Uniprot).20,21 KM2076 is predicted to not detect a putative C terminus β cut Klotho (KL2 domain). The serum 130 kD band was estimated on the basis of murine rKlotho isoform 1 standard as a density and thus likely represents the α cut protein ECD. The rKlotho protein standard was obtained from a CHO cell line that stably expressed the full-length Klotho (CHOKL, Isoform 1), obtained as a gift from Kyowa Hakko Kogyo Co. Ltd.22
Urine Klotho was measured by adding urine to 4× lithium dodecyl sulfate sample buffer containing a final concentration of 100 mM dithiothreitol. The boiled samples were run on a 4%–14% Bis-Tris gel (NuPage; Invitrogen), immunoblotted using clone KM2076 antibody, and reported as density of the 130-kD band.
Plasma renin and aldosterone and urinary aldosterone concentrations were measured by chemiluminescence immunoassay (Bioscientia, Ingelheim, Germany). Urinary total metanephrine and normetanephrine (comprising free and conjugated compounds) were measured by HPLC after acid hydrolysis, and urinary free cortisol was measured by liquid chromatography–mass spectrometry.
The serum calcification propensity test (T50) was performed using a Nephelostar nephelometer (BMG Labtech, Offenburg, Germany).23 Serum oxidized LDL and SOD1 were measured by ELISA, antioxidative capacity and serum lipid peroxide concentration were measured photometrically, and urinary 8-oxo-2-deoxy guanosine was measured by ELISA (all: Biovis, Limbur, Offheim, Germany).
After enrollment, subjects received advice to ingest a stable self-selected diet.
All values given in “Results” are the means of three measurements made on these three separate, consecutive days in each period.
Investigators were blinded to treatment assignment of each study subject throughout the study.
The protocol was approved by the ethics committee of both Cantons of Basel (Switzerland).
Methods and safety considerations of the vitamin D dosing are described in the Supplemental Material.
Statistical Analyses
Sample size determination was empiric without consideration of power or effect size. Values given are means with 95% confidence intervals (95% CIs) for the cardiovascular outcome parameters. All other values are mean±SD or SEM, as indicated. Statistical significance was tested with ANOVA for repeated measures for intragroup comparison and with one-way ANOVA for intergroup comparisons.
Results
Study population: 53 human subjects were screened and 20 were included into the study at 1:1 randomization (HP group/LP group). All randomized subjects completed the study. Reasons for exclusion were: Loss of interest in participation due to occupational duties (n=9), study perceived as too long and too demanding (n=21), vitamin D deficiency (n=1), and concomitant medications (n=2). Subject characteristics are listed in Supplemental Table 1. Compliance rate (inspection of unused agents) for the ingestion of oral study drugs (NaPO4, lanthanum, and NaCl) was 96% in the HP group and 95% in the LP group.
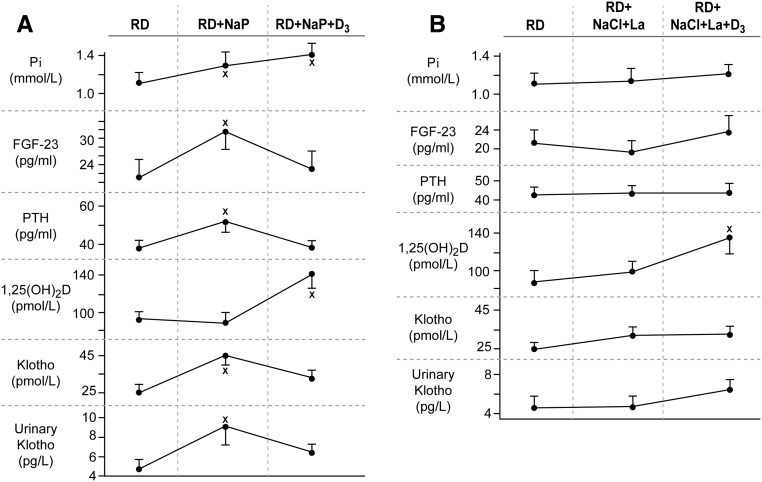
HP induced a significant intra- and intergroup rise in fasting plasma phosphate concentration (+0.19±0.09 mmol/L, P=0.03, Figure 2A, Table 1) and 24-hour mean urinary phosphate excretion rate (+21.0±7.2 mmol/24 hr, P<0.01, Table 2). Neither value was further affected significantly by the D3 supplement. The LP diet’s lanthanum supplement decreased urinary phosphate excretion nonsignificantly (−5.9±2.3 mmol/24 hr) without affecting fasting plasma phosphate concentration. In contrast to HP, D3 in the LP group increased urinary phosphate excretion significantly (+6.8±3.1 mmol/24 hr, P=0.04). Table 2 also shows that 24-hour urinary sodium excretion rates were not different among HP and LP groups, confirming the protocol’s intent for a similar daily sodium load throughout the study among both groups.
Figure 2.
High phosphate diet increases FGF-24, PTH, and both plasma and urinary Klotho, while low phosphate diet has no effect. (A) Effect of HP diet: Baseline (RD), after 6 weeks of HP diet (RD+NaP), and 5 weeks after supplemental vitamin D3 (600,000 U in one single intramuscular dose) and continued HP diet. (B) Effect of LP diet: Baseline (RD), after 6 weeks of ingestion of the phosphate binding substance lanthanum (750 mg per os three times a day) with supplemental NaCl, and 5 weeks after supplemental vitamin D3 (600,000 U in one single i.m. dose) and continued lanthanum and NaCl administration. *P at least <0.05 compared with baseline (RD). La, lanthanum; NaCl, sodium chloride; NaP, neutral sodium phosphate (0.55 mmol/kg body wt phosphate per day); PTH, parathyroid hormone; RD, regular diet.
Table 1.
Fasting mean plasma electrolyte, uric acid, urea, creatinine, and blood bicarbonate concentrations in HP and LP groups without and with vitamin D3 at baseline, W6, and W11
| Period/Group | Na+ | K+ | Cl− | HCO3− | Catot | Ca++ | Pi | Mg | Uric Acid (μmol/L) | Urea | Creatinine (μmol/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HP baseline | 141±6 | 4.1±0.6 | 101±9 | 24.8±1.5 | 2.30±0.34 | 1.19±0.25 | 1.11±0.32 | 0.84±0.28 | 295±34 | 4.40±0.70 | 67.5±17.6 |
| LP baseline | 141±6 | 4.1±0.6 | 101±12 | 24.5±2.5 | 2.30±0.62 | 1.19±0.22 | 1.12±0.34 | 0.82±0.31 | 296±47 | 4.41±0.95 | 67.6±18.6 |
| HP W6 | 142±9 | 4.3±0.6 | 100±9 | 25.8±3.6 | 2.30±0.65 | 1.20±0.19 | 1.30a±0.47 | 0.80±0.35 | 283±53 | 4.17±1.15 | 65.2±18.9 |
| LP W6 | 141±6 | 4.2±0.6 | 101±4 | 24.5±3.2 | 2.30±0.50 | 1.19±0.29 | 1.15±0.39 | 0.85±0.38 | 282±69 | 4.29±1.25 | 66.0±18.2 |
| HP plus D3 W11 | 141±9 | 4.3±0.6 | 101±4 | 25.7±2.3 | 2.31±0.49 | 1.18±0.39 | 1.41b±0.45 | 0.8±0.33 | 279±60 | 4.37±1.45 | 67.5±18.1 |
| LP plus D3 W11 | 141±6 | 4.4±0.6 | 100±3 | 24.9±2.8 | 2.30±0.60 | 1.18±0.25 | 1.22±0.38 | 0.8±0.41 | 284±62 | 4.21±1.44 | 66.3±19.0 |
Data are mean±SD, values are millimoles per liter unless indicated otherwise. HP, HP group; LP, LP group; W, week; Catot, total calcium.
P=0.03 compared with own baseline (intragroup) and 0.03 compared with LP (intergroup).
P=0.02 compared with baseline and 0.03 compared with LP.
Table 2.
Twenty-four-hour urinary excretion rates of electrolytes, creatinine, and albumin in HP and LP groups without and with vitamin D3 at baseline, W6, and W11
| Period/Group | Na+ | K+ | Cl− | Ca | Pi | Mg | Creatinine | Albumin (mg/24 h) | Body Weight (kg) |
|---|---|---|---|---|---|---|---|---|---|
| HP baseline | 119±8 | 55±4 | 106±8 | 3.4±0.2 | 20.5±2.2 | 3.3±0.3 | 7.8±1.1 | 5.2±2.2 | 64.5±5.6 |
| LP baseline | 117±7 | 53±5 | 107±7 | 3.3±0.2 | 21.9±2.4 | 3.2±0.4 | 7.6±1.2 | 5.3±2.4 | 63.7±4.8 |
| HP W6 | 145a±9 | 59±6 | 97±6 | 2.8±0.1 | 41.5b±4.1 | 3.2±0.2 | 7.1±1.0 | 3.4±2.3 | 65.1±5.8 |
| LP W6 | 149a±8 | 52±5 | 139c±8 | 3.1±0.3 | 14.6±1.8 | 3.3±0.2 | 7.1±1.2 | 5.0±2.5 | 64.5±4.4 |
| HP plus D3 W11 | 142a±10 | 59±4 | 102±7 | 3.0±0.2 | 42.5b±3.4 | 3.3±0.3 | 6.5±0.9 | 4.3±2.6 | 64.6±5.1 |
| LP plus D3 W11 | 149a±9 | 57±5 | 133c±7 | 3.0±0.2 | 21.4d±2.9 | 3.3±0.2 | 6.6±0.9 | 2.5±2.4 | 64.4±4.5 |
Data are mean±SD, values are millimoles (milliequivalents per 24 h unless indicated otherwise). HP, HP group; LP, LP group; W, week.
P<0.03 compared with own baseline.
P<0.01 for intra- and intergroup comparisons.
P<0.02 compared with own baseline.
P=0.04 for comparison of LP plus D3 to LP without D3.
Figure 2, A and B, and Table 3 show that HP induced significant intra- and intergroup increases in the serum concentrations of PTH, FGF-23, and αKlotho and urinary Klotho, whereas LP without and with D3 had no effect. In response to vitamin D3, serum 25(OH)D and 1,25(OH)2D increased significantly in both groups, whereas mean serum PTH and FGF-23 decreased in the HP group. Both PTH and 1,25(OH)2D have been shown to increase FGF-23 serum concentration.24,25 The baseline values for urinary phosphate excretion of approximately 21 mmol/24 hr are similar to reported United States and European subjects on self-selected diets.26,27
Table 3.
Mean serum concentrations of phosphate-regulating hormones and urinary excretion of Klotho under HP and LP dietary conditions without and with supplemental D3
| Group/Period | Serum 25(OH)D (nmol/L) | Serum 1,25(OH)2D (pmol/L) | Serum iPTH (pg/ml) | Serum FGF-23 (pg/ml) | Serum αKlotho (pmol/L) | Urinary αKlotho (ng/g Creatinine) |
|---|---|---|---|---|---|---|
| HP baseline | 63.0±6.4 | 92.4±8.1 | 41.4±4.8 | 21.2±3.8 | 24.6±3.7 | 7.9±1.8 |
| LP Baseline | 63.5±7.4 | 93.7±8.4 | 41.8±3.9 | 21.3±2.9 | 24.6±2.9 | 7.2±1.5 |
| HP W6 | 68.3±5.8 | 89.1±9.9 | 51.7a±6.2 | 31.4a±3.6 | 45.0a±5.2 | 12.6a±3.0 |
| LP W6 | 67.3±7.6 | 99.4±10.4 | 42.4±4.4 | 19.1±2.4 | 31.6±4.6 | 7.1±1.3 |
| HP plus D3 W11 | 113.6b±12.4 | 141.6b±15.2 | 41.3c±4.5 | 23.1c±3.6 | 32.0c±4.7 | 7.8±2.0 |
| LP plus D3 W11 | 104.9b±14.2 | 132.9b±14.8 | 43.1±5.2 | 22.8±3.0 | 31.8±4.4 | 6.2±1.3 |
Values are mean±SEM. iPTH, intact parathyroid hormone; HP, high phosphate group; LP, low phosphate group; W, week.
P<0.02 compared with own baseline, and <0.025 for comparison to LP group.
P<0.01 in comparison with baseline and HP/LP without D3 supplementation.
P<0.03 for the comparison of HP without to HP with D3 supplemented.
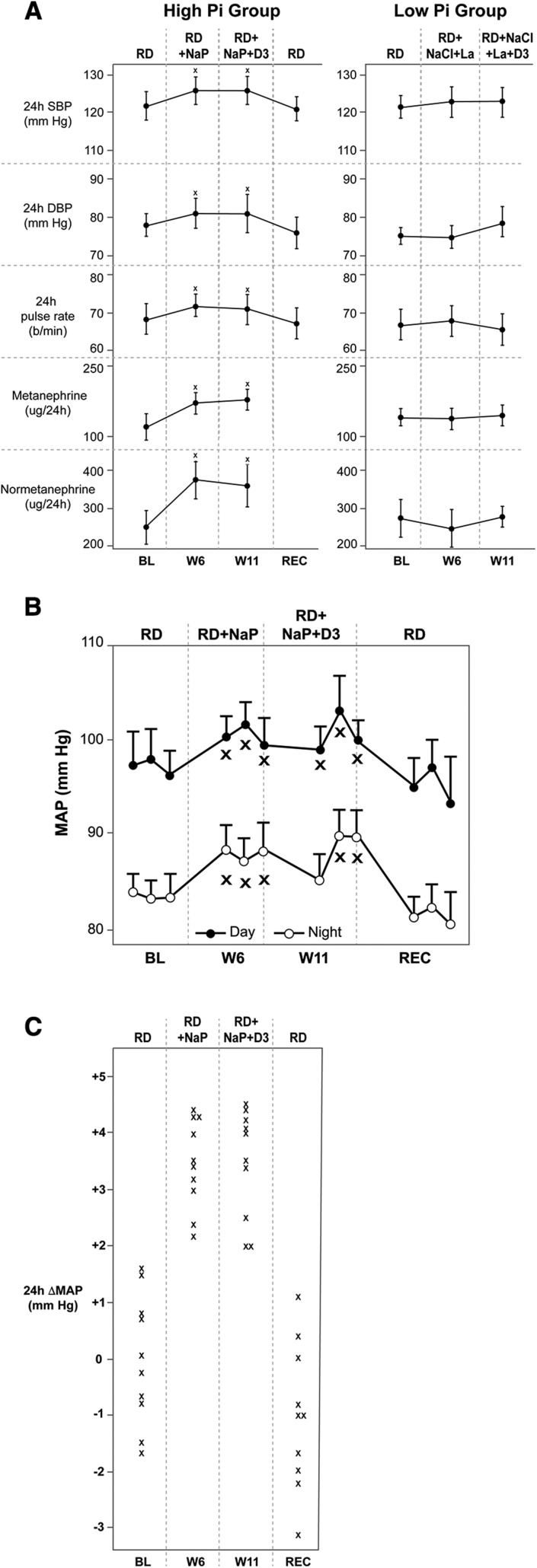
Figure 3A and Table 4 illustrate that HP induced significant inter- and intragroup increases in both mean 24-hour SBP and DBP and mean 24-hour pulse rate, whereas LP had no effect. HP increased 24-hour mean SBP by +4.1 mm Hg (95% CI, 2.1 to 6.1), 24-hour mean DBP by +3.2 (95% CI, 1.2 to 5.2) mm Hg, and mean 24-hour pulse rate by +4.0 (95% CI, 2.0 to 6.0) beats/min. Because these increases were resistant to further change by supplemental vitamin D3, subjects in the HP group were restudied and reversibility of short-term dietary phosphate–induced increases in BP and pulse rate was documented. Figure 3B shows the circadian rhythm of mean arterial pressure. Sympathoadrenergic activity, as assessed by 24-hour urinary excretion of metanephrine and normetanephrine, was stimulated by HP: Urinary metanephrine and normetanephrine excretion increased by 54 (95% CI, 28 to 70) and 122 (95% CI, 85 to 159) µg/24 hr, respectively, whereas 24-hour urinary excretion rates of aldosterone, free cortisol, and plasma renin/aldosterone concentration were not affected by either HP or LP (Figure 3A, Table 4). Figure 3C shows a scatter plot of the individual mean 24-hour arterial BP changes in the HP group.
Figure 3.
HP diet increases mean 24-hour SBP, DBP, and pulse rate with preserved circadian oscillation, and in parallel with significant increases in urinary metanephrine and normetanephrine excretion rates. Vitamin D does not reverse the hypertensiongenic effect in the short term (5 weeks). (A) See Figure 2 legend for description of periods. All values are means of three consecutive daily measurements during the last 3 days of each period. Recovery values are from measurements 2 months after week 11 to show reversibility and with the subjects on regular diet only. *P at least <0.05 for both intra- and intergroup comparisons. (B) Effect of HP diet on day and night mean arterial BP and mean pulse rates. *P at least <0.05 for the comparisons to both baseline and recovery. (C) Per subject changes in MAP in the HP group. BL, baseline; D3, vitamin D3; HP, HP diet; La, lanthanum; LP, LP diet; MAP, mean arterial pressure; NaCl, sodium chloride; NaP, neutral sodium phosphate; PTH, parathyroid hormone; RD, regular diet; REC, recovery; W6 and W11, week 6 and 11, respectively.
Table 4.
Mean 24-h SBP and DBP and pulse rate as well as mean 24-h urinary excretion rates of metanephrine, normetanephrine, aldosterone, and cortisol and mean plasma concentrations of renin and aldosterone under LP and HP dietary conditions without and with supplemental D3
| Variable | 24-h SBP (mm Hg) | 24-h DBP (mm Hg) | 24-h Mean Pulse Rate (Beats/Min) | Metanephrine (μg/24 h) | Metanephrine (μg/gr Creatinine) | Normetanephrine (μg/24 h) | Normetanephrine (μg/g creatinine) | Aldosterone (nmol/24 h) | Free Cortisol (nmol/24 h) | Plasma Renin (ng/L) | Plasma Aldosterone (nmol/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HP baseline | 122 (118 to 126 | 78 (75 to 81) | 68 (64 to 72) | 147±86 | 18.5±11.2 | 252±107 | 33.2±14.1 | 61±53 | 24±10 | 9.42±6.80 | 555±245 |
| LP Baseline | 122 (119 to 127) | 76 (74 to 78) | 67 (63 to 71) | 141±107 | 18.3±14.2 | 271±119 | 35.6±14.6 | 70±44 | 26±13 | 9.73±7.92 | 579±265 |
| HP W6 | 126a (123 to 129) | 81a (77 to 85) | 72 (69 to 75)a | 201a±145 | 28.0a±20.3 | 374a±147 | 52.2a±19.9 | 55±41 | 29±15 | 8.98±8.31 | 525±279 |
| LP W6 | 123 (119 to 127) | 75 (72 to 78) | 68 (64 to 72) | 132±91 | 18.3±13.0 | 241±130 | 33.8±18.3 | 64±51 | 23±15 | 9.23±8.82 | 536±226 |
| HP plus D3 W11 | 126a (122 to 130) | 81a (76 to 86) | 71 (67 to 75)a | 198a±139 | 29.8a±21.2 | 358a±139 | 54.0a±21.3 | 68±48 | 31±19 | 8.10±8.62 | 507±207 |
| LP plus D3 W11 | 123 (119 to 127) | 79 (75 to 83) | 66 (62 to 70) | 144±63 | 19.5±8.9 | 280±132 | 42.4±20.0 | 75±51 | 27±13 | 7.78±7.51 | 505±221 |
Values are means plus 95% CIs (BP and pulse rate) and mean±SD (endocrine parameters). HP, HP group; LP, LP group; W, week.
P<0.03 for intergroup and intragroup comparisons.
As illustrated in Table 5, in both HP and LP groups without and with vitamin D, no measurable effects on endothelial function and/or arterial elasticity were found.
Table 5.
Effects of low (upper panel) and high Pi dietary intake (lower panel) without and with vitamin D3 on parameters of endothelial function and arterial elasticity
| Endothelial and Arterial Parameters | Baseline | W6 | W11 (Plus D3) | Recovery |
|---|---|---|---|---|
| Low Pi dietary intake | ||||
| Reactive hyperemia index | 1.67 (1.30 to 2.04) | 1.53 (1.30 to 1.86) | 1.76 (1.40 to 2.12) | ND |
| AI, % | −10.6 (−2.0 to −19.2) | −7.4 (−2 to −12.8) | −10.7 (−5.7 to 16.4) | ND |
| PWV, m/s | 5.1 (4.4 to 5.8) | 5.3 (4.5 to 6.1) | 5.1 (4.4 to 5.8) | ND |
| High Pi dietary intake | ||||
| Reactive hyperemia index | 1.93 (1.48 to 2.38) | 1.84 (1.60 to 2.08) | 1.86 (1.50 to 2.22) | 1.92 (1.70 to 2.14) |
| AI, % | −10.1 (−2.0 to 18.2) | −8.1 (−3.2 to −13.0) | −4.9 (+1.0 to 10.8) | −7.8 (0 to 15.6) |
| PWV, m/s | 5.7 (4.7 to 6.7) | 5.8 (4.9 to 6.7) | 5.7 (4.8 to 6.6) | 5.8 (4.9 to 6.7) |
Values are means with 95% CIs in brackets. Recovery measurements (2 mo after the high-Pi plus D3 period+1 week) were only performed in the high-Pi group. D3, vitamin D3; W, week; Pi, phosphorus.
With the exception of increases in lipid peroxide serum concentration and urinary excretion rate of 8-oxo-2’-deoxy-guanosine, modulation of dietary Pi loading did not affect markers of cardiovascular risk, oxidative stress, and senescence (Table 6). No adverse events were reported.
Table 6.
Effect of HP and LP dietary conditions without and with vitamin D3 on fasting markers of cardiovascular risk, oxidative stress, and aging as well as the calcifying propensity of blood (T50)
| Variable | Insulin (IU/L) | HOMA-IR | Glucose (mmol/L) | HbA1C (%) | hsCRP (mg/dl) | Fibrinogen (mmol/L) | D-Dimers (μg/ml) | Homocysteine (μmol/L) | Albumin (g/L) | Triglycerides (mmol/L) | Total Cholesterol (mmol/L) | oxLDL (ng/ml) | Antiox. Capacity (μmol/L) | Lipid Peroxides (μmol/L) | Superoxide Dismutase (ng/ml) | 8-oxo-2deoxy guanosine (urine) (μg/L) | T50a (Min) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HP baseline | 9.2±1.4 | 1.71±0.17 | 3.99±0.33 | 5.2±0.2 | 1.38±0.11 | 2.9±0.2 | 0.22±0.06 | 11.0±1.4 | 46.0±1.1 | 0.99±0.09 | 4.44±0.20 | 385±27 | 253±22 | 385±38 | 4.26±0.31 | 3.39±0.21 | 340±32 |
| LP baseline | 9.4±1.2 | 1.74±0.15 | 4.02±0.31 | 5.2±0.2 | 1.42±0.12 | 2.9±0.2 | 0.24±0.07 | 10.8±1.5 | 45.9±1.2 | 1.00±0.07 | 4.45±0.23 | 419±30 | 256±25 | 398±33 | 4.27±0.27 | 3.39±0.22 | 300±34 |
| HP W6 | 8.2±0.9 | 1.44±0.14 | 3.87±0.38 | 5.1±0.3 | 2.10±0.16 | 2.9±0.1 | 0.20±0.05 | 12.2±1.3 | 46.9±1.0 | 0.95±0.06 | 4.51±0.20 | 362±28 | 236±22 | 521b±42 | 4.84±0.30 | 5.55b±0.41 | 305±40 |
| LP W6 | 9.1±1.1 | 1.64±0.14 | 3.92±0.35 | 5.2±0.3 | 1.58±0.11 | 2.9±0.2 | 0.26±0.06 | 10.9±1.2 | 46.8±0.9 | 0.93±0.07 | 4.50±0.22 | 397±32 | 234±24 | 478b±44 | 4.72±0.26 | 5.98b±0.42 | 274±29 |
| HP plus D3 W11 | 9.4±1.2 | 1.92±0.24 | 4.16±0.25 | 5.1±0.2 | 1.39±0.12 | 2.8±0.1 | 0.28±0.05 | 12.0±1.3 | 46.0±0.9 | 0.97±0.06 | 4.50±0.24 | 435±33 | 240±21 | 449b±47 | 3.83±0.36 | 5.42b±0.44 | 308±39 |
| LP plus D3 W11 | 9.3±1.0 | 1.82±0.18 | 4.04±0.29 | 5.1±0.2 | 1.79±0.14 | 2.8±0.1 | 0.30±0.06 | 12.3±1.2 | 46.3±1.0 | 0.95±0.05 | 4.6±0.23 | 439±37 | 241±19 | 448b±45 | 4.43±0.41 | 6.00b±0.47 | 259±27 |
Values are mean±SEM. HOMA-IR, homeostatis model assessment insulin resistance; HbA1C, glycosylated hemoglobin; hsCRP, high-sensitivity C-reactive protein; oxLDL, oxidized low density lipoprotein; Antiox., antioxidant; W, week.
n=7 in both groups.
P<0.05 for the intragroup comparison with baseline.
Discussion
These are—to our knowledge—the first reported hemodynamic data from a prospective dietary phosphate interventional study in humans with normal renal function. The principal finding is that increasing the systemic phosphate load by an amount sufficient to significantly increase fasting plasma phosphate concentration induces significant increases in both SBP and DBP as well as pulse rate. The effect is experimentally specific to the ingested phosphate because the subjects were well controlled for the increase in sodium load. These results are in accordance with all but one28 report in normotensive and spontaneously hypertensive rats (both with normal renal function), that an HP diet elevated BP and augmented the exercise pressor reflex function.29–31 Our studies also offer an explanation—at least in part—of the mechanism of the phosphate-induced hypertensinogenic effect: Although plasma renin/aldosterone concentrations and 24-hour excretion rates for both aldosterone and free cortisol were unchanged, 24-hour urinary excretion rates of metanephrine and normetanephrine increased significantly, explaining the additional observation of significantly increased mean 24-hour pulse rate. Even small increases in sympathetic nervous system activity may be sufficient to cause significant hypertension because an HP diet in mice has been reported to potentiate phenylephrine-induced vasoconstriction in ex vivo aortic rings.32 In addition, increased extracellular phosphate concentration, per se, has also been demonstrated to result in vasoconstriction in murine aortic rings in vitro.33 It will be of interest to investigate whether phosphate exerts its sympathetic effect via a primary central or peripheral stimulation of the sympathetic nervous system and whether the effect of phosphate is direct or mediated via unknown endocrine/paracrine factors that are modulated by phosphate intake. The finding of increases in both PTH and FGF-23 in the HP group, yet persistence of the hypertensinogenic and heart rate effects when PTH and FGF-23 levels had normalized, suggests that at least those effects in tandem may not be pivotal hemodynamic mediators for the present results.
The basis for sympathetic activation by an HP diet remains to be determined. One potential mechanism for phosphate-induced sympathetic activation is the presence of phosphate-responsive NaPi2-like transporters in the rat amygdaloid region of the central nervous system as identified by immunocytochemistry.34 Infusion of very low (20 nM) phosphate concentrations into the third ventricle resulted in a profound suppression of NaPi-2 protein expression as compared with vehicle perfusion, providing at least an afferent arm for potential systemic phosphate effects on brain/sympathetic function.
Although not investigated in this study, an additional explanation for phosphate-induced autonomic increase in BP is phosphate-induced arterial or visceral inflammation35 resulting in local sympathetic afferent activation.36 Human vascular smooth muscle cells when incubated for 9 days in an HP medium produced increased levels of reactive oxygen and nitrogen species as well as inflammatory cytokines (e.g., TNFα, IL6, and ICAM-1), a finding not modulated by the presence of physiologic 1,25(OH)2D concentrations.35 Peripheral vascular or visceral inflammation provides an afferent autonomic signal that can activate efferent/systemic sympathetic outflow.36 Notably, however, we did not find an effect of HP on high-sensitivity C-reactive protein serum concentration (Table 6).
The phosphate-induced sympathetic activity in this study did not induce detectable changes in arterial elasticity as analyzed by PWV and AI, confirming and extending the previous observation that acute and short-term increases of dietary phosphate did not affect PWV in non-CKD subjects.35 In contrast to the findings in this chronic study, acute phosphate loading in humans has been reported to impair endothelial function by disruption of the nitric oxide pathway.37,38 It is possible that a more chronic phosphate exposure induces adaptive or regulatory mechanisms able to restore endothelial function.
The phosphate-induced increase in BP appears to depend on chronicity of phosphate loading. In a previous acute study of neutral phosphate loading (intravenously and enterally) for 36 hours, also with appropriate Na load control, we did not detect significant differences in 24-hour BP measurements and pulse rates during phosphate loading and in the first 24 hours thereafter (unpublished data, R. Scanni et al.).17 Thus, delineation of the exact time course of the hypertensinogenic effect of dietary phosphate and of increased sympathetic tone may yield additional insight into the mechanisms involved.
Cardiovascular risk markers and markers of oxidative stress (“aging”) were not affected by modulating dietary phosphate or by the supplementation of vitamin D3. The exception was the increase of lipid peroxide serum concentration and urinary 8-oxo-2-deoxyguanosine in both LP and HP groups that could, however, be caused by the increase in sodium intake in both LP and HP.39
Significance of Observed Increase in BP and Pulse Rate
The morbidity and mortality implications of phosphate-induced BP elevations with a mean increment in SBP of approximately 4 mm Hg and DBP of approximately 3 mm Hg are substantial. Each 20 mm Hg increment in SBP and 10 mm Hg increment in DBP is associated with a doubling in the risk of death caused by stroke, heart disease, or other vascular disease.40,41 The general population risk ratio (RR) data for elevated pulse rate are also highly significant and independent of age.36 On the basis of a recent meta-analysis of 1.2 million persons aged 25–90, the RR for resting pulse rate for both cardiovascular (CV) and all-cause mortality exhibited a linear rise for values above 45 beats per minute (bpm) (RR, 1.08, 1.09 per 10-bpm increment, respectively, P<0.01, each), attributed to sympathetic nervous system activity.42 Thus, although this study lacks middle-aged and elderly subjects that exhibit the highest CV event rates, strong observational data support both BP and pulse rate CV morbidity and mortality effects even for small increments and, where data are available, across all adult age groups for both sexes.
It is also important to point out that the controlled increase in phosphate load induced in this study (renal excretion increased from a mean of 615 to 1245 mg per day, or from 20.5 to 41.5 mmol/d, see Table 3) is not excessive, but is well within the range that a majority of a representative United States general population consumes each day (National Health And Nutrition Examination Survey III).6
Modulators of Phosphate Homeostasis
FGF-23/PTH/1,25(OH)2D
In response to HP, serum phosphate, PTH, FGF-23, and α-Klotho and urinary Klotho increased significantly, whereas 1,25(OH)2D serum concentrations did not change. After supplementation with 600,000 U vitamin D3, 25(OH)D and 1,25(OH)2D increased significantly suggesting that, at least in part, D3 period elevations in 1,25(OH)2D during both arms were driven by rising 25(OH)D levels. PTH and FGF-23 (and serum and urine Klotho) decreased significantly in the D3 period of HP. PTH stimulates and FGF-23 inhibits 1α hydroxylase and both 1,25(OH)2D and PTH have been shown to increase FGF-23.24,25 Thus, under the HP conditions of these experiments, our D3 period results are best explained by a dominant effect of PTH on FGF-23/Klotho because FGF-23 and serum and urine Klotho fell in parallel with falling PTH concentrations, despite continued HP, a further significant increase in plasma phosphate concentration, and persistently high 1,25(OH)2D levels. These changes in aggregate lend further support to the notion that complete adaptation to phosphate loading requires PTH.17,43
It is possible that the sympathetic activation observed herein is responsible in part for the FGF-23 increase, because acute dosing with the β adrenergic agonist, isoproterenol, produced large increases in femur FGF-23 expression and administration of the β blocker, propranolol, prevented the circadian increase in femur FGF-23 expression.44 Propranolol pretreatment, however, had no effect on HP-diet–induced femur FGF-23 expression. The mechanism of β adrenergic control of FGF-23 production was attributed to a cAMP response element in the FGF-23 promotor as assessed in osteoblastic UMR-106 cells.44
Klotho
The hyperphosphatemic increase in serum and urinary Klotho as well as FGF-23 represent the first demonstration in humans of a phosphate-driven increase in Klotho and suggest that elevated Klotho levels reflect a chronic adaptation to phosphate inasmuch as acute phosphate loading in humans documented an increase in serum FGF-23 but found no change in serum Klotho.38 Recombinant Klotho administration to FGF-23 knockout mice is reported to exhibit a phosphaturic effect,45 albeit with n=4, raising the possibility that proximal tubule effects of klotho are not confined to its role as coreceptor with membrane FGFR1c for circulating FGF-23 agonism.46 The present results with elevated Klotho and FGF-23 levels leave open the question as to whether both of the reported proximal tubule effects of the Klotho response to hyperphosphatemia [i.e., phosphaturia and decreased 1,25(OH)2D] are dependent on or independent of increased FGF-23 in humans. The elevations in serum and urinary Klotho levels in response to hyperphosphatemia in humans reinforce the mounting evidence for Klotho’s key role as a homeostatic phosphatonin and, potentially, for homeostatic beneficial effects in multiple organs.
The elevation of serum 1,25(OH)2D after D3 administration did not detectably stimulate intestinal phosphate absorption in HP and induced only a modest stimulatory effect under LP conditions. Thus, the effect of vitamin D on intestinal phosphate absorption in humans is small, compatible with the small effect found in rats.12
In conclusion, this study demonstrates that chronic, i.e., weeks of high dietary phosphate intake as sodium phosphate increases sympathoadrenergic activity and consequently increases SBP, DBP, and pulse rate in young humans with normal renal function. Thereby, it provides at least one important explanation for the association of dietary phosphate intake and/or plasma phosphate concentrations with increased cardiovascular morbidity and mortality, irrespective of renal function. These conclusions, although of high public health importance, require support of larger studies in more diverse populations.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Mrs. Julia Dober (study coordinator) for administrative help in writing the protocol and scheduling the visits and Jitka O'Neill (study nurse) for help with the blood sampling and urine handling. The authors thank Dr. Orson Moe and collaborators, i.e., Johanne Pastor, for analyzing serum iFGF-23 and α-klotho in serum and urine. They also thank the University of Texas Southwestern George M. O’Brien Kidney Research Core Center (P30DK079328) services for the resources that were needed to perform these analyses. They are also grateful to Andreas Pasch for measuring the calcifying propensity of serum.
Studies were performed with financial support from a grant to R.K. from the Swiss National Science Foundation, National Center of Competence in Research (NCCR kidney.ch).
J.M. cared for the study subjects and helped to perform and to analyze 24-hour ambulatory BP measurements as well as endothelial and arterial function/elasticity tests. R.S. helped to write the protocol and to recruit the volunteers. L.B. supervised the biochemical analysis and helped in analyzing the data. H.N.H. helped design the study and write both the protocol and the manuscript. R.K. designed and supervised the study and wrote the protocol and manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017121254/-/DCSupplemental.
References
- 1.Raggi P, Cooil B, Callister TQ: Use of electron beam tomography data to develop models for prediction of hard coronary events. Am Heart J 141: 375–382, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Chen NX, Moe SM: Vascular calcification: Pathophysiology and risk factors. Curr Hypertens Rep 14: 228–237, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32[Suppl 3]: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Calvo MS, Moshfegh AJ, Tucker KL: Assessing the health impact of phosphorus in the food supply: Issues and considerations. Adv Nutr 5: 104–113, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang AR, Lazo M, Appel LJ, Gutiérrez OM, Grams ME: High dietary phosphorus intake is associated with all-cause mortality: Results from NHANES III. Am J Clin Nutr 99: 320–327, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foley RN: Phosphate levels and cardiovascular disease in the general population. Clin J Am Soc Nephrol 4: 1136–1139, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB Sr, Gaziano JM, et al.: Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 167: 879–885, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA. Serum phosphate and left ventricular hypertrophy in young adults: The coronary artery risk development in young adults study. Kidney Blood Press Res 32: 37–44, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G; Cholesterol And Recurrent Events Trial Investigators : Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 112: 2627–2633, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Chang AR, Grams ME: Serum phosphorus and mortality in the Third National Health and Nutrition Examination Survey (NHANES III): Effect modification by fasting. Am J Kidney Dis 64: 567–573, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizzoli R, Fleisch H, Bonjour JP. Role of 1,25-dihydroxyvitamin D3 on intestinal phosphate absorption in rats with a normal vitamin D supply. J Clin Invest 60: 639–647, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giovannucci E, Liu Y, Hollis BW, Rimm EB: 25-hydroxyvitamin D and risk of myocardial infarction in men: A prospective study. Arch Intern Med 168: 1174–1180, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al.: Vitamin D deficiency and risk of cardiovascular disease. Circulation 117: 503–511, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginde AA, Scragg R, Schwartz RS, Camargo CA Jr: Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J Am Geriatr Soc 57: 1595–1603, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Semba RD, Houston DK, Bandinelli S, Sun K, Cherubini A, Cappola AR, et al.: Relationship of 25-hydroxyvitamin D with all-cause and cardiovascular disease mortality in older community-dwelling adults. Eur J Clin Nutr 64: 203–209, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scanni R, vonRotz M, Jehle S, Hulter HN, Krapf R: The human response to acute enteral and parenteral phosphate loads. J Am Soc Nephrol 25: 2730–2739, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parati G, Stergiou G, O’Brien E, Asmar R, Beilin L, Bilo G, et al.; European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability : European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens 32: 1359–1366, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson IB, Prasad K, Hall IR, Thomas A, MacCallum H, Webb DJ, et al.: Increased central pulse pressure and augmentation index in subjects with hypercholesterolemia. J Am Coll Cardiol 39: 1005–1011, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Hu MC, Shi M, Zhang J, Addo T, Cho HJ, Barker SL, et al.: Renal production, uptake, and handling of circulating αKlotho. J Am Soc Nephrol 27: 79–90, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato Y, Arakawa E, Kinoshita S, Shirai A, Furuya A, Yamano K, et al.: Establishment of the anti-Klotho monoclonal antibodies and detection of Klotho protein in kidneys. Biochem Biophys Res Commun 267: 597–602, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, et al.: Secreted Klotho protein in sera and CSF: Implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett 565: 143–147, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Pasch A, Farese S, Gräber S, Wald J, Richtering W, Floege J, et al.: Nanoparticle-based test measures overall propensity for calcification in serum. J Am Soc Nephrol 23: 1744–1752, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T: PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: A bone parathyroid feedback loop. Am J Physiol Renal Physiol 299: F882–F889, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Barthel TK, Mathern DR, Whitfield GK, Haussler CA, Hopper HA 4th, Hsieh JC, et al.: 1,25-Dihydroxyvitamin D3/VDR-mediated induction of FGF23 as well as transcriptional control of other bone anabolic and catabolic genes that orchestrate the regulation of phosphate and calcium mineral metabolism. J Steroid Biochem Mol Biol 103: 381–388, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Palomino HL, Rifkin DE, Anderson C, Criqui MH, Whooley MA, Ix JH: 24-hour urine phosphorus excretion and mortality and cardiovascular events. Clin J Am Soc Nephrol 8: 1202–1210, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nygaard B, Frandsen NE, Brandi L, Rasmussen K, Oestergaard OV, Oedum L, et al.: Effects of high doses of cholecalciferol in normal subjects: A randomized double-blinded, placebo-controlled trial. PLoS One 9: e102965, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bindels RJ, van den Broek LA, Hillebrand SJ, Wokke JM: A high phosphate diet lowers blood pressure in spontaneously hypertensive rats. Hypertension 9: 96–102, 1987 [DOI] [PubMed] [Google Scholar]
- 29.Bozic M, Panizo S, Sevilla MA, Riera M, Soler MJ, Pascual J, et al.: High phosphate diet increases arterial blood pressure via a parathyroid hormone mediated increase of renin. J Hypertens 32: 1822–1832, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Suzuki Y, Mitsushima S, Kato A, Yamaguchi T, Ichihara S.. High-phosphorus/zinc-free diet aggravates hypertension and cardiac dysfunction in a rat model of the metabolic syndrome. Cardiovasc Pathol 23: 43–49, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Mizuno M, Mitchell JH, Crawford S, Huang CL, Maalouf N, Hu MC, et al.: High dietary phosphate intake induces hypertension and augments exercise pressor reflex function in rats. Am J Physiol Regul Integr Comp Physiol 311: R39–R48, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Six I, Maizel J, Barreto FC, Rangrez AY, Dupont S, Slama M, et al.: Effects of phosphate on vascular function under normal conditions and influence of the uraemic state. Cardiovasc Res 96: 130–139, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Six I, Okazaki H, Gross P, Cagnard J, Boudot C, Maizel J, et al.: Direct, acute effects of Klotho and FGF23 on vascular smooth muscle and endothelium. PLoS One 9: e93423, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulroney SE, Woda CB, Halaihel N, Louie B, McDonnell K, Schulkin J, et al.: Central control of renal sodium-phosphate (NaPi-2) transporters. Am J Physiol Renal Physiol 286: F647–F652, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Martínez-Moreno JM, Herencia C, de Oca AM, Díaz-Tocados JM, Vergara N, Gómez-Luna MJ, et al.: High phosphate induces a pro-inflammatory response by vascular smooth muscle cells and modulation by vitamin D derivatives. Clin Sci (Lond) 131: 1449–1463, 2017 [DOI] [PubMed] [Google Scholar]
- 36.Jänig W: Sympathetic nervous system and inflammation: A conceptual view. Auton Neurosci 182: 4–14, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Stevens KK, Denby L, Patel RK, Mark PB, Kettlewell S, Smith GL, et al.: Deleterious effects of phosphate on vascular and endothelial function via disruption to the nitric oxide pathway. Nephrol Dial Transplant 32: 1617–1627, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shuto E, Taketani Y, Tanaka R, Harada N, Isshiki M, Sato M, et al.: Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol 20: 1504–1512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, et al.: Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol 14: 2775–2782, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee : Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation 135: e146–e603, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawes CM, Vander Hoorn S, Rodgers A; International Society of Hypertension : Global burden of blood-pressure-related disease, 2001. Lancet 371: 1513–1518, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Zhang D, Shen S, Qi X. Resting heart rate and all-cause and cardiovascular mortality in the general population: A meta-analysis. CMAJ 188: E53–E63, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas L, Bettoni C, Knöpfel T, Hernando N, Biber J, Wagner CA: Acute adaption to oral or intravenous phosphate requires parathyroid hormone. J Am Soc Nephrol 28: 903–914, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawai M, Kinoshita S, Shimba S, Ozono K, Michigami T: Sympathetic activation induces skeletal Fgf23 expression in a circadian rhythm-dependent manner. J Biol Chem 289: 1457–1466, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, et al.: Klotho: A novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24: 3438–3450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wright JD, An SW, Xie J, Yoon J, Nischan N, Kohler JJ, et al.: Modeled structural basis for the recognition of α2-3-sialyllactose by soluble Klotho. FASEB J 31: 3574–3586, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.