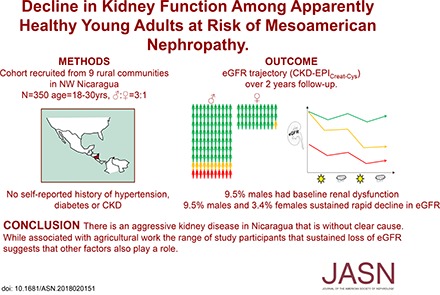
Abstract
Background Epidemic levels of CKD of undetermined cause, termed Mesoamerican nephropathy in Central America, have been found in low- and middle-income countries. We investigated the natural history of, and factors associated with, loss of kidney function in a population at high risk for this disease.
Methods We conducted a 2-year prospective, longitudinal study with follow-up every 6 months in nine rural communities in northwestern Nicaragua and included all men (n=263) and a random sample of women (n=87) ages 18–30 years old without self-reported CKD, diabetes, or hypertension. We used growth mixture modeling to identify subgroups of eGFR trajectory and weighted multinomial logistic regression to examine associations with proposed risk factors.
Results Among men, we identified three subpopulations of eGFR trajectory (mean baseline eGFR; mean eGFR change over follow-up): 81% remained stable (116 ml/min per 1.73 m2; −0.6 ml/min per 1.73 m2 per year), 9.5% experienced rapid decline despite normal baseline function (112 ml/min per 1.73 m2; −18.2 ml/min per 1.73 m2 per year), and 9.5% had baseline dysfunction (58 ml/min per 1.73 m2; −3.8 ml/min per 1.73 m2 per year). Among women: 96.6% remained stable (121 ml/min per 1.73 m2; −0.6 ml/min per 1.73 m2 per year), and 3.4% experienced rapid decline (132 ml/min per 1.73 m2; −14.6 ml/min per 1.73 m2 per year; n=3 women). Among men, outdoor and agricultural work and lack of shade availability during work breaks, reported at baseline, were associated with rapid decline.
Conclusions Although Mesoamerican nephropathy is associated with agricultural work, other factors may also contribute to this disease.
Keywords: CKDu, Endemic nephropathy, eGFR decline, MeN, CKD of unknown etiology
CKD of undetermined cause (CKDu), also termed Mesoamerican nephropathy (MeN) in Central America, has led to the deaths of tens of thousands of young adults in rural Nicaragua and El Salvador.1,2 Cross-sectional studies have shown low (<60 ml/min per 1.73 m2) eGFR at a prevalence between 2% and 50% among the population of lowland agricultural communities in the region.3–6 Forms of CKDu occur in other tropical climates, with reports of high prevalence in Sri Lanka7,8 (where similar but not identical histopathologic findings have been reported9), India,10 and Egypt,11 although whether this represents the same disease entity remains unclear.
Men from communities affected by MeN predominantly work in agriculture, primarily sugar production from cane. Agricultural activity in this industry is concentrated in the dry season, which in Nicaragua, occurs between November and May. Although a leading hypothesis in Mesoamerica is that the disease relates to heat stress, a number of other causes, including agrichemicals, infection, and heavy metals, have been proposed.1,12–14
Empirical evidence for causes of CKDu has to date been limited to identification of factors associated with either reduced eGFR in cross-sectional studies3,15,16 or loss of eGFR across the harvest season in two workplace-based follow-up studies.17,18 Given the potential for reverse causation (i.e., reduced eGFR resulting in changes in exposure) and misclassification of exposures and/or outcome in the cross-sectional designs along with the nongeneralizability and the substantial loss to follow-up that occurred in the longitudinal workplace studies, evidence on risk factors for and evolution of CKDu is extremely limited.19
Our aim was to investigate the natural history of disease, specifically early loss of kidney function, along with risk factors and urinary markers (albumin-to-creatinine ratio [ACR] and neutrophil gelatinase–associated lipocalin [NGAL]) associated with decline in eGFR. Therefore, we conducted a community-based longitudinal study of an initially apparently healthy young rural population in northwest Nicaragua.
Methods
Cohort
Both local and United Kingdom–based institutional review boards approved the study, and participants provided written informed consent. The rationale and description of the study design have been published elsewhere.20 Briefly, this was a 2-year longitudinal, community-based study following 350 participants ages 18–30 years old in the Leon and Chinandega regions of Nicaragua (Figure 1). After engagement work, we performed a census of all adults ages 18–30 years old in nine rural communities. Because we were specifically interested in associations with early kidney injury in MeN, all potential participants with a self-reported diagnosis of CKD, diabetes, or hypertension were excluded. All remaining men (because men have been reported to suffer more CKDu) and women selected at random (in numbers leading to a men-to-women ratio of 3:1) were invited to take part. Participants were predominantly recruited in November 2014, with an additional 7% recruited in May 2015, because recruitment targets had not been met in November.
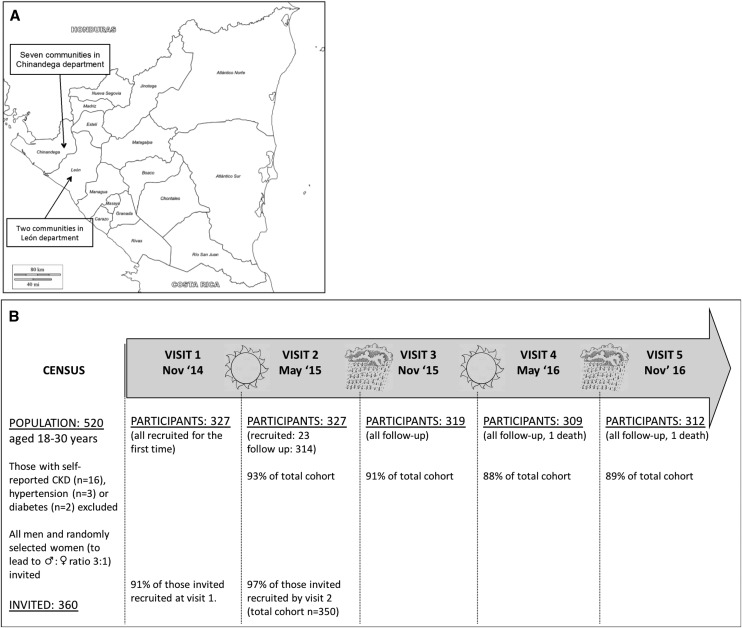
Figure 1.
Study participants were recruited from nine communities in Northwest Nicaragua and study retention rates were high. (A) Location of the nine study communities in Nicaragua. (B) Cartoon showing the study timeline along with population, recruitment, and follow-up numbers. Two participants died from ESRD.
Procedures
Questionnaire data, clinical measurements, and biosamples were collected at baseline and then every 6 months until November 2016. Participants were asked to respond to questions on demography, occupational history and current job, lifestyle factors, and symptoms. Urinary tract infection was recorded where participants reported a clinical diagnosis (which is common in this part of Nicaragua), typically without urinalysis or microbiologic confirmation. Body weight was measured with minimal clothes using electronic scales (Seca, Birmingham, United Kingdom), and height was measured using a portable stadiometer (Seca). BP and heart rate were measured in a sitting position using a calibrated digital sphygmomanometer (Omron, Kyoto, Japan) after 5 minutes of quiet seated rest. A mean of three measurements was recorded. Participants were asked to attend fasted first thing in the morning (before work) in an attempt to reduce within- and between-person variation in serum creatinine.
Biochemical Methods
Serum creatinine and cystatin C were both measured in a single batch using quality control referenced to international standards (for creatinine, isotope dilution mass spectrometry–quantified National Institute of Standards and Technology Standard Reference Material 967). eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula combining creatinine and cystatin C.21 ACR along with semiquantitative protein and specific gravity by test stick were performed in baseline urine samples thawed for the first time. In addition, 55 samples (thawed for a second time) selected using a nested patient-control approach were analyzed for NGAL.
Statistical Methods
The collection and categorization of exposure variables are described in Supplemental Material. Because eGFR trajectories clustered in discrete subgroups (Supplemental Figures 1 and 2) and differently between sexes, we used growth mixture modeling (GMM) separately in men and women to empirically derive latent classes of eGFR trajectory.22 The GMM is a longitudinal finite mixture model that allows identification of unobserved latent classes of individuals following similar progression of the outcome over time without imposing a priori constraints on the levels of eGFR or rates of eGFR change (or the proportion of participants experiencing any class of change). Each individual’s probability of belonging to a particular latent class is derived entirely from the observed eGFR measurements, with individual departures from the mean trajectory within each class represented by random effects. We primarily used the Bayesian Information Criterion to determine the optimal number of classes as suggested in this setting.23 The GMM was estimated by maximum likelihood using an expectation maximization algorithm, with 95% confidence intervals (95% CIs) for the mean rate of eGFR decline derived using conventional SEM.
Each individual was assigned a probability of each class (eGFR trajectory) and then for the purposes of the descriptive figure, tables, and urinary findings, allocated to the highest probability group.
To test whether proposed causal exposures (alcohol or nonsteroidal anti-inflammatory drug use, occupational factors, heat stress, agrochemical exposure, fever, dysuria, or water quantity/quality/source in men only) were associated with rapidly declining eGFR trajectory, we conducted age- and educational level–adjusted analyses using probability-weighted logistic regression (with weighting according to the participant’s probability of each eGFR trajectory as per the GMM), examining exposures individually using stable with preserved eGFR trajectory as a reference. Associations where the 95% CI of the odds ratio (OR) did not include unity were interpreted as significant. We also performed a sensitivity analysis using exposures assessed at visit 2 (only in those men recruited at visit 1) and rapid decline given the seasonal variation in occupational exposures. Those with baseline dysfunction were not the primary focus of this study, but another analysis additionally exploring associations between risk factors and this eGFR trajectory was also performed using probability-weighted logistic regression (Supplemental Material).
Differences in urinary markers in each eGFR trajectory group (defined on the basis of the highest probability as above) were investigated either in the whole population for ACR or using a nested patient-control approach in the case of NGAL. Differences between groups were explored using ANOVA with the Dunnett post hoc test. Positive and negative predictive values were calculated for urinary NGAL for the rapid decline versus stable group.
Results
Cohort and Follow-Up
Five hundred twenty adults ages 18–30 years old were identified in the study communities. After exclusion of 4% of the potential participants because of self-reported CKD, diabetes, or hypertension, 350 participants (of the 360 invited after random selection of eligible women; 97%) were included in the study.20 Overall, participants attended a total of 1581 study visits over the 2-year follow-up (92% of planned visits). Two participants died from ESRD during the study period. The cohort is described in Figure 1 and Table 1.
Table 1.
Selected demographic, lifestyle, and occupational characteristics of the study cohort
| Characteristic | Overall, n=350 | Men, n=263 | Women, n=87 |
|---|---|---|---|
| Personal and lifestyle factors | |||
| Age, yr, mean (SD) | 23.9 (3.7) | 23.7 (3.8) | 24.2 (3.6) |
| Educational level, n (%) | |||
| Illiteracy | 18 (5.1) | 18 (6.8) | 0 (0) |
| Primary school | 176 (50.3) | 133 (50.6) | 43 (49.4) |
| Secondary school | 138 (39.5) | 100 (38.0) | 38 (43.7) |
| Higher education | 18 (5.1) | 12 (4.6) | 6 (6.9) |
| Body mass index, median (IQR) | 22.7 (21.0–25.0) | 22.4 (20.8–24.1) | 24.5 (21.9–30.0) |
| Systolic BP, mm Hg, median (IQR) | 117 (109–124) | 119 (111–125) | 109 (103–119) |
| Diastolic BP, mm Hg, median (IQR) | 68 (63–73) | 68 (63–74) | 68 (63–72) |
| Household income, Córdobas per 1 mo, median (IQR) | 6000 (4000–9200) | 6000 (4000–10,000) | 5120 (3380–8144) |
| Family history of CKD, n (%) | |||
| Yes | 165 (47.1) | 126 (47.9) | 39 (44.8) |
| No | 185 (52.9) | 137 (52.1) | 48 (55.2) |
| Annual alcohol consumption, g, median (IQR) | 0·0 (0–849) | 82.9 (0–1350) | 0.0 (0–0) |
| Smoking pack-year, median (IQR) | 0·0 (0–0) | 0·0 (0–1) | 0.0 (0–0) |
| NSAID use ever, n (%) | |||
| Never | 58 (16.6) | 49 (18.6) | 9 (10.3) |
| Occasionally | 251 (71.7) | 185 (70.3) | 66 (75.9) |
| Regularly | 31 (8.9) | 23 (8.8) | 8 (9.2) |
| Daily | 10 (2.8) | 6 (2.3) | 4 (4.6) |
| Water sources, n (%) | |||
| Piped water | 186 (53.1) | 139 (52.9) | 47 (54.0) |
| Dug well | 126 (36.0) | 98 (37.2) | 28 (32.2) |
| Drilled well | 38 (10.9) | 26 (9.9) | 12 (13.8) |
| Water hardness, n (%) | |||
| Soft | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Moderately hard | 97 (27.7) | 67 (25.4) | 30 (34.5) |
| Hard | 160 (45.7) | 123 (46.8) | 37 (42.5) |
| Very hard | 93 (26.6) | 73 (27.8) | 20 (23.0) |
| Total liquid in last 24 h, median (IQR) | 5.0 (3.7–6.3) | 5.6 (4.2–6.7) | 3.6 (2.5–4.5) |
| Occupational factors | |||
| Current occupation, n (%) | |||
| Sugarcane | 55 (15.7) | 45 (17.1) | 10 (11.5) |
| Banana work | 14 (4.0) | 13 (4.9) | 1 (1.1) |
| Other agricultural work | 115 (32.9) | 109 (41.5) | 6 (6.9) |
| Commerce | 14 (4.0) | 5 (1.9) | 9 (10.3) |
| Construction | 10 (2.9) | 10 (3.8) | 0 (0) |
| Fishing | 7 (2.0) | 7 (2.7) | 0 (0) |
| Homeworker | 54 (15.4) | 0 (0) | 54 (62.1) |
| Student | 6 (1.7) | 4 (1.5) | 2 (2.3) |
| Unemployed | 51 (14.6) | 49 (18.6) | 2 (2.3) |
| Other occupationsa | 24 (6.8) | 21 (8.0) | 3 (3.5) |
| Main sugarcane role (if ever worked in sugarcane), n (%) | |||
| Cane cutter | 81 (23.2) | 81 (30.8) | 0 (0) |
| Seed cutter | 56 (16.3) | 56 (21.3) | 0 (0) |
| Seeder | 67 (19.2) | 47 (17.9) | 21 (24.1) |
| Cane cleaner | 26 (7.4) | 17 (6.5) | 9 (10.4) |
| Pesticide applicator | 4 (1.1) | 4 (1.5) | 0 (0) |
| Cane irrigator | 8 (2.3) | 8 (3.0) | 0 (0) |
| Driver | 4 (1.1) | 4 (1.5) | 0 (0) |
| Never worked in sugarcane | 103(29.4) | 46 (17.5) | 57 (65.5) |
| Current or previous banana work, n (%) | |||
| Yes | 56 (16.0) | 47 (17.9) | 9 (10.3) |
| No | 294 (84.0) | 216 (82.1) | 78 (89.7) |
| Years in sugarcane, mean (SD) | 2.2 (2.8) | 2.8 (2.8) | 0.67 (1.7) |
| Years in agricultural, mean (SD) | 3.6 (4.4) | 4.3 (4.5) | 1.2 (3.3) |
| Work carried out,b n (%) | |||
| Indoors | 136(38.9) | 69 (26.2) | 67 (77.0) |
| Outdoors | 214 (61.1) | 194 (73.8) | 20 (23.0) |
| Work in a hot environment,b n (%) | |||
| Irregularly | 137 (39.2) | 92 (35.0) | 45 (51.7) |
| Regularly | 74 (21.1) | 57 (21.7) | 17 (19.5) |
| Frequently | 139 (39.7) | 114 (43.3) | 25 (28.8) |
| Always | 0 (0) | 0 (0) | 0 (0) |
| Shade availability,b n (%) | |||
| Yes | 254 (72.6) | 190 (72.2) | 64 (73.6) |
| No | 96 (27.4) | 73 (27.8) | 23 (26.4) |
| Duration of breaks, min,b median (IQR) | 20 (10–30) | 15.0 (10–30) | 30.0 (20–60) |
| Physical effort at work,c n (%) | |||
| Did not work | 15 (4.3) | 14 (5.3) | 1 (1.2) |
| Slight | 142 (40.6) | 100 (38.0) | 42 (48.3) |
| Moderate | 155 (44.2) | 119 (45.3) | 36 (41.4) |
| Hard | 38 (10.9) | 30 (11.4) | 8 (9.2) |
| Glyphosate use,b,d n (%) | |||
| Yes | 77 (22.0) | 77 (29.3) | 0 (0) |
| No | 273 (78.0) | 186 (70.7) | 87 (100.0) |
| Paraquat use,b,d n (%) | |||
| Yes | 44 (12.6) | 44 (16.7) | 0 (0) |
| No | 306 (87.4) | 219 (83.3) | 87 (100.0) |
| Cypermethrin use,b,d n (%) | |||
| Yes | 75 (21.4) | 73 (27.7) | 2 (2.3) |
| No | 275 (78.6) | 190 (72.2) | 85 (97.7) |
| Methomyl use,b,d n (%) | |||
| Yes | 12 (3.4) | 12 (4.6) | 0 (0) |
| No | 338 (96.6) | 251 (94.4) | 87 (100.0) |
| Clinical history/symptoms | |||
| Heat/dehydration symptoms,b n (%) | |||
| Yes | 240 (68·6) | 175 (66·5) | 65 (74·7) |
| No | 110 (31·4) | 88 (33·5) | 22 (25·3) |
| UTI in the previous year, n (%) | |||
| Yes | 91 (26.0) | 56 (21.3) | 35 (40.2) |
| No | 259 (74.0) | 207 (78.7) | 52 (59.8) |
| Weight loss,b n (%) | |||
| Yes | 63 (18.0) | 55 (20.9) | 8 (9.2) |
| No | 287 (82.0) | 208 (79.1) | 79 (90.8) |
| Dysuriab | |||
| Yes | 94 (26.9) | 72 (27.4) | 22 (25.3) |
| No | 256 (73.1) | 191 (72.6) | 65 (74.7) |
| Feverb | |||
| Yes | 36 (10.3) | 32 (12.2) | 4 (4.6) |
| No | 314 (89.7) | 231 (87.8) | 83 (95.4) |
| Study visits and outcome | |||
| Initial serum creatinine, mg/dl, median (IQR) | 0.81 (0.70–0.90) | 0.84 (0.77–0.94) | 0.63 (0.57–0.68) |
| Final serum creatinine, mg/dl, median (IQR) | 0.81 (0.70–0.90) | 0.91 (0.80–1.03) | 0.64 (0.57–0.72) |
| Initial cystatin C, mg/L, median (IQR) | 0.82 (0.74–0.92) | 0.85 (0.77–0.95) | 0.72 (0.67–0.80) |
| Final cystatin C, mg/L, median (IQR) | 0.84 (0.76–0.94) | 0.88 (0.80–1.01) | 0.72 (0.67–0.80) |
| Initial eGFR, ml/min per 1.73 m2, median (IQR) | 118.3 (106.6–125.4) | 116.2 (102.4–124.6) | 122.0 (116.3–127.2) |
| Final eGFR, ml/min per 1.73 m2, median (IQR) | 113.1 (99.4–122.3) | 110.4 (92.5–120.1) | 120.2 (110.6–126.6) |
eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation on the basis of creatinine and cystatin c. Questionnaire data before recoding are presented in Supplemental Table 1. IQR, interquartile range. NSAID, nonsteroidal anti-inflammatory drug; UTI, diagnosed with a urinary tract infection typically without microbiologic or dipstick confirmation.aOther occupations include teacher, painter, shoemaker, security, manufacturing operator, and barber.
Over the last 6 months.
Over the last week.
Data were collected at the second visit.
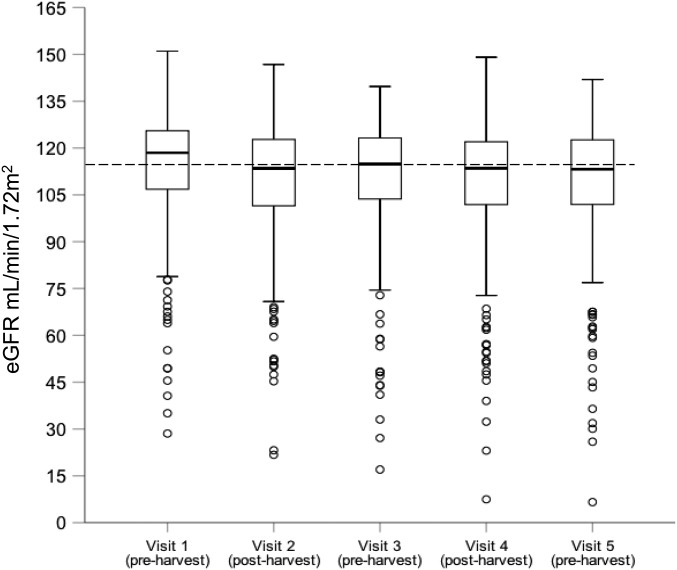
The median eGFR in men was 116.2 ml/min per 1.73 m2 (interquartile range [IQR], 102.4–124.6) at baseline, and 110.4 ml/min per 1.73 m2 (IQR, 92.5–120.5) at the end of follow-up. The corresponding figures for women were 122.0 ml/min per 1.73 m2 (IQR, 116.3–127.2) at baseline and 120.2 ml/min per 1.73 m2 (IQR, 110.6–126.6) at the end of follow-up. The eGFR varied by season (Figure 2), with a median of 116.0 ml/min per 1.73 m2 (IQR, 102.7–123.8) at the end of the rainy season (November; i.e., before sugarcane harvest, all years combined) compared with 113.4 ml/min per 1.73 m2 (IQR, 100.8–122.4) at the end of the dry season (May; i.e., after sugarcane harvest, all years combined). This effect was greatest in those participants with lower eGFRs, but it was also present in those with stable kidney function (Supplemental Table 2).
Figure 2.
Median eGFR was lower postharvest than preharvest. Box and whisker plot of eGFR across the study population. The dashed line represents the median of all eGFR values in the population across the study.
eGFR Trajectory Groups
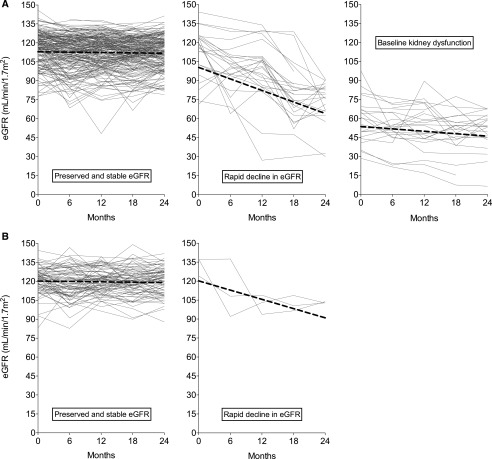
Using GMM, we identified three different subgroups in men and two subgroups in women on the basis of the model intercept (baseline eGFR) and slope (change in eGFR over time). Among men (Figure 3A), the majority (81%) of men had preserved and stable eGFR; however, 9.5% (n=25) had baseline kidney dysfunction (eGFR of approximately 60 ml/min at recruitment), and another 9.5% experienced rapid decline in eGFR (with a mean loss of 18 ml/min per 1.72 m2 per year) despite preserved eGFR at baseline. Almost all of the women (Figure 3B) had preserved and stable eGFR, but 3.4% (n=3) also experienced rapid decline (with a mean loss of 14 ml/min per 1.72 m2 per year). No differences were seen between communities in the proportions of participants in these subgroups.
Figure 3.
A substantial proportion of men and a small number of women experience rapid decline in eGFR. Individual eGFR values over time stratified by trajectory subgroup. (A) Three subgroups were identified in 263 men, and (B) two subgroups were identified in 87 women. Each line represents the individual eGFR of a single participant. Each participant was allocated to the group of highest probability derived from the growth mixture model. Coefficients for the three groups of men: preserved and stable eGFR (n=213; intercept [mean eGFR at baseline], 113.3 ml/min per 1.73 m2; 95% confidence interval [95% CI], 111.3 to 115.3; slope [mean eGFR decline over time], −0.6 ml/min per 1.73 m2 per year; 95% CI, 0.0 to −0.9); rapid decline in eGFR (n=25; intercept, 109.5 ml/min per 1.73 m2; 95% CI, 99.1 to 119.9; slope, −18.2 ml/min per 1.73 m2 per year; 95% CI, −13.5 to −22.9); and baseline dysfunction (n=25; intercept, 55.6 ml/min per 1.73 m2; 95% CI, 48.5 to 62.7; slope, −3.8 ml/min per 1.73 m2 per year; 95% CI, −0.7 to −6.9). Coefficients for the two groups women: preserved and stable eGFR (n=84; intercept, 120.5 ml/min per 1.73 m2; 95% CI, 118.1 to 122.9; slope, −0.6 ml/min per 1.73 m2 per year; 95% CI, 0.2 to −1.4). We also identified a small number with rapid decline in kidney function (n=3; intercept, 127.5 ml/min per 1.73 m2; 95% CI, 119.3 to 135.7; slope, −14.6 ml/min per /1.73 m2 per year; 95% CI,−7.5 to −21.7).
Baseline sociodemographics, occupational history, occupational exposures, lifestyle factors, and symptoms stratified by the assigned kidney trajectory groups are presented in Supplemental Tables 2 and 3. The frequencies of indoor work and availability of shade were both lower in the rapidly declining subgroup. Of the three women who fell into the rapid decline group, one had worked in (nonsugarcane) agriculture, and two worked exclusively at home.
Adjusted Associations with Rapid Decline Trajectory
Baseline age– and educational level–adjusted probability-weighted associations with the rapid decline in eGFR trajectory in men using the preserved and stable trajectory as the reference are presented in Table 2. Outdoor work (OR, 10.35; 95% CI, 1.35 to 79.24), (nonsugarcane) agricultural work (OR, 3.57; 95% CI, 1.14 to 11.13), and lack of shade available during work breaks (OR, 3.74; 95% CI, 1.59 to 8.76) were associated with this outcome. However, we found no evidence for associations between rapid decline and years of work in sugarcane or agriculture; self-reported physical effort in the last week at work; self-reported occupational heat or agrochemical exposure over last 6 months; alcohol consumption, self-reported fluid consumption, or water quality or source; heat/dehydration-related symptoms; or use of nonsteroidal anti-inflammatory drugs.
Table 2.
Age- and education level–adjusted associations of rapid decline in eGFR by baseline exposure in study participants who were men
| Characteristic | Rapid Decline in eGFRa | |
|---|---|---|
| OR | 95% CI | |
| Alcohol consumption | ||
| Any | 1.69 | 0.70 to 4.10 |
| None | Reference | Reference |
| NSAID use | ||
| Daily/regularly | 1.28 | 0.34 to 4.74 |
| Never/occasionally | Reference | Reference |
| Water sources | ||
| Piped water | 0.79 | 0.34 to 1.81 |
| Dug well/drilled well | Reference | Reference |
| Water hardness | ||
| Soft/moderately hard | 1.21 | 0.47 to 3.11 |
| Hard/very hard | Reference | Reference |
| Total liquid in last 24 h, L | ||
| >5.0 | 1.01 | 0.43 to 2.38 |
| ≤5.0 | Reference | Reference |
| Current occupation | ||
| Sugarcane | 1.51 | 0.31 to 7.29 |
| Agricultural work | 3.57 | 1.14 to 11.13 |
| Other occupations/EIP | Reference | Reference |
| Main sugarcane role (if ever worked in sugarcane) | ||
| Cane/seed cutter | 2.15 | 0.57 to 8.06 |
| Seeder | 1.82 | 0.40 to 8.20 |
| Other cane jobs | 0.94 | 0.14 to 6.08 |
| Never worked in sugarcane | Reference | Reference |
| Current or historical banana work | ||
| Yes | 1.77 | 0.60 to 5.18 |
| No | Reference | Reference |
| Years in sugarcane | 1.02 | 0.87 to 1.19 |
| Years in agriculture | 0.99 | 0.89 to 1.09 |
| Work carried outb | ||
| Outdoors | 10.35 | 1.35 to 79.24 |
| Indoors | Reference | Reference |
| Work in a hot environmentb | ||
| Regular/frequently | 0.46 | 0.20 to 1.06 |
| Irregularly | Reference | Reference |
| Shade availabilityb | ||
| No | 3.74 | 1.59 to 8.76 |
| Yes or inside | Reference | Reference |
| Duration of breaks,b min | ||
| ≤10 | 1.86 | 0.80 to 4.33 |
| >10 | Reference | Reference |
| Physical effort at workc | ||
| Moderate/hard | 1.40 | 0.59 to 3.32 |
| None/slight | Reference | Reference |
| Agrochemicalsb,d | ||
| Yes | 1.70 | 0.72 to 4.03 |
| No | Reference | Reference |
| Heat/dehydration symptomsb | ||
| Yes | 1.40 | 0.55 to 3.55 |
| No | Reference | Reference |
| Dysuriab | ||
| Yes | 1.18 | 0.48 to 2.89 |
| No | Reference | Reference |
| Feverb | ||
| Yes | 2.41 | 0.80 to 7.27 |
| No | Reference | Reference |
Agricultural work includes all nonsugarcane agricultural work. OR, odds ratio; 95% CI, 95% confidence interval; NSAID, nonsteroidal anti-inflammatory drug; EIP, economically inactive population.
Rapid decline versus preserved and stable eGFR. Probability weighted according to the results of the growth mixture model.
Over the last 6 months.
Over the last week.
Data were collected at the second visit and included glyphosate, cypermethrin, paraquat, and methomyl.
We were concerned that the questionnaire administered at baseline might fail to capture important occupational exposures, because for most participants, it was conducted 6 months after the harvest season. Therefore, we conducted a sensitivity analysis (men recruited at the November visit only; n=213) examining the association with the same rapid decline eGFR trajectory as above and occupational exposures, hydration variables, and heat-related symptoms captured at the second study visit (May 2015; immediately after harvest) (Supplemental Table 4). At this time point, no associations were detected between working outdoors or lack of shade and rapid decline in eGFR trajectory (although very few participants were not exposed). There was an association between both those working in a sugarcane cutting role (OR, 3.84; 95% CI, 1.17 to 12.58) and those reporting fever over the last 6 months (OR, 5.77; 95% CI, 2.03 to 16.33) and rapid decline trajectory, but in line with the baseline exposure analysis, no associations were observed between self-reported measures of heat exposure, combined heat-related symptoms, or fluid intake and outcome (Supplemental Tables 5 and 6).
Urinary Findings
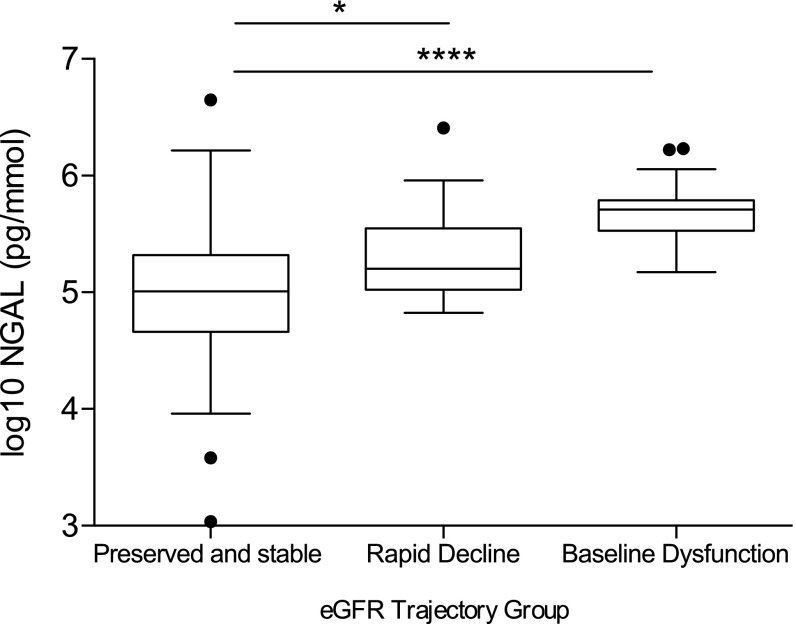
No associations were found between dipstick proteinuria, specific gravity, or ACR and eGFR trajectory subgroups (Tables 3 and 4). Urinary NGAL levels among men differed between the three groups tested (Figure 4). The positive and negative predictive values of NGAL≥5.5 pg/mmol for rapid decline were 28.5% and 62.5%, respectively.
Table 3.
Description of urinary findings at baseline by assigned eGFR trajectory groups in men
| Urine Findings | Overall, n=263 | Preserved and Stable eGFR, n=213 | Rapid Decline in eGFR, n=25 | Baseline Dysfunction, n=25 |
|---|---|---|---|---|
| Urinary specific gravity, n (%) | ||||
| ≤1020 | 256 (97.3) | 207 (97.2) | 24 (96.0) | 25 (100.0) |
| >1020 | 7 (2.7) | 6 (2.8) | 1 (4.0) | 0 (0) |
| Protein, n (%) | ||||
| Negative | 224 (85.2) | 181 (85.0) | 22 (88.0) | 21 (84.0) |
| Trace | 25 (9.5) | 19 (8.9) | 2 (8.0) | 4 (16.0) |
| Positive | 14 (5.3) | 13 (6.1) | 1 (4.0) | 0 (0) |
| ACR, mg/g, n (%) | ||||
| ≥30 | 15 (5.7) | 11 (5.2) | 0 (0) | 4 (16.0) |
| <30 | 248 (94.3) | 201 (94.8) | 25 (100.0) | 21 (84.0) |
Participants were assigned to the group with the highest probability in the growth mixture model. P values were NS by Fishers exact test for differences by group. ACR, albumin-to-creatinine ratio.
Table 4.
Description of urinary findings at baseline by assigned eGFR trajectory groups in women
| Urine Findings | Overall, n=87 | Preserved and Stable eGFR, n=84 | Rapid Decline in eGFR, n=3 |
|---|---|---|---|
| Urinary specific gravity, n (%) | |||
| ≤1020 | 81 (93.1) | 79 (94.1) | 2 (66.7) |
| >1020 | 6 (6.9) | 5 (5.9) | 1 (33.3) |
| Protein, n (%) | |||
| Negative | 70 (80.5) | 68 (81.0) | 2 (66.7) |
| Trace | 13 (14.9) | 12 (14.3) | 1 (33.3)) |
| Positive | 4 (4.6) | 4 (4.7) | 0 (0) |
| ACR, mg/g, n (%) | |||
| ≥30 | 9 (10.3) | 9 (10.7) | 0 (0) |
| <30 | 78 (89.7) | 75 (89.3) | 3 (100.0) |
Participants were assigned to the group with the highest probability in the growth mixture model. Given the small number in some cells, no statistical tests were performed. ACR, albumin-to-creatinine ratio.
Figure 4.
Urinary NGAL concentrations were higher in men with baseline kidney dysfunction and those who experienced rapid decline in eGFR. Box and whisker plot of urinary neutrophil gelatinase–associated lipocalin (NGAL)/creatinine concentrations by assigned eGFR trajectory group among male study participants. Lines indicate medians. Boxes are interquartile ranges. Whiskers indicate 1.5× interquartile ranges. Dots are outlying values. Stable group, n=55; rapid decline group, n=25; baseline dysfunction, n=24. *P=0.03 using ANOVA followed by the Dunnett multiple comparisons test (using the stable and preserved eGFR group as the reference); ****P≤0.001 using ANOVA followed by the Dunnett multiple comparisons test (using the stable and preserved eGFR group as the reference).
Discussion
This is the first community-based cohort study from an area with high reported prevalence of MeN and the first longitudinal study of at least moderate size with follow-up of >6 months in an area at high risk of disease. Even after excluding those with self-reported CKD, 9.5% of the apparently healthy men (but no women) in the study had evidence of baseline renal dysfunction. Rapid loss of eGFR from normal baseline levels was found in another 9.5% of men and 3.4% of women. Among men, risk factors at baseline for rapid decline included working outdoors, agricultural work, and lack of shade availability, but none of the other questionnaire responses aimed at capturing heat stress, time-accumulated occupation, or other proposed causes of MeN were associated with the outcome at baseline. Because of small numbers, we were unable to examine associations in women.
Other important findings from our study include the cyclical annual fluctuation in renal function across the entire population, with the average eGFR approximately 2.5-ml/min per 1.73 m2 lower after the dry (harvest) season compared with 6 months earlier. Furthermore, although there were no differences in albuminuria between those with different kidney function trajectories, urinary NGAL was substantially higher among those with baseline dysfunction and marginally elevated in the rapid decline group.
Although CKDu has been anecdotally reported as an aggressive disease,1 the rate of loss of kidney function in those in the rapid decline group who make up almost 10% of the unselected population of young men in our study is, to our knowledge, without precedent. Even compared with eGFR decline in other forms of CKD seen in clinic populations, the observed loss of kidney function is alarming. Although a recent biopsy study that enrolled patients with established CKDu reported a rate of decline in eGFR of 7.0 ml/min per 1.73 m2 per year among men with a history of work in the sugarcane,24 there have been no longitudinal studies that have examined medium- or long-term (>1-year) changes in kidney function in the at-risk population. The rate of eGFR decline has been explored in more detail in other forms of CKD; for example, a longitudinal study in 55 clinic patients with diabetic nephropathy from Belgium reported that approximately 15% of patients suffered severe decline in kidney function (defined as eGFR loss >4 ml/min per 1.73 m2 per year).25 Most recently, Boucquemont et al.26 examined eGFR decline in a patient population with CKD in France using a similar latent class-based modeling approach to that used in this analysis. This study reported severe eGFR decline in only 0.6% of patients (approximately 50 ml/min per 1.73 m2 over almost 6 years). Therefore, our study findings underline the unique and severe nature of kidney disease in this region.
The associations with rapid decline trajectory in men suggest that occupation (outdoor agricultural work) is an important risk factor for loss of kidney function, consistent with previous reports.18 The temporary nature of work in this population makes distinguishing relationships between specific occupations and eGFR loss challenging; however, it is interesting to note that neither time-accumulated sugarcane work nor agricultural work were associated with outcome. Furthermore, the association between lack of available shade at baseline and rapid decline trajectory suggests that working environment may play an important role in disease evolution either by (not reducing) solar exposure or as a surrogate for generally poor occupational conditions. Consistent with this and in line with previous crossharvest studies,17 we identified an association between rapid decline and a cane/seed cutting role (a job role that has been associated with particularly hot working conditions) in a sensitivity analysis examining associations with exposures assessed postharvest.
The abscence of an association between variables aimed at capturing heat stress (self-reported physical effort the previous week at work and both work in very hot environment and combined dehydration/heat stress symptoms in the last 6 months) and the outcome measure, both at baseline and in the sensitivity analysis with exposures assessed at visit 2 raises further questions. Although self-reported measures of thermal sensation and physical exertion have been shown to robustly capture acute physiologic heat stress,27 our (similar) instruments (and/or our combined measure of heat symptoms) may not be valid in the rural Nicaraguan population, or they may not reflect time-accumulated heat stress. Alternatively, we may have had inadequate power to detect heat stress as a partial contributor to eGFR decline, or otherwise, it may be that nonheat-related occupational exposures promote the development of CKDu. Finally, the association between self-reported fever over the previous 6 months at the second study visit and the rapid decline trajectory might support a proposed infective/inflammatory contributor to MeN,28 although this finding was from a sensitivity analysis and should be treated with caution.
In summary, our data do not provide clear evidence for a cause of the disease. Along with occupation, the importance of nonoccupational factors is supported by (1) the range of jobs undertaken by the men experiencing rapid decline and (2) the 3.4% of women in our study who also showed a rapid loss of eGFR. As others have suggested,2 separate initiating and exacerbating factors should be considered, such as in other forms of CKD. For example, the progression of kidney disease due to known causes (e.g., diabetes or GN) can be exacerbated by episodes of volume depletion. Therefore, the possibility of an initial (currently unknown) subclinical insult, which is then exacerbated by the harsh working conditions, might explain the increased rates of eGFR loss and excess of advanced disease in men.
Although other studies have identified changes in urinary biomarkers in sugarcane workers over the harvest season in Mesoamerica,29 none have examined associations with subsequent eGFR loss over the medium term. There were no associations between dipstick proteinuria or ACR and eGFR trajectory group. Although albuminuria is a strong risk factor for renal decline in most populations, this is consistent with previous reports from Mesoamerica, where patients with established CKDu show only low-grade proteinuria.6,24,30 Urinary NGAL levels were substantially raised in those with baseline dysfunction, but levels in the rapid decline group overlapped with the stable group, making this test poorly predictive at an individual level.
Finally, it is worth noting the seasonal variation of eGFR in the population. Other studies (unrelated to CKDu) have described similar seasonal differences in renal function31,32; whether this variation is in any way related to the factors that cause MeN is unclear, but this finding does need to be considered when interpreting the change in eGFR reported in crossharvest studies.5,18 Ideally, any future longitudinal biomarker study should be of >1 year in duration to ensure that small falls in eGFR do not reflect cyclical seasonal changes.
Our study has several strengths. Overall response rates were high, and the eGFR was estimated using robust methods. We excluded those with self-report of diabetes and hypertension in an attempt to focus our study on eGFR decline due to MeN, and the prospective nature of our study enabled us to identify those with aggressive disease without necessarily meeting definitions for CKD. Furthermore, we excluded those with established renal disease (either by self-report from the study as a whole or by examining only those with preserved eGFR at baseline for the risk factor analysis), and hence, we could overcome issues associated with reverse causation.
Our study also has limitations. We did not formally exclude diabetes in our participants. Although often undiagnosed,33 the prevalence of diabetes is low in Nicaraguans of this age group,34 and none of those in the rapid decline group showed albuminuria (or glycosuria; data not presented), making an underlying diabetic lesion highly unlikely. We also relied on self-report to quantify the majority of occupational and environmental exposures. Although questionnaire-based assessments are useful instruments, none of them have been validated in the Nicaraguan population; therefore, some exposures may be prone to misclassification. The study took place in a confined geographical area, which limits generalizability. Resources restricted our study to a moderate sample size, and we had to alter our statistical approach. We were nonetheless able to detect a number of strong associations with eGFR trajectory, but the analytical change did lead to a reduction in power. Therefore, we would have expected to identify associations with a primary cause of disease that had been reliably captured by questionnaire but may have missed weaker associations, particularly with contributing exposures. The baseline dysfunction group is unrepresentative due to selection criteria (those with established CKD were intentionally excluded at recruitment) and possibly survivor bias (due to the small number of deaths in this group), and the nature of the study design means that the relationship between rapid decline in eGFR and hard outcomes could not be described. However, we hope to perform extended follow-up to investigate the longer-term outcomes in the cohort. Finally, the CKD-EPI formula has not been validated for this population, although because we were interested in within-person change in eGFR, this is unlikely to be of major importance.
In conclusion, this is the first community-based cohort study that describes the natural history of eGFR in those at risk of MeN. Almost 10% of apparently healthy young men and 3.4% of young women showed a marked decline in kidney function. Additional studies with at least 1-year of follow-up are needed to understand the causes of this decline, including the risks associated with outdoor (agricultural) work. Efforts to identify biomarkers of this early loss of eGFR rather than established disease are essential to gain a better understanding of etiology as well as to identify the population(s) that would benefit from interventions. A combined multidisciplinary approach is called for in partnership with the affected communities and local employers to address this devastating disease.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors would like to thank the participants and each of the community leaders for their support during the data collections across study visits. We would also like to thank the interview team, drivers, phlebotomists, and staff of the Research Centre for Health, Work and Environment, National Autonomous University of Nicaragua at Leon for their assistance during the study.
The study was supported by a grant from the Colt Foundation, UK. In addition, the Dutch National Postcode Lottery provided funding to Solidaridad to support a proportion of the fieldwork costs.
No funding source was involved in any part of the study design or the decision to submit the manuscript for publication.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018020151/-/DCSupplemental.
References
- 1.Ordunez P, Saenz C, Martinez R, Chapman E, Reveiz L, Becerra F: The epidemic of chronic kidney disease in Central America. Lancet Glob Health 2: e440–e441, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Wegman D, Crowe J, Hogstedt C, Jakobsson K, Wesseling C: Mesoamerican nephropathy: Report from the second international research workshop on MeN. In: SALTRA, Vol. 33: 1–193, edited by Central American Institute for Studies on Toxic Substances (IRET-UNA) and Program on Work, Environment and Health in Central America, Heredia, Costa Rica, SALTRA/IRET-UNA, 2016 [Google Scholar]
- 3.Orantes CM, Herrera R, Almaguer M, Brizuela EG, Núñez L, Alvarado NP, et al.: Epidemiology of chronic kidney disease in adults of Salvadoran agricultural communities. MEDICC Rev 16: 23–30, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Ordunez P, Martinez R, Reveiz L, Chapman E, Saenz C, Soares da Silva A, et al.: Chronic kidney disease epidemic in Central America: Urgent public health action is needed amid causal uncertainty. PLoS Negl Trop Dis 8: e3019, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peraza S, Wesseling C, Aragon A, Leiva R, García-Trabanino RA, Torres C, et al.: Decreased kidney function among agricultural workers in El Salvador. Am J Kidney Dis 59: 531–540, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Torres C, Aragón A, González M, López I, Jakobsson K, Elinder CG, et al.: Decreased kidney function of unknown cause in Nicaragua: A community-based survey. Am J Kidney Dis 55: 485–496, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Jayatilake N, Mendis S, Maheepala P, Mehta FR; CKDu National Research Project Team : Chronic kidney disease of uncertain aetiology: Prevalence and causative factors in a developing country. BMC Nephrol 14: 180, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ministry of Health Nutrition and Indigenous Medicine Medical Statistics Unit : Annual Health Bulletin of Sri Lanka 2015, Colombo, Sri Lanka, Ministry of Health, Nutrition and Indigenous Medicine, 2017 [Google Scholar]
- 9.Wijkström J, Jayasumana C, Dassanayake R, Priyawardane N, Godakanda N, Siribaddana S, et al.: Morphological and clinical findings in Sri Lankan patients with chronic kidney disease of unknown cause (CKDu): Similarities and differences with Mesoamerican Nephropathy. PLoS One 13: e0193056, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajapurkar MM, John GT, Kirpalani AL, Abraham G, Agarwal SK, Almeida AF, et al.: What do we know about chronic kidney disease in India: First report of the Indian CKD registry. BMC Nephrol 13: 10, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Minshawy O, Ghabrah T, El Bassuoni E: End-stage renal disease in Tabuk Area, Saudi Arabia: An epidemiological study. Saudi J Kidney Dis Transpl 25: 192–195, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Correa-Rotter R, Wesseling C, Johnson RJ: CKD of unknown origin in Central America: The case for a Mesoamerican nephropathy. Am J Kidney Dis 63: 506–520, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riefkohl A, Ramírez-Rubio O, Laws RL, McClean MD, Weiner DE, Kaufman JS, et al.: Leptospira seropositivity as a risk factor for Mesoamerican Nephropathy. Int J Occup Environ Health 23: 1–10, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valcke M, Levasseur ME, Soares da Silva A, Wesseling C: Pesticide exposures and chronic kidney disease of unknown etiology: An epidemiologic review. Environ Health 16: 49, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Donnell JK, Tobey M, Weiner DE, Stevens LA, Johnson S, Stringham P, et al.: Prevalence of and risk factors for chronic kidney disease in rural Nicaragua. Nephrol Dial Transplant 26: 2798–2805, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wesseling C, Aragón A, González M, Weiss I, Glaser J, Rivard CJ, et al.: Heat stress, hydration and uric acid: A cross-sectional study in workers of three occupations in a hotspot of Mesoamerican nephropathy in Nicaragua. BMJ Open 6: e011034, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laws RL, Brooks DR, Amador JJ, Weiner DE, Kaufman JS, Ramírez-Rubio O, et al.: Changes in kidney function among Nicaraguan sugarcane workers. Int J Occup Environ Health 21: 241–250, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wesseling C, Aragón A, González M, Weiss I, Glaser J, Bobadilla NA, et al.: Kidney function in sugarcane cutters in Nicaragua--A longitudinal study of workers at risk of Mesoamerican nephropathy. Environ Res 147: 125–132, 2016 [DOI] [PubMed] [Google Scholar]
- 19.González-Quiroz M, Caplin B, Pearce N, Nitsch D: What do epidemiological studies tell us about chronic kidney disease of undetermined cause in Meso-America? A systematic review and meta-analysis. Clin Kidney J: sfx136, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.González-Quiroz M, Camacho A, Faber D, Aragón A, Wesseling C, Glaser J, et al.: Rationale, description and baseline findings of a community-based prospective cohort study of kidney function amongst the young rural population of Northwest Nicaragua. BMC Nephrol 18: 16, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al.; CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boucquemont J, Heinze G, Jager KJ, Oberbauer R, Leffondre K: Regression methods for investigating risk factors of chronic kidney disease outcomes: The state of the art. BMC Nephrol 15: 45, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nylund KL, Asparouhov T, Muthén BO: Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Struct Equ Modeling 14: 535–569, 2007 [Google Scholar]
- 24.Wijkström J, González-Quiroz M, Hernandez M, Trujillo Z, Hultenby K, Ring A, et al.: Renal morphology, clinical findings, and progression rate in mesoamerican nephropathy. Am J Kidney Dis 69: 626–636, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Goderis G, Van Pottelbergh G, Truyers C, Van Casteren V, De Clercq E, Van Den Broeke C, et al.: Long-term evolution of renal function in patients with type 2 diabetes mellitus: A registry-based retrospective cohort study. BMJ Open 3: e004029, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boucquemont J, Loubère L, Metzger M, Combe C, Stengel B, Leffondre K; NephroTest Study Group : Identifying subgroups of renal function trajectories. Nephrol Dial Transplant 32[Suppl 2]: ii185–ii193, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Chan AP, Yang Y: Practical on-site measurement of heat strain with the use of a perceptual strain index. Int Arch Occup Environ Health 89: 299–306, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Fischer RSB, Vangala C, Truong L, Mandayam S, Chavarria D, Granera Llanes OM, et al.: Early detection of acute tubulointerstitial nephritis in the genesis of Mesoamerican nephropathy. Kidney Int 93: 681–690, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Laws RL, Brooks DR, Amador JJ, Weiner DE, Kaufman JS, Ramírez-Rubio O, et al.: Biomarkers of kidney injury among nicaraguan sugarcane workers. Am J Kidney Dis 67: 209–217, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gracia-Trabanino R, Domínguez J, Jansà JM, Oliver A: [Proteinuria and chronic renal failure in the coast of El Salvador: Detection with low cost methods and associated factors]. Nefrologia 25: 31–38, 2005 [PubMed] [Google Scholar]
- 31.Masugata H, Senda S, Inukai M, Himoto T, Murao K, Hosomi N, et al.: Seasonal variation in estimated glomerular filtration rate based on serum creatinine levels in hypertensive patients. Tohoku J Exp Med 224: 137–142, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Ranucci M, Castelvecchio S, La Rovere MT; Surgical and Clinical Outcome Research (SCORE) Group : Renal function changes and seasonal temperature in patients undergoing cardiac surgery. Chronobiol Int 31: 175–181, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Barcelo A, Gregg EW, Gerzoff RB, Wong R, Perez Flores E, Ramirez-Zea M, et al.; CAMDI Collaborative Study Group : Prevalence of diabetes and intermediate hyperglycemia among adults from the first multinational study of noncommunicable diseases in six Central American countries: The Central America Diabetes Initiative (CAMDI). Diabetes Care 35: 738–740, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iniciativa Centroamericana de Diabetes (CAMDI) : Encuesta de Diabetes, Hipertensión y Factores de Riesgo de Enfermedades Crónicas, Villa Nueva, Guatemala, Organización Panamericana de la Salud, 2009 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.