Abstract
Background
We previously reported that mutations in the anillin (ANLN) gene cause familial forms of FSGS. ANLN is an F-actin binding protein that modulates podocyte cell motility and interacts with the phosphoinositide 3-kinase (PI3K) pathway through the slit diaphragm adaptor protein CD2-associated protein (CD2AP). However, it is unclear how the ANLN mutations cause the FSGS phenotype. We hypothesized that the R431C mutation exerts its pathogenic effects by uncoupling ANLN from CD2AP.
Methods
We conducted in vivo complementation assays in zebrafish to determine the effect of the previously identified missense ANLN variants, ANLNR431C and ANLNG618C during development. We also performed in vitro functional assays using human podocyte cell lines stably expressing wild-type ANLN (ANLNWT) or ANLNR431C.
Results
Experiments in anln-deficient zebrafish embryos showed a loss-of-function effect for each ANLN variant. In human podocyte lines, expression of ANLNR431C increased cell migration, proliferation, and apoptosis. Biochemical characterization of ANLNR431C-expressing podocytes revealed hyperactivation of the PI3K/AKT/mTOR/p70S6K/Rac1 signaling axis and activation of mTOR-driven endoplasmic reticulum stress in ANLNR431C-expressing podocytes. Inhibition of mTOR, GSK-3β, Rac1, or calcineurin ameliorated the effects of ANLNR431C. Additionally, inhibition of the calcineurin/NFAT pathway reduced the expression of endogenous ANLN and mTOR.
Conclusions
The ANLNR431C mutation causes multiple derangements in podocyte function through hyperactivation of PI3K/AKT/mTOR/p70S6K/Rac1 signaling. Our findings suggest that the benefits of calcineurin inhibition in FSGS may be due, in part, to the suppression of ANLN and mTOR. Moreover, these studies illustrate that rational therapeutic targets for familial FSGS can be identified through biochemical characterization of dysregulated podocyte phenotypes.
Keywords: focal segmental glomerulosclerosis, ANILLIN, ER-STRESS, MTOR, RAC1, AKT
FSGS is the most common primary glomerular disorder causing ESKD in the United States.1 Despite current therapies, approximately 50% of patients with nephrotic FSGS develop ESKD within 10 years of initial diagnosis.2 It is, therefore, critical to improve our understanding of the pathobiology of the disease to devise more effective treatment strategies.
Studies of familial forms of FSGS suggest that disease results from injury or loss of glomerular epithelial cells (i.e., podocytes).3 Podocytes are terminally differentiated cells that maintain the structural integrity of the glomerular filtration barrier. This function is highly dependent on the dynamic regulation of the podocyte actin cytoskeleton. The phosphoinositide 3-kinase (PI3K)/AKT signaling pathway is an essential regulator of podocyte actin cytoskeletal dynamics that has been shown to participate in slit diaphragm signaling through interactions with proteins, such as CD2-associated protein (CD2AP), nephrin, and podocin.4 Pathogenic mutations in CD2AP cause FSGS through mechanisms that involve the disruption of PI3K/AKT signaling, highlighting the importance of this pathway in the pathobiology of podocyte dysfunction in FSGS.5
We previously identified disease-causing mutations (c.1852G>T: p.Gly618Cys[G618C] and c.1291C>T: p.Arg431Cys[R431C]) in familial FSGS, the latter of which is in the F-actin binding domain of anillin (ANLN). ANLNR431C induced hypermotility in podocytes and disrupted the critical interaction between ANLN and CD2AP. To gain a better understanding of the role of ANLNR431C and ANLNG618C in the development of FSGS, we examined the effects of this mutation in vivo through mRNA rescue experiments in anln-deficient zebrafish embryos and established that both mutations cause a loss of function during development. In complimentary in vitro studies, we showed that ANLNR431C overexpression hyperactivates AKT, mTOR, p70S6 Kinase, and Rac1 signaling in podocytes and that pharmacologic inhibition of mTOR and Rac1 attenuates ANLNR431C-induced hypermotility and hyperproliferation. Additionally, we showed that ANLNR431C-induced hyperactivation of mTOR induces endoplasmic reticulum (ER) stress and podocyte apoptosis and that this effect was attenuated by pharmacologic inhibition of mTOR, glycogen synthase kinase 3β (GSK-3β), and calcineurin phosphatase (Cn). Finally, we determined that endogenous ANLN and mTOR protein expressions are downregulated by the Cn/NFAT pathway. Together, these findings suggest that ANLNR431C disrupts podocyte cytoskeletal dynamics and promotes podocyte apoptosis through dysregulation of the PI3K/AKT/mTOR/p70S6/Rac1 pathway. Furthermore, we identify the Cn/NFAT signaling pathway as a regulator of endogenous mTOR and ANLN expression and provide novel mechanistic insights into the beneficial clinical effects of Cn inhibitors in some forms of FSGS.
Methods
Transient Suppression of anln and In Vivo Complementation Assays in Zebrafish
We designed a splice blocking morpholino (MO) targeting the donor site of exon 5 of the anln ortholog in zebrafish (Supplemental Figure 1A) and performed a transient suppression experiment as described in Supplemental Material. We validated the transient suppression experiment using CRISPR/Cas9 genome editing of the anln locus in zebrafish (Supplemental Material).
Conditionally Immortalized Human Podocyte Culture
Conditionally immortalized human podocytes were cultured and harvested as described.6
Lentiviral Constructs and Infection
Standard molecular cloning methods were used for stable transfection of all cell lines (Supplemental Material).7
Immunofluorescence
Conditionally immortalized human podocytes were differentiated and stained using standard protocols (Supplemental Material).
Rac1 and RhoA Activity Assay
GTP-bound Rac1 and RhoA were analyzed using PAK and Rhotekin-Rho binding domain bead assays (Supplemental Material).
Targeted Inhibition
For inhibition experiments, stock inhibitors were made in DMSO, and dilution was carried out as described in Supplemental Material.
Migration Assay
Scratch wound assays were used as described in Supplemental Material.
Proliferation Assay
A cell counting colorimetric assay was used for the cell proliferation studies as described in Supplemental Material.
Apoptosis Assay
Serum starvation method was used to induce apoptosis in experimental podocyte lines as described in Supplemental Material.
In Silico Modeling
The ANLNR431C variant was assessed for its effects on the secondary structure of the protein. The in silico prediction program I-TASSER (http://zhanglab.ccmb.med.umich.edu/I-TASSER/) was used to generate the ANLNWT and ANLNR431C images.8
Actin Bundling/Polymerization Assay
HEK 293T cells were grown in DMEM + 5% FBS in T75 flasks at 37°C until 85% confluency and transfected using Lipofectamine 2000 with plasmids containing CMV-turbo GFP (tGFP), CMV-ANLNWT-tGFP, or CMV-hANLNR431C-tGFP; 65 μl of Lipofectamine 2000 (Thermo-Fisher) was combined with 1.7 ml of Opti-MEM (Gibco) and allowed to incubate at room temperature for 5 minutes before combining with 1.7 ml of Opti-MEM containing 26 μg of plasmid DNA. After 20 minutes of incubation, the mixture was added to 15 ml of DMEM with 5% FBS. Cells were analyzed using fluorescence microscopy between 40 and 48 hours to ensure adequate transfection levels, and then, they were harvested at 48 hours and lysed using a syringe in 400 μl of 20 mM HEPES and 20 mM NaCl buffer. Supernatant was collected after spinning for 3 minutes at 14,000×g at 4°C and analyzed using immunoblotting to ensure equivalent levels of exogenous ANLN expression between samples. Bundling reactions including appropriate positive and negative controls were performed using the Actin Binding Protein Biochem Kit (Cytoskeleton Inc.) per the manufacturer’s instruction. Next, 30 μl of experimental lysate was combined with 50 μl of purified human nonmuscle F-actin stock or an F-actin buffer and incubated for 30 minutes at room temperature. After spinning at 14,000×g for 1 hour, the supernatant was collected, and the F-actin pellet was mixed with 30 μl of water and 30 μl of 2× Laemmli reducing sample buffer and analyzed using immunoblotting with an F-actin mouse mAb (1:500; Novus Biologicals).
Statistical Analyses
Zebrafish larval batches were compared using chi-squared tests. All in vitro data are represented as the mean±SEM. Group differences were assessed by the t test with unequal variances. One-way ANOVA with a Tukey HSD post hoc test was used to determine the differences between means where there are three or more groups. Statistical significance was established at P<0.05.
Results
In Vivo Complementation Studies in Zebrafish Indicate That Missense ANLN Variants Result in a Loss of Function
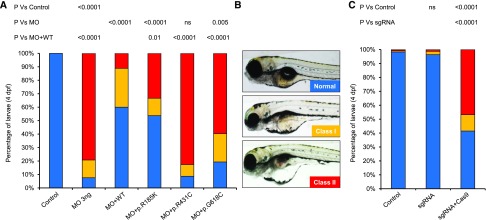
We have shown that transient suppression of anln in zebrafish larvae recapitulates aspects of FSGS pathology.7 We designed a splice blocking MO targeting the exon 5 splice donor (e5i5) (Supplemental Figure 1A) and injected it into WT zebrafish embryos (3, 6, and 9 ng); we observed disruption of mRNA splicing in anln e5i5 morphants (Supplemental Figure 1, B and C). Furthermore, we observed a dose-dependent response of e5i5 MO; larval batches scored live at 4 days postfertilization displayed ocular and pericardial edema (P<0.001 versus controls; n=63–81 per batch, repeated) (Supplemental Figure 1D). Next, we coinjected 3 ng MO with 150 pg of human ANLNWT mRNA and scored larval batches for edema phenotypes in three phenotypic classes. Whereas 92% of e5i5 morphant larvae displayed edema phenotypes, an equivalent dose of MO with ANLNWT mRNA improved phenotypes significantly (P<0.001 versus MO; n=42–56 per injection batch, repeated) (Figure 1, A and B).
Figure 1.
Transient suppression and CRISPR/Cas9 genome editing of anillin (anln) demonstrates loss-of-function phenotypes for two human FSGS mutations. (A) Zebrafish larvae injected with 3 ng morpholino (MO) alone or with 150 pg ANLN mRNA were scored live at 4 days postfertilization (dpf) for edema as a proxy for glomerular filtration defects: class 1 (mild), mild ocular and pericardial edema; class 2 (severe), pronounced ocular, pericardial, and yolk sac edema (B). Statistical calculations were performed using a chi-squared test (n=42–56 per batch, repeated). p.G618C, p.Gly618Cys; p.R185K, p.Arg185Lys (likely benign variant because of common incidence in healthy control populations, allele frequency 0.6480; dbSNP ID: rs572020591); p.R431C, p.Arg431Cys; WT, wild type. (B) Representative live lateral images of zebrafish larvae at 4 dpf show different phenotypic classes. (C) CRISPR/Cas9 F0 mutants mimic anln morphant phenotypes. Qualitative scoring of zebrafish larvae at 4 dpf on the basis of objective phenotypic criteria (B). Statistical differences were compared using a chi-squared test (n=41–75 per batch, repeated). sgRNA, single-guide RNA.
To complement our transient suppression reagents,7 we introduced small insertions and deletions into the Danio rerio anln locus using CRISPR/Cas9 genome editing. We designed a single-guide RNA (sgRNA) targeting exon 8 (Supplemental Figure 1A) and injected 100 pg sgRNA with 200 pg Cas9 protein into WT zebrafish embryos. Heteroduplex analysis showed high mosaicism in F0 mutants (100% of clones had insertions and deletions; n=5 embryos) (Supplemental Figure 1, E and F). Importantly, anln F0 mutants recapitulated morphant phenotypes at 4 days postfertilization, and 59% of larvae displayed edema (P<0.001; n=41–75 per batch, repeated) (Figure 1, B and C); larvae injected with sgRNA alone were indistinguishable from controls (Figure 1C).
Next, we used in vivo complementation studies to test the direction of allele effect of ANLNR431C and ANLNG618C.8 We have used renal function readouts previously to both inform variant pathogenicity and indicate whether deleterious effects are likely to be loss of function, dominant negative, or gain of function.6,9 We compared the ANLNWT rescue efficiency with that of either FSGS-associated variant or a likely benign variant, ANLNR185K. We observed that ANLNR185K improved the anln e5i5 MO-induced pathology (P<0.001 versus MO; n=42–56, repeated); both mutant mRNAs rescued at efficiencies significantly worse than ANLNWT (P<0.001), ANLNR431C was not significantly different from MO, and ANLNR618C was modestly improved in comparison with MO alone (Figure 1, A and B). Heterologous expression of human ANLN mRNAs in embryo batches produced no phenotypes that differed from uninjected controls (n=53–75, repeated) (Supplemental Figure 2), arguing against any dominant negative or toxic effects. Together, our in vivo data reinforce the involvement of anln in podocyte integrity and indicate that ANLNR431C and ANLN618C confer a loss-of-function effect.
Overexpression of ANLNR431C Alters Podocyte F-Actin Organization In Vitro
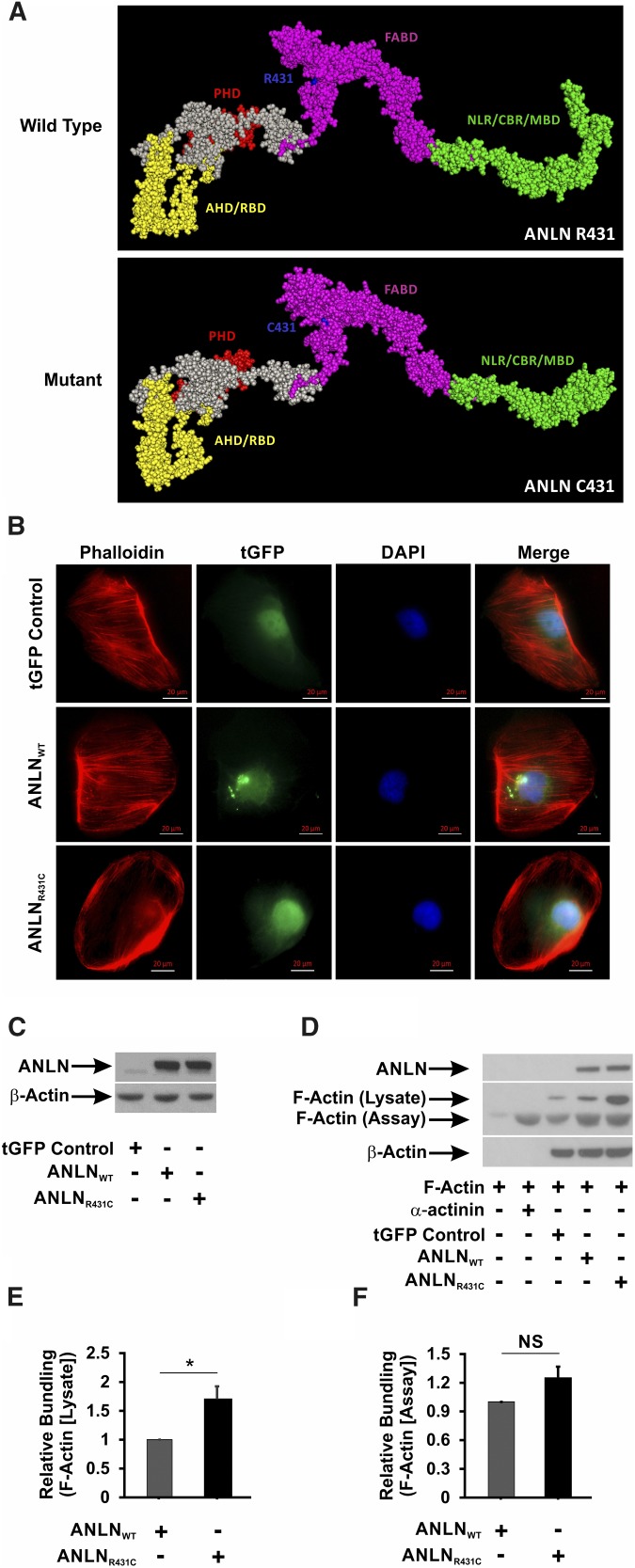
ANLN is known to bind directly to F-actin filaments and induce formation of F-actin bundles at epithelial cell junctions.10 CD2AP is a multifunctional adaptor protein that assembles intermediates of the PI3K/AKT pathway for signaling at the slit diaphragm.4,11 We previously showed that the R431C mutation disrupts the interaction between ANLN and CD2AP. In silico modeling of the effect of the mutation on ANLN secondary structure suggests that introduction of a cysteine residue at amino acid 431 distorts the architecture of the F-actin binding domain and the CD2AP binding region (Figure 2A). On the basis of these findings, we postulated that ANLNR431C overexpression would disrupt F-actin bundling and organization in podocytes. We used a lentiviral delivery system to generate immortalized human podocyte lines stably expressing tGFP control, tGFP-ANLNWT (ANLNWT), and tGFP-ANLNR431C (ANLNR431C). By immunofluorescence imaging, we observed well organized bundles of filamentous actin in regular array throughout the cytoplasm in tGFP-ANLNWT–expressing podocytes and tGFP controls (Figure 2B). Conversely, there was prominent disruption of F-actin organization in ANLNR431C-overexpressing podocytes with mislocalization of disorganized aggregates to the periphery of the cytoplasmic compartment. Equivalent ANLN overexpression was confirmed by immunoblot in ANLNWT- and ANLNR431C-expressing lines relative to the endogenous ANLN expression in the control line (Figure 2C). These findings suggest that ANLNR431C may induce podocyte injury through disruption of actin bundling and organization in podocytes. To test this hypothesis, we performed F-actin bundling/polymerization assays in HEK 293T cells transiently transfected with plasmids expressing tGFP control, tGFP-ANLNWT, and tGFP-ANLNR431C (Figure 2, D–F, Supplemental Figure 3). In Figure 2D, we confirm F-actin bundling/polymerization in the presence of purified α-actin under cellfree conditions. In the presence of HEK 293T cell lysates, we detected a doublet of F-actin. There was no significant difference in ANLNWT and ANLNR431C in the bundling/polymerization of F-actin in the lower molecular weight band (Figure 2, D and F); however, there was a significant difference in F-actin bundling/polymerization at the higher molecular weight (Figure 2, D and E), suggesting that the difference in F-actin bundling/polymerization observed in ANLNR431C-overexpressing HEK 293T cells may be dependent on the interaction of ANLN with other F-actin–associated proteins.
Figure 2.
The R431C mutation distorts the secondary structure and F-actin bundling activity of ANLN. (A) In silico modeling of the effect of R431C mutation on the secondary structure of ANLN reveals that the introduction of a cysteine residue at amino acid position 431 distorts the architecture of the F-actin binding domain (FABD) and the CD2-associated protein binding region (CBR). AHD, Anillin Homology Domain; MBD, Myosin Binding Domain; NLR, Nuclear Localization Region; PHD, Pleckstrin Homology Domain; RBD, Rho A Binding Domain. (B) ANLNWT and ANLNR431C cells were stained with a Phalloidin antibody to visualize F-actin. GFP-tagged ANLN expression can be seen in the nucleus of the three lines. Phalloidin staining showed F-actin fibers extending from end to end in regular array throughout the cell in the ANLNWT cell line compared with ANLNR431C-overexpressing podocytes that showed a paucity of F-actin staining, with small aggregates present mostly in the periphery. (C) Western blot analysis of lentiviral-infected GFP control, turbo GFP (tGFP)–tagged ANLNWT, and tGFP-tagged ANLNR431C immortalized podocyte cell lines for ANLN and β-actin protein levels. Both experimental lines displayed similar levels of tGFP-ANLN, whereas endogenous ANLN is visible in a lower band. (D and E) Actin bundling/polymerization assays were performed with or without the addition of assay-specific nonmuscle F-actin. Transfected HEK 293 cell lines overexpressing ANLNR431C displayed increased bundling/polymerization of lysate-associated F-actin (upper band) compared with the wild-type ANLN (P=0.03). (D and F) There was no difference in relative bundling/polymerization of assay-specific purified F-actin (lower band) between the two ANLN cell lines (P=0.08). *P=0.03.
Overexpression of ANLNR431C Induces Hyperproliferation in Podocytes and Activation of the PI3K/AKT/mTOR/Rac1 Signaling Axis
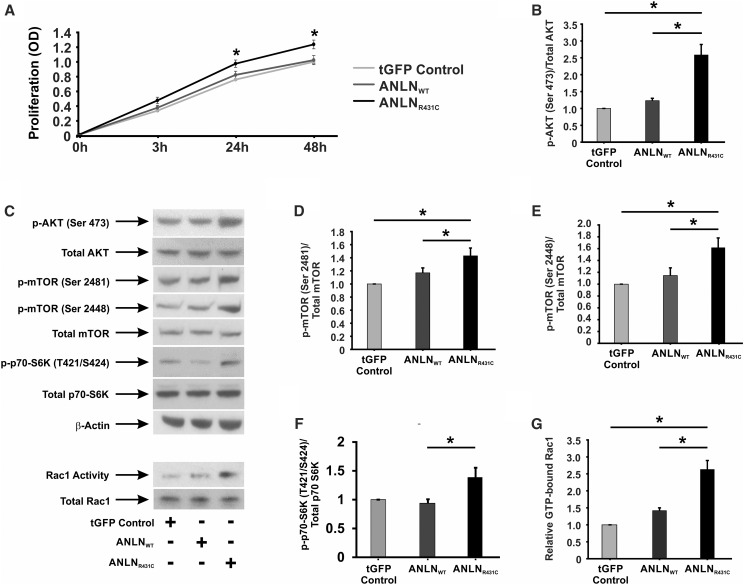
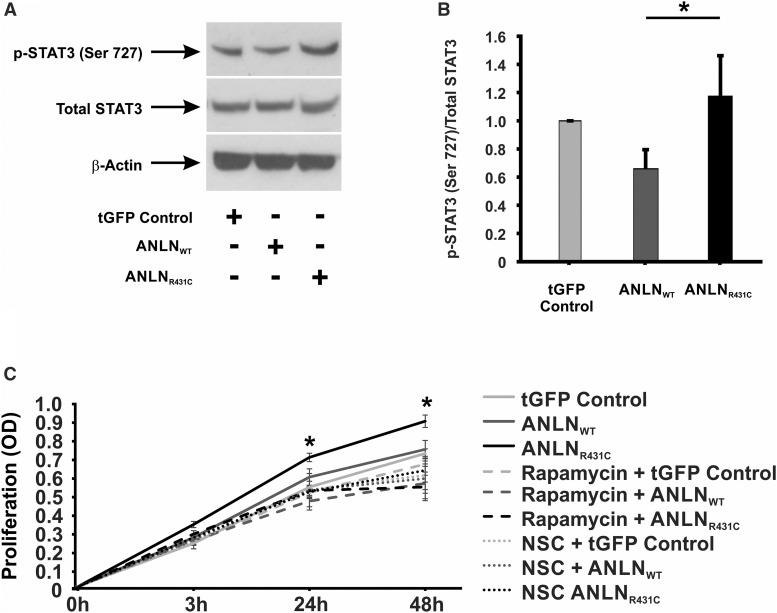
ANLN is recognized as a driver of cellular proliferation in various forms of cancer,12–15 and it is upregulated in HIV-associated nephropathy (HIVAN), a phenotype characterized by podocyte hyperproliferation and apoptosis.7,16–18 On the basis of these findings, we postulated that ANLNR431C may cause podocyte dysfunction through hyperproliferation. Cell proliferation assays revealed a significant increase in ANLNR431C podocyte proliferation relative to ANLNWT and tGFP controls at 24 and 48 hours (P=0.02 and P=0.001, respectively) (Figure 3A). To understand the biochemical basis for the observed phenotype, we evaluated the activation state of signaling intermediates of the PI3K/AKT/mTOR pathway. In ANLNR431C-overexpressing podocytes, levels of activated AKT (phospho-AKT S473) were significantly elevated relative to ANLNWT-overexpressing and tGFP control podocytes (P=0.03 and P=0.004, respectively) (Figure 3, B and C). Additionally, phosphorylation of mTOR at serine residues 2448 and 2481 (considered indicators of rapamycin-sensitive, mTORC1-specific and total mTOR activity, respectively19,20) was increased in ANLNR431C-overexpressing podocytes relative to ANLNWT-overexpressing and tGFP control podocytes (P=0.05 and P=0.05, respectively) (Figure 3, B, D, and E). Hyperactivation of mTOR was also reflected in the increased activation of p70 S6 Kinase, a downstream effector kinase of mTOR, in ANLNR431C podocytes (Figure 3, C and F). The p70S6 kinase is a downstream effector kinase of mTOR that modulates actin cytoskeletal dynamics through direct activation of Rac1 and F-actin binding.21 Because we previously established that ANLNR431C induces podocyte hypermotility,7 we evaluated the activation state of the Rho GTPases Rac1 and RhoA as the potential mediators of both hyperproliferation and hypermotility in ANLNR431C-overexpressing podocytes given their known roles in both cellular processes.22,23 In ANLNR431C podocytes, Rac1 was significantly upregulated relative to ANLNWT and tGFP control podocytes (P=0.004) (Figure 3, C and G). There was no significant difference observed in RhoA activation between ANLNWT and ANLNR431C cell lines (P=0.10) (Supplemental Figure 4). Next, we evaluated the activation state of the transcription factor Signal Transducer and Activator of Transcription 3 (STAT3). STAT3 is indirectly activated by Rac1 and has been identified as the principal driver of podocyte proliferation in HIVAN.24–26 In ANLNR431C-overexpressing podocytes, we showed that activation of STAT3 was significantly upregulated relative to ANLNWT-overexpressing podocytes (P<0.01) (Figure 4, A and B), suggesting that ANLNR431C-induced podocyte hyperproliferation may be driven by the mTOR-dependent activation of STAT3 through Rac1. To test this possibility, we evaluated podocyte proliferation in the presence of selective pharmacologic inhibitors of mTOR and Rac1. The mTOR inhibitor rapamycin and the Rac1 inhibitor NSC23766 both eliminated the increased podocyte proliferation in ANLNR431C-overexpressing podocytes at 24 and 48 hours relative to ANLNWT-overexpressing podocytes (P=0.99, P=0.78, P=0.53, and P=0.98, respectively) (Figure 4C). Together, these data show that the ANLNR431C mutation induces podocyte hyperproliferation through inappropriate upregulation of PI3K/AKT/mTOR/Rac1/STAT3 signaling.
Figure 3.
AnillinR431C (ANLNR431C)-expressing podocytes display an increase in proliferation and activation of the phosphoinositide 3-kinase (PI3K)/AKT/mTOR/Rac1 signaling axis. (A) Proliferation assays were performed on control, ANLNWT, and ANLNR431C podocyte cell lines using a CCK colorimetric assay with data collected at 0, 3, 24, and 48 hours. ANLNR431C podocytes display significantly increased proliferation at the 24- and 48-hour time points (n=13; P=0.02 and P=0.001, respectively). (B) Western blot quantification revealed a significant difference between GFP and experimental cell lines as well as a significant increase in the ANLNR431C-expressing podocytes compared with ANLNWT podocytes when exposed to phosphorylated AKT S473 (n=6; P=0.03). (C) Representative Western blots are depicted for phospho-AKT S473, total AKT, phospho-mTOR S2481, phospho-mTOR S2448, total mTOR, phospho–p70-S6K T424/S421, total p70-S6K, b-actin, GTP-bound Rac1, and total Rac1. (D–F) This same pattern of increased activation in ANLNR431C compared with ANLNWT was revealed when examining mTOR phosphorylation at serine 2481 and serine 2448 as well as the downstream effector kinase p70-S6K at Thr424/Ser421 (n=6, P=0.05; n=5, P=0.05; n=5, P=0.02, respectively). (G) Rac1 activity assays using a PAK bead pulldown assay to analyze GTP-bound active Rac1 showed an increase in Rac1 activity in ANLNR431C-expressing podocytes compared with ANLNWT (n=3; P<0.01). tGFP, turbo GFP. *P<0.05.
Figure 4.
AnillinR431C (ANLNR431C)-induced podocyte hyperproliferation is driven by mTOR-dependent activation of Signal Transducer and Activator of Transcription 3 (STAT3) through Rac1. (A) Representative Western blots are depicted for phospho-Stat3 S727, total Stat3, and β-actin in the turbo GFP (tGFP) control, ANLNWT, and ANLNR431C podocyte cell lines. (B) Western blot quantification revealed a significant increase in Stat3 activation in ANLNR431C podocyte cell lines compared with ANLNWT (P<0.01). (C) Quantification of proliferation using a CCK colorimetric assay showed that the increased proliferation in the ANLNR431C podocyte line at the 24- and 48-hour time points was rescued with 100 nM rapamycin (P=0.99 and P=0.78, respectively) or 80 μM NSC-23766 (P=0.53 and P=0.98, respectively). Twelve wells per time point per sample (n=4). *P<0.05.
Overexpression of ANLNR431C Induces Hypermotility in Podocytes
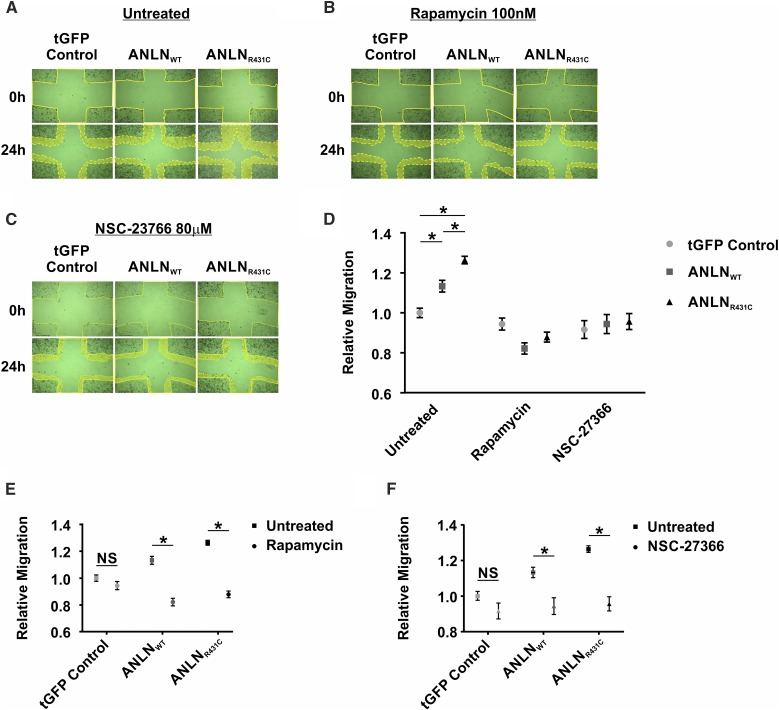
We previously reported that ANLNR431C induces hypermotility in podocytes.7 This finding supports a similar role for ANLN in the migration and invasiveness of various malignancies.27 In this study, we examined the biochemical basis for the increased migratory activity in ANLNR431C-overexpressing podocytes. As described above, PI3K/AKT/mTOR/Rac1 signaling and podocyte motility were significantly upregulated in mutant podocytes compared with the wild type (Figure 5, A–D). Hypermotility in ANLNR431C-overexpressing podocytes was significantly attenuated in the presence of inhibitors of this pathway, including rapamycin and Rac1 inhibitor NSC-23766 (P=0.32 and P=0.98, respectively) (Figure 5, A–D, Supplemental Figure 5). Both rapamycin and NSC-23766 treatment resulted in a significant reduction of motility in ANLNWT (P<0.001 and P=0.003, respectively) and ANLNR431C podocytes (P<0.001 and P<0.001, respectively) while leaving control cells relatively unaffected (P=0.15 and P=0.10, respectively) (Figure 5, E and F). These findings suggest that the hypermotility phenotype in ANLNR431C-overexpressing podocytes is driven by inappropriate activation of the PI3K/AKT/mTOR/Rac1 signaling and that targeting components of this pathway may be a viable strategy in cases of ANLN overexpression or mutation.
Figure 5.
AnillinR431C (ANLNR431C) dysregulated motility can be rescued with inhibitors of mTOR and Rac1 activity. (A–C) Representative images at 0 and 24 hours for GFP control–, ANLNWT-, and ANLNR431C-expressing podocytes when exposed to (A) vehicle control (DMSO), (B) 100 nM rapamycin, and (C) 80 μM NSC-23766 in a scratch wound healing assay. (D) Quantification of podocyte migration in GFP control–, ANLNWT-, and ANLNR431C-expressing podocytes when exposed to vehicle control (DMSO), mTOR inhibitor (rapamycin), or Rac1 inhibitor (NSC-23766). There is significant increase in motility of ANLNR431C cells at baseline compared with ANLNWT cells; this increased motility was rescued by inhibition of mTOR (P=0.32) or Rac1 (P=0.98; n=20 for each condition). (E and F) Quantification of migration revealed a significant decrease in motility in ANLNWT and ANLNR431C cells after treatment with either (E) rapamycin (P<0.001 and P<0.001, respectively) or (D) NSC-23766 (P=0.003 and P<0.001, respectively). tGFP, turbo GFP. *P value <0.05.
Overexpression of ANLNR431C Induces ER Stress and Apoptosis in Podocytes
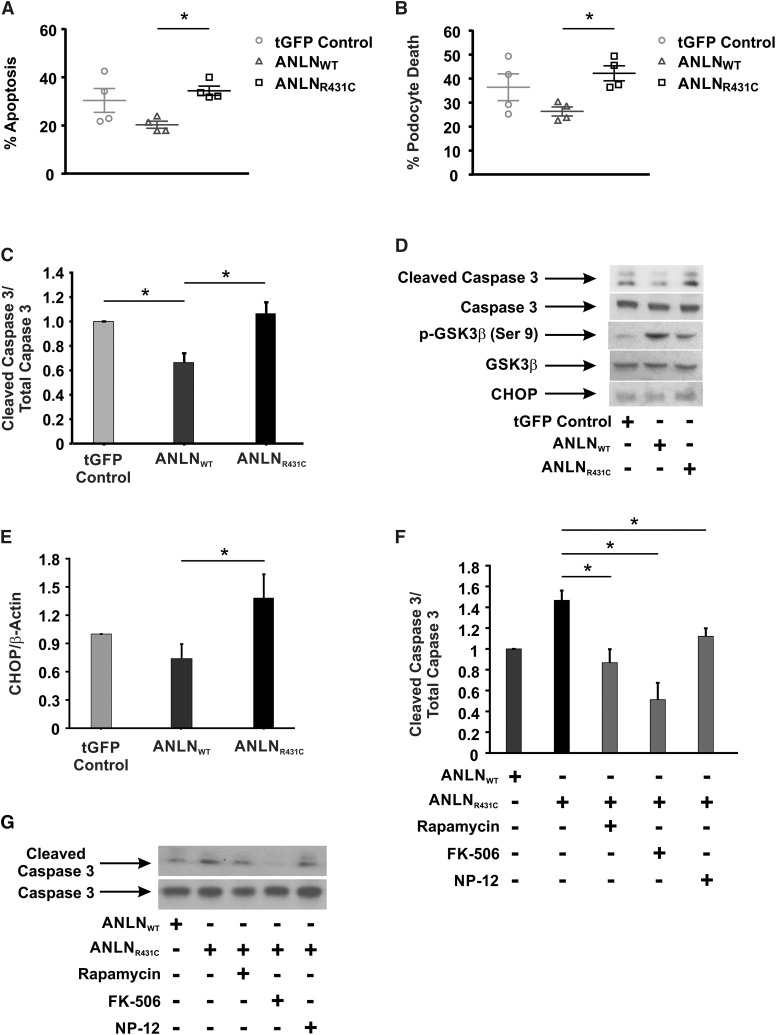
We previously showed that ANLN expression was upregulated in human collapsing glomerulopathy and the murine model of HIVAN.7 Because podocyte apoptosis is a characteristic feature of HIVAN,28 we determined if ANLNR431C overexpression induced apoptosis in podocytes. We subjected tGFP control–, ANLNWT-, and ANLNR431C-overexpressing podocytes to serum deprivation to induce apoptosis. Overexpression of ANLNR431C induced a significant increase in apoptosis and total cell death (P=0.03 and P=0.04, respectively) relative to the ANLNWT-overexpressing cell line (Figure 6, A and B). Caspase 3 cleavage was also significantly increased (P=0.01) in the mutant cell line (Figure 6, C and D). Because PI3K/AKT is widely recognized as a prosurvival pathway, we sought to characterize the biochemical basis for the increased susceptibility of ANLNR431C-overexpressing podocytes to apoptosis. We postulated that the ANLNR431C-induced hyperactivation of mTOR may cause ER stress and podocyte apoptosis.29 To test this, we evaluated the activation state of GSK-3β, a downstream target of AKT and regulator of the death-inducing transcription factor C/EBP homologous protein (CHOP).30 We observed a decrease in inhibitory phosphorylation of GSK-3β and an increase in CHOP in ANLNR431C-overexpressing podocytes relative to ANLNWT-overexpressing podocytes (Figure 6, D and E). The dephosphorylation/activation of GSK-3β is regulated by Protein Phosphatase 2A (PP2A) and Cn. Because both have been shown to modulate ER stress–induced apoptotic signaling, we postulated that inhibition of Cn or PP2A would suppress GSK-3β dephosphorylation/activation and promote cell survival. We showed that caspase 3 cleavage was significantly attenuated in ANLNR431C-overexpressing podocytes in the presence of the Cn inhibitor FK-506 and the GSK-3β inhibitor NP-12 (Figure 6, F and G). The PP2A inhibitor Calyculin A did not inhibit ER stress–induced apoptosis (data not shown). Rapamycin also significantly reduced apoptosis in ANLNR431C-overexpressing podocytes (Supplemental Figure 6), consistent with previous reports of the protective roles of mTOR and GSK-3β inhibition against ER stress and apoptosis (Supplemental Figure 6).31 Taken together, these data suggest that ANLNR431C induces ER stress and apoptosis in podocytes through hyperactivation of mTOR.
Figure 6.
Overexpression of anillinR431C (ANLNR431C) induces endoplasmic reticulum (ER) stress and apoptosis in podocytes. (A and B) Apoptosis was examined in the control and experimental podocyte cell lines after 48 hours of serum starvation using Annexin V and 7-AAD staining. FACS analysis revealed increased apoptosis and total cell death in the ANLNR431C-expressing podocytes compared with the wild type. (C and D) Cell lysates from control, ANLNWT, and ANLNR431C podocyte cell lines exposed to 48 hours of serum starvation were examined for activation of caspase 3 apoptotic signaling. Cleaved caspase expression was significantly increased in the ANLNR431C cell line compared with the ANLNWT. (D) Representative Western blots are shown for cleaved caspase 3, total caspase 3, and β-actin expression. (C) Quantification of Western blot analysis shows a decrease in caspase 3 apoptotic signaling in ANLNWT cells compared with GFP controls (P=0.03) but an increase in ANLNR431C compared with ANLNWT cells (n=4; P=0.01). (D) Representative Western blots images showing expression of cleaved caspase 3, total caspase 3, phosphoglycogen synthase kinase 3β (phospho–GSK-3β) S9, total GSK-3β, and C/EBP homologous protein (CHOP) in serum-starved turbo GFP (tGFP) control–, ANLNWT-, and ANLNR431C-expressing podocytes. (E) Quantification of CHOP expression revealed a significant increase in CHOP expression in ANLNR431C compared with ANLNWT cells (n=3; P=0.04). (F) Twenty-four–hour treatment of the serum-starved cells with 100 nM mTOR inhibitor rapamycin, 1 μM calcineurin phosphatase (Cn) inhibitor FK-506, or 2.5 μM GSK-3β inhibitor NP-12 was able to significantly reduce cleaved caspase 3 in ANLNR431C podocytes (P=0.02, P<0.01, and P=0.05, respectively) (n=3). Representative blots for each treatments as well as control ANLNWT- and ANLNR431C-expressing podocytes are depicted in G. *P value <0.05.
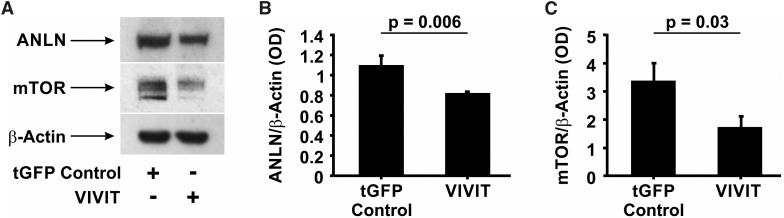
Calcineurin Inhibitors May Ameliorate ANLN-Mediated Disease by Targeting ANLN and mTOR Expression
Recent studies suggest that mTOR inhibitors may be useful for the treatment of FSGS.32 However, the use of rapamycin has been associated with significant nephrotoxicity, especially in patients with renal disease.33–35 To identify additional candidate therapeutic targets, we interrogated putative MTOR and ANLN promoter sequences to identify regulatory elements that might operate downstream of pharmacologically modifiable pathways. We identified multiple candidate NFAT binding sequences in both putative promoters and posited that inhibition of the Cn/NFAT signaling pathway could effectively attenuate ANLN-induced podocyte injury through downregulation of endogenous mTOR and ANLN. To examine this, we established a conditionally immortalized human podocyte cell line overexpressing GFP-tagged VIVIT, a peptide inhibitor of the interaction between Cn and NFAT.36 We determined that VIVIT-overexpressing podocytes exhibited significantly reduced expression of both ANLN (P<0.01) (Figure 7, A and B) and mTOR (P=0.03) (Figure 7, A and C), suggesting that endogenous expression of both genes is regulated by Cn/NFAT. Coupled with the finding that FK-506 attenuated caspase 3 cleavage in ANLNR431C-expressing podocytes, this suggests that Cn inhibitors may be a viable alternative to rapamycin for treatment of mTOR-mediated injury and apoptosis in ANLNR431C-expressing podocytes. Additionally, these results support the hypothesis that therapies targeting the reduction of mTOR expression (i.e., tacrolimus or cyclosporin A) may be as effective as therapies that reduce mTOR activity (i.e., rapamycin) in the management of ANLNR431C-induced podocyte injury and FSGS. All uncropped original Western blot images are shown in Supplemental Figure 7.
Figure 7.
Calcineurin inhibitors may ameliorate anillin (ANLN)-mediated disease by targeting ANLN and mTOR expression. (A) Representative Western blot images of ANLN and mTOR protein amounts in GFP control– and VIVIT-expressing podocytes. (B and C) Quantification of Western blots revealed a significant decrease in (B) ANLN and (C) mTOR protein in VIVIT-expressing podocytes compared with GFP control podocytes (P<0.01 and P=0.03, respectively). tGFP, turbo GFP.
Discussion
We previously identified missense mutations in ANLN as a cause of familial FSGS and found that the ANLNR431C mutation induced podocyte dysmotility as well as disruption of a critical interaction with CD2AP,7 which induces downstream PI3K/Akt signaling.8 On the basis of these observations, we postulated that ANLNR431C may cause podocyte injury and loss through disruption of actin cytoskeletal dynamics, prosurvival signaling, and proliferation; we, therefore, examined the biochemical basis of the pathologic phenotypes induced by ANLNR431C mutation in cultured podocytes.
First, we validated our previous findings that anln deficiency in zebrafish larvae disrupts the glomerular filtration barrier,7 with (1) an additional transient knockdown reagent targeting an independent site of the locus and (2) CRISPR/Cas9 genome editing of the anln locus. Furthermore, we used in vivo complementation studies to show the pathogenicity of the previously reported ANLNR431C and ANLNG618C mutations in zebrafish and found that both variants cause a loss of function during development.
Second, we showed that podocytes expressing ANLNR431C exhibit hyperactivation of the PI3K/AKT/mTOR/Rac1 signaling pathway, which drives the dysmotility phenotype that we previously reported.7 Notably, RhoA activity was also upregulated in cell lines overexpressing mutant ANLN compared with controls. Although RhoA is known to inhibit Rac1-mediated cell migration through retraction of actomyosin-mediated lamellipodial extensions, its upregulation in ANLNR431C-induced hypermotility may reflect a bistable system of mutually antagonistic cytoskeletal regulation that underlies podocyte motility in epithelial cells.37,38 Furthermore, we found that pharmacologic inhibition of mTOR and Rac1 ameliorates dysmotility in ANLNR431C-overexpressing podocytes, suggesting that mTOR and Rac1 may be viable therapeutic targets for phenotype correction in ANLNR431C-mediated podocyte injury. These findings are in agreement with previous reports of increased Rac1 and mTOR activation in biopsies from patients with FSGS, and the ability of these inhibitors to correct the Rac1 and mTOR overexpression induced glomerular disease in mice.32,39,40
In addition to its role in the dysregulation of podocyte motility, we showed that the ANLNR431C-induced hyperactivation of PI3K/AKT/mTOR/Rac1 signaling also induced an upregulation of podocyte proliferation, which was ameliorated by pharmacologic inhibitors of mTOR and Rac1. Because Rac1 indirectly activates the proproliferative transcription factor STAT3, we examined the role of STAT3 in ANLNR431C-induced podocyte hyperproliferation. Our findings suggest that ANLNR431C induces proliferation through the amplification of STAT3-mediated proproliferative gene expression. These findings are similar to those of previous studies, which showed that decreased STAT3 activation was protective against inappropriate cell cycle re-entry and the development of HIVAN in mice.25
Interestingly, we also observed an increase in apoptosis in ANLNR431C-overexpressing podocytes. This increase in cell death was unexpected given the hyperactivation of PI3K/AKT signaling, which is widely recognized as a prosurvival pathway. However, this finding can be explained by the inappropriate activation of mTOR signaling in ANLNR431C-overexpressing podocytes. It is well recognized that upregulation of mTOR can lead to ER stress and apoptosis through induction of the unfolded protein response.29 ER stress–induced death signaling can proceed through a number of pathways, culminating in the expression of transcriptional activators of apoptotic signaling, such as CHOP.41 GSK-3β is a regulator of CHOP expression that is negatively regulated by phosphorylation at serine 9 (Ser 9) by AKT.30 Although we did not anticipate the decrease in inhibitory GSK-3β phosphorylation at Ser 9 that we observed in our ANLNR431C-overexpressing podocytes, this might be explained by the concomitant activation of protein phosphatases such as Cn, which activate GSK-3β through dephosphorylation of Ser 9. In these studies, we found that GSK-3β activation and CHOP expression were induced in ANLNR431C-overexpressing podocytes. Moreover, caspase 3 cleavage was significantly attenuated in ANLNR431C-overexpressing podocytes treated with inhibitors of mTOR (rapamycin) and GSK-3β (NP-12). The calcineurin inhibitor (Cn) FK-506 was also effective in attenuating caspase 3 cleavage, which may be due to inhibition of GSK-3β dephosphorylation or the downregulation of endogenous mTOR expression. The identification of Cn inhibitors as negative modulators of mTOR and ANLN expression is novel and may have broader implications for our understanding of the use of Cn inhibitors in proteinuric kidney disease.
In addition to broadening our understanding of the therapeutic benefits of Cn inhibitors, these studies are consistent with previous reports that mTOR inhibitors have beneficial effects in FSGS.32 Although the use of mTOR inhibitors in FSGS remains controversial, our studies suggest that mTOR is a central contributor to the pathogenesis of podocyte dysfunction in some forms of FSGS. As we progress toward an era of personalized medicine, individual assessment of mTOR activity in patients with FSGS may inform the targeted use of mTOR activity inhibitors (i.e., rapamycin) or mTOR expression inhibitors (i.e., FK-506 or cyclosporin A) for certain types of FSGS.
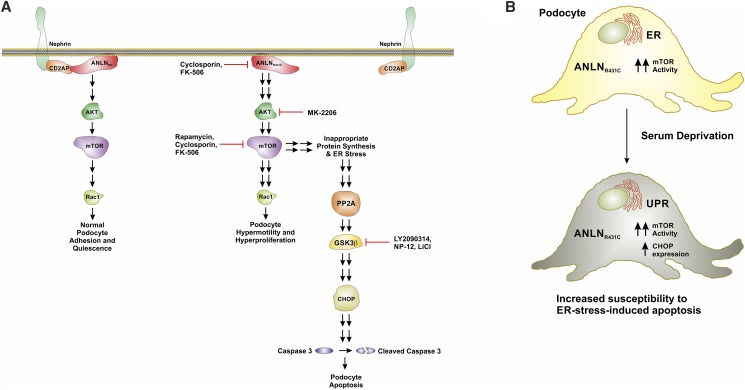
In summary, we report that the loss-of-function ANLNR431C mutation may exert its deleterious effects on podocyte function and viability through multiple derangements of PI3K/AKT/mTOR/Rac1 signaling (Figure 8). Through the identification and biochemical characterization of ANLNR431C-induced podocyte injury phenotypes, we have identified discrete molecular targets (i.e., ANLN, mTOR, and GSK-3β) that may be pharmacologically manipulated to ameliorate podocyte dysfunction in a form of hereditary FSGS. Although further characterization of the effects of these therapies in a representative in vivo model of disease will be required to assess their potential clinical utility, this study highlights the value of rigorous biochemical characterization of the molecular mechanisms of familial nephrotic syndromes for the identification of potential therapeutic targets.
Figure 8.
The R431C mutation induces hyperproliferation, hypermotility and ER stress-induced apoptosis in podocytes. (A) In healthy podocytes, ANLN modulates actin cytoskeletal dynamics at the slit diaphragm and facilitates prosurvival signaling through the phosphoinositide 3-kinase (PI3K)/AKT signaling pathway. The ANLN interaction with CD2-associated protein (CD2AP) is essential to these functions. The R431C mutation disrupts the ANLN-CD2AP interaction, producing (1) dysregulated F-actin polymerization and bundling, (2) hyperactivation of the PI3K/AKT/mTOR/p70S6K/Rac1 signaling axis, and (3) activation of endoplasmic reticulum (ER) stress–induced apoptosis. Specifically, the R431C mutation distorts the secondary structure of ANLN at the F-actin binding domain (Figure 2), which impairs F-actin polymerization and bundling in podocytes. These changes alter podocyte foot process architecture at the slit diaphragm, contributing to the development of proteinuria.7 The R431C mutation also disrupts the ANLN-CD2AP interaction through distortion of the CD2-associated protein binding region (Figure 2). This uncoupling further impairs F-actin cytoskeletal dynamics and alters podocyte prosurvival signaling through the PI3K/AKT signaling pathway. Hyperactivation of the PI3K/AKT/mTOR/p70S6K/Rac1 axis enhances Rac1-mediated cytoskeletal remodeling, contributing to distortion of foot process architecture. Additionally, increased Rac1 activity induces activation of the transcriptional regulator Signal Transducer and Activator of Transcription 3 (Figure 4), which promotes podocyte proliferation. In a parallel process, the hyperactivation of mTOR induces ER stress. ER stress promotes the upregulation of calcineurin phosphatase (Cn) activity. Cn dephosphorylates/activates glycogen synthase kinase 3β (GSK-3β), which activates C/EBP homologous protein (CHOP) expression in the context of ER stress. Activation of CHOP induces caspase 3–mediated apoptotic signaling. The loss-of-function effect of the R431C mutation in vivo likely reflects the effect of R431C-induced podocyte apoptosis. R431C-induced apoptosis is attenuated by inhibition of PI3K, AKT, mTOR, GSK-3β, and Cn in vitro. (B) The R431C mutation causes podocyte dysfunction through hyperactivation of PI3K/AKT/mTOR signaling and induces podocyte apoptosis through activation of mTOR-driven ER stress. PP2A, Protein Phosphatase 2A.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants 1K08DK111940-01 (to G.H.) and 5R01DK098135 (to R.A.G.) and the American Society of Nephrology/Amos Medical Faculty Development Program/Robert Wood Johnson Foundation. We acknowledge support from the Animal Models Core (Core A) and Renal Genomics Core (Core B) of the Duke O'Brien Center for Kidney Research supported by NIH NIDDK grant P30DK096493. R.F.S. is supported by grant R01 DK087707 and Veterans Affairs Merit Review BX002984.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017121338/-/DCSupplemental.
References
- 1.Hall G, Gbadegesin RA: Translating genetic findings in hereditary nephrotic syndrome: The missing loops. Am J Physiol Renal Physiol 309: F24–F28, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korbet SM: Treatment of primary FSGS in adults. J Am Soc Nephrol 23: 1769–1776, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Tryggvason K, Patrakka J, Wartiovaara J: Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387–1401, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Huber TB, Hartleben B, Kim J, Schmidts M, Schermer B, Keil A, et al.: Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol 23: 4917–4928, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gigante M, Pontrelli P, Montemurno E, Roca L, Aucella F, Penza R, et al.: CD2AP mutations are associated with sporadic nephrotic syndrome and focal segmental glomerulosclerosis (FSGS). Nephrol Dial Transplant 24: 1858–1864, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Hall G, Gbadegesin RA, Lavin P, Wu G, Liu Y, Oh EC, et al.: A novel missense mutation of Wilms’ Tumor 1 causes autosomal dominant FSGS. J Am Soc Nephrol 26: 831–843, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gbadegesin RA, Hall G, Adeyemo A, Hanke N, Tossidou I, Burchette J, et al.: Mutations in the gene that encodes the F-actin binding protein anillin cause FSGS. J Am Soc Nephrol 25: 1991–2002, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J, Zhang Y: Protein structure and function prediction using I-TASSER. Curr Protoc Bioinforma 52: 5.8.1–5.8.15, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niederriter AR, Davis EE, Golzio C, Oh EC, Tsai IC, Katsanis N: In vivo modeling of the morbid human genome using Danio rerio. J Vis Exp 78: 50338, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D, Chadha GK, Feygin A, Ivanov AI: F-actin binding protein, anillin, regulates integrity of intercellular junctions in human epithelial cells. Cell Mol Life Sci 72: 3185–3200, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang VW, Brieher WM: FSGS3/CD2AP is a barbed-end capping protein that stabilizes actin and strengthens adherens junctions. J Cell Biol 203: 815–833, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall PA, Todd CB, Hyland PL, McDade SS, Grabsch H, Dattani M, et al.: The septin-binding protein anillin is overexpressed in diverse human tumors. Clin Cancer Res 11: 6780–6786, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Olakowski M, Tyszkiewicz T, Jarzab M, Król R, Oczko-Wojciechowska M, Kowalska M, et al.: NBL1 and anillin (ANLN) genes over-expression in pancreatic carcinoma. Folia Histochem Cytobiol 47: 249–255, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Suzuki C, Daigo Y, Ishikawa N, Kato T, Hayama S, Ito T, et al.: ANLN plays a critical role in human lung carcinogenesis through the activation of RHOA and by involvement in the phosphoinositide 3-kinase/AKT pathway. Cancer Res 65: 11314–11325, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Shimizu S, Seki N, Sugimoto T, Horiguchi S, Tanzawa H, Hanazawa T, et al.: Identification of molecular targets in head and neck squamous cell carcinomas based on genome-wide gene expression profiling. Oncol Rep 18: 1489–1497, 2007 [PubMed] [Google Scholar]
- 16.Bódi I, Abraham AA, Kimmel PL: Apoptosis in human immunodeficiency virus-associated nephropathy. Am J Kidney Dis 26: 286–291, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Barisoni L, Kriz W, Mundel P, D’Agati V: The dysregulated podocyte phenotype: A novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 10: 51–61, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Barisoni L, Bruggeman LA, Mundel P, D’Agati VD, Klotman PE: HIV-1 induces renal epithelial dedifferentiation in a transgenic model of HIV-associated nephropathy. Kidney Int 58: 173–181, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Rosner M, Siegel N, Valli A, Fuchs C, Hengstschläger M: mTOR phosphorylated at S2448 binds to raptor and rictor. Amino Acids 38: 223–228, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Soliman GA, Acosta-Jaquez HA, Dunlop EA, Ekim B, Maj NE, Tee AR, et al.: mTOR Ser-2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action. J Biol Chem 285: 7866–7879, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ip CKM, Cheung ANY, Ngan HYS, Wong AST: p70 S6 kinase in the control of actin cytoskeleton dynamics and directed migration of ovarian cancer cells. Oncogene 30: 2420–2432, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Moore KA, Sethi R, Doanes AM, Johnson TM, Pracyk JB, Kirby M, et al.: Rac1 is required for cell proliferation and G2/M progression. Biochem J 326: 17–20, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filić V, Marinović M, Faix J, Weber I: A dual role for Rac1 GTPases in the regulation of cell motility. J Cell Sci 125: 387–398, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Simon AR, Vikis HG, Stewart S, Fanburg BL, Cochran BH, Guan KL: Regulation of STAT3 by direct binding to the Rac1 GTPase. Science 290: 144–147, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Feng X, Lu T-C, Chuang PY, Fang W, Ratnam K, Xiong H, et al.: Reduction of Stat3 activity attenuates HIV-induced kidney injury. J Am Soc Nephrol 20: 2138–2146, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu L, Dai Y, Xu J, Mallipattu S, Kaufman L, Klotman PE, et al.: Deletion of podocyte STAT3 mitigates the entire spectrum of HIV-1-associated nephropathy. AIDS 27: 1091–1098, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou W, Wang Z, Shen N, Pi W, Jiang W, Huang J, et al.: Knockdown of ANLN by lentivirus inhibits cell growth and migration in human breast cancer. Mol Cell Biochem 398: 11–19, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Ross MJ, Martinka S, D’Agati VD, Bruggeman LA: NF-kappaB regulates Fas-mediated apoptosis in HIV-associated nephropathy. J Am Soc Nephrol 16: 2403–2411, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reiling JH, Sabatini DM: Increased mTORC1 signaling UPRegulates stress. Mol Cell 29: 533–535, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Meares GP, Mines MA, Beurel E, Eom T-Y, Song L, Zmijewska AA, et al.: Glycogen synthase kinase-3 regulates endoplasmic reticulum (ER) stress-induced CHOP expression in neuronal cells. Exp Cell Res 317: 1621–1628, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marchand B, Arsenault D, Raymond-Fleury A, Boisvert F-M, Boucher M-J: Glycogen synthase kinase-3 (GSK3) inhibition induces prosurvival autophagic signals in human pancreatic cancer cells. J Biol Chem 290: 5592–5605, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zschiedrich S, Bork T, Liang W, Wanner N, Eulenbruch K, Munder S, et al.: Targeting mTOR signaling can prevent the progression of FSGS. J Am Soc Nephrol 28: 2144–2157, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boratyńska M, Banasik M, Watorek E, Falkiewicz K, Patrzałek D, Szyber P, et al.: Conversion to sirolimus from cyclosporine may induce nephrotic proteinuria and progressive deterioration of renal function in chronic allograft nephropathy patients. Transplant Proc 38: 101–104, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Letavernier E, Bruneval P, Mandet C, Duong Van Huyen J-P, Péraldi M-N, Helal I, et al.: High sirolimus levels may induce focal segmental glomerulosclerosis de novo. Clin J Am Soc Nephrol 2: 326–333, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Marti HP, Frey FJ: Nephrotoxicity of rapamycin: An emerging problem in clinical medicine. Nephrol Dial Transplant 20: 13–15, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Yu H, van Berkel TJC, Biessen EAL: Therapeutic potential of VIVIT, a selective peptide inhibitor of nuclear factor of activated T cells, in cardiovascular disorders. Cardiovasc Drug Rev 25: 175–187, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Ridley AJ: Rho GTPase signalling in cell migration. Curr Opin Cell Biol 36: 103–112, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byrne KM, Monsefi N, Dawson JC, Degasperi A, Bukowski-Wills J-C, Volinsky N, et al.: Bistability in the Rac1, PAK, and RhoA signaling network drives actin cytoskeleton dynamics and cell motility switches. Cell Syst 2: 38–48, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robins R, Baldwin C, Aoudjit L, Côté J-F, Gupta IR, Takano T: Rac1 activation in podocytes induces the spectrum of nephrotic syndrome. Kidney Int 92: 349–364, 2017 [DOI] [PubMed] [Google Scholar]
- 40.Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, et al.: Modification of mineralocorticoid receptor function by Rac1 GTPase: Implication in proteinuric kidney disease. Nat Med 14: 1370–1376, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Guo Y, Tang J, Jiang J, Chen Z: New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin (Shanghai) 46: 629–640, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.