Abstract
BACKGROUND
The prognosis of patients with recurrent World Health Organization (WHO) grade IV malignant glioma is dismal, and there is currently no effective therapy. We conducted a dose-finding and toxicity study in this population of patients, evaluating convection-enhanced, intratumoral delivery of the recombinant nonpathogenic polio–rhinovirus chimera (PVSRIPO). PVSRIPO recognizes the poliovirus receptor CD155, which is widely expressed in neoplastic cells of solid tumors and in major components of the tumor microenvironment.
METHODS
We enrolled consecutive adult patients who had recurrent supratentorial WHO grade IV malignant glioma, confirmed on histopathological testing, with measurable disease (contrast-enhancing tumor of ≥1 cm and ≤5.5 cm in the greatest dimension). The study evaluated seven doses, ranging between 107 and 1010 50% tissue-culture infectious doses (TCID50), first in a dose-escalation phase and then in a dose-expansion phase.
RESULTS
From May 2012 through May 2017, a total of 61 patients were enrolled and received a dose of PVSRIPO. Dose level −1 (5.0×107 TCID50) was identified as the phase 2 dose. One dose-limiting toxic effect was observed; a patient in whom dose level 5 (1010 TCID50) was administered had a grade 4 intracranial hemorrhage immediately after the catheter was removed. To mitigate locoregional inflammation of the infused tumor with prolonged glucocorticoid use, dose level 5 was deescalated to reach the phase 2 dose. In the dose-expansion phase, 19% of the patients had a PVSRIPO-related adverse event of grade 3 or higher. Overall survival among the patients who received PVSRIPO reached a plateau of 21% (95% confidence interval, 11 to 33) at 24 months that was sustained at 36 months.
CONCLUSIONS
Intratumoral infusion of PVSRIPO in patients with recurrent WHO grade IV malignant glioma confirmed the absence of neurovirulent potential. The survival rate among patients who received PVSRIPO immunotherapy was higher at 24 and 36 months than the rate among historical controls.
Despite aggressive therapy, survival after a diagnosis of World Health Organization (WHO) grade IV malignant glioma is usually less than 20 months,1–3 and patients with recurrence usually survive less than 12 months.4 For decades, research efforts have focused on advancing surgical and radiation therapies, chemotherapy, and targeted agents. These strategies are plagued by a lack of consistent improvement in survival, poor distribution in the central nervous system (CNS), acute systemic toxic effects, and long-term toxic effects in the CNS and bone marrow. Since malignant gliomas have bland mutagenomic profiles and lack substantial T-cell infiltration, immune-checkpoint blockade is not a viable approach.5 We examined the therapeutic potential of PVSRIPO, a live attenuated poliovirus type 1 (Sabin) vaccine with its cognate internal ribosome entry site replaced with that of human rhinovirus type 2.6
The foreign internal ribosome entry site of PVSRIPO causes neuronal incompetence — a failure to recruit host ribosomes, translate viral genomes, and propagate in neurons6–9 — and ablates neurovirulence.10 PVSRIPO tropism is determined by CD155,11 a high-affinity ligand for the T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibition motif domains.12 In solid tumors, CD155 is broadly up-regulated on malignant cells13,14 and expressed in antigen-presenting cells. PVSRIPO infection of neoplastic cells in vitro results in lethal cytotoxic effects and activates innate antiviral interferon responses.15 Antigen-presenting cells are natural poliovirus targets after oral infection in vivo.16 PVSRIPO causes chronic, sublethal infection of antigen-presenting cells in vitro, eliciting sustained proinflammatory cytokine responses and activation of the function of antigen-presenting cells, which enables T-cell stimulation in pre-clinical in vitro assays.15 Tumor cytotoxic effects, interferon-dominant activation of antigen-presenting cells, and the profound inflammatory response to poliovirus may counter tumor-induced immunosuppression and instigate antitumor immunity.15
Here we report the results of a clinical trial of intratumoral infusion of PVSRIPO by convection-enhanced delivery to overcome limitations imposed by the blood–brain barrier.17,18 PVSRIPO was granted breakthrough-therapy designation by the Center for Biologics Evaluation and Research of the Food and Drug Administration in May 2016.
METHODS
OBJECTIVES
The primary objective of the clinical trial was to determine the toxicity profile and phase 2 dose for intratumoral convection-enhanced delivery (targeted delivery of therapeutic agents to the CNS with a pressure gradient through a catheter) of PVSRIPO in patients with recurrent WHO grade IV malignant glioma. The secondary objective was to estimate overall survival among these patients, as compared with a historical control group. Exploratory objectives were to define PVSRIPO-induced imaging changes and explore tumor-associated biomarkers that may predict response.
POPULATION OF PATIENTS
We enrolled consecutive adult patients who had a recurrence of a single supratentorial WHO grade IV malignant glioma, confirmed on histopathogical testing, with contrast-enhancing tumor of at least 1 cm and no more than 5.5 cm in the greatest dimension. Patients had to have a Karnofsky performance score of at least 70 (on a scale from 0 to 100, with higher numbers indicating better functional status). Details of the inclusion and exclusion criteria are provided in Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org. A historical control group of patients who had been treated at Duke University Medical Center and who would have met the eligibility criteria for PVSRIPO if the study had been available at the time of their disease progression was identified. A description of the historical control group is provided separately (see the Supplementary Methods Section in the Supplementary Appendix). Furthermore, we compared outcomes with an approved treatment approach, NovoTTF-100A (so-called tumor treatment fields; alternating electrical current applied to the head),19 while taking into account confounding factors.
STUDY DESIGN AND OVERSIGHT
The study was designed by the authors, seven of whom now own equity in the start-up company, Istari Oncology. Patents for PVSRIPO have been licensed from Duke University to Istari Oncology. Before these commercial arrangements were in place, the study had been approved by the institutional review board at Duke University. The study was conducted in accordance with the provisions of the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference on Harmonisation. Because of conflict of interest and intellectual property considerations, an external data and safety monitoring board oversaw the conduct of the study. All the patients provided written informed consent. The authors vouch for the accuracy and completeness of the data and its analysis and confirm that the study was conducted as outlined in the protocol, available at NEJM.org. No one who is not an author contributed to the writing of the manuscript.
For confirmation of viable malignant glioma and for genomic analyses, a stereotactic biopsy was performed before the PVSRIPO infusion; no additional tumor resection was carried out. Immediately after biopsy, a catheter (Vygon PIC-030, Sophysa) was implanted into the tumor.
PVSRIPO was manufactured by the Biopharmaceutical Development Program and SAIC at the National Cancer Institute–Frederick. Patients received an infusion of PVSRIPO by means of convection-enhanced delivery over a period of 6.5 hours, at a rate of 500 μl per hour, with the use of a Medfusion 3500 or 3010 infusion pump (Smiths Medical ASD) and infusion catheter and infusion tubing (PIT 400, Sophysa). The volume of the delivered inoculum was 3.25 ml.
A dose-escalation trial of PVSRIPO was planned. One patient each was to be enrolled at dose level 1 (108 50% tissue-culture infectious doses [TCID50]), dose level 2 (3.3×108 TCID50), dose level 3 (109 TCID50), and dose level 4 (3.3×109 TCID50) and 21 patients at dose level 5 (1010 TCID50) in the dose-escalation phase. During the course of the trial, in the dose-expansion phase, the dose was gradually reduced to dose level 2 (3.3×108 TCID50), then to dose level −1 (5.0×107 TCID50), and finally to dose level −2 (107 TCID50).
TOXICITY EVALUATION
Patients were continuously monitored for toxic effects during the study. Adverse events were categorized and graded according to the Common Toxicity Criteria for Adverse Events, version 4.03, of the National Cancer Institute. Dose-limiting toxic effects were defined as any toxic effects of grade 3 or 4 that were not reversible within 2 weeks or any death that was considered by the investigators to be related to treatment. Any serious autoimmune toxic effects of grade 2 or higher, particularly those affecting vital systems (e.g., cardiac, renal, or CNS), were considered to be dose-limiting toxic effects if they occurred within 2 weeks after PVSRIPO infusion. Events that were not considered by the investigators to be dose-limiting toxic effects are listed separately (see the Supplementary Methods Section in the Supplementary Appendix).
IMAGING ANALYSIS
Magnetic resonance imaging (MRI) scans were obtained at screening, within 4 hours after the completion of the infusion, at 4 and 8 weeks after the infusion and then every 8 weeks for 1 year, and afterward at an interval selected by the treating physician. Imaging techniques and the data obtained are described in the Supplementary Methods Section in the Supplementary Appendix.
STATISTICAL ANALYSIS
In describing the adverse events that occurred in patients who received PVSRIPO, the highest-grade event of each type of event was summarized. Overall survival was defined as the time from PVSRIPO infusion until death. For patients who were alive at the time of analysis, survival time was censored at the date of last follow-up. For the patients who received PVSRIPO and the historical controls, the Kaplan–Meier estimator was used to describe the distribution of survival time. SAS software, version 9.4 (SAS Institute), was used for all the analyses.
Additional descriptions of the statistical design of the study are provided in the Supplementary Methods in the Supplementary Appendix. It is too early to evaluate the statistical hypothesis of survival at 24 months; the analyses presented in this article are descriptive and do not include formal statistical tests.
RESULTS
CHARACTERISTICS OF THE PATIENTS AND CONTROLS
From May 2012 through May 2017, a total of 61 consecutive patients with recurrent WHO grade IV malignant glioma were treated during the study. The demographic and clinical characteristics of the patients who received PVSRIPO and the historical control group differed with respect to extent of resection at diagnosis and previous treatment failure with bevacizumab (Table 1). A total of 41% of the patients who received PVSRIPO and 38% of the historical controls were women.
Table 1.
Demographic and Clinical Characteristics of the Patients and Historical Controls.*
| Characteristic | Patients Who Received PVSRIPO (N = 61) | Historical Controls (N = 104) |
|---|---|---|
| Sex — no. (%) | ||
| Female | 25 (41) | 39 (38) |
| Male | 36 (59) | 65 (62) |
| Median age at PVSRIPO treatment or eligibility (range) — yr | 55 (20–75) | 55 (23–77) |
| Karnofsky performance status — no. (%)† | ||
| 100 | 2 (3) | 8 (8) |
| 90 | 42 (69) | 64 (62) |
| 80 | 16 (26) | 30 (29) |
| 70 | 1 (2) | 2 (2) |
| Extent of maximal resection at diagnosis — no. (%)‡ | ||
| Gross total resection | 47 (77) | 69 (66) |
| Subtotal resection | 14 (23) | 25 (24) |
| Biopsy | 0 | 10 (10) |
| No. of previous progressions — no. (%) | ||
| 1 | 45 (74) | 85 (82) |
| 2 | 12 (20) | 11 (11) |
| 3 | 2 (3) | 8 (8) |
| 4 | 2 (3) | 0 |
| Previous treatment failure with bevacizumab — no. (%) | ||
| No | 47 (77) | 61 (59) |
| Yes | 14 (23) | 43 (41) |
| IDH1 R132 status at diagnosis — no. (%)‡ | ||
| Nonmutant | 45 (74) | 23 (22) |
| Mutant | 7 (11) | 4 (4) |
| Unknown | 9 (15) | 77 (74) |
| TERT status at diagnosis — no. (%)‡ | ||
| Nonmutated | 15 (25) | 0 |
| Mutated | 39 (64) | 0 |
| Unknown | 7 (11) | 104 (100) |
| MGMT gene-promoter methylation status — no. (%) | ||
| At diagnosis‡ | ||
| Methylated | 17 (28) | 18 (17) |
| Unmethylated | 24 (39) | 17 (16) |
| Unknown | 20 (33) | 69 (66) |
| At PVSRIPO treatment | ||
| Methylated | 14 (23) | NA |
| Unmethylated | 36 (59) | NA |
| Unknown | 11 (18) | NA |
The demographic and clinical characteristics of the group of patients who received the recombinant nonpathogenic polio–rhinovirus chimera (PVSRIPO) and the historical control group differed with respect to extent of resection at diagnosis (P = 0.02) and previous treatment failure with bevacizumab (P = 0.03). Percentages may not total 100 because of rounding. MGMT denotes methylated O6-methylguanine–DNA methyltransferase, NA not available, and TERT the gene encoding telomerase reverse transcriptase.
Karnofsky performance status is assessed on a scale of 0 to 100, with higher numbers indicating better functional status.
This assessment was made at the diagnosis of malignant glioma of World Health Organization grade IV.
DOSE-LIMITING TOXIC EFFECTS AND ADVERSE EVENTS
In the dose-escalation phase of the study, one patient each received dose level 1, 2, and 3; two patients received dose level 4; and four received dose level 5. The adverse events according to dose level in the dose-escalation phase are reported in Table 2. In the dose-expansion phase of the study, dose level 2 included 6 patients, dose level −1 included 31 patients, and dose level −2 included 15 patients (Table 3).
Table 2.
Adverse Events Attributable to PVSRIPO, According to Dose Level, in the 9 Patients in the Dose-Escalation Phase.*
| Event, According to Body System and Grade | PVSRIPO Dose Level | ||||
|---|---|---|---|---|---|
| Level 1 (N = 1) | Level 2 (N = 1) | Level 3 (N = 1) | Level 4 (N = 2) | Level 5 (N = 4) | |
| number of patients | |||||
| General disorder or administration-site condition | |||||
|
| |||||
| Fatigue, grade 1 | — | — | — | 1 | 1 |
|
| |||||
| Gait disturbance, grade 1 | — | — | 1 | — | — |
|
| |||||
| Nervous system disorder | |||||
|
| |||||
| Cognitive disturbance, grade 2 | — | — | 1 | — | — |
|
| |||||
| Dysphasia | |||||
|
| |||||
| Grade 1 | — | — | — | — | 1 |
|
| |||||
| Grade 2 | — | — | 1 | — | — |
|
| |||||
| Headache, grade 1 | 1 | — | — | 1 | — |
|
| |||||
| Paresthesia | |||||
|
| |||||
| Grade 1 | — | 1 | — | — | — |
|
| |||||
| Grade 2 | — | — | — | 1 | 1 |
|
| |||||
| Pyramidal tract syndrome† | |||||
|
| |||||
| Grade 2 | — | — | — | — | 1 |
|
| |||||
| Grade 3 | — | — | — | 1 | 1 |
|
| |||||
| Seizure | |||||
|
| |||||
| Grade 1 | — | — | — | — | 3 |
|
| |||||
| Grade 2 | — | — | 1 | — | — |
|
| |||||
| Psychiatric disorder: confusion, grade 1 | — | — | — | — | 1 |
|
| |||||
| Total no. of patients with an event | 1 | 1 | 1 | 1 | 3 |
In the dose-escalation phase, dose level 1 was 108 50% tissue-culture infectious doses (TCID50), dose level 2 was 3.3×108 TCID50, dose level 3 was 109 TCID50, dose level 4 was 3.3×109 TCID50, and dose level 5 was 1010 TCID50.
Pyramidal tract syndrome was defined as hemiparesis.
Table 3.
Adverse Events Attributable to PVSRIPO, According to Grade, in the 52 Patients in the Dose-Expansion Phase.*
| Body System and Adverse Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| number of patients (percent) | |||||
| Eye disorder | |||||
|
| |||||
| Blurred vision | 1 (2) | — | — | — | — |
|
| |||||
| Diplopia | 1 (2) | — | — | — | — |
|
| |||||
| Focusing difficulty | 1 (2) | — | — | — | — |
|
| |||||
| Visual field cut or hemianopia | 8 (15) | 2 (4) | — | — | — |
|
| |||||
| Gastrointestinal disorder | |||||
|
| |||||
| Nausea | 5 (10) | — | — | — | — |
|
| |||||
| Vomiting | 3 (6) | — | — | — | — |
|
| |||||
| General disorder or administration-site condition | |||||
|
| |||||
| Fatigue | 4 (8) | 2 (4) | — | — | — |
|
| |||||
| Gait disturbance | 4 (8) | — | 1 (2) | — | — |
|
| |||||
| Nervous system disorder | |||||
|
| |||||
| Cerebral edema | — | — | — | 1 (2) | — |
|
| |||||
| Cognitive disturbance | 12 (23) | 1 (2) | — | — | — |
|
| |||||
| Dysphasia | 7 (13) | 8 (15) | — | — | — |
|
| |||||
| Dystonia | — | — | 1 (2) | — | — |
|
| |||||
| Facial muscle weakness | 1 (2) | — | — | — | — |
|
| |||||
| Headache | 12 (23) | 14 (27) | 1 (2) | — | — |
|
| |||||
| Intracranial hemorrhage | 1 (2) | — | — | — | — |
|
| |||||
| Paresthesia | 7 (13) | — | — | — | — |
|
| |||||
| Pyramidal tract syndrome† | 14 (27) | 8 (15) | 4 (8) | — | — |
|
| |||||
| Seizure | 19 (37) | 2 (4) | 1 (2) | — | 1 (2) |
|
| |||||
| Psychiatric disorder | |||||
|
| |||||
| Confusion | 3 (6) | 5 (10) | 1 (2) | — | — |
|
| |||||
| Delusions | — | — | 1 (2) | — | — |
|
| |||||
| Hallucinations | 1 (2) | — | — | — | — |
|
| |||||
| Renal and urinary disorder: urinary incontinence | 1 (2) | — | — | — | — |
|
| |||||
| Vascular disorder: hypertension | — | 1 (2) | — | — | — |
|
| |||||
| Total no. of patients with an event‡ | 14 (27) | 23 (44) | 9 (17) | 0 | 1 (2) |
In the dose-expansion phase, 6 patients received dose level 2 (3.3×108 TCID50), 31 received dose level −1 (5.0×107 TCID50), and 15 received dose level −2 (107 TCID50).
Pyramidal tract syndrome was defined as hemiparesis.
Each patient is included only once in the total row under the grade level that represents their highest-grade event.
One dose-limiting toxic effect, a grade 4 intracranial hemorrhage immediately after catheter removal, occurred in the last patient who was treated at dose level 5 (Table S3 in the Supplementary Appendix). After surgical evacuation of the hemorrhage, the patient had right hemiparesis and aphasia. Histopathological analysis of tissue that was obtained with debulking of the hemorrhage did not reveal evidence of vascular alterations, viral activity, or inflammatory events related to PVSRIPO infusion, which had been completed 5 hours earlier. The patient remained alive more than 57.5 months after PVSRIPO infusion (see below) with moderate residual right hemiparesis and aphasia. After this event, one additional patient was treated at dose level 4 before the study shifted to the dose-expansion phase.
Tissue from a resection (obtained at first diagnosis or at the time of previous disease progression) in all 43 patients who were enrolled in this study and for whom resection specimens were available stained positive on CD155 immunohistochemical testing (Fig. S1 in the Supplementary Appendix). PVSRIPO infusion was not associated with evidence of encephalomyelitis, poliomyelitis, meningitis, or systemic autoimmune reactions in any patient at any dose level. Positive antipoliovirus (type 1)–neutralizing antibody titers (>1:8) increased after boost immunization with IPOL, an inactivated polio vaccine, in all the patients (Fig. S2 in the Supplementary Appendix). Tests of infectious PVSRIPO shedding in stool were negative in all the patients, in line with Investigational New Drug–directed shedding studies involving nonhuman primates.10 Two deaths occurred during the trial. One patient (who received dose level −2) had a seizure related to cerebral edema that was probably related to autopsy-confirmed tumor progression at 4.8 months after the PVSRIPO infusion. Since PVSRIPO causation could not be ruled out at the time of the events, the events were attributed to PVSRIPO as grade 4 cerebral edema and grade 5 seizure (Table 3). Another patient (who received dose level −1) died 10.5 months after the PVSRIPO infusion from complications of an intracranial hemorrhage while receiving anticoagulation and bevacizumab; this event was attributed to bevacizumab (Table S2 in the Supplementary Appendix).
Among all the patients who received PVSRIPO, 69% had a grade 1 or 2 event that was attributed to PVSRIPO as their most severe adverse event (Tables 2 and 3). Adverse events that affected more than 20% of the patients in the dose-expansion phase included headache (in 52% of the patients), the pyramidal tract syndrome (hemiparesis) (in 50%), seizure (in 45%), dysphasia (in 28%), and cognitive disturbance (in 25%) (Table 3). Less common adverse events that were attributed to PVSRIPO (occurring in 10 to 20% of the patients in the dose-expansion phase) included hemianopia (in 19%), confusion (in 18%), paresthesia (in 13%), fatigue (in 12%), nausea (in 10%), and gait disturbance (in 10%). In the dose-expansion phase, 19% of the patients had a PVSRIPO-related adverse event of grade 3 or higher. Adverse events that were considered by the investigators to be related to PVSRIPO were dependent on the specific area of the cerebrum that was exposed to the localized inflammatory reaction associated with PVSRIPO. A summary of all the adverse events in the dose-escalation and the dose-expansion phases of the study, regardless of attribution, is provided in Table S3 in the Supplementary Appendix.
TREATMENT OUTCOMES
The median follow-up of the patients who received PVSRIPO was 27.6 months (95% confidence interval [CI], 20.5 to 41.1). All but 1 patient in the historical control group are known to have died (the remaining patient was lost to follow-up). The median overall survival among all 61 patients who received PVSRIPO was 12.5 months (95% CI, 9.9 to 15.2), which was longer than the 11.3 months (95% CI, 9.8 to 12.5) in the historical control group and the 6.6 months in the Novo-TTF-100A treatment group.19
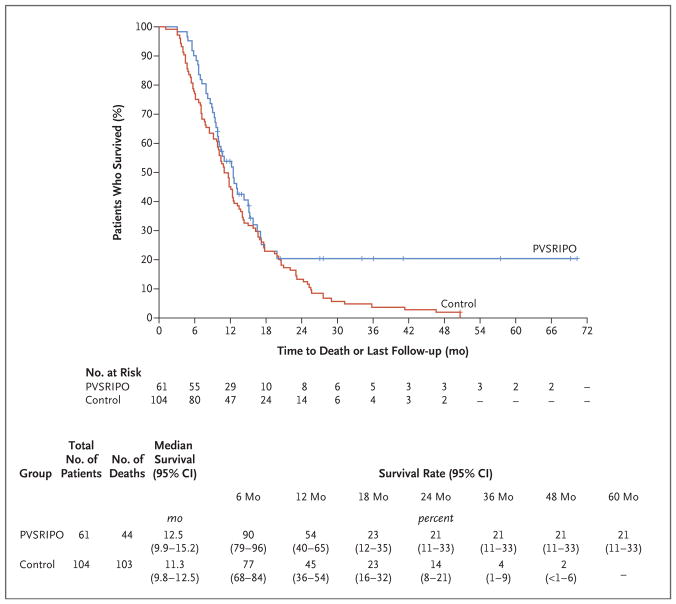
However, overall survival among the patients who received PVSRIPO reached a plateau beginning at 24 months, with the overall survival rate being 21% (95% CI, 11 to 33) at 24 months and 36 months, whereas overall survival in the historical control group continued to decline, with overall survival rates of 14% (95% CI, 8 to 21) at 24 months and 4% (95% CI, 1 to 9) at 36 months (Fig. 1). A sensitivity analysis evaluating the effect of including patients in the historical control group who only underwent biopsy revealed that their inclusion had no effect on survival estimates (Table 1, and Fig. S3 in the Supplementary Appendix). In comparison, the use of NovoTTF-100A in patients with recurrent glioblastoma led to an overall survival rate of 8% at 24 months and of 3% at 36 months. It is too early to evaluate our statistical hypothesis of survival at 24 months, because only 20 of the 31 patients at dose level −1 were treated with PVSRIPO more than 24 months before the data-cutoff date of March 20, 2018.
Figure 1. Overall Survival among Patients Who Received PVSRIPO and Historical Controls.
Tick marks indicate censored data. PVSRIPO denotes recombinant nonpathogenic polio–rhinovirus chimera.
Because patients who have tumors with the IDH1 R132 mutation are thought to have a survival advantage, we examined whether long-term survivors who have tumors with the IDH1 R132 mutation disproportionately contributed to the overall survival in the entire group. Survival analyses involving only the patients who received PVSRIPO whose tumors were confirmed to have nonmutant IDH1 R132 (Table 1) revealed a median overall survival of 12.5 months among the 45 patients with nonmutant IDH1 R132 and 12.5 months among all 61 patients who received PVSRIPO. Moreover, the overall survival rate at 24 months and 36 months was 21% among the 45 patients with nonmutant IDH1 R132 and among all 61 patients who received PVSRIPO. These findings are consistent with reports that IDH1 R132 status has no bearing on survival among patients with recurrent glioblastoma.20
A median cross-sectional area of tumor of 873 mm2, measured at the largest dimension, was observed among all the patients in the study. Figure S4 in the Supplementary Appendix shows the results regarding survival according to the cross-sectional area of tumor (<873 mm2 vs. ≥873 mm2). Results regarding survival according to Karnofsky performance status are presented in Figure S5 in the Supplementary Appendix.
IMAGING
As with other types of immunotherapy, complete and partial responses to PVSRIPO were easily recognizable, but assessment of tumor progression was difficult. Initially, most patients had increased fluid-attenuated inversion recovery (FLAIR; a method to prevent cerebrospinal-fluid suppression of lesion detection) signal abnormalities on MRI that receded over time. From the standpoint of disease shown on contrast enhancement, an initial increase in lesion size that was associated with polycystic degradation (“soap bubble” appearance) was observed in all the patients (Figs. S8 and S10 in the Supplementary Appendix). This finding frequently entailed enhancing-disease extension into previously nonenhancing infiltrative disease, followed by tumor contraction (Fig. S8 in the Supplementary Appendix). Depending on the size of the tumor, imaging changes that were suggestive of inflammatory tissue responses (an increase in the extent of FLAIR abnormalities and an increase in the size of enhancing disease with a soap-bubble appearance) were evident up to 12 months after the administration of PVSRIPO.
During the dose-escalation phase, imaging changes that were suggestive of localized inflammation were managed with glucocorticoids if they were associated with neurologic symptoms. Given the protracted course of such peritumoral inflammation before tumor contraction, symptomatic patients had to continue taking glucocorticoids for extended periods, which exposed them to substantial side effects. To mitigate peritumoral inflammation, the PVSRIPO dose was de-escalated gradually to dose level 2 and then to dose levels −1 and −2. For patients with neurologic symptoms, glucocorticoids were limited to a maximum of 4 mg of dexamethasone daily, and a short course of bevacizumab, at a dose of 7.5 mg per kilogram administered intravenously every 3 weeks, was empirically initiated and continued for as long as needed to control the symptoms from the localized inflammatory reaction.
As of March 20, 2018, eight patients had a durable radiographic response of the treated tumor. Of these eight patients, two patients had a complete response and remained alive at more than 70.4 months and more than 15.1 months after the PVSRIPO infusion (Fig. 2, and Fig. S7 in the Supplementary Appendix). Three patients had stable to partial radiographic responses for 60 months, 34 months, and 26 months each (Figs. S8 through S10 in the Supplementary Appendix). Three of the eight patients received a short course of bevacizumab at a dose of 7.5 mg per kilogram, administered intravenously every 3 weeks, and were without signs of active tumor and without use of additional treatment at more than 34.1 months, more than 27.1 months, and more than 15.4 months after the PVSRIPO infusion. FLAIR images corresponding to all the MRIs shown in Figures 2 and 3 and in Figures S7 through S10 are provided in Figure S11 in the Supplementary Appendix.
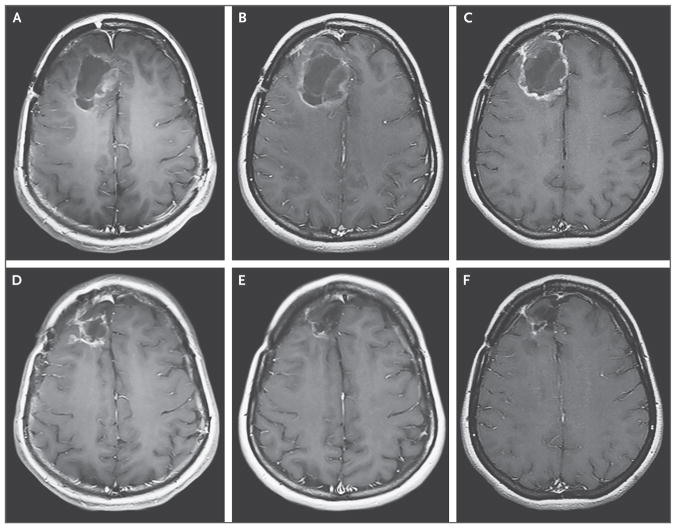
Figure 2. Axial T1-Weighted MRI, Obtained with the Use of Contrast Material, of the First Patient Treated in the Study, at Dose Level 1.
Panel A shows the baseline MRI of this patient, who received dose level 1 (108 50% tissue-culture infectious doses [TCID50]). Panel B shows the expansion of tumor 2 months after the PVSRIPO infusion. Panel C shows the initial tumor contraction 6 months after the infusion, and Panels D and E show the results at 12 months and 24 months, respectively. Panel F shows the contraction of the resection cavity and the treated tumor 58 months after the infusion.
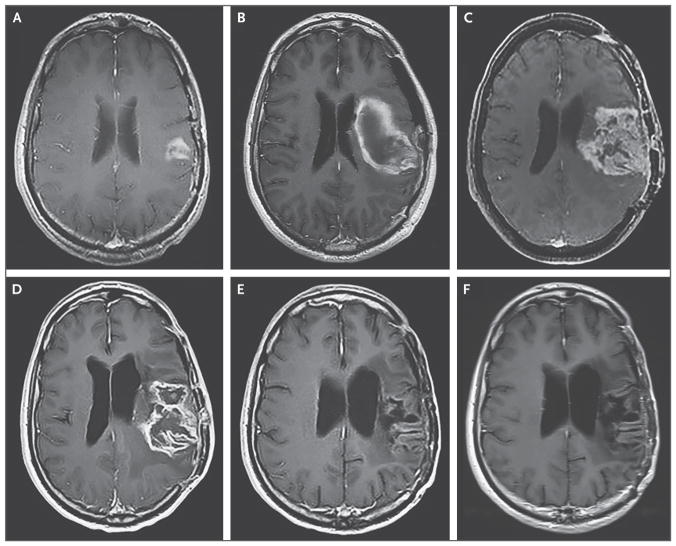
Figure 3. Axial T1-Weighted MRI, Obtained with the Use of Contrast Material, of the Eighth Patient Treated in the Study, at Dose Level 5.
Panel A shows the baseline MRI of this patient, who received dose level 5 (1010 TCID50). Panel B shows the image obtained after surgery for evacuation of intracranial hemorrhage after the removal of the infusion catheter. Panel C shows the image obtained at the time of disease progression, 7 months after the PVSRIPO infusion. Panel D shows the image obtained after the first cycle of lomustine, and Panel E the image obtained at the completion of nine cycles of lomustine. Panel F shows the results 1 year after the discontinuation of lomustine and 32 months after the PVSRIPO infusion.
TREATMENTS AFTER PVSRIPO
As of March 20, 2018, a total of 4 patients had undergone reoperation and 34 patients had received bevacizumab (7.5 mg per kilogram every 3 weeks) after the administration of PVSRIPO to mitigate peritumoral inflammation. This practice was initiated only in the dose-expansion phase. Patients were considered to have discontinued the study when tumor progression was observed on histologic testing or when the treating physician thought that it was in the patient’s best interest to escalate therapy beyond bevacizumab at a dose of 7.5 mg per kilogram every 3 weeks. For details regarding the interventions that were received by patients after the PVSRIPO infusion, see Table S4 in the Supplementary Appendix. Information regarding survival among patients who received bevacizumab during the study, as compared with those who did not, and on survival among patients who received any bevacizumab after PVSRIPO infusion, as compared with those who did not, is provided in Figure S6 in the Supplementary Appendix.
A relevant finding was first observed in the patient who had a dose-limiting toxic effect at dose level 5 (hemorrhage on catheter removal; see above) (Fig. 3A and 3B). At 7 months after the PVSRIPO infusion, the patient had radiographic and histopathological evidence of recurrence of a WHO grade IV malignant glioma (Fig. 3C). Lomustine therapy was then initiated. After the first cycle of lomustine, cystic degeneration of the lesion was observed (Fig. 3D). At the completion of more than 12 months of lomustine therapy, the patient had a complete response and remained disease-free for an additional 20 months and remained alive more than 57.5 months after the hemorrhage that occurred after the PVSRIPO infusion. After this observation, 37 patients with radiographic evidence of possible disease progression were treated with temozolomide, lomustine, or other agents. At least 11 patients had radiographic signs of cystic tumor degradation and a rapid decline in tumor volume. This phenomenon typically occurred after the first cycle of chemotherapy (Table S4 in the Supplementary Appendix).
DISCUSSION
We report the results of a phase 1 clinical trial with dose expansion of intratumoral delivery of PVSRIPO in patients with recurrent WHO grade IV malignant glioma. A total of 61 patients underwent successful infusion of PVSRIPO, and there was no evidence of viral neuropathogenicity or virus shedding. At the time of this writing, the survival rate at 24 months and 36 months was 21% (95% CI, 11 to 33), with patients remaining alive more than 70 months, more than 69 months, and more than 57 months after the PVSRIPO infusion.
In the dose-expansion phase, the protocol was revised to limit the use of glucocorticoids at the time of eligibility and during the study. This decision was made in order to protect potential mechanisms of PVSRIPO immunotherapy15 and to avoid side effects of prolonged glucocorticoid use. PVSRIPO did not induce systemic autoimmune reactions; rather, patients had neurologic symptoms owing to what we think was peritumoral inflammation related to the location of the infused tumor. To mitigate this situation, we used bevacizumab to reverse edema rapidly and to treat symptoms.21,22 The role of bevacizumab in promoting immunotherapy23 mechanistically complements preclinical evidence of PVSRIPO-instigated antitumor immunity.15 We therefore amended the protocol to allow the use of bevacizumab at a dose of 7.5 mg per kilogram of body weight, administered intravenously every 3 weeks, in patients in whom intervention in excess of 4 mg daily of dexamethasone was considered to be useful. Typically, bevacizumab at a dose of 10 mg per kilogram, administered intravenously every 2 weeks, or a dose of 15 mg per kilogram, administered intravenously every 3 weeks, is used in the United States to treat patients with recurrent WHO grade IV malignant glioma. Because the goal of the administration of bevacizumab was to control symptoms of locoregional inflammation and not to treat the tumor itself, we decided to use a lower dose that has been shown to be effective in managing symptoms of radiation necrosis24 and limiting the incidence of bevacizumab-related complications.
Clinical and radiographic responses were observed after the first cycle of chemotherapy administered for tumor progression that occurred after the PVSRIPO infusion (Fig. 3). Early preliminary tests of immune-cell frequencies in the periphery suggest that there is a reduction of immunosuppressive regulatory T cells with the onset of homeostatic reconstitution of effector T cells at the nadir (4 weeks after the administration of lomustine). Potential beneficial effects stemming from such events are probably lost with standard multicycle chemotherapy, because sustained lymphodepletion dampens immune capacity. These data were the rationale for the development of our ongoing phase 2, randomized trial of PVSRIPO alone or in combination with single-cycle lomustine in patients with recurrent WHO grade IV malignant glioma (ClinicalTrials.gov number, NCT02986178).
Tumor, autopsy, and immune-monitoring specimens were obtained from patients during the study. Preliminary results from 14 brain specimens obtained during autopsy of patients who received PVSRIPO showed the presence of WHO grade IV malignant glioma in all the patients.
In this clinical trial, we identified a safe dose of PVSRIPO when it was delivered directly into intracranial tumors. Of the 35 patients with recurrent WHO grade IV malignant glioma who were treated more than 24 months before March 20, 2018, a total of 8 patients remained alive as of that cutoff date. Two patients were alive more than 69 months after the PVSRIPO infusion. Further investigations are warranted.
Supplementary Material
Acknowledgments
Funded by the Brain Tumor Research Charity and others; ClinicalTrials.gov number, NCT01491893.
Supported by grants from the Brain Tumor Research Charity, the Tisch family through the Jewish Communal Fund, Circle of Service Foundation, Uncle Kory Foundation, the Department of Defense (W81XWH-16-1-0354), and the National Institutes of Health (R35CA197264, P01CA154291, P50CA190991, R01CA124756, and R01NS099463), and by Angels among Us and the Asness family (both in support of Brain Tumor Research).
We thank Michael Brown, Susan Boulton, Chevelle Bullock, Christina Cone, Elena Dobrikova, Kristen Foss, Rachel Hesler, Jenny Jackman, Eric Lipp, Zachary McKay, Elizabeth Miller, and Stevie Threatt; the members of the data and safety monitoring board (John M. Cunningham [University of Chicago], A. William Blackstock, Jr. [Wake Forest University], Michael Vogelbaum [Cleveland Clinic], Kenneth R. Hess [M.D. Anderson Cancer Center], Jeremy Rich [University of California San Diego], and Ryan J. Sullivan [Massachusetts General Hospital]); and the participants and their families, who made possible the successful conduct of the trial.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy–temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–22. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314:2535–43. doi: 10.1001/jama.2015.16669. [DOI] [PubMed] [Google Scholar]
- 4.Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15:943–53. doi: 10.1016/S1470-2045(14)70314-6. [DOI] [PubMed] [Google Scholar]
- 5.Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gromeier M, Alexander L, Wimmer E. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc Natl Acad Sci U S A. 1996;93:2370–5. doi: 10.1073/pnas.93.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, Wimmer E. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc Natl Acad Sci U S A. 2000;97:6803–8. doi: 10.1073/pnas.97.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merrill MK, Dobrikova EY, Gromeier M. Cell-type-specific repression of internal ribosome entry site activity by double-stranded RNA-binding protein 76. J Virol. 2006;80:3147–56. doi: 10.1128/JVI.80.7.3147-3156.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merrill MK, Gromeier M. The double-stranded RNA binding protein 76:NF45 heterodimer inhibits translation initiation at the rhinovirus type 2 internal ribosome entry site. J Virol. 2006;80:6936–42. doi: 10.1128/JVI.00243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobrikova EY, Goetz C, Walters RW, et al. Attenuation of neurovirulence, bio-distribution, and shedding of a poliovirus:rhinovirus chimera after intrathalamic inoculation in Macaca fascicularis. J Virol. 2012;86:2750–9. doi: 10.1128/JVI.06427-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendelsohn CL, Wimmer E, Racaniello VR. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56:855–65. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- 12.Stengel KF, Harden-Bowles K, Yu X, et al. Structure of TIGIT immunoreceptor bound to poliovirus receptor reveals a cell-cell adhesion and signaling mechanism that requires cis-trans receptor clustering. Proc Natl Acad Sci U S A. 2012;109:5399–404. doi: 10.1073/pnas.1120606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandramohan V, Bryant JD, Piao H, et al. Validation of an immunohistochemistry assay for detection of CD155, the poliovirus receptor, in malignant gliomas. Arch Pathol Lab Med. 2017;141:1697–704. doi: 10.5858/arpa.2016-0580-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol. 2008;9:603–15. doi: 10.1038/nrm2457. [DOI] [PubMed] [Google Scholar]
- 15.Brown MC, Holl EK, Boczkowski D, et al. Cancer immunotherapy with recombinant poliovirus induces IFN-dominant activation of dendritic cells and tumor antigen-specific CTLs. Sci Transl Med. 2017;9(408):eean4220. doi: 10.1126/scitranslmed.aan4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen L, Chen CY, Huang D, et al. Pathogenic events in a nonhuman primate model of oral poliovirus infection leading to paralytic poliomyelitis. J Virol. 2017;91(14):e02310–16. doi: 10.1128/JVI.02310-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sampson JH, Brady M, Raghavan R, et al. Colocalization of gadolinium-diethylene triamine pentaacetic acid with high-molecular-weight molecules after intracerebral convection-enhanced delivery in humans. Neurosurgery. 2011;69:668–76. doi: 10.1227/NEU.0b013e3182181ba8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sampson JH, Brady ML, Petry NA, et al. Intracerebral infusate distribution by convection-enhanced delivery in humans with malignant gliomas: descriptive effects of target anatomy and catheter positioning. Neurosurgery. 2007;60(Suppl 1):ONS89-98. doi: 10.1227/01.NEU.0000249256.09289.5F. [DOI] [PubMed] [Google Scholar]
- 19.Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48:2192–202. doi: 10.1016/j.ejca.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Mandel JJ, Cachia D, Liu D, et al. Impact of IDH1 mutation status on outcome in clinical trials for recurrent glioblastoma. J Neurooncol. 2016;129:147–54. doi: 10.1007/s11060-016-2157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vredenburgh JJ, Cloughesy T, Samant M, et al. Corticosteroid use in patients with glioblastoma at first or second relapse treated with bevacizumab in the BRAIN study. Oncologist. 2010;15:1329–34. doi: 10.1634/theoncologist.2010-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wefel JS, Cloughesy T, Zazzali JL, et al. Neurocognitive function in patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13:660–8. doi: 10.1093/neuonc/nor024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79:1487–95. doi: 10.1016/j.ijrobp.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.