Abstract
Purpose
Radiation therapy (RT) for gastric mucosa–associated lymphoid tissue (MALT) lymphoma is challenging because of variation in the stomach’s position, size, and shape. We investigated the interfractional changes in stomach location, consequent dosimetric effects, and impact of daily computed tomography image guidance RT (CT-IGRT).
Methods and materials
Twelve patients treated for gastric MALT lymphoma with intensity modulated radiation therapy, using a breath-hold technique and restriction of oral intake, were studied retrospectively. The planning target volume (PTV) comprised a 0.5 to 1.0 cm expansion of the stomach. The prescription dose was 30 Gy in 15 to 20 fractions. CT-IGRT was performed daily using CT-on-Rails. Dosimetry was calculated on 229 daily CT images after bony versus CT-based soft tissue alignment, and doses delivered to the target and adjacent structures were compared with the treatment plan. Target coverage was expressed as the percent of the clinical target volume (CTV) and PTV receiving ≥95% of the prescribed dose (V95%).
Results
The average change in stomach volume was −12.4% (range, −47.6% to 38.6%). The average shift required for target coverage was 1.0 cm (maximum, 2.2 cm). With CT-based alignment to the stomach, the average V95% was 98.5% for CTV and 94.9% for PTV; with bony alignment, these values were 94.5% and 90.4%, respectively (P < .01 for CTV and PTV). With bony alignment, the PTV V95% was ≤90% in 4 patients (33%) over the course of treatment and was as low as 72.5% for 1 fraction. The kidney position varied with respect to the stomach and bony anatomy. Consequently, the dose to the left kidney was higher based on daily CT scans than on planning scans. Dose to other organs at risk did not vary significantly.
Conclusions
Substantial interfractional variation in stomach volume was observed, despite treatment with breath-hold and restriction of oral intake. Daily CT-IGRT improved target coverage, enabling excellent coverage despite the use of small PTV margins.
Introduction
Target motion is an important concern when radiation therapy (RT) is used to treat cancers involving mobile intra-abdominal organs.1–5 In the case of gastric mucosa–associated lymphoid tissue (MALT) lymphoma, challenges include (1) respiratory-induced movement of the stomach by up to 3 cm6,7; (2) variation in the shape, size, and location of the stomach because of intrinsic organ motion4,6–8; and (3) differences in stomach filling and distension. These uncertainties can greatly affect target localization and the accuracy of treatment delivery.
Significant effort has been devoted to account for respiratory-induced intra-abdominal organ motion.9–12 One approach is to add an adequate margin to the clinical target volume (CTV), so the involved site is encompassed within the treatment volume throughout the normal respiratory cycle8,13; however, the resulting large planning target volume (PTV) may cause the delivery of excess dose to surrounding normal structures.3,14 Techniques that reduce the effect of organ motion during RT include the use of external respiratory restraining devices,9 gating,15–17 and breath-hold.18 For example, Wagman et al found that the PTV margin could be reduced from 2 to 1 cm if patients held their breath, rather than breathing freely, during RT for liver tumors.18 The stomach, like the liver, is an upper abdominal organ prone to respiratory-induced motion, suggesting that breath-hold should provide a similar benefit; however, the stomach is also prone to changes in size, shape, and positioning, unrelated to respiration, which complicates the use smaller PTV margins, even when a breath-hold technique is employed.
With image-guided RT (IGRT), computed tomography (CT) imaging performed immediately before RT ensures accurate target localization.3,19 In the treatment of gastric MALT lymphoma, CT-IGRT provides information about the shape and position of the stomach before each fraction, so patient positioning can be adjusted appropriately. Cone beam CT (CBCT) is the most widely available type of CT-IGRT unit; however, CT-on-Rails (CTOR) produces higher quality images for intra-abdominal soft tissue organs.20
At our institution, we treat gastric MALT lymphoma with intensity modulated RT (IMRT), using (1) a breath-hold technique, (2) daily CTOR, (3) restriction of oral intake before RT, and (4) a nongaseous diet throughout the course of RT. The aims of this study were to assess the daily variation in stomach volume with this approach, to analyze the consequent dosimetric effects, and to evaluate the effect of CT-IGRT on the accuracy of RT delivery.
Materials and methods
Patients
Twelve patients treated for gastric MALT lymphoma with definitive RT at our institution were studied retrospectively. All patients were advised to follow a nongaseous diet throughout the duration of treatment. Patients were also instructed to take nothing by mouth for at least 4 hours before simulation and daily treatment.
RT planning
Patients performed an inspiration breath-hold using a respiratory monitoring device and feedback system (Varian Real-time Position Management System, Palo Alto, CA). During CT simulation, 3 breath-hold CT scans were obtained for each patient to confirm consistency of the breath-hold. Planning CT images were acquired with 2.5-mm slice thickness. No intravenous or oral contrast was used.
The CTV, defined as the entire stomach, was contoured on breath-hold CT images (planning CT).21 An internal CTV was created to encompass the entire stomach on all 3 breath-hold scans. A 0.5- to 1.0-cm margin was added to the internal CTV to create a PTV, at the discretion of the treating physician.
A total radiation dose of 30 Gy, delivered at 1.5 to 2.0 Gy per fraction, was prescribed to cover the PTV. IMRT plans were generated with the Pinnacle treatment planning system (Pinnacle,3 Philips Medical Systems, Fitchburg, WI) using 5 to 7 coplanar 6-MV photon beams. In all plans, >95% of the PTV was covered by the prescription dose. Plans were optimized to deliver the lowest possible dose to adjacent organs at risk (OARs). The bilateral kidney volume receiving 15 Gy was limited to less than one-third (33%).
Radiation treatment
Before each fraction of RT, CT-IGRT was performed using a high-speed in-room CT scanner (CT-on-Rails, GE Medical Systems, Milwaukee, WI) integrated with the Varian Exact Targeting System (Varian Medical Systems). In-house 3-dimensional CT-CT alignment software was used to align daily CT images with the reference planning CT images.22 The regions of interest (CTV and OARs), the prescription isodose line, and the 95% isodose line were overlaid on the daily-acquired CT images to evaluate target coverage. If a shift of >1.0 cm was required for CTV coverage, the treating physician reviewed the alignment and made appropriate shifts before treatment. During the CT scan and RT treatment, patients maintained a breath-hold at the level established at the time of simulation. The typical treatment time was 20 minutes from CT image acquisition to completion of RT delivery.
Volume and shift analysis
Daily shifts applied during treatment in the left-right, anteroposterior, and superoinferior directions to ensure CTV coverage were recorded. To further investigate the effect of these shifts, the stomach and OARs were contoured on each daily treatment CT scan following the Radiation Therapy Oncology Group guidelines.21 This “daily CTV” was expanded, using the same margin that was used for treatment planning, to generate a “daily PTV.” These daily contours were overlaid on the reference planning CT, based on a bony alignment fusion. The bony alignment–based fusion represented the patient positioning that would be achieved using 2-dimensional orthogonal imaging. The daily stomach and kidney positions were compared between the planning CT and daily treatment CT scans.
Dosimetric analysis
The treatment plan was recalculated on all daily treatment CT scans, using alignment to bone and to the CTV (stomach), to investigate the dosimetric differences. The percent volume of the CTV and PTV covered by the 95% isodose line (V95%), and the mean dose and percent volume of OARs receiving 25, 15, and 5 Gy (V25Gy, V15Gy, and V5Gy) were compared with the treatment plans.
Statistical analysis
Dosimetric data were compared using the independent samples t test. A P value <.05 was considered to be statistically significant. Statistical analyses were performed using SPSS software (version 22.0, IBM Corp, Armonk, NY).
Results
Interfractional variation in stomach location
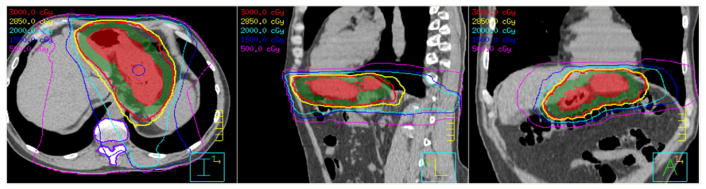
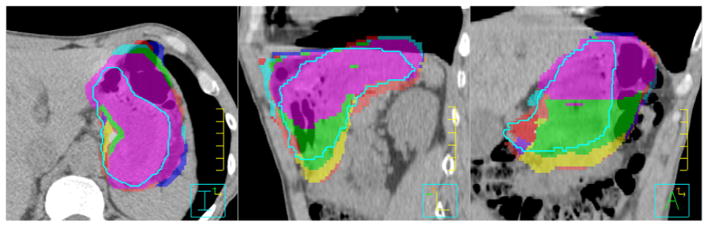
This cohort comprised 12 patients treated with IMRT and CT-IGRT. Figure 1 shows a representative stomach IMRT plan. A total of 229 daily CT scans were included in the analyses. Table 1 summarizes the stomach volumes on the 12 planning and 229 daily CT scans for all patients. The average stomach volume on planning scans was 264.3 cm3 and on daily scans was 222.6 ± 82.5 cm3. The average change in volume was −12.4% (range, −47.6% to 38.6%). In Fig 2, the daily stomach volumes are overlaid on the planning CT, based on bony alignment, to illustrate the variation in stomach positioning during the course of treatment for a representative patient (patient 6).
Figure 1.
A representative intensity modulated radiation therapy plan in axial (left), sagittal (middle), and coronal (right) views. Colorwash: Red, clinical target volume (CTV; stomach); green, planning target volume (0.5–1.0 cm expansion of CTV). Isodose lines: red, 30 Gy; yellow, 28.5 Gy; cyan, 20 Gy; blue, 15 Gy; magenta, 5 Gy.
Table 1.
Stomach volumes and absolute shifts relative to bony setup by CT-IGRT
| Patient | CTV to PTV margin (cm) | Reference stomach volume (cm3) | Daily stomach volume | CT-IGRT shift | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Mean ± SD (cm3) | Range (cm3) | Mean ± SD (cm) | Range (cm) | |||
| 1 | 0.5 | 200.0 | 190.6 ± 15.6 | 157.8–222.2 | 1.1 ± 0.5 | 0.3–2.1 |
| 2 | 0.5 | 253.0 | 212.0 ± 46.4 | 159.4–350.6 | 1.1 ± 0.4 | 0.6–2.0 |
| 3 | 0.5 | 184.5 | 108.7 ± 11.9 | 89.1–134.8 | 1.0 ± 0.4 | 0.4–1.6 |
| 4 | 0.5 | 161.0 | 108.3 ± 14.5 | 95.2–123.9 | 0.2 ± 0.1 | 0.1–0.3 |
| 5 | 0.5 | 676.2 | 428.7 ± 60.6 | 368.0–489.3 | 0.7 ± 0.2 | 0.4–1.2 |
| 6 | 0.5 | 187.9 | 151.8 ± 3.4 | 148.7–155.4 | 0.7 ± 0.2 | 0.4–1.1 |
| 7 | 1.0 | 202.1 | 170.7 ± 15.5 | 139.4–190.4 | 0.6 ± 0.4 | 0.2–1.9 |
| 8 | 1.0 | 250.4 | 250.0 ± 26.8 | 194.4–287.5 | 1.8 ± 0.3 | 1.3–2.2 |
| 9 | 1.0 | 227.7 | 240.3 ± 20.4 | 215.1–273.2 | 1.1 ± 0.4 | 0.4–2.0 |
| 10 | 1.0 | 364.5 | 430.6 ± 106.4 | 321.4–534.0 | 1.2 ± 0.4 | 0.6–2.1 |
| 11 | 1.0 | 162.7 | 153.6 ± 17.6 | 137.2–171.9 | 0.8 ± 0.3 | 0.5–1.4 |
| 12 | 1.0 | 301.9 | 221.3 ± 58.7 | 171.7–286.2 | 0.8 ± 0.3 | 0.3–1.3 |
CT-IGRT, computed tomography image guidance radiation therapy; CTV, clinical target volume; PTV, planning target volume; SD, standard deviation.
Figure 2.
Daily stomach volumes (colorwash) overlaid on the planning computed tomography image after bony alignment compared with the reference stomach volume (light blue contour) in axial (left), sagittal (middle), and coronal (right) views for patient 6.
Relative to bony structures, daily shifts were required before treatment to align the CTV with the stomach. The shifts required for each patient are summarized in Table 1. The mean shift required was 1.0 cm ± 0.6 cm. The maximum shift performed per patient ranged from 0.3 to 2.2 cm. Six patients (50%) had mean shifts of ≥1.0 cm, and 1 patient (patient 8) required shifts of >1.0 cm for all treatments. Daily shifts were made in all directions; in the left-right, anteroposterior, and superoinferior directions, the absolute shifts were 0.4 ± 0.5 cm, 0.5 ± 0.4 cm, and 0.5 ± 0.4 cm, respectively.
Interfractional variation in kidney location
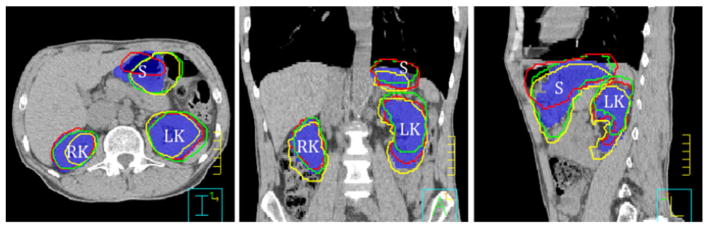
Table 2 summarizes the daily differences in left kidney positioning. Relative to bony structures, the change in left kidney location measured 0.0 ± 0.3 cm (range, −0.5 to 0.9 cm), −0.3 ± 0.5 cm (range, −1.4 to 0.6 cm), and −0.6 ± 1.3 cm (range, −2.2 to 1.3 cm) in the left-right, anteroposterior, and superoinferior directions, respectively. The change in right kidney positioning was similar. Additionally, Table 2 shows the location of the left kidney relative to the stomach on the planning CT and the daily CT scans for each patient. In 8 patients (67%), the mean distance between the left kidney and the stomach was less on the daily treatment scans than on the planning scan. The kidney was up to 1.1 cm closer to the stomach (patient 1). Figure 3 illustrates the daily changes in kidney location relative to the stomach for a representative patient (patient 12).
Table 2.
Daily left kidney shift relative to bony anatomy and distance between centroids of the left kidney and stomach
| Patient | Daily left kidney shift relative to bony reference (cm) (mean ± SD) | Left kidney-stomach distance (cm) | |
|---|---|---|---|
|
| |||
| Plan | Daily (mean ± SD) | ||
| 1 | 1.8 ± 0.6 | 9.6 | 8.5 ± 0.3 |
| 2 | 2.0 ± 0.8 | 8.6 | 8.5 ± 0.7 |
| 3 | 1.3 ± 0.3 | 6.5 | 6.1 ± 0.9 |
| 4 | 0.8 ± 0.7 | 10.4 | 10.2 ± 0.4 |
| 5 | 0.6 ± 0.3 | 10.5 | 10.6 ± 0.4 |
| 6 | 1.0 ± 0.3 | 10.8 | 10.8 ± 0.2 |
| 7 | 1.8 ± 0.5 | 13.3 | 12.8 ± 0.4 |
| 8 | 0.4 ± 0.3 | 10.6 | 11.2 ± 0.4 |
| 9 | 0.6 ± 0.1 | 7.9 | 7.6 ± 0.6 |
| 10 | 0.9 ± 0.2 | 12.6 | 11.9 ± 0.6 |
| 11 | 1.3 ± 0.2 | 7.9 | 7.0 ± 0.1 |
| 12 | 0.8 ± 0.3 | 12.1 | 12.9 ± 0.8 |
Abbreviation as in Table 1.
Figure 3.
Daily left kidney (LK), right kidney (RK), and stomach (S) volumes overlaid on the planning computed tomography image after bony alignment in axial (left), sagittal (middle), and coronal (right) views for patient 12. Blue colorwash, reference stomach and kidney volumes; contours, daily stomach and kidney volumes (red, day 1; green, day 8; and yellow, day 15).
Daily CTV coverage using CT-IGRT
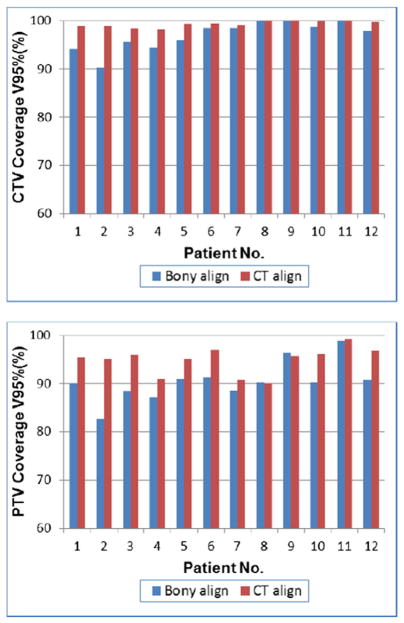
We assessed how changes in stomach volume, shape, and location influenced target coverage. First, we evaluated the target coverage that would have been achieved if patients were positioned using bony alignment only. Figure 4 demonstrates CTV coverage on the planning scan and on the daily CT scans that would have been achieved using bony alignment for a representative patient (patient 1). In this patient, the daily CTV V100% and V95% were as low as 75.0% and 84.1%, respectively. As shown in Fig 5, with bony alignment, the PTV V95% over the entire course of treatment was ≤90% in 4 patients (33%).
Figure 4.
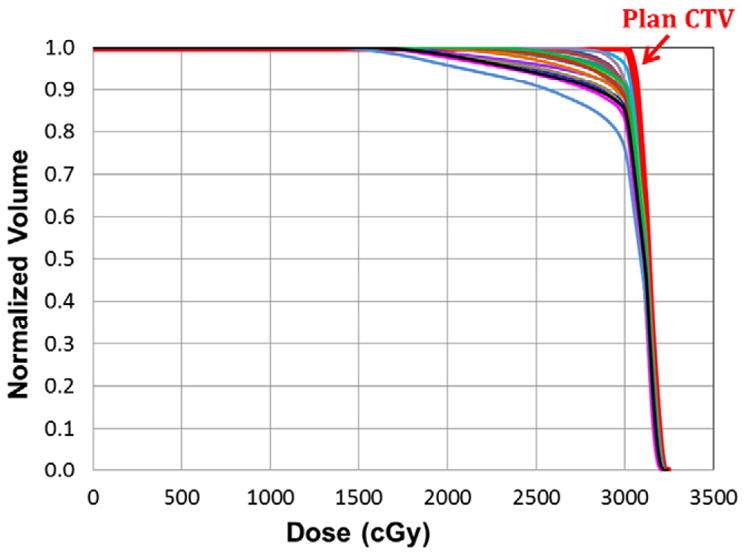
Dose-volume histogram of the stomach from the planning computed tomography scan (red) and daily computed tomography scans (other colors) after bony alignment for patient 1. CTV, clinical target volume.
Figure 5.
Mean clinical target volume (CTV) receiving ≥95% of the prescribed dose (CTV V95%) and mean planning target volume (PTV) receiving ≥95% of the prescribed dose (PTV V95%) for each patient with bony alignment (blue) versus computed tomography (CT)-based alignment to soft tissue (red) over the entire course of treatment.
We compared the target coverage that was achieved with CT-IGRT–based alignment to the stomach to the coverage that would have been observed with bony alignment only. As demonstrated in Fig 5, CT-IGRT was associated with improved target coverage. Over the entire course of therapy, the mean CTV V95% was 98.5% (range, 97.9%–99.2%) with CT-IGRT–based alignment versus 94.5% (range, 90.1%–99.1%) with bony alignment (P < .01). Similarly, the mean PTV V95% was 94.9% (range, 90.1%–99.2%) versus 90.4% (range, 82.5%–98.9%), respectively (P < .01). In the most extreme example, patient 2, the mean CTV V95% was 98.8% versus 90.1% and PTV V95% was 95.1% versus 82.5% with CT-IGRT–based alignment versus bony alignment, respectively. For this patient, coverage during 1 fraction would have been as low as 79.5% (CTV V95%) and 72.5% (PTV V95%) with bony alignment.
Dosimetric analysis of OARs
The mean dose, V5Gy, V15Gy, and V25Gy for OARs are tabulated in Table 3. For the liver, spleen, and heart, these doses were within 1.0 Gy or 5% of the planned dose with both bony alignment and CT-based alignment to the stomach. Conversely, the change in location of the kidneys relative to the bones and stomach affected their exposure in some patients. Compared with the expected dose from the treatment plan, the right kidney mean dose increased by 11.0% with bony alignment and by 2.9% with CT-IGRT; the left kidney mean dose increased by 37% and 39%, respectively. In all patients, the bilateral kidney D33% was maintained at <15 Gy; however, in 1 patient the left kidney D33% was >15 Gy. In this patient, the distance between the left kidney and stomach was relatively small, and a larger PTV expansion (1 cm) was used.
Table 3.
Dose to organs at risk, presented as mean dose and mean percent volume receiving 5, 15, and 25 Gy
| OAR | Plan | Bony alignment | CT-IGRT alignment | |
|---|---|---|---|---|
| Right kidney | V5Gy (%) | 19.1 | 21.9 | 19.7 |
| V15Gy (%) | 4.2 | 6.1 | 6.3 | |
| V25Gy (%) | 0.1 | 0.2 | 0.5 | |
| Mean (Gy) | 3.5 | 3.9 | 3.6 | |
| Left kidney | V5Gy (%) | 22.5 | 31.0 | 32.1 |
| V15Gy (%) | 6.5 | 12.0 | 12.2 | |
| V25Gy (%) | 2.5 | 4.9 | 5.7 | |
| Mean (Gy) | 4.3 | 5.9 | 6.0 | |
| Liver | V5Gy (%) | 55.1 | 55.4 | 53.5 |
| V15Gy (%) | 22.8 | 24.1 | 22.6 | |
| V25Gy (%) | 11.5 | 13.0 | 11.4 | |
| Mean (Gy) | 9.7 | 10.0 | 9.3 | |
| Spleen | V5Gy (%) | 77.3 | 77.8 | 78.5 |
| V15Gy (%) | 48.0 | 45.1 | 52.2 | |
| V25Gy (%) | 16.9 | 14.5 | 19.3 | |
| Mean (Gy) | 14.7 | 14.0 | 15.4 | |
| Heart | V5Gy (%) | 6.4 | 4.6 | 6.2 |
| V15Gy (%) | 2.1 | 1.7 | 2.0 | |
| V25Gy (%) | 0.0 | 0.0 | 0.0 | |
| Mean (Gy) | 1.4 | 1.0 | 1.3 |
OARs, organs at risk; V5Gy, percent volume of OARs receiving 5 Gy; V15Gy, percent volume of OARs receiving 15 Gy; V25Gy, percent volume of OARs receiving 25 Gy. Other abbreviation as in Table 1.
Clinical outcomes
In this cohort, no instance of local disease relapse was observed at a median follow-up of 50 months.
Discussion
Definitive RT for gastric MALT lymphoma is highly effective23; however, treatment delivery can be complicated by significant inter- and intrafractional target motion. At our institution, we reduce this motion with a breath-hold technique, restriction of oral intake before treatment, and a nongaseous diet throughout the course of therapy. Even with this approach, we noted significant day-to-day variation in stomach volume. Interestingly, stomach volumes tended to be smaller at the time of treatment than the time of simulation. Typically, daily treatments were scheduled for the early morning, so patients had not eaten since the previous night. We hypothesize that this factor explains the smaller stomach volumes at the time of treatment. Despite the marked variability in stomach volume that was observed, daily image guidance with CTOR enabled excellent target coverage (CTV V95% >98%), even with the use of small PTV margins (0.5–1 cm). Furthermore, in this cohort, no instance of local relapse was observed, suggesting that the use of smaller margins did not compromise tumor control when CTOR was used to confirm target coverage.
Historically, PTV expansions of 2 to 3 cm have been recommended, based on CT and fluoroscopic assessments of stomach motion.3,4,14 More recently, researchers have shown that the use of a breath-hold technique and daily image guidance reduces stomach motion and improves setup accuracy in patients treated for gastric cancer. With this approach, PTV margins of 0.5 to 1 cm were shown to be sufficient.17 Our findings corroborate that 0.5- to 1-cm margins are adequate to ensure coverage of the stomach if careful motion management and image guidance are used. In our cohort, daily volumetric image guidance significantly improved coverage of the stomach when these reduced PTV expansions were used. We compared the target coverage that would have been achieved with bony alignment to the target coverage that was achieved using CT-based alignment to the stomach. With bony alignment, the CTV V95% was as low as 79.5%, despite the use of a breath-hold technique and restriction of oral intake before treatment. With CTOR, the mean CTV V95% was >98% for all patients over the course of therapy. We conclude that daily CT image guidance is essential to ensure adequate target coverage in the treatment of gastric MALT lymphoma when reduced PTV expansions and IMRT, which generates steep dose gradients, are used.
CT guidance was useful to evaluate motion not only of the stomach, but also of adjacent OARs. Specifically, we found that the kidneys moved with respect to both the stomach and bony anatomy when we compared their location on daily CT scans to the planning scan. Consequently, the delivered kidney dose was higher than expected based on the planning scan. As a result, CT guidance should be used to localize not only the target, but also adjacent OARs. Furthermore, the observed variation in kidney location suggests that a planning risk volume, created by expanding the kidneys with adequate margin (eg, 1 cm), may be useful as an avoidance structure.
Although CBCT is used commonly for 3-dimensional image guidance, we use CTOR in this clinical setting at our institution. CTOR offers 2 advantages over CBCT. First, the CTOR is a diagnostic quality CT scanner. We do not use diagnostic protocols to reduce radiation exposure from image guidance procedures; however, the image quality is similar to that of simulation CT scans. CBCT provides worse soft tissue contrast than CTOR because of greater scatter; therefore, in our experience, CTOR enables better soft tissue alignment for the treatment of gastric MALT lymphoma. A second benefit of CTOR is the longer longitudinal scan range, which enables evaluation of nearby critical structures, such as the kidneys and heart.
A strength of this study is the large number of daily scans that were available for comparative dosimetric analyses. Although only 12 randomly selected patients were included, each patient received 15 to 20 fractions of RT; thus, the dose distribution was calculated using a total of 229 daily scans. Our conclusions are based upon paired dose comparisons between the 12 treatment plans and 229 daily scans.
Patients treated for gastric MALT lymphoma have excellent prognoses and long life expectancies23; therefore, minimizing the risk of treatment-related toxicity carries particular weight in this population. To this end, the use of small PTV expansions is desirable to reduce the dose to adjacent OARs.8,17 Historically, large PTV expansions of at least 2 cm were used to ensure coverage of the stomach. In our cohort, an increase in the CTV-to-PTV expansion from 1 to 2 cm would result in the volume receiving the prescription dose almost doubling and a significantly higher integral dose. The dose to normal tissues and the consequent treatment-related toxicity, therefore, would be greater. To enable the use of small expansions while maintaining PTV coverage, with IMRT, which generates steep dose gradients, accurate target localization is of critical importance. In this clinical setting, the target is soft tissue, which cannot be visualized with portal images. Furthermore, given the dramatic interfractional changes in the stomach’s shape, location, and volume, bony alignment alone is inadequate to ensure target coverage. Daily CT guidance, in combination with a breath-hold technique and restriction of oral intake before treatment, enables excellent target coverage with small PTV expansions (0.5–1.0 cm). We recommend this approach to for the treatment of gastric MALT lymphoma.
Footnotes
Conflicts of interest: None.
References
- 1.Dabaja BS, Perrin KJ, Romaguera JE, et al. Successful treatment of a free-moving abdominal mass with radiation therapy guided by cone-beam computed tomography: A case report. J Med Case Rep. 2010;4:329–332. doi: 10.1186/1752-1947-4-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallman JL, Mori S, Sharp GC, Lu HM, Hong TS, Chen GT. A four-dimensional computed tomography analysis of multiorgan abdominal motion. Int J Radiat Oncol Biol Phys. 2012;83:435–441. doi: 10.1016/j.ijrobp.2011.06.1970. [DOI] [PubMed] [Google Scholar]
- 3.Johnson ME, Pereira GC, El Naqa IM, et al. Determination of planning target volume for whole stomach irradiation using daily megavoltage computed tomographic images. Pract Radiat Oncol. 2012;2:e85–e88. doi: 10.1016/j.prro.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe M, Isobe K, Uno T, et al. Intrafractional gastric motion and interfractional stomach deformity using CT images. J Radiat Res. 2011;52:660–665. doi: 10.1269/jrr.11018. [DOI] [PubMed] [Google Scholar]
- 5.Wysocka B, Kassam Z, Lockwood G, et al. Interfraction and respiratory organ motion during conformal radiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys. 2010;77:53–59. doi: 10.1016/j.ijrobp.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 6.Gierga DP, Brewer J, Sharp GC, et al. The correlation between internal and external markers for abdominal tumors: Implications for respiratory gating. Int J Radiat Oncol Biol Phys. 2005;61:1551–1558. doi: 10.1016/j.ijrobp.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe M, Isobe K, Takisima H, et al. Intrafractional gastric motion and interfractional stomach deformity during radiation therapy. Radiother Oncol. 2008;87:425–431. doi: 10.1016/j.radonc.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Matoba M, Oota K, Toyoda I, et al. Usefulness of 4D-CT for radiation treatment planning of gastric MZBCL/MALT. J Radiat Res. 2012;53:333–337. doi: 10.1269/jrr.11127. [DOI] [PubMed] [Google Scholar]
- 9.Goitein M. Organ and tumor motion: an overview. Semin Radiat Oncol. 2004;14:2–9. doi: 10.1053/j.semradonc.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Murphy MJ. Tracking moving organs in real time. Semin Radiat Oncol. 2004;14:91–100. doi: 10.1053/j.semradonc.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Giraud P, Houle A. Respiratory gating for radiotherapy: Main technical aspects and clinical benefits. ISRN Pulmonol. 2013;2013:13. doi: 10.1684/bdc.2010.1143. [DOI] [PubMed] [Google Scholar]
- 12.Keall P, Mageras GS, Balter JM, et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med Phys. 2006;33:3874–3900. doi: 10.1118/1.2349696. [DOI] [PubMed] [Google Scholar]
- 13.van der Geld YG, Senan S, van Sörnsen de Koste, et al. A four-dimensional CT-based evaluation of techniques for gastric irradiation. Int J Radiat Oncol Biol Phys. 2007;69:903–909. doi: 10.1016/j.ijrobp.2007.06.062. [DOI] [PubMed] [Google Scholar]
- 14.Della Biancia C, Hunt M, Furhang E, Wu E, Yahalom J. Radiation treatment planning techniques for lymphoma of the stomach. Int J Radiat Oncol Biol Phys. 2005;62:745–751. doi: 10.1016/j.ijrobp.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Giraud P, Yorke E, Jiang S, et al. Reduction of organ motion effects in IMRT and conformal 3D radiation delivery by using gating and tracking techniques. Cancer Radiothér. 2006;10:269–282. doi: 10.1016/j.canrad.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Mageras GS, Yorke E. Deep inspiration breath hold and respiratory gating strategies for reducing organ motion in radiation treatment. Semin Radiat Oncol. 2004;14:65–75. doi: 10.1053/j.semradonc.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Hu W, Ye J, Wang J, Xu Q, Zhang Z. Incorporating breath holding and image guidance in the adjuvant gastric cancer radiotherapy: A dosimetric study. Radiat Oncol. 2012;7:98. doi: 10.1186/1748-717X-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagman R, Yorke E, Ford E, et al. Respiratory gating for liver tumors: use in dose escalation. Int J Radiat Oncol Biol Phys. 2003;55:659–668. doi: 10.1016/s0360-3016(02)03941-x. [DOI] [PubMed] [Google Scholar]
- 19.Ma CMC, Paskalev K. In-room CT techniques for image-guided radiation therapy. Med Dosim. 2006;31:30–39. doi: 10.1016/j.meddos.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Boda-Heggemann J, Lohr F, Wenz F, Flentje M, Guckenberger M. kV cone-beam CT-based IGRT: A clinical review. Strahlenther Onkol. 2011;187:284–291. doi: 10.1007/s00066-011-2236-4. [DOI] [PubMed] [Google Scholar]
- 21.Jabbour SK, Hashem SA, Bosch W, et al. [Accessed March 13, 2017];Upper abdominal normal organ contouring guidelines. doi: 10.1016/j.prro.2013.06.004. Available at:, http://www.rtog.org/CoreLab/ContouringAtlases/UpperAbdominalNormalOrganContouringConsensusGuidelines.aspx. [DOI] [PMC free article] [PubMed]
- 22.Zhang L, Dong L, Court L, et al. TU-EE-A4-05: Validation of CT-assisted targeting (CAT) software for soft tissue and bony target localization. Med Phys. 2005;32:2106. [Google Scholar]
- 23.Schechter NR, Portlock CS, Yahalom J. Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone. J Clin Oncol. 1998;16:1916–1921. doi: 10.1200/JCO.1998.16.5.1916. [DOI] [PubMed] [Google Scholar]