SUMMARY
Cohesin extrusion is thought to play a central role in establishing the architecture of mammalian genomes. However, extrusion has not been visualized in-vivo and thus its functional impact and energetics are unknown. Using ultra-deep Hi-C we show that loop domains form by a process that requires cohesin ATPases. Once formed however, loops and compartments are maintained for hours without energy input. Strikingly, without ATP we observe the emergence of hundreds of CTCF-independent loops that link regulatory DNA. We also identify architectural “stripes”, where a loop anchor interacts with entire domains at high frequency. Stripes often tether super-enhancers to cognate promoters and in B cells they facilitate Igh transcription and recombination. Stripe anchors represent major hotspots for topoisomerase-mediated lesions, which promote chromosomal translocations and cancer. In plasmacytomas, stripes can deregulate Igh-translocated oncogenes. We propose that higher organisms have coopted cohesin extrusion to enhance transcription and recombination, with implications for tumor development.
Graphical abstract

INTRODUCTION
The application of DNA proximity ligation and its derivatives has shown that chromosomes contain contact domains, also known as topologically associated domains (TADs) (Dixon et al., 2012; Hou et al., 2012; Lieberman-Aiden et al., 2009; Nora et al., 2012; Rao et al., 2014; Sexton et al., 2012; van Berkum and Dekker, 2009). One way these domains can form is as a byproduct of looping between DNA sequences that recruit the architectural protein CTCF and its partner the SMC cohesin complex (Rao et al., 2014; Rowley and Corces, 2016). Crucially, the CTCF binding motifs at loop domains are largely found in a convergent orientation – i.e., they point at one another (Rao et al., 2014). To explain this phenomenon, it has been proposed that loops form by a process of extrusion (Figure 1A, (Alipour and Marko, 2012; Fudenberg et al., 2016; Nasmyth, 2001; Nichols and Corces, 2015; Sanborn et al., 2015)). In this model, a single or a pair of physically tethered cohesin rings is loaded on chromatin by the Nipbl-Mau4 complex. The rings then slide in opposite directions until they are stopped by CTCF bound to inward-oriented motifs.
Figure 1. Cohesin translocation requires ATP and Smc ATPases.
(A) Schematics representing the extrusion model of nuclear topology. Cohesin rings are loaded at Nipbl+ sites, extrude along DNA, and halt at CTCF+ anchor sites in the convergent orientation. (B) Venn diagram showing the distribution of Rad21 ChIP-Seq peaks relative to CTCF and Nipbl. (C) ChIP-Seq tracks of CTCF, Nipbl, and Rad21 in the presence and absence of ATP in activated B cells. (D) Histograms showing global Rad21 (left) or CTCF (right) occupancy at Nipbl+ loading sites in oligomycin-treated (black) and untreated (red) activated B cells. (E) ChIP-Seq profiles of transduced Smc3-biotag WT and ATPase mutants in activated B cells. (F) Occupancy of Smc3 WT and mutants at loading and anchor sites.
Anomalies in CTCF motifs lead to oncogene deregulation in some tumors (Flavahan et al., 2016; Hnisz et al., 2016), and defects in limb development in humans and mice (Lupianez et al., 2015). Acute, global disruption of loop domains genome-wide also lead to modest but significant changes in transcription (Nora et al., 2017; Rao et al., 2017). Contact domains thus are believed to represent structural and functional units of genome organization that minimize spurious cross-talk of unrelated elements across boundaries (Dekker and Mirny, 2016; Denker and de Laat, 2016).
Exactly how nuclear architecture facilitates the tethering of gene regulatory elements has not been fully resolved. One possibility is that contact domains confine functional interactions to dedicated nuclear volumes (Di Pierro et al., 2016; Hnisz et al., 2017). In these microenvironments, genes and their distant enhancers could be simply brought together by diffusion. A second possibility is that architecture plays a more direct role, tethering regulatory DNA to their targets via point-to-point looping. However, while thousands of loops have been identified by Hi-C studies, their numbers are surprisingly small relative to the putative enhancer elements in the human genome.
Notably, the extrusion model predicts a third mechanism by which nuclear topology could regulate transcription. In principle, when cohesin loading takes place adjacent to a CTCF anchor motif, one extrusion subunit might be arrested, while its partner continues to slide along chromatin. In this setting, the CTCF-anchored element would form rapid, transient contacts with an entire genomic interval. This “asymmetric” extrusion mechanism is appealing as it could explain how promoters might track along arrays of enhancers spread across hundreds of kilobases, such as super-enhancers (SEs). A similar tracking mechanism was recently proposed to facilitate V(D)J recombination in developing lymphocytes (Hu et al., 2015b). However, these ideas have not been fully explored because of our inability to track cohesin translocation in vivo.
Another key puzzle underlying the loop extrusion model is how cohesin rings slide the vast distances that are required to form large loop domains. There is considerable debate about the possible source of energy for such a process. One possibility is that cohesin slides via diffusion without energy input, although this would be relatively inefficient given the long distances involved. Alternatively, the sliding might be facilitated by active processes, such as ATP-driven motors including transcription or DNA replication (Busslinger et al., 2017; Davidson et al., 2016). Recent studies for instance have shown that RNA polymerases can push cohesin rings along DNA in vitro (Davidson et al., 2016), and that the distribution of Rad21 in the mammalian genome depends in part on transcription (Busslinger et al., 2017).
We have recently reported marked changes in genome architecture as transcriptionally quiescent G0 B cells enter the cell cycle (Kieffer-Kwon et al., 2017). Concomitant with a global amplification of the transcriptome and activating epigenetic marks, we showed that loop domains double in number. Because of these massive gains, B cell activation provides a powerful system for dissecting the pathways underlying nuclear architecture. Using this model, we here shed light on the dynamics and physiological impact of cohesin translocation.
RESULTS
ATP depletion biases cohesin localization towards Nipbl loading sites
We recently showed that ATP depletion reverses the nuclear topology of activated B cells towards the G0 state, resulting in fewer loops and intra-domain interactions (Kieffer-Kwon et al., 2017). One possible explanation is that a reduction in energy input may interfere with the translocation of cohesin along DNA (Figure 1A). To test this idea, we began by examining cohesin (Rad21) occupancy by ChIP-Seq.
In activated B cells, 86% of all Rad21 peaks were associated either with CTCF, Nipbl, or both (Figure 1B). We also found a strong correlation between the strength of Nipbl and CTCF binding, and that of cohesin (Figure S1A). Next, we treated the cells for 2h with oligomycin, which reduced ATP levels by ~90% (Kieffer-Kwon et al., 2017). Whereas the overall levels of cohesin remained essentially the same upon ATP depletion (Figure S1B), its distribution across the genome changed markedly. Specifically, Rad21 signals were stronger at Nipbl loading sites and weaker at CTCF anchor sites relative to controls (p < 2.2e-16, Figure 1C and 1D). This indicates either improper cohesin loading, or a defect in cohesin mobility from loading to anchor sites. Notably, similar profiles were observed in G0, consistent with low ATP levels in these cells (Figure S1C, (Kieffer-Kwon et al., 2017)).
We repeated the above analysis after blocking DNA replication (using hydroxyurea, a potent inhibitor of DNA replication) or RNA PolII elongation (using flavopiridol, Figure S1D). To determine the extent to which cohesin mobility was affected, we computed the ratio of Rad21 signals at CTCF+ anchors vs. Nipbl+ loading sites. There was a small reduction on cohesin-CTCF co-localization upon transcriptional inhibition and no effect when replication was blocked (Figure S1E). Taken together the data are consistent with the notion that cohesin translocation from loading to anchor sites requires ATP, with little or no input from replication or transcription.
Smc ATPase mutations impair cohesin mobility along DNA
A recent report revealed that condensin, another SMC complex, functions as a motor, deriving energy from its Smc2-4 ATPase domains to translocate along DNA (Terakawa et al., 2017). To test whether cohesin ATPases play a similar role in vivo, we transduced activated B cells with Smc3 variants: wild type or ATPase Walker A motif (K38A) and Walker B motif (E1144Q) mutants (Beckouet et al., 2016; Hu et al., 2011; Ladurner et al., 2014). Chromatin occupancy of transduced Smc3s was distinguished from endogenous ones by biotagging their C-terminus and coinfecting B cells with retroviruses expressing the biotin ligase BirA (Figure 1E schematics, (Nakahashi et al., 2013)). ChIP-Seq analysis showed a marked reduction in K-A and E-Q binding across the genome (Figure S1F), consistent with the notion that ATPase domains facilitate cohesin loading (Hu et al., 2011). At the same time, particularly at CTCF sites adjacent to multiple loading sites, mutant cohesin complexes colocalized with CTCF anchors (Figure 1E), indicating that their mobility is not entirely blocked, either because of their pairing to endogenous proteins or residual ATPase activity. The analysis revealed that only ~50–60% of mutant complexes reached CTCF anchors relative to what was expected based on wild type controls (Figure 1F and S1E). Interestingly, this ratio was comparable to the defect observed in G0 or oligomycin-treated cells. These data demonstrate that cohesin requires its intrinsic ATPase activity to reach CTCF anchors. Whether this is due to impaired elongation or a combination of elongation and loading defects awaits further biochemical analysis.
Establishment of loop domains requires ATP but not replication or transcription
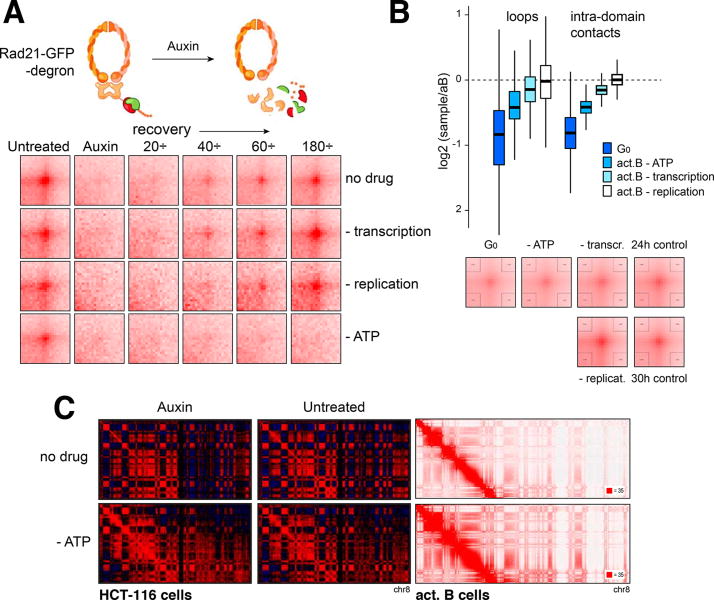
Next, we explored the effect of ATP depletion on the formation of loop domains. To do so, we employed an auxin-inducible degron system in HCT-116 cells (Natsume et al., 2016), where loop domains are entirely eliminated upon degradation of Rad21(-GFP), but readily re-form once auxin is withdrawn (Rao et al., 2017). To examine the effects of various conditions on loop re-formation, we combined auxin treatment with inhibition of ATP synthesis, transcription or DNA replication. We performed in situ Hi-C on untreated, and 0’, 20’, 40’, 60’ or 180’ time points following auxin removal, and used Aggregate Peak Analysis (APA, (Rao et al., 2014)) to examine the composite signal of loops genome-wide. Consistent with previous results (Rao et al., 2017), loop domains re-formed to nearly their original levels within three hours after auxin removal (Figure 2A, upper row). Notably, inhibition of transcription or DNA replication had no appreciable effect. By contrast, oligomycin treatment completely prevented the re-formation of loop domains (Figure 2A, lowest row), even as cohesin recovered to roughly untreated levels (Figure S1G). This demonstrates that the establishment of loop domains requires ATP.
Figure 2. Creation of loop domains but not their maintenance requires ATP.
(A) APA analysis of HCT-116 cells, where Rad21 is temporarily degraded and allowed to recover in the absence of ATP, transcription, or replication. (B) Box and APA plots showing Hi-C signals at loops or intra-domain interactions under various conditions relative to 24h activated B cells. Conditions were: G0, ATP-, transcription-, or replication-inhibited B cells. Data are represented as mean ± SEM. (C) Compartments in HCT-116 and activated B cells ± oligomycin. HCT cells were also ± Auxin.
Loop domains and compartments are maintained for hours in the absence of energy input
We next examined the role of continual energy input, transcription or DNA replication on the maintenance of genome architecture. We did this by stimulating B cells in the presence of oligomycin, flavopiridol or hydroxyurea, and generated 1 billion contact Hi-C maps for each condition. We found that inhibiting DNA replication did not affect the strength of loops or intra-domain interactions, blocking transcription had a modest effect, and inhibiting ATP synthesis showed the greatest effect (Figure 2B, bar graph). Nevertheless, the differences were small and APA signals were pronounced throughout, implying that once formed, most architectural loops can persist for several hours, even in the absence of ATP synthesis (Figure 2B, S1H, and STAR Methods). This is consistent with recent kinetic studies showing very long chromatin residence time for cohesin and CTCF (Kieffer-Kwon et al., 2017; Rhodes et al., 2017). It is important to note, however, that while the above estimates reflect the passive decay of loops, cohesin unloading may similarly be disrupted by oligomycin treatment, and thus the half-life of a loop domain under normal physiological conditions may be shorter.
Notably, none of the treatments (in B cells or HCT-116 degron cells) affected the presence of compartments (Figure 2C and not shown), indicating that compartments can also be preserved for several hours in the absence of ATP, DNA replication, or transcription. Taken together, the results are consistent with a model where loop extrusion requires ATP, at least in part because cohesin ATPases drive cohesin loading and/or sliding. By contrast, loop domains and compartments, once formed, can persist for hours without continual energy input.
Stripes of enhanced contact probability are consistent with loop extrusion
The physiological impact of loop extrusion has been unclear due to our inability to visualize cohesin mobility in vivo. However, visual inspection of ultra-deep Hi-C maps with Juicebox (Robinson et al., 2018) revealed “stripes” where a single locus (the “stripe anchor”, Figure 3A) forms frequent contacts with a contiguous genomic interval (the “stripe domain”). Stripes frequently appeared along the edges of domains with abundant cohesin loading and ranged from a few to hundreds of Kb (Figure 3A).
Figure 3. Characterization of architectural stripes.
(A) Examples of stripes (black arrowheads) in Bcl6 and Basp1 domains in activated B cells. CTCF and Nipbl tracks are shown below. “a” single-stripe domain; “b” double-stripe domain; “c” conventional loop domain. (B) Bar graph illustrates the percentage of stripe anchors associated with CTCF binding motifs. The pie chart shows the percentage of motifs facing the stripe. Data are represented as mean ± SEM. (C) Violin plot shows insulation scores for stripe and stripe partner anchors. (D) Upper schematic depicts stripe formation at the Basp1 domain based on the extrusion model. Composite graph shows Nipbl profiles at loop domains displaying double (blue), left (red), or right stripes (green), or at no stripes (grey). (E) Hi-C matrices show the loss of stripes in ZF9-11 cells at loci where stripe anchors fail to recruit CTCF. (F) Molecular dynamic simulations of Hi-C data where cohesin is loaded near the left (first domain) or right anchor CTCF anchors (third domain).
Stripes were observed in all cell types from which high-resolution contact maps have been produced by in situ Hi-C, including human lymphoblastoid cells, mouse ES cells and CH12 B cells (Figure S2A). By developing an algorithm (Zebra) that searches for pixel tracks of higher interaction frequency (Figure S2B), and manually curating the results, we identified a total of 3,901 stripes in activated B cells.
We found that stripe anchors often coincide with loop anchors. Thus, loop domains can be associated with one (example “a” in Figure 3A) or two stripes (example “b”). Consistent with the convergent rule for loop formation, ~90% of stripe anchors are associated with CTCF motifs facing the stripe domain (Figure 3B). Notably, 32% of stripe anchors exhibit multiple inward-oriented CTCF motifs, compared to only 15% for ordinary loops. In this orientation, stripe anchors are well positioned to arrest extrusion complexes. Conversely, nearly 60% of stripes extended past loop anchors into neighboring domains (e.g. Figure S2A), consistent with a model where many loop anchors do not arrest cohesin extrusion absolutely. Consistent with this idea, CTCF binding and insulation strength is enhanced at stripe anchors as compared to loop anchors that are not associated with stripes (Figure 3C and S2C). The key implication is that the ability to arrest cohesin efficiently by one or multiple CTCF sites, facilitates stripe formation. To test this idea, we weakened CTCF binding affinity in CH12 cells by mutating CTCF zinc fingers (ZFs) 9, 10, and 11 with CRISPR-Cas9. Consistent with our previous findings (Nakahashi et al., 2013), recruitment of ZF9-11 mutants was defective at sites carrying upstream motifs (Figure S2D). Furthermore, in situ Hi-C and ChIA-PET showed a correlation between these changes and loop formation (Figure S3A). Among the 574 loops that were lost, 47 were associated with stripes, and the corresponding stripe signals were markedly decreased (Figure 3E and S3B–C (p < 2.2e-16)).
We found that 97% of stripe domains exhibited Nipbl and Rad21 recruitment. Strikingly, a global analysis revealed a tendency for the cohesin loading factor Nipbl to aggregate near the stripe anchors (p < 7e-7, Figure 3D and S3D). Furthermore, Rad21 profiles closely followed the Nipbl distribution (Figure S3E).
Thus, architectural stripes form in mammalian cells at domains where one or more strong CTCF anchor motifs point toward a nearby cohesin loading area (Figure 3D schematics). In this configuration, which we dubbed a ‘loop gun’, the extrusion model predicts that as cohesin complexes are loaded, one subunit is arrested for an extended period of time at the proximal CTCF boundary, while the other slides across the domain. This asymmetric extrusion then manifests in Hi-C maps as horizontal and vertical lines (i.e. streaks of pixels of enhanced signal relative to the local background).
We tested this model by running molecular dynamic simulations of loop guns, in which pairs of cohesin rings initiate chromatin extrusion at Nipbl loading sites until they are arrested by inward-pointed CTCF motifs. The modeling confirmed that the presence of a strong Nipbl peak near a CTCF loop anchor creates stripes of cohesin translocation across entire domains (Figure 3F and S3F).
Stripes tether active regulatory DNA
Having defined stripes as prototypes of cohesin extrusion, we next explored their physiological impact. We first examined the link between stripes and transcription, based on the observation that Nipbl occupancy overlaps with regulatory elements (Kagey et al., 2010). Indeed, 79% of stripe domains (3,076 of 3,901) were associated with active enhancers, including conventional and SEs, compared to 47% for random regions. Notably, of the 996 SEs identified in activated B cells (Kieffer-Kwon et al., 2013), 66% (657) overlapped with stripe domains (36% overlapped with random intervals). Furthermore, SEs were enriched near stripe anchors and thus closely followed the overall distribution of stripes (Figure 4A–C). Histone acetylation (e.g., H3K27Ac), which demarcates active enhancers, also displayed a similar correlation (Figure S4A). Conversely, poised enhancers were in general not associated with stripes (Figure S4B). Of note, contacts between regulatory elements linked by stripes were significantly higher than those not associated with stripes within the same domains (Figure 4D). We conclude that stripes often tether regulatory DNA at SEs and that, except for loops (Figure 4D), their interactions are among the highest in the genome.
Figure 4. Stripes form preferentially at SEs and play a role in transcription and recombination of Ig genes.
(A) Composite graph showing SEs at loop domains carrying left (red) or right (green) stripes. (B) Contact matrix showing stripes at the Bach2 SE locus. (C) A nearly 3Mb-size stripe covers the Igh locus in B cells. Igh SE elements (hs3a, 1–2, 3b, and 4), Nipbl, and CTCF are shown. Arrowheads denote the orientation of CTCF motifs. CTCF tracks for WT, SA−/−, and SApartial mutants are shown. (D) Box plot shows Hi-C signals at loops (grey) and active regulatory elements linked by stripes (red) or not associated with stripes (white). Interactions were normalized based on non-regulatory DNA Hi-C signals in the same domain. p for all differences were 2.2e-16. (E) Hi-C2 analysis of the Igh locus in SA+/+ and SA−/− CH12 B cells. Black arrowhead shows loss of stripe signals. (F) Igα germline transcripts in SA+/+, SApartial and SA−/− activated cells. (G) Representative IgA recombination in WT and SA−/− cells. All data are represented as mean ± SEM.
We found little overlap in the number and distribution of stripes between B cells and ES cells (Figure S4C), presumably because enhancer profiles fluctuate considerably in response to developmental changes in CpG methylation (Kieffer-Kwon et al., 2013). Consistent with this view, genomic sites associated with stripes in B cell SEs were markedly demethylated relative to ES cells (p = 1e-3, Figure S4DE). Thus, stripes appear during development in conjunction with demethylation of cell-specific enhancers.
Loop extrusion activates IgCh transcription and recombination
The observation that asymmetric extrusion can tether promoters and enhancers suggests an additional mechanism by which nuclear architecture can influence gene expression. To validate this idea, we turned to the Igh contact domain, which displays one of the most prominent stripes in B lymphocytes, spanning ~2.7Mb in length (Figure 4C). Its so-called “superanchor” (SA, (Aiden and Casellas, 2015; Benner et al., 2015)), consists of 10 CTCF binding motifs pointing towards the Igh SE, which exhibits extensive Nipbl loading in activated B cells (Figure 4C). Conversely, in ES cells, the SE is inactive, fails to recruit Nipbl, and no stripe is observed (Figure S5A).
To explore the impact of Igh stripe-mediated contacts, we deleted all 10 CTCF motifs from the Igh SA in CH12 B cells (SA−/−). Mutant and control cells were cultured in the presence of TGF-β, CD40L and IL-4, which activates expression of the deaminase AID and the B cell receptor Igα gene. First, we examined the effect of SA deletion on nuclear architecture. Using Hi-C2 (Sanborn et al., 2015), we found the stripe signal was greatly attenuated (Figure 4E). Next, we examined the effect of SA deletion on recombination. Under normal conditions, the presence of AID and Igα accessibility brings about efficient Igμ → Igα class switch recombination (CSR). Strikingly, CSR was reduced by half in SA−/− cells (p = 1e-8, Figure 4G and S5C). Moreover, we observed a modest but statistically significant decrease in Igα expression (p = 3e-4, Figure 4F). Transcription of AID or other CSR factors was not altered (Figure S5B).
As an additional control, we deleted 6 of 10 CTCF motifs from the SA (Figure 4C, SApartial). Consistent with the above findings, stripe signals, Igα transcription, and CSR were reduced in SApartial cells, although not to the levels seen in SA−/− clones (Figure 4F and S5C–D). Thus, deletion of the Igh stripe anchor weakens functional interactions between the 3’ SE and Igα, leading to a reduction in transcription and CSR.
Loop extrusion facilitates oncogene deregulation
The Igh SE frequently deregulates expression of translocated oncogenes in B cell tumors (Gostissa et al., 2009; Kovalchuk et al., 2012). To determine whether extrusion contributes to tumorigenesis we deleted the Igh SA in a mouse plasmacytoma (PCT 7134, (Kovalchuk et al., 2012)). This tumor carries a homozygous reciprocal translocation (T(12;15), Figure 5A) that juxtaposes Igμ 6.6Kb upstream of Myc exon 1, positioning the Myc promoter ~165Kb from the Igh SE (Figure 5B).
Figure 5. A role for loop extrusion in tumor development.
(A) Karyotype analysis of plasmacytoma 7134 carrying a t(12;15) translocation. (B) Schematic depicts the translocated domain. (C) Hi-C2 analysis of t(12;15) in SA+/+ and SA−/− cells. (D) Transcriptional analysis of genes upstream and downstream of the translocation breakpoint. (E) Proliferation of SA+/+ and SA−/− PCT cells. (F) Distribution of TOP2B induced damage in activated B cells relative to Rad21 and CTCF occupancy at loop anchors. (G) DNA damage profiles at double (red line), single (green line) or nostripe (back line) domains. (H) Extent of DNA damage in domains with no stripes (0) or with stripes up to 300Kb or 500Kb in length. Data are represented as mean ± SEM.
Hi-C2 analysis showed that the Igh SA forms a distinct architectural stripe that reaches Myc and Pvt1 (Figure 5C, left matrix). Following Igh SA deletion the stripe disappeared (Figure 5C, right matrix), and 4C-Seq corroborated the result (Figure S6A). Decreased Igh stripe contacts at the Myc locus correlated with a significant loss in Myc and Pvt1 expression (p < 1e-8, Figure 5D). Concurrently, genes upstream of Igh SE (Tmem121, Rik, Crip1, and Crip2) increased in expression (Figure 5D and S6B), and this correlated with increased interactions between their promoters and the SE (Figure S6A). This observation is consistent with the proposal that the SA insulates neighboring non-Ig genes from the Igh SE (Birshtein, 2012). For genes positioned further upstream of Crip2 (e.g. Mta1), we did not detect any significant changes in transcription (Figure S6B). The decrease in Myc expression impacted proliferation, as the number of SA−/− cells decreased by ~40% relative to untargeted tumor control (Figure 5E). We conclude that cohesin extrusion associated with Igh SE contributes to the deregulation of translocated oncogenes in B cell tumors.
Stripe anchors are hotspots for DNA damage
Recent studies suggest another mechanism whereby loop extrusion might be implicated in tumor development. CTCF loop anchors actively recruit type II topoisomerases (TOP2B, (Uuskula-Reimand et al., 2016)), which typically relieves torsional stress by inducing DNA breaks in response to replication or transcription. At loop anchors, we have shown that TOP2B also creates DNA lesions, and in a manner proportional to cohesin occupancy (Canela et al., 2017). One explanation is that as chromatin is fed through the extrusion complex, it creates topological constraints on DNA that are alleviated by topoisomerases. This idea is supported by the fact that TOP2B lesions accumulate just outside CTCF loop anchors, following the expected orientation of cohesin translocation along DNA (Figure 5F). Importantly, these lesions frequently occur at breakpoint clusters commonly translocated in human cancer (Canela et al., 2017).
Because stripes occur primarily at domains with extensive cohesin translocation, we surmised that stripe anchors must be particularly vulnerable to TOP2B-induced breaks. We tested this idea by mapping TOP2B cleavage sites in activated B cells cultured with etoposide, an inhibitor that prevents religation of TOP2B-induced breaks. A composite analysis showed DNA breaks to be twice as frequent at loop domains carrying two stripes than at ordinary domains (Figure 5G, red vs. back lines). Furthermore, at single-stripe domains, DNA damage accumulated preferentially at the stripe anchor (p < 7e-7, Figure 5G, green line). Interestingly, we found a direct correlation between stripe length and the extent of DNA damage (Figure 5H), consistent with the model that the farther extrusion complexes must travel, the greater the accumulation of knots at stripe anchors. We conclude that stripe anchors are prime genomic hotspots for TOP2B-mediated lesions.
Loop extrusion realigns regulatory DNA clusters into functional interactions
We found it noteworthy that while 1,556 loops are downregulated in ATP-depleted B cells, nearly the same number (1,394) are gained. Hi-C maps revealed that these new loops often emerge within domains associated with architectural stripes. Figures 6A–B and S6C show representative examples, where stripes originating at Hs3st1, Foxo1, Trib, Myc, Stt3, and mir155 are depleted upon oligomycin treatment while new loops appear in their place. Interestingly, only ~50% of these loops recruit CTCF at both anchors vs. 86% for ATP-dependent ones (Figure 6C). Furthermore, when the new loop anchors do overlap with CTCF, the binding motifs are less likely to be in the convergent orientation (62%), compared to loops that are lost when ATP is depleted (91%, p < 1.7e-6, Figure 6C).
Figure 6. Loops extrusion untangles regulatory DNA hubs.
(A–B) Examples of enhancer-enhancer loop formation in the absence of cohesin extrusion in ATP-depleted B cells relative to activated B cells (72h). (C) Bar graph characterizes gained and lost loops in oligomycin-treated cells relative to CTCF binding, motif orientation, and overlap with active regulatory DNA. (D) ChIP-Seq tracks showing promoters and enhancers (H3K4me1highH3K4me3low), Nipbl, Rad21, and CTCF.
Notably, anchors of ATP-independent loops were enriched in epigenetic modifications that demarcate accessibility (ATAC-Seq+), including active enhancers and promoters (Figure S6D and S6C). In total, 71% of gained loops tethered active regulatory DNA, compared to 30% for lost loops (p < 4e-9, odd ratio = 5.9, Figure 6C). In this respect, loops created by ATP-depletion are reminiscent of the long-range interactions that form between active SEs in cohesin-depleted cells (Rao et al., 2017).
Thus, in the absence of continual energy input DNA regulatory elements aggregate in 3D nuclear space, leading to enhancer-enhancer loops among others. We propose that cohesin translocation modifies the spatial neighborhood of these regulatory elements, reducing interactions between enhancers that lie nearby in 1D, while favoring functional associations with long-distant promoters.
DISCUSSION
The proposal that chromatin loops form via cohesin extrusion has successfully explained a wide range of observations about nuclear architecture. Here, we clarify the ATP dependence of multiple phenomena associated with nuclear architecture: Nipbl and cohesin localization, loop formation, and loop and compartment maintenance. The resulting data greatly clarifies the energetic constraints on loop extrusion models. The data is consistent with a model in which extrusion relies on cohesin’s intrinsic ATPase activity to load or slide and form loops. Once formed however, loops can persist for hours in the absence of energy input.
In addition, we confirm a key prediction of the extrusion model: the existence of stripes which can be explained by asymmetric extrusion, in which one cohesin subunit is arrested, while the other continues to slide along the domain. We show that stripe formation is facilitated by a loop gun configuration, in which strong CTCF anchor motifs point toward nearby Nipbl cohesin loading areas. Using B cell activation and antibody gene recombination as model systems, we propose that stripes play a role in transcription, rearrangements, and tumor development.
Energetics of Nuclear Architecture
While many aspects of the loop extrusion model have been carefully tested, the energy requirements are poorly understood. Here we greatly clarify this issue.
Cohesin subunits are believed to be recruited to chromatin by the Nipbl-Mau4 complex. ATP hydrolysis by cohesin ATPases then fuels the assembly of Scc1-Smc1-Smc3 tripartite rings onto DNA. Consistent with this model, we find that transduced Smc3 ATPase mutants display a reduced affinity for chromatin in B cells.
No motors have been specifically linked with cohesin sliding or loop extrusion in vivo. Several possibilities have been explored. Experiments in yeast and mammalian cells have shown that transcription can relocate cohesin over long distances (Busslinger et al., 2017; Glynn et al., 2004; Gullerova and Proudfoot, 2008; Hu et al., 2015a; Lengronne et al., 2004). However, blocking transcription has little impact on the frequency of TOP2β cleavage at CTCF anchors (Canela et al., 2017), which likely occurs in response to loop extrusion. Others have proposed that DNA replication might also play a role (Ke et al., 2017). Our data show that cohesin extrusion occurs essentially independent of replication or transcription.
An obvious alternative to RNA or DNA polymerases is that cohesin translocation is driven by Smc ATPases themselves, as was recently shown for condensin (Terakawa et al., 2017). In support of this idea, some in vitro studies revealed that purified cohesin only requires ATP to move along DNA (Kanke et al., 2016). However, others have reported conflicting results using a similar in vitro model (Davidson et al., 2016; Stigler et al., 2016). Our in vivo studies now show that cohesin complexes carrying ATPase mutantions do reach CTCF anchors, although at diminished levels. One possibility is that this activity stems from residual ATP hydrolysis by the mutant subunit, perhaps when paired to a wild type subunit (Smc3MT/Smc1WT). Alternatively, and more interestingly, mutant complexes may homodimerize with wild type complexes (Smc3MT/Smc1WT:Smc3WT/Smc1WT) as predicted by the “hand-cuff” model of loop extrusion. Regardless of the mechanism at play, the results raise the interesting possibility that cohesin has intrinsic motor activity which facilitates its ability to slide along chromatin and extrude loops. This possibility is consistent with our degron experiments, which show conclusively that loop domains require ATP for establishment. However, additional experiments are required to directly test this hypothesis.
A side question is the source of ATP driving cohesin activity. In addition to ATP from mitochondria, recent studies have identified a pool of nuclear ATP generated via hydrolysis of poly(ADP-ribose) (Wright et al., 2016). However, this pathway is unlikely to activate cohesin in most cells, as it appears to be confined to differentiation steps during embryogenesis or breast cancer tumors exposed to high levels of hormones (Miguel Beato, personal communication).
We have also shown that not all processes associated with nuclear architecture require ATP. Treatment of B cells with oligomycin demonstrates that loop domains can be maintained for hours without energy input. This is consistent with a model where, once it has been arrested, the extrusion complex does not require ATP to continue tethering pairs of loci. Moreover, ATP depletion does not eliminate compartmentalization, demonstrating that once they have been established, compartments do not require ATP.
Transcriptional impact of loop extrusion
Various models have been put forward to explain the role of topology in gene expression. In the 1980s, the lac repressor was proposed to tether distantly located repressor sequences by DNA looping (Majumdar and Adhya, 1984). This same mechanism later explained transcriptional activation in mammalian cells, where PolII, Mediator and transcription factors bridge regulatory elements separated by vast distances. However, there are far more putative promoter-enhancer interactions than peaks seen by Hi-C (Rao et al., 2014). Thus, stable point-to-point looping may not be the dominant mechanism underlying functional interactions in mammalian cells.
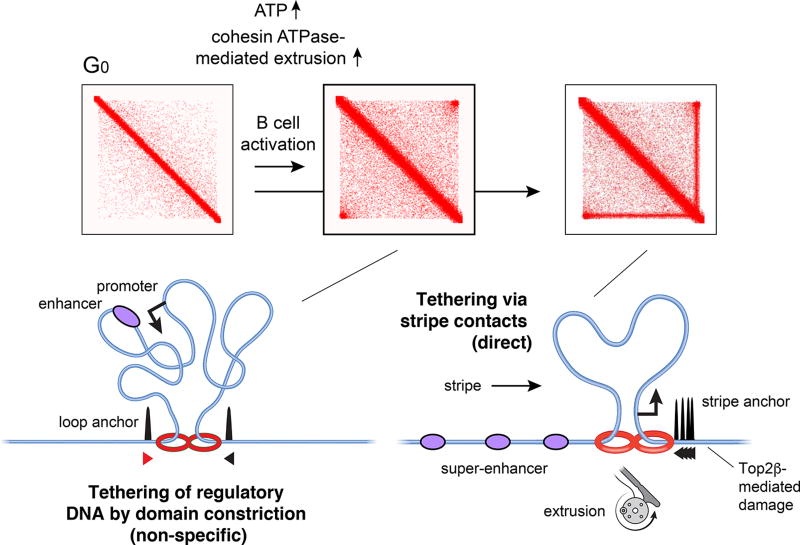
One possibility are transient interactions between regulatory DNA within loop domains (Hnisz et al., 2017). In this model, point-to-point interactions are not absolutely required to facilitate distal regulation; intra-interval proximity, combined with stochastic mixing, would be sufficient (Figure 7). When, during B cell activation, loop domains become more prominent, promoter-enhancer interactions also become more frequent, potentially contributing to transcriptional upregulation (Kieffer-Kwon et al., 2017). In this case, however, functional associations are not preferential since all sequences interact more frequently with one another upon domain constriction (Figure 7).
Figure 7. Transcriptional regulation by loop extrusion.
Based on the new and our recent findings (Kieffer-Kwon et al., 2017), we propose two mechanisms whereby nuclear architecture enhances transcription. First, as B cells enter the cell cycle, ATP synthesis increases, fueling cohesin ATPase-driven extrusion. Loop domains are thus constrained and intra-domain interactions (including regulatory DNA interactions) are increased “non-specifically”. Second, at sites with extensive loop extrusion, stripe contacts can directly tether promoters to enhancers, including SEs by a reeling in mechanism. As shown in Figure 6, this activity may disengage enhancer-enhancer loops and realigns them into functional interactions involving promoters near stripe anchors. In consequence of extensive extrusion, stripe anchors are hotspots for topoisomerase-induced damage.
Our data now propose a stripe model by which nuclear architecture can tether regulatory DNA. In this model, promoters or enhancers bound by CTCF near a strong loop anchor arrest a single extrusion subunit, while its partner continues to slide in the opposite direction. In this configuration, the CTCF-anchored element “reels in” cognate elements scattered over long distances (Figure 7). The model thus can explain how regulatory elements interact at SEs, which can span hundreds of kilobases of chromatin. Because of their ability to recruit high levels of Nipbl and their tendency to aggregate near domain boundaries, SEs are particularly well suited to form stripes. At the same time, it is important to point out that stripes are not unique to SEs.
A New Class of Loops Between Regulatory Elements
We have found that in the absence of ATP, regulatory elements form cliques, in which elements that lie Kb apart along the contour of the chromosome form loops with one another. It is reasonable to think that this feature might interfere with transcriptional activation, as tightly associated enhancers may be less likely to activate promoters. Thus, loop extrusion may ensure that, rather than interacting among themselves, enhancers are available to pair with cognate promoters.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to, and will be fulfilled by the Lead Contact, Rafael Casellas (rafael.casellas@nih.gov).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Source of cell lines and mouse models used in the study is reported in the Key Resourse Table. All mouse experiments were approved by the NIAMS Animal Care and Use Committee.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| FITC anti-IgA | Southern Biotech | Cat# 1040-02 |
| anti-CD180 (RP105) | BD PharMingen | Cat# 552128 |
| anti-CD40 | eBioscience | Cat # 160400186 |
| H3K27Ac | Abcam | Cat# ab4729 |
| RAD21 | Abcam | Cat# ab992 |
| Nipbl | Bethyl Laboratories | Cat# A301-779A |
| CTCF | Millipore | Cat# 07-729 |
| CTCF for ChIA-PET | Abcam | Cat# ab70303 |
| PolII | Abcam | Cat# ab817 |
| Actin | N/A | N/A |
| beta-tubulin | Sigma | Cat# T8328 |
| SNRP70 | Sigma | Cat# SAB2102255 |
| Bacterial and Virus Strains | ||
| pSpCas9(BB)-2A-GFP (PX458) | Addgene | Cat #48138 |
| pX330-U6-Chimeric_BB-CBh-hSpCas9 | Cong et al., 2013 | Cat #42230 |
| Z9-11 donor vector | This paper | N/A |
| pMy-BirA-T2A-eGFP | Nakahashi et al., 2013 | N/A |
| pMy-Smc3_Biotag- T2A-mOrange | This paper | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| hTGFbeta | R & D Systems | Cat#240-B-002 |
| IL-4 from mouse | Sigma | Cat# SRP3211 |
| LPS | Sigma | Cat# L2630 |
| FBS | Gemini | Cat# 100-500 |
| RPMI 1640 | Invitrogen (GIBCO) | Cat#R7388 |
| penicillin/streptomycin | Invitrogen (GIBCO) | Cat#15070063 |
| 2-beta mercaptoethanol | Invitrogen (GIBCO) | Cat# 21985-023 |
| recombinant murine IL6 | PeproTech | Cat#216-16 |
| puromycin | Sigma | Cat#P8833 |
| Flavopiridon | Sigma | Cat# F3055 |
| hydroxyurea | Sigma | Cat# H8627 |
| Oligomycin A | Sigma | Cat# 75351 |
| Micrococcal Nuclease | NEB | Cat# M0247S |
| 2-Deoxy-D-glucose | Sigma | Cat# D8375 |
| 37% formaldehyde | Sigma | Cat# F1635 |
| Glycine | Sigma | Cat#50046 |
| DpnII | NEB | Cat#R0543S |
| Csp6i | Fermentas | Cat# ER0211 |
| Tn5 transposase | Nextera kit, Illumina | N/A |
| Critical Commercial Assays | ||
| EasySep Mouse B Cell Isolation Kit | Stemcell technologies | Cat#19854 |
| Genomic extraction kit | Biotool | Cat#B4001 |
| ATP determination kit | Thermo Fisher | Cat#A22066 |
| Ovation Ultralow Library System V2 | Nugen | Cat#344 |
| PicoProbeAcetyl-CoA Fluorometric Assay Kit | BioVision | Cat#K317 |
| ChIP DNA clean and concentrator | ZymoResearch | Cat # 11379 |
| RNAeasy kit | QIAGEN | Cat# 74104 |
| DNeasy blood and tissue kit | QIAGEN | Cat #69504 |
| Epitect Bisulfite kit | QIAGEN | Cat# 59104 |
| MinElute PCR purification kit | QIAGEN | Cat# 28004 |
| Nucleofector Kit V | Lonza | Cat# VCA-1003 |
| Deposited Data | ||
| Raw and analyzed data | This paper | GEO: GSE82144 and GEO: GSE98119 |
| Experimental Models: Cell Lines | ||
| Mouse: murine CH12 ZF9-11 mutant | This paper | N/A |
| Mouse: primary splenic B cells | This paper | N/A |
| E14 tg2A mouse embryonic stem cells | ATCC | Cat # CRL-1821 |
| Mouse PCT 7134 plasmacytoma cell lines | Kovalchuk et al., 2012 | N/A |
| Experimental Models: Organisms/Strains | ||
| C57BL6 | Jackson Laboratory | JX664 |
| Oligonucleotides | ||
| SA_screening pcr primer F: CCACACGTGTGCTCACTTC | This paper | N/A |
| SA_screening pcr primer R: CTGCATACAAGGGCTGCAAG | This paper | N/A |
| ZF9-11 sgRNA CTGCGACAAGACCTTCCGCC | This paper | N/A |
| ZF9-11 screening pcr For1: ATGAGAAGCGCTTCAAGTGTGAC | This paper | N/A |
| ZF9-11 screening pcr Rev1: TACTCTCAGCCTACTCAAGTCAT | This paper | N/A |
| ZF9-11 screening pcr For2: TCAGGAGCGGCACATGATCAT | This paper | N/A |
| ZF9-11 screening pcr Rev2: TGTAACTGAAGATCAAGTGTGTGCT | This paper | N/A |
| SA_gRNA 1: GCGCTGCAATGGGAACCA | This paper | N/A |
| SA_gRNA 2: CCTTGAGTTAACCTGGGACCA | This paper | N/A |
| SApartial_gRNA1: GCGCTGCAATGGGAACCA | This paper | N/A |
| SApartial_gRNA2: CACCTGAGCACTACGCTGTGG | This paper | N/A |
| SApartial_screening F: CCACACGTGTGCTCACTTC | This paper | N/A |
| SApartial_screening R: AGGAAGATTGAGAACCACTGAGCT | This paper | N/A |
| 4C 3RR_DpnII GCTTATCTGTAAAGAATGGGTC | This paper | N/A |
| 3RR_Csp6i GGCCTTAGAAGGCTCTGTAC | This paper | N/A |
| Software and Algorithms | ||
| sgRNA CRISPR Design software | http://crispr.mit.edu |
◦ Mice
Resting splenic B-cells were isolated from 6–8 weeks old C57BL6/J mouse spleen by negative selection (Stemcell technologies) and were activated for 24–72h with LPS (50 gg/ml; Sigma), IL-4 (2.5 ng/ml; Sigma), and anti-CD180 (RP105) antibody (0.5 µg/ml, BD PharMingen).
◦ Cell lines
CH12 B lymphoma cell line was maintained and passaged every 2 days in RPMI 1640 supplemented with 10% FBS (Gemini), 1% penicillin/streptomycin (Invitrogen), 55 µM 2–3 mercaptoethanol (Invitrogen). E14 tg2A mouse embryonic stem cells were maintained as described in PMID:18555785. PCT 7134 plasmacytoma cell lines were generated as described in Kovalchuk et al 2012, (where Igm is juxtaposed 6.6Kb upstream of myc exon 1). Cells were maintained and passaged every 2 days in RPMI 1640 supplemented with 10%FBS, 1% penicillin/streptomycin, 55µM 2-β mercaptoethanol and 10 pg/ml of recombinant murine IL6 (PeproTech).
All cells were maintained at 37°C and 5% CO2 in a humidified incubator.
METHOD DETAILS
◦ CSR and flow cytometry
For CSR assays CH12 WT and SA partial and full deleted cell lines were activated for 6–72h with hTGFbeta (1ng/ml), IL-4 (5 ng/ml; Sigma) and anti-CD40 (5 ng/ml; eBioscience). After 72h cells were harvested and stained for surface expression of IgA using FITC-conjugated anti-IgA antibody (Southern Biotech).
◦ Smc3_WT and mutants B Cell Transduction
Mouse splenic B lymphocytes were preactivated overnight in the presence of 0.5 mg/ml of aCD180 (RP105) antibody. At 24 hrs cells were transduced with Smc3 WT or mutants (K38A, E1144Q) vectors (pMy-Smc3_biotag-T2A-mOrange) and pMy-BirA-T2A-eGFP by centrifugation for 90 min at 2,500 rpm, at 32C. B cell media was supplemented with 50 gg/ml of LPS, 2.5 ng/ml of IL-4, and 0.5 µg/ml of aCD180. At 32 hrs, cells were diluted to O.lxlO.cells per ml. Seventy-two hours after first infection, B cells were harvested and GFP/mOrange double positives were cell sorted using a BD FACSAria III (Becton Dickinson).
◦ Activated B cell transcription and replication inhibition
Resting splenic B-cells were activated for 20 or 30hrs and respectively treated 4hrs with flavopiridol (1µM, SIGMA) or 10mM hydroxyurea (HU, sigma).
◦ ChIP-seq
Cultured cells were fixed with 1% formaldehyde (Sigma) for 10' at 37°C. Fixation was quenched by addition of glycine (Sigma) at a final concentration of 125 mM. Twenty million fixed cells were washed with PBS and stored at −80°C until further processing or resuspended in 1 mL of RIPA buffer (10 mM Tris pH 7.6, 1 mM EDTA, 0.1% SDS, 0.1% sodium deoxycholate, 1% Triton X-100, 1 × Complete Mini EDTA free proteinase inhibitor (Roche)). Sonication was performed using Branson sonifier at amplitude 35%, 12 cycles of 20” sonication and 30” of pause. For native chip, chromatin was digested with Mnase (Sigma) in digestion buffer (50 mM Tris-HCl, pH7.6, 1 mM CaCl2, 0.2% Triton X-100, butyrate 5 mM) for 5′ at 37° C and dialyzed against RIPA buffer for 2hrs at 4° C. Five microgram of respective antibody was incubated with 40 µL of Dynabeads Protein A (or G) for 40 min at room temperature. Antibody-bound beads were added to 500 µL of sonicated or Mnase-digested chromatin, incubated at 4°C overnight, and washed twice with RIPA buffer, twice with RIPA buffer containing 0.3M NaCl, twice with LiCl buffer (0.25 M LiCl, 0.5% Igepal-630, 0.5% sodium deoxycholate), once with TE (pH 8.0) plus 0.2% Triton X-100, and once with TE (pH 8.0). For Smc3 WT and Mutants ChIP-Seq experiments, biotinylated samples were incubated with 40 pl of Dynabeads M-280 Streptavidin Beads (Invitrogen) overnight at 4°C in RIPA buffer. Beads were washed twice with Wash buffer 1 (2% [v/v] SDS), once with Wash buffer 2 (0.1% [v/v] deoxycholate, 1% [v/v], once with Wash buffer 3 (250 mM LiCl, 0.5% [v/v] NP-40, 0.5% [v/v] deoxycholate, 1 mM EDTA, and 10 mM Tris-HCl [pH 8.1]), and then twice with TE buffer (10 mM Tris-HCl [pH 7.5] and 1 mM EDTA). Crosslinking was reversed by incubating the beads at 65°C for 4 hr in the presence of 0.3% SDS and 1 mg/ml Proteinase K. ChIP DNA was purified by ChIP DNA clean and concentrator column (Zymo research). Library was prepared in Ovation SP Ultralow library system (Nugen). 50 cycles of sequencing data were acquired on HiSeq2000 or 2500 (Illumina). Antibodies used for ChIP-Seq are listed in the Key Resources Table.
◦ RNA-seq
Total RNA from 106 of WT or ZF9-11 CH12 cells, PCT 7134 plasmacytoma cell lines and mES was isolated by Trizol extraction. mRNA was then isolated and the standard RNA-Seq library preparation was performed following Illumina's RNA-Seq protocol v2.
◦ Western blot
Protein from CH12 nuclear and cytoplasmic fractions was transferred onto PVDF membranes and resolved by SDS-PAGE gel electrophoresis. Antibodies used for western blotting are listed in the Key Resources Table.
◦ In situ HiC
A detailed protocol to generate in situ HiC libraries including sequence alignment can be obtained at PMID:25497547. For the Hi-C2, we followed the procedure outlined in PMID:26499245 with minor modifications.
◦ B cell Acute ATP depletion
For acute ATP depletion, resting splenic B-cells were 20h-activated and shifted to glucose deficient media for 2h then, 2-Deoxy-D-glucose (5mM, Sigma) and Oligomycin (126nM, Sigma) were added to the culture for another 1.5–2h before the harvest. ATP level was measured by ATP determination kit (Thermo Fisher Scientific)
◦ Bi-Seq
Genomic DNA was isolated from 5×106 cells using Qiagen DNeasy blood and tissue kit. Libraries were prepared following whole-genome bisulfite sequencing for methylation analysis guide from Illumina (15021861_B) with slight modifications. Briefly, 5 µg of genomic DNA was sheared and blunt-ended with End-It DNA end repair kit (Epicenter) and A-tailed with Taq DNA polymerase (Invitrogen) in the presence of 200mM of dATP for 40 min at 70°C. Illumina compatible adaptors (5' PGATXGGAAGAGXGGTTXAGXAGGAATGXXGAG,5’ AXAXTXTTTXXXTAXAXGAXGXTXTTXXGATXT where X is a methylated cytosine) were then ligated with T4 DNA ligase (Enzymatics). Adapter-ligated DNA of 275–350 bp was isolated by 2% agarose gel electrophoresis, and sodium bisulfite conversion performed on it using the Epitect Bisulfite kit (Qiagen). Bisulfite converted DNA was divided in three tubes and PCR amplified for 6 cycles by PfuTurbo Cx hotstart DNA polymerase (Stratagene). The reaction products were purified using the MinElute PCR purification kit (Qiagen) then separated by 2% agarose gel electrophoresis and purified from the gel using the MinElute gel purification kit (Qiagen).
◦ END_Seq
The END-Seq method was performed as described inPMID:28735753.
◦ CRISPR/Cas9 mediated genome editing
Both CH12 and PCT 7134 plasmacytoma cell lines SA−/− and SApartial knock out and CTCF ZF9-11 knock in mutants were generated using CRISPR/Cas9 technique. Suitable sgRNA targets were identified using Optimized CRISPR Design (http://crispr.mit.edu) and cloned into pSpCas9(BB)-2A-GFP (PX458) and pX330-U6-Chimeric_BB-CBh-hSpCas9 (Addgene) as directed in PMID:24157548. For SA−/− a pair of gRNAs (GCGCTGCAATGGGAACCA and CCTTGAGTTAACCTGGGACCA) and SApartial (GCGCTGCAATGGGAACCA and CACCTGAGCACTACGCTGTGG) were cloned in pX458 and introduced into CH12 cell line using Nucleofector Kit V according to the manufacturer's instructions (Lonza). Twenty-four hours post-nucleofection, GFP positive cells were collected using a BD FACSAriaIII (Becton Dickinson) flow cytometer and seeded at a density of 1 cell/well in 96-well plates. Screening for full or partial deletions of SA region used oligonucleotides are listed in the Key Resources Table.
CH12 ZF9-11 mutant was generated replacing endogenous CTCF exons 7 to 10 with a cDNA carrying point mutations in ZF 9, 10 and 11 (as shown in our previous paper Nakahashi H et al 2013). A single sgRNA (CTGCGACAAGACCTTCCGCC) cloned in px330 vector and a DNA donor vector were introduced into CH12 as above. The donor vector included 500–1500 bp homology arms and a PGK_ Puromycin cassette to select for targeted clones. After 36h cells were treated with puromycin (0.8 µg/ml, Sigma). The day after puromycin was wash out and limiting dilution was performed in fresh media. Individual clones were picked and genomic DNA was extracted (Biotool™). Genotyping and sequencing were done by PCR using several locus-specific pairs of primers listed in the Key Resources Table.
◦ Stripe simulations
Molecular dynamics simulations were run as in PMID:26499245 using HOOMD-blue. Each polymer contains 1500 beads representing 1.5Mb of chromatin. Simulations were run for 150,000 timesteps with only Lennard-Jones intermonomeric forces and then for 800,000 timesteps with 6 extrusion complexes. Simulations were run with temperature at 1.75, 1.5, 1.25, or 1. Extrusion complex processivity was set to 400kb, gamma was set to 1.75, Lennard-Jones strength (epsilon) was set to 0.3. Between 300 and 350 replicates were run for each condition.
NIPBL-stimulated cohesin loading was modeled by increasing the on-rate of extrusion complexes at the two simulated NIPBL sites. Specifically, each monomer was assigned a weight proportional to the probability of initiating extrusion at that position; binding of the extrusion complex was no longer uniformly random. Binding of NIPBL within a loop domain shifted the large majority of extrusion complex loading to that position.
◦ 4C-Seq
The 4C assay was performed as previously described in PMID:22961246 with minor modifications. Ten million of CH12 B cell line and PCT 7134 plasmacytoma cell lines were crosslinked in 2% formaldehyde at 37°C for 10 min. The reaction was quenched by the addition of glycine (final concentration of 0.125 M). Cells were then washed with cold PBS and lysed (10 mM Tris-HCl, pH 8.0, 10 mM NaCl, 0.2% NP-40, 1× complete protease inhibitors [Roche]) at 4°C for 1h. Nuclei were incubated at 65°C for 30 min, 37°C for 30 min in 500 pl of restriction buffer (New England BioLabs DpnII buffer) containing 0.3% SDS. To sequester SDS, Triton X-100 was then added to a final concentration of 1.8%. DNA digestion was performed with 400 U of DpnII (New England Biolabs) at 37°C overnight. After heat inactivation (65°C for 30 min), the reaction was diluted to a final volume of 7 ml with ligation buffer containing 100 U T4 DNA Ligase (Roche) and incubated at 16°C overnight. Samples were then treated with 500 pg Proteinase K (Ambion) and incubated overnight at 65°C to reverse formaldehyde crosslinking. DNA was then purified by phenol extraction and ethanol precipitation. For circularization, the ligation junctions were digested with Csp6I (Fermentas) at 37°C overnight. After enzyme inactivation and phenol extraction, the DNA was religated in a 7 ml volume (1,000 U T4 DNA Ligase, Roche). Three micrograms of 4C library DNA was amplified with Expand Long template PCR System (Roche). Thermal cycle conditions were DNA denaturing for 2 min at 94°C, followed by 30 cycles of 15 s at 94°C, 1 min at 58°C, 3 min at 68°C, and a final step of 7 min at 68°C. Bait was amplified with inverse PCR primers as follows: 3RR with DpnII: _4C 5′-GCTTATCTGTAAAGAATGGGTC-3′, 3RR_Csp6i 5′-GGCCTTAGAAGGCTCTGTAC-3′. 4C-amplified DNA was microsequenced with the Illumina platform.
◦ GRO-seq
For global run-on and library preparation, nuclei were extracted from 20 million cells and after run-on reaction the RNA was extracted with Trizol LS Reagent (Life Technologies, Carlsbad, CA, USA). RNA was treated with TURBO DNase (Life Technologies), fragmented using RNA Fragmentation Reagents (Life Technologies) and purified by running through P-30 column (Bio-Rad, Hercules, CA, USA). The 3' end of the fragmented RNA was dephosphorylated with T4 polynucleotide kinase (PNK; New England Biolabs, Ipswich, MA, USA) followed by heat-inactivation. Dephosphorylation reactions were purified using anti-BrdU beads (SantaCruz Biotech, Santa Cruz, CA, USA) and precipitated overnight. Poly(A)-tailing and cDNA synthesis were performed the next day as described (Kaikkonen et al). However, for reverse transcription an oligo allowing custom barcoding during final amplification was used: /5Phos/GATCGTCGGACTGTAGAACTCTGAAC/iSp18/TCAGACGTGTGCTCTTCCGATCTTTTTTTTTTTTTTTTTTTTVN (IDT). After cDNA synthesis, Exonuclease I (New England Biolabs) was used to catalyze the removal of excess oligo. The DNA–RNA hybrid was purified using ChIP DNA Clean & Concentrator Kit (Zymo Research Corporation, Irvine, CA, USA), RNaseH treated and circularized. The libraries were amplified for 11 – 14 cycles with oNTI201-primer: 5′ -AATGATACGGCGACCACCGACAGGTTCAGAGTTCTACAGTCCGACG-3′ and a barcode specific primer oNTI200-index: 5 ′ -CAAGCAGAAGACGGCATACGAGATXXXXXXGTGACTGGAGTTCAGACGTGT GCTCTTCCGATCT (barcode XXXXXX underlined). The final product was ran on EX-gel (Thermo Fisher), purified and cleaned up as above.
◦ ChromRNA-seq
Chromatin RNA fraction was prepared from 3× 106 cells following the method previously described described (Pandya-Jones & Black, RNA 2009) with some modifications. The pellet was lysed in a cytoplasm lysis buffer (20mM Hepes-KOH ph 7.6, 2mM MgCl2, 10% glycerol, 0.1% NP40, 0.5mM DTT with protease inhibitor, phosphatase inhibitor and RNase inhibitor). The lysate was then layered on top of a sucrose buffer in order to isolate the nuclei fraction from the cytoplasmic one (10mM Hepes-KOH pH7.6, 10 mM NaCl, 1.5 mM MgCl2, 10% glycerol, 0.5 mM EDTA, 0.5 mM DTT, 34% sucrose w/v with protease inhibitor, phosphatase inhibitor and RNase inhibitor). The nuclei fraction was resuspended and lysed in a nuclear lysis buffer (10 mM Hepes-KOH pH 7.6, 100 mM NaCl, 0.5 mM EDTA, 50% glycerol, 0.5 mM DTT with protease inhibitor, phosphatase inhibitor and RNase inhibitor). Chromatin was precipitated by adding a Urea containing buffer (50 mM HEPES pH 7.6, 7.5 mM MgCl2, 0.2 mM EDTA, 0.6M NaCl, 2M urea, 2% NP-40, 2mM DTT).
The chromatin and the chromatin associated RNA were extracted using Trizol followed by a column extraction for RNA (RNAeasy kit from Qiagen) followed by DNAse treatment and a phenol-ethanol extraction for the protein isolation. The purity of the chromatin fraction was verified by western blot using the following antibodies: beta-tubulin (T8328, Sigma), SNRP70 (SAB2102255, Sigma) and H3K27Ac (ab4729, Abcam). Twenty five nanograms of RNA was used for RNA library preparations. These were carried out according to Ovation RNA-Seq Systems (NuGEN). Deep sequencing was performed using Hiseq3000.
◦ Long read ChlA-PET
The CTCF long-read ChIA-PET (ChIA-PET v2) libraries were performed as described in Tang et al., 2017. Briefly, approximately 100–200 million cells were harvested and fixed by 30 ml of 1.5 mM EGS (ethylene glycol bis[succinimidylsuccinate]) in PBS buffer for 45 min at room temperature. Next, formaldehyde was added to final concentration of 1% to cross-link the cells for another 20 min at room temperature and then neutralized with 0.125 M glycine. The cross-linked cells were lysed by cell lysis buffer and nuclear lysis buffer. Chromatin was obtained and subjected to fragmentation with an average length of 300 bp by sonication. The anti-CTCF polyclonal antibody (Abcam, ab70303) was used to enrich CTCF-bound chromatin fragments. ChIP DNA on beads was used for ChIA-PET library preparation. After performing the end-repair and A-tailing using T4 DNA polymerase (NEB) and Klenow enzyme, the ChIP DNA ends were proximity-ligated by the single biotinylated bridge-linker: Forward strand: 5'-[5Phos]CGCGATATC/iBIOdT/TATCTGACT-3', Reverse strand: 5'-[5Phos]GTCAGATAAGATATCGCGT-3', with the 3' nucleotide T over-hanging on both strands. Proximity ligation DNA was reverse cross-linked and fragmented and added sequencing adaptors simultaneously by using Tn5 transposase (Nextera kit, Illumina). DNA fragments contained the bridge linker at ligation junctions were captured by Streptividin beads, and used as templates for PCR amplification. These DNA products were then subjected to size-selection and paired-end sequencing (2×150 bp) using Illumina Hi-Seq 2500.
◦ HCT-116 degron system
HCT-116 RAD21-degron cells were cultured in McCoy’s 5A medium supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin, and 100ug/ml streptomycin at 37C with 5%CO2. Degradation of the AID-tagged RAD21 was induced by the addition of 500uM indole-3-acetic acid (IAA; Sigma Aldrich). In situ Hi-C experiments were performed as described above.
For auxin withdrawal, cells were treated or not with IAA, in the presence or not of specific inhibitors. After 6 hours treatment with 500um IAA, the cells were trypsinized, washed twice in fresh media and replated in 6-well plates in fresh media (with or without inhibitors) and the AID-tagged RAD21 degradation was measured at various time points (0, 1 and 4 hours) by flow cytometry.
For experiments with inhibitors, 300nM of Flavopiridol was added either together with 500um IAA or after 5hrs of IAA treatment. Thymidine (to inhibit DNA replication) was added at 2mM 18hrs prior IAA treatment and maintained throughout the experiment. For oligomycin/2DG experiments, the culture media was switched to glucose-free two hours after starting auxin treatment (still maintaining auxin treatment), and then treated with 40mM 2DG, 2uM oligomycin for either one and two hours prior to auxin withdrawal. All inhibitors were maintained during the auxin withdrawal.
◦ Bioinformatics
Software packages used:
-
-
Bedtools 2.17 to 2.25 (PMID: 20110278)
-
-
bedops/2.4.3 to 2.4.14 (PMID: 22576172)
-
-
Bowtie 1.1.1 (PMID: 19261174)
-
-
CASAVA 1.8.0 to 1.8.2
-
-
DESeq2 R package 1.10.1 (PMID: 20979621)
-
-
Fastqc 0.10.1 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc)
-
-
ggplot2 R package 0.9.3
-
-
MACS 1.4.2 and 2.0.10 (PMID: 18798982)
-
-
MaSC.pl Version 1.2.1 (PMID: 23300135)
-
-
picard 1.7.9 (http://picard.sourceforge.net)
-
-
R 2.15 to 3.3.2
-
-
samtools 0.1.18 to 1.2
-
-
UCSC Browser (PMID: 12045153)
-
-
gsnap version 2013-05-09 (PMID: 20147302)
-
-
htseq (PMID: 25260700)
-
-
Juicebox (PMID: 27467250)
-
-
Juicer (PMID: 27467249)
-
-
UCSC utilities 308–311
-
-
perl 5.18.2 (http://www.perl.org)
◦ Previously published data sets used in this study
Mouse G0 and activated B cell Bi-Seq data (SRX347820, SRX370339, SRX370352, SRX370353), DHS hotspots and footprints definition, annotation of DHS regions into Promoters and Enhancers (SRP029721, (PMID: 24360274)) Super-Enhancer definition, Regulatory clusters (groups of DHS elements, (PMID: 25483777).
QUANTIFICATION AND STATISTICAL ANALYSIS
◦ Processing of deep-sequencing data
Reads were sequenced using the illumina HiSeq 2000, 2500 or 3000, following the manufacturer's instructions. The standard pipeline (1.8.2) was used for image analysis and base calling. Data quality was inspected with fastqc. For 4C-seq, HiC-seq and ChIA-PET, we performed paired-end sequencing with 50-bp, 100-bp, 151-bp read-length, respectively. For others, we conducted single-end sequencing of 50-bp read-length.
◦ CTCF ChlA-PET Processing
The ChIA-PET data was processed by a customized ChIA-PET v2 data processing pipeline as described in (PMID: 26686651). Briefly, the bridge linker was scanned in each pair-end tag (PET) sequences and only the PETs with bridge linkers were used for downstream analysis. After trimming the bridge linkers, the flanking sequences of linker were mapped to the mouse reference genome mm9 using bwa-mem with default parameter settings (PMID: 20080505). Only the uniquely aligned PETs with mapping quality more than 30 were retained. PCR duplicates were removed using the MarkDuplicates tool of the Picard Tools library.
Each PET was categorized as either a self-ligation PET (two ends of the same DNA fragment) or inter-ligation PET (two ends from two different DNA fragments in the same chromatin complex) regarding to the genomic span between the two ends of a PET or origin of the two ends of a PET from two different chromosomes. PETs with two ends originating from the same chromosome and a genomic span less than 8 kb were classified as self-ligation PETs. Self-ligation PETs were used as a proxy for ChIP fragments since they were derived in a manner analogous to ChIP-Seq mapping for protein binding sites. PETs with two ends originating from the same chromosome and a genomic span more than 8 kb were classified as inter-ligation PETs. PETs with the two ends mapped to two different chromosomes were also classified as inter-ligation PETs. The inter-ligation PETs reflect the long-range chromatin interaction mediated by protein of interest. To accurately represent the frequency of interaction between two loci, both ends of inter-ligation PETs were extended by 500 bp along the reference genome, and PETs overlapping at both ends (with extension) were clustered together as one PET cluster. Individual inter-ligation PETs didn’t merged as PET clusters were referred as singletons. Singleton is similar to Hi-C data in a function to reflect high-order chromatin topology (Tang et al., 2015).
All uniquely mapped and non-redundant reads, including self-ligation and inter-ligation were used to pileup the CTCF binding coverage along the chromosomes for visualization. Also, all of these reads were applied to the MACS pipeline (version 1.4.2) for protein factor binding peak identification with default parameters. PET clusters with 3 or more PETs are referred to as interactions or connections and showed at the genome browser.
◦ 4C-seq Processing
Each read pair was tested for the presence of a perfect match to the respective bait primer as well as the bait spacer between the end of the primer and the restriction sites used in the corresponding experiment. These flanking sequences other than the restriction site were trimmed and the remainder was aligned against the mouse genome (July 2007; NCBI37/mm9) with Bowtie with the following command line options: ‘-X 500 −p 3 −v2−k2 −m1—phred64-quals -sam’, which reported all unique alignments with at most two mismatches. Alignments were then matched up with restriction sites and assigned to a DPNII/CSP6i fragment. Fragments were combined into 100-kb non-overlapping windows to determine the total number of 4C reads per fragment, and the fraction of restriction fragments for which 4C reads were found. Those procedures were carried out by the house-made python program. Processing of window-level data and visualization were conducted with R packages.
◦ Alignment of deep-sequencing data
Sequence reads were aligned to the mouse genome (July 2007; NCBI37/mm9) using bowtie with flags -S -m 1 -a --best --strata -n 2, and aligned reads were selected with samtools view -S -b -F4 and sorted. For GRO-seq, additionally two nucleotides from the 5' end were trimmed before aligning with option ‘—tirm5 2’.
◦ Visualization of density tracks
For PolII ChIP-seq track, we generated a single-nucleotide resolution coverage tracks to see the effect of ATP depletion in detail. Insert sizes were determined using MaSC. For high-resolution coverage tracks, individual sample alignment files were converted to bed format (bamToBed), and deduplicated, allowing maximally 2 reads at the same position with awk. Then tags were extended by the insert size, sorted and merged with bedops, and counted using genomeCoverageBed. The resulting bedGraph file was converted to bigWig with bedGraphToBigWig. For ChromRNA-seq and GRO-seq, strand specific density tracks were generated by using bedtools genomecov program with a normalizing scale factor to calculate rpm. For others, density tracks were generated using custom software based on the samtools library to count the number of reads in 100 bp windows normalized to window size and library size to obtain densities in units of reads per kb region per million mapped reads (rpkm) across the genome. Up to 2 redundant reads were allowed. Tracks were smoothed and sometimes log-transformed in the UCSC genome browser.
◦ RNA-seq data processing
Reads were aligned to the mouse genome (mm9) with gsnap without detecting splice junctions de novo (--novelsplicing=0). Existing splice junctions from RefSeq annotation were taken into account (--use-splicing=/path/to/mm9.splices.iit). Output files were filtered to remove unaligned reads and any alignments with a mapping quality less than 20. Reads were mapped to RefSeq genes with htseq-count -m intersection-nonempty and rlog-transformed counts, fold-change and adjusted p-values were calculated using the R package DESeq2. For some analysis, we calculated the rpkm from the counts directly, instead of DESeq2. Very low expressed genes (~ 10%) were filtered out to reduce noises in fold change calculation for comparison between the lost and unchanged loop domains in CH12 WT and MT cells.
◦ Composite Rad21 ChIP-Seq analysis
All Rad21, CTCF and Nipbl de-duplicated ChIP-seq algnment files and input alignment files are provided in MACS2 for the peak calling with default options. If the Rad21 peak regions are overlapped with only CTCF peaks in activated B cells, we called those as “anchors”. If the Rad21 peak regions are overlapped with only Nipbl peaks in activated B cells, we called those as “loading”. After extending the reads to the expected fragment size from MACS2, Rad21 signals were counted in a single nucleotide resolution around the peak centers of the activated B cell Rad21 sample. Each count is added 0.1 pseudo counts and then normalized with the number of mapped reads after deduplication. Trimmed mean values (+/− 10%) on each position were plotted. All plotting was done with the R package ggplot2.
◦ Composite Polll ChIP-Seq analysis
Single resolution rpkm values are calculated around the +/− 500 bp on non-overlapping TSSs by the home-made script. Enhancer sites are defined by DHS signals which are not overlapped with promoters and are similar both in activated and resting B cells as in accompanying paper. PolII signals on overlapping 10nt windows by sliding 1 nt were counted to get a single nucleotide resolution around the center of enhancer signals. After adding 0.1 pseudo counts, rpkm values are calculated. Trimmed mean values (+/− 10%) on each position around the middle point of peak were plotted. All plotting was done with the R package ggplot2.
◦ Rad21, Nipbl, CTCF peak overlap and dynamics of their binding
MACS2 was used for peak calling with corresponding input files and 0.001 p-value cutoff for each ChIPseq. Two peaks are considered overlapping if peak intervals are overlapped with each other. For peak numbers in overlapped regions, largest numbers were picked up to show in Venn diagram.
To show dynamics, shifted fragments by half of the fragment size (inferred by MACS2) were counted in each peak interval and their rpkm values are calculated where library size is the number of unique reads in each experiment. If a Rad21 peak is overlapped with more than one peaks of Nipbl or CTCF, mean rpkm value were taken. In the same manner, a Nipbl peak is overlapped with more than one CTCF peaks, mean rpkm value were taken. Three outlier Nipbl peaks were removed in plot. To show the scatterplot in the log2 scale for CTCF peaks, 0 rpkm (Rad21 or Nipbl peaks which are not overlapped with CTCF peaks) were converted into 1 which is smaller than the minimum value of rpkm of CTCF peaks. Top 5% outlier CTCF peaks rpkm values were set to the 95% value of them. All plotting was done with the R package ggplot2.
◦ Motif annotation (core, upstream, downstream)
Motif annotation was performed with fimo as previously described PMID: 23707059. Default setting were used for the core motif, while the command was modified to fimo -- text --motif 1 --thresh 0.001 for up- and downstream motives, to ensure all motives were found. Spacing cutoffs for UC, UCD, CD classification have been described PMID: 23707059.
◦ Hi-C data analysis
The following sections describe downstream computational analysis of Hi-C data. In each case, the input was normalized or un-normalized ligation frequency matrices, loop or domain calls obtained using juicer software.
aB cell specific loop domains for Nipbl analysis
Among the common and cell-type specific loop domains between G0 cells and normal activated B cells, we selected the empty loop domains which do not include inside loop domains, and of which anchors have convergent CTCF bindings sites confirmed through ChIP-seq. We called peaks for the Nipbl from the G0 cells to sum the read counts at Nipbl binding sites or to count Nipbl peaks normalized by the domain size within the loop domain including anchors. We also analyzed Nipbl binding sites at normal activated B cells. Same analysis on those loops were conducted with the Nipbl ChIP-seq in ATP depleted activated B cells.
Intra-domain interactions in CH12 WT compared to ZF9-11 MT
First, we converted the two-dimensional loop annotation into one-dimensional loop region annotation (from the start to the end of loop domains). By using bedtools intersect, we selected the unchanged and lost loop domains of CH12 WT. After sorting the result files, we merged loop domains within each category to deal with the overlapped loop domains by the bedtools merge -i command. Intra-domain intensity was calculated by summing all the contacts within the merged loop domains excluding the first three diagonals in the Hi-C contact matrices in 5-kb resolution, and then the ratio was calculated between WT and MT. For mRNA analysis, the lost loop-domains are further divided into two groups according to the intra-domain intensity ratio between WT and MT.
Insulation score
We adopted the method of computing the insulation score (PMID: 24185899). We defined an insulation score (IS) of an anchor, as the ratio of the average interaction strength at both sides of the central bin of the anchor to the interaction strength across the central bin of the anchor. IS was computed for a fixed genomic distance S. We considered Hi-C matrices at a resolution of 10kb and S was measured in the number of off-diagonals in the Hi-C matrix. S was set to 8.
To compute the interaction strength across the anchor, we considered a square of 3×3 pixels whereby the central pixel (i) represents the interactions between a pair of bins crossing the loop anchor and (ii) is located at the off-diagonal S. We summed the Hi-C signal in this square (X’ij). Next, we moved the 3×3 square by 11 bins (parameter D) in either the 5’prime direction (left inside, L’ij) or the 3’prime direction (right inside, R’ij) and summed the Hi-C signal in these positions.
We computed the insulation score as follows:
X’ij - the sum of the normalized Hi-C signal in the central square
D - distance (in the number of 10 kb bins) between midpoints of the central and left square, and between right and central square. Here, D=11
L’ij - the sum of the normalized Hi-C signal in the left square
R’ij - the sum of the normalized Hi-C signal in the right square
ISij - insulation score at pixel of coordinates i and j corresponding to the midpoint of the central square.
We empirically set D=11 and S=8 parameters based both on the assessment of the composite profiles of interactions in loop and stripe domains as well as on manual inspection of Hi-C matrices.
Automated identification ofstripes
All the analyses described in this section were performed using raw ligation frequency matrices and the normalized matrices generated using juicer software (the .hic files). The matrices were exported to a .txt format from the .hic files using the dump function of juicer. The stripe calling was implemented and performed in R using custom functions.
To identify stripes, we searched for instances whereby consecutive pixels displayed signal higher relative to the surrounding area. We searched for 3 and 5 prime stripes sparely.
As stripe anchors are typically 10–30kb wide, we considered in situ Hi-C data at a resolution of 10kb. We focused on bin pairs (pixels) separated by less than 3Mb. We removed pixels with an overall low signal coverage (<10 normalized read pairs). Given a complex nature of the signal at any pixel forming the stripe (Supplementary Figure S2B), to enhance the discovery, we followed an approach combining local data binning and the estimation of pixel-specific enrichment relative to its local neighborhood.
We computed local signal at the pixel in two directions (horizontal and vertical), summed the signal of the pixel and the signal of 2 preceding and 2 following pixels in the two orthogonal directions, and defined these two sums as the pixel’s horizontal and vertical signal.
In mathematical terms - the observed, locally binned signal at a pixel Xij, in the vertical and horizontal directions were defined as follows:
where:
Xij - the raw Hi-C ligation count in between bins i and j.
We evaluated the enrichment of Hi-C signal in the vertical and horizontal zones with respect to the in the pixels’ neighborhood. To represent the local neighborhood, we set four intervals adjacent to the pixel (Supplementary Figure S2B). To reduce the influence of outlier pixels on the estimate of the expected signal strength, we computed the medians (M) of the normalized signal as follows:
where,
S′i,j — the normalized Hi-C signal.
We also computed the median values of the local normalized background and median biases of pixel’s signal in the testing procedure.
where,
3 and 5 prime stripes were defined depending on whether they appeared as horizontal (3-prime stripe) or vertical lines (5-prime stripe) in the Hi-C contact map. We searched for 3-and 5-prime stripes separately. Below, we outline the methodology, taking as an example the identification of 3-prime stripes (Supplementary Figure S2B).
In the 3-prime stripe, for each pixel, the pixel’s local horizontal signal is higher than both the top and bottom aggregates. We inferred the expected signal strength given the local aggregates as follows:
where, that summarizes to the bases inferred during the iterative normalization (matrix balancing).
S′ - denotes the normalized Hi-C signal.
We tested whether the observed raw signal was higher than the expected values ( and ) with Poisson statistics (two tests per pixel).
We obtained a binary matrix where entries = 1 denoted pixels for which P-val.<0.05 and Fold change >1.1 for both comparisons from the above test. We next, searched for rows whereby an uninterrupted stretch of 6 pixels>0 could be identified. For such rows, we clustered the identified stretches of pixels into bigger ones. In cases where two consecutive stretches were separated by less than 5 pixels, the two were merged into a bigger stretch. This operation was continued until a stretch separated by more than 5 pixels from the current one was encountered. The remote stretch was a seed for an analogous clustering until no new seed was identified. Following this step, we obtained a list of putative stripes for a matrix. We considered only stripes which were at least 9 pixels long. We adopted an analogous procedure to identify 5-prime stripes, here we considered the vertical Left and Right zones and columns of the binary matrix instead of rows.
As depicted in Supplementary Figure S2B, we defined row coordinates of stripes as anchors of 3-primes stripes and column coordinates as domains of 3-prime stripes. Inverse was set in the case of 5-prime stripes (not shown).
Processing of stripe calls
The 3- and 5-prime stripe calls from Automated identification of stripes were exported to a .txt file and manually curated using juicebox. Sub-compartments, as judged from the chromosome-wide interaction matrix, were removed by considering matrices at 5kb resolution (estimated FDR = 30–40%). As outlined in the paper, stripe anchors corresponded almost perfectly to loop anchors. Our stripe identification algorithm was not designed to omit loop pixels from the stripe domain coordinates. To this end, loop coordinates were first extended by ±10kb and 10kb pixels corresponding to these enlarged areas removed from the stripe domain coordinates. In the final step, pixels were locally clustered (3- and 5-prime stripes were considered separately).
Comparison ofstripe calls between cell types
We considered anchors of processed stripes (Processing of stripe calls). To remove redundancy, we merged the overlapping stripe anchors in each condition. Anchors from two conditions were deemed as common if their genomic coordinates overlapped. The same procedure was applied when comparing stripe domains.
Comparison of stripe signal between B cell types
We considered stripe and loop coordinates identified in the activated B cell condition. Since the total sequencing depths varied between libraries, we considered the contact probabilities instead of the normalized read counts for comparisons. We took interaction matrices at the resolution of 10kb and computed the sum of the contact probabilities for pixels in each stripe (stripe signal). Finally, we computed the log2 of the ratio of the stripe signal in various conditions (G0, Myc−/− cells, oligomycin treated activated B cells) to stripe signal in the activated B cell condition. Random stripes were generated as outlined below in the section Assessment of the enrichment of chromatin features at stripe domains.
Comparison ofstripe signal between Chl2 wt and ZF9-11 mutant cells
We considered a list of manually curated stripe calls in the wt Chl2 Hi-C experiment. We removed the pseudo count transformation of the balanced Hi-C matrices and worked directly on the probability matrices. First, we identified loops lost in the mutant cells (loop signal FCwt/mut>1.5). Next, we computed the sum of the Hi-C signal for each stripe in both conditions. We then defined a list of potentially affected stripes as follows. We focused on stripes for which anchors overlapped genomic positions losing both CTCF and Rad21 binding in the mutant cells (FDR=1%, FCwt/mut>3). We further identified stripes for which the 5’ or the 3’prime or both anchors overlapped anchors of loops diminished in the mutant cells. Stripes that did not match both criteria (did not correspond to lost loops and did not diminish CTCF and Rad21 binding) were conceded as a control population.
Imbalance in Nipbl binding at the two anchors of stripes
We considered four groups of loops: (i) loops where none of the anchors produced a stripe (ordinary loops), (ii) loops where both anchors produced a stripe (both), (iii) loops for which only the 5-prime anchor produced a stripe (left) and, (iv) loops for which only the 3’ anchor produced a stripe (right). Next, we defined loop anchors as genomic intervals starting at the outermost position of the loop anchor and ending 20kb towards the interior of the domain. We identified Nipbl peaks in these intervals. For each loop, we then computed the cumulative MACS score of Nipbl peaks in the two loop anchor intervals separately (Nipbl left and right scores). We considered only those loops, for which we detected Nipbl peak at least one of the anchors. Finally, we computed the log2 of the ratio of Nipbl left to right score. Prior to the computation of the logarithm, we added a pseudo score=1 to each of the two anchors, to avoid the division by 0.
Estimation of the strength of contacts between regulatory element with respect to the intra-domain background
We used Hi-C data generated using B cells activated for 72h. We identified contact domains with the arrowhead algorithm as implemented in juicer (PMID: 25497547). This algorithm allows to annotate nested domains (i.e., domains that contain other domains inside). As this could interfere with our subsequent analyses, we considered only domains for which boundaries did not enclose any smaller domain.
We considered the normalized (balanced) Hi-C interaction maps at the resolution of 5kb. We removed interactions spanning less than 10kb and focused only on the intra-domain contacts. As regulatory elements, we considered 5kb bins overlapping at least one Dnasel hypersensitivity site (DHS, the coordinates of DHS sites were expanded by 5kb in both directions) and an active regulatory element (active promoter, active enhancer or super-enhancer; these elements were defined in the section Chromatin features - definition). As not regulatory elements, we considered bins that did not overlap: DHS nor regulatory element (neither active not inactive) nor CTCF nor Rad21 nor H3K27me3 nor H3K9m3 enriched regions.
For each regulatory element pair located in the same domain, we then computed the log2 of the ratio of the Hi-C signal between the bins overlapping both regulatory partners to the average signal between non-regulatory element pairs at the same genomic distance within the same domain. To make sure our estimation of the ratio is robust, we considered only ratios whereby we detected at least 3 non-regulatory element bin pairs at the corresponding genomic distance within the domain. Finally, we stratified the ratios according to whether both bins overlapped loop anchors (‘Loop’ class), at one of the bins overlapped stripe anchor (‘Stripe’ class) or none of the bins overlapped neither loop nor stripe anchor (‘Other’ class).
Relationship between the length of the stripe and DNA damage at stripe anchor
We used Hi-C data generated using B cells activated for 72h. We considered a set of non-redundant stripe anchors. To obtain the cumulative stripe width for each anchor, we summed-up the widths of stripe domains associated with it. We then divided the stripe anchors into categories according to the genomic span of the stripe domain. For each category, we assessed the fraction of stripe anchors that overlapped a double strand brake measured by END-seq (PMID: 27477910). We considered genomic intervals centered on the midpoint of each of the nonredundant stripe anchors extended by 15kb in both directions).
Epigenetic signature and cis regulatory element enrichment at stripes
This analysis was stratified based on whether we analyzed stripe domains or stripe anchors chromatin signature. Stripe domains anchors and anchors were defined as illustrated in Supplementary Figure S2B.
Chromatin features definition
Promoters, enhancers and super enhancers are defined by the previous activated B cells data (PMIDs: 25483777 and 24360274). Enhancers which have a little or no H3K27ac levels (less than 20%) are defined as the poised enhancers. Promoters which have higher Groseq signals (more than 40%) are defined as the active promoters. Inactive promoters are defined by the promoters which have no overlapped DHS signals. Active regulatory elements include active promoters, active enhancers and super enhancers.
Assessment of the enrichment of chromatin features at stripe domains
To produce the bar graph in Figure S5B, we considered three enhancer classes: active (H3K27ac+), and poised (H3K27ac−). We recorded the number of stripe domains overlapping an element and the number of stripe domains that did not overlap that class of elements. In parallel, we generated a population of random stripes. These random intervals were equal in size and genomic distance to stripe domains. The random stripes did not-overlap any stripe domain. We then referred the enrichment of regulatory elements in stripe domains to that obtained for random stripe domains.
Analysis of the directionality of CTCF at stripe anchors
We obtained CTCF motif directionality for CTCF peaks identified in the activated B cell condition using juicebox. To assess for the directionality of CTCF peak at stripe anchors, we considered only instances whereby all the peaks overlapping the anchor were attributed a CTCF motif.
Scaled loop domain profiles of ChIP-seq peaks and regulatory element enrichments
Loop domain was defined as the genomic interval demarcated by the start and the end of a loop (loops were obtained from HiCUPPS). For each loop we searched for an overlap with stripes. First, we identified stripes, based on the overlap between their anchor the loop anchors. We considered loop’s left anchor to find the overlapping horizontal stripes and loops’ right anchor to find the overlapping vertical stripes. We kept the overlapping instances for further filtering. Loops with left stripe were defined when loop left anchors overlapped a horizontal stripe anchor and the stripe domain was within the loop domain. The converse was done for loops associated with right stripes. Loop domains were next divided into 100 equally sized intervals (for each loop, Linterval_size=S) and ten bin of size S were added in silico to the start and end positions of the loop domain region (region start=loop start-10xS; region end=loop end+10xS). This resulted in 120 intervals for each loop. The overlap between ChIP-seq peak or a regulatory element in each of the intervals was scored. Y axes depict the % of loops in each class, with an overlap with a ChIP-seq peak or a regulatory element.
◦ Hi-C2 data processing and normalization
Hi-C2 in the Ch12 cell line
We used juicer to prepossess the libraries and obtain raw ligation frequency matrices at the resolution of 5 kb. We observed similar number of inter-chromosomal reads in each of the libraries, we therefore worked only with chromosome12 in the subsequent steps. The raw interaction matrix corresponding to chromosome 12 was retrieved from the .hic files (using the Mapq threshold of 30) using juicebox dump observed NONE command. Then the file was imported to R and using custom script, a chromosome-wide contact matrix suitable for normalization by balancing was created, as suggested in (PMID: 26499245). The number of in silico spike ins (Sins) was estimated by computing median of the chromosome-wide per-bin coverage of the local HiC matrix at the targeted region (IgH). Then the number of reads per bin pair resulting in the final coverage of each of the 5kb bin equal to Sins was sampled using Hi-C map obtained in the wt Ch12 cells (excluding the targeted region from the procedure). This resulted in a Hi-C matrix whereby all the rows had similar overall coverage. This matrix was then and normalized using Knight-Ruiz balancing algorithm with the juicebox pre −r 5000 command.
Hi-C2 in the 7134-tumor cell line
We first generated the fasta file corresponding to the tumor genome. We considered mm9 genome assembly and included chromosomes 1–11 and 13,14,16–19 and chromosome × without modifications. Based on our previous sequencing data, we assembled the translocated chromosome by connecting chromosome 12 piece 1–114,658,552 to chromosome 15: 61,810,260 onwards (chr12T15). We generated BWA index file and juicer to prepossess the libraries and obtain raw ligation frequency matrices at the resolution of 5 kb. We exported the raw interaction frequency matrix corresponding to this chromosome to R. To obtain the spike-in matrix for the normalization purpose we aligned one of our high density in situ Hi-C (activated B cells) against the 7134 tumor genome and processed as outlined in the section above. We exported the chromosome – wide matrix for chr12T15 and normalized it with juicebox using Knight-Ruiz balancing algorithm with the juicebox pre −r 5000 command.
Supplementary Material
(A) Scatter plot shows a correlation between Nipbl and Rad21 signals genome wide. The datapoints are color coded based on CTCF signals. Each point represents the integrated value of ChIP-Seq signals at Rad21 peaks. (B) Western blot showing expression of Rad21 (migrating at ~100KDa) and Actin control in activated B cells that are untreated or treated with oligomycin. (C) Histograms display the global distribution of Rad21 at Nipbl+ loading and CTCF+ anchor sites in G0 (red) vs. activated B cells (red). (D) Cell cycle analysis (DAPI) of B cells activated for 30h in the presence of hydroxyurea (red histogram) or untreated (blue histogram). Right ChIP-Seq tracks show a representative example of PolII profiles in the presence (upper) or absence (lower) of transcription. (E) Cohesin “relative translocation” computed as the ratio of Rad21 or Smc3 ChIP-Seq signals at anchors/loading sites under the indicated conditions. (F) Box plot comparing ChIP-Seq signals for WT Smc3, or mutants carrying E-Q or K-A substitutions. p (WT vs. E-Q or K-A) = 2.2e-16. (G) FACS analysis of Rad21-GFP recovery in HCT-116 cohesin degron cells where replication, transcription, or ATP synthesis was inhibited. Data are represented as mean ± SEM.
(A) Examples of stripes (black arrowheads) in in situ Hi-C maps from human B lymphoblastoid cell lines (left), mouse ES cells (middle), or mouse CH12 B cells (right). (B) Computational approach for stripe detection. Stripes appear as streaks of pixels displaying enriched signal as compared to the local background (top left panel, black box). For each pixel separated by less than 3Mb (not shown), six local neighborhoods were defined which either included the pixel (vertical and horizontal zones) or were situated around it (Top, Bottom, Left and Right zones; top right panel). Raw signal in the vertical and horizontal zone was aggregated. Medians of the normalized signal in all the zones computed. For each pixel, four expected interaction strengths were obtained – given the Top, Bottom the Left and Right signal medians. Poisson statistics was used to assess whether the vertical or horizontal signal of the pixel was significantly higher than the local signal. Then stripes were identified and clustered as follows. For each pixel, four values of fold changes and four associated P-values were obtained from the step above. The 5’ and 3’ stripe identification (vertical and horizontal respectively) was separated at this stage. In the horizontal stripe identification (bottom left panel, stripes from the left-hand side of the domains), the horizontal to Top and the horizontal to Bottom comparisons were considered. Pixels with FC > 1.1 and p-value < 0.05 were retained and clustered to obtain stripes. Stripe calls were manually filtered using Juicebox visualization tool. In the final step, loop contacts were removed from consideration (bottom right panel). An analogous approach was used to identify 5’ stripes, whereby the vertical to Left and vertical to Right ratios and p-values were considered (not shown). (C) Comparison of CTCF signals at left (blue line) and right (red line) anchors of loop (left graph) or stripe (right graph) domains, as determined by ChIP-Seq from activated B cells. Data are represented as mean ± SEM. (D) Representative example of defective binding of ZF9–11 mutant to upstream (U)-containing core (C) sites. ChIP-Seq values are plotted as reads per kilobase per million (rpm). Right violin plots: Rad21 signals (ZF9-11/WT) at binding sites carrying various combinations of core (C), upstream (U) and downstream (D) motifs.
(A) Upper: APA analysis showing the cumulative Hi-C signals at loops where CTCF is either unchanged or >3 fold depleted in mutant cells. Lower: representative ChIA-PET example showing the loss of loops in ZF9-11 cells at loci where CTCF binding is reduced. (B) Example of loop domain and stripe loss when CTCF ZF9-11 fails to bind to loop anchors. (C) Stripe (Hi-C) signals associated with anchors that display defective CTCF binding in ZF9-11 cells (red line) or no defect (black line). (D) Box plot shows Nipbl signals at loop anchors (+20Kb). Anchors were classified as not associated or associated with a stripe, either at the left, right, or both boundaries (double). Data are represented as mean ± SEM. (E) Composite profile of Rad21 binding at loop domains stratified based on the overlap with double, right, and no stripes as classified in activated B cells. Each loop domain identified with HiCUPPS was divided into 100 equally sized intervals and the overlap with Rad21 assessed (extra 10 intervals were added to the start and end positions). Y-axis depicts the % of loop domains in each class. (F) Molecular dynamic simulation of Hi-C data (temperature = 1.75) where cohesin is loaded near the left CTCF anchor (upper left domain) or right anchor (lower right domain)
(A) Composite profile of H3K27Ac signals at loop domains stratified based on their overlap with stripes (double, left, right, or absent). Each loop domain identified with HiCUPPS was divided into 100 equally sized intervals and the overlap with H3K27Ac assessed (extra 10 intervals were added to the start and end positions). Y-axis depicts the % of loops in each class, with an overlap with a H3K27Ac peak. (B) Bar graph depicting the enrichment of SEs, active conventional enhancer, and poised enhancers within stripe domains as compared to randomly generated domains of equal size and genomic distance. Poised enhancers were defined as H3K4me1+H3K4me3-H3K27Ac-. (C) Venn diagram showing the overlap between stripe anchors in activated B cells and ES cells. (D) Box plot compares DNA demethylation between activated B cells and ES cells in cell-type specific stripes associated with SEs. Data are represented as mean ± SEM. (E) Hi-C contact matrices show stripes associated with the Foxp1 SE in ES cells (left map) and activated B cells (right map). CTCF and Nipbl ChIP-Seq tracks and the location of the SEs are shown below. CpG demethylation was calculated as activated B - ES cell Bis-Seq signals.
(A) Hi-C matrices comparing contacts at the Igh domain in ES cells and B cells. ChIP-Seq tracks show the distribution of H3K27Ac, Nipbl, Rad21, or CTCF at the two loci. (B) Gene expression (mRNA-Seq) comparison of CSRdriving genes (The 53 CSR genes were described in (Xu et al., 2012); correlation coefficient = 0.98. (C) Percentage of Igμ → Igα class switch recombination in SA+/+, SApartial, and SA−/− cells (in triplicate). Data are represented as mean ± SEM. (D) Hi-C2 analysis comparison between SApartial (upper) and wild type CH12 B cells (lower half).
(A) 4C-Seq profiles of Igh SE interactions in the plasmacytoma line 7134. The region shown encompasses the chromosome breakpoint surrounding of Igh and Myc translocated regions in wild-type (SA+/+) and superanchor deleted (SA−/−) cells. (B) ChromRNA-Seq profiles at the translocated region in WT and SA−/− 7134 plasmacytoma cells. ChromRNA-Seq reads on both plus and minus strand are shown (top and bottom strands respectively). (C) Examples of loops gained at promoters upon ATP depletion in activated B cells.
Cohesin extrusion requires cohesin’s intrinsic ATPase activity
Extensive loading of cohesin near CTCF anchors creates architectural stripes
Architectural stripes facilitate cognate promoter-enhancer interactions
Stripe anchors are prime sites of tumor-inducing TOP2β DNA breaks
Cohesin continually extrudes loops of chromatin in vivo, relying on ATP to fuel the process.
Acknowledgments
We thank Kim Nasmyth, who independently noticed stripes in Hi-C data, for his valuable input on the manuscript. Megan Laycock for proofreading the manuscript. Gustavo Gutierrez for technical assistance. This work was supported by NIAMS, NCI, and NIAID funding and NIH Helix Systems (http://helix.nih.gov). Supported by a Paul and Daisy Soros Fellowship, a Fannie and John Hertz Foundation Fellowship, and a Cornelia de Lange Syndrome Foundation Grant to S.S.P.R. and an NIH New Innovator Award (1DP2OD008540-01), an NSF Physics Frontiers Center Award (PHY-1427654), the NHGRI Center for Excellence for Genomic Sciences (HG006193), the Welch Foundation (Q-1866), an NVIDIA Research Center Award, an IBM University Challenge Award, a Google Research Award, a Cancer Prevention Research Institute of Texas Scholar Award (R1304), a McNair Medical Institute Scholar Award, an NIH Encyclopedia of DNA Elements Mapping Center Award UM1HG009375, and the President’s Early Career Award in Science and Engineering to E.L.A. Work at CTBP was supported by NSF PHY-1427654, NSF-CHE-1614101, and Welch Foundation C-1792.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
D.L., E.L.A., A.N. and R.C. designed experiments.
L.V., A.P., S.S.P.R., S.N., K-R.K-K., N.P., A.O., L.B., X.L., A.K., S.-C.H., S.N., I.M., N.D., M.S., A.C. and E.S. performed experiments.
A.P., S.S.P.R., S.J., A.L.S., M.D.P., R.R.C., J.N.O., W.R., Y.M. and Z.T. analyzed data.
R.C. and E.L.A. wrote the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
CONTACT FOR REAGENT AND RESOURCE SHARING
Any requests may be directed to Rafael Casellas (rafael.casellas@nih.gov).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Source of cell lines used in the study is reported in the Key Resources Table. Mouse experiments were approved by NIAMS ACUC.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical significance was determined by Student T test using R.
References
- Aiden EL, Casellas R. Somatic Rearrangement in B Cells: It's (Mostly) Nuclear Physics. Cell. 2015;162:708–711. doi: 10.1016/j.cell.2015.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alipour E, Marko JF. Self-organization of domain structures by DNA-loop-extruding enzymes. Nucleic Acids Res. 2012;40:11202–11212. doi: 10.1093/nar/gks925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckouet F, Srinivasan M, Roig MB, Chan KL, Scheinost JC, Batty P, Hu B, Petela N, Gligoris T, Smith AC, et al. Releasing Activity Disengages Cohesin's Smc3/Sccl Interface in a Process Blocked by Acetylation. Mol Cell. 2016;61:563–574. doi: 10.1016/j.molcel.2016.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner C, Isoda T, Murre C. New roles for DNA cytosine modification, eRNA, anchors, and superanchors in developing B cell progenitors. Proc Natl Acad Sci U S A. 2015;2:12776–12781. doi: 10.1073/pnas.1512995112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birshtein BK. The role of CTCF binding sites in the 3' immunoglobulin heavy chain regulatory region. Front Genet. 2012;3:251. doi: 10.3389/fgene.2012.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busslinger GA, Stocsits RR, van der Lelij P, Axelsson E, Tedeschi A, Galjart N, Peters JM. Cohesin is positioned in mammalian genomes by transcription, CTCF and Wapl. Nature. 2017;544:503–507. doi: 10.1038/nature22063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canela A, Maman Y, Jung S, Wong N, Callen E, Day A, Kieffer-Kwon KR, Pekowska A, Zhang H, Rao SSP. Genome Organization Drives Chromosome Fragility. Cell. 2017;170:507–521 e518. doi: 10.1016/j.cell.2017.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson IF, Goetz D, Zaczek MP, Molodtsov MI, Huis In 'tVeld PJ, Weissmann F, Litos G, Cisneros DA, Ocampo-Hafalla M, Ladurner R, et al. Rapid movement and transcriptional re-localization of human cohesin on DNA. EMBO J. 2016;35:2671–2685. doi: 10.15252/embj.201695402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J, Mirny L. The 3D Genome as Moderator of Chromosomal Communication. Cell. 2016;164:1110–1121. doi: 10.1016/j.cell.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker A, de Laat W. The second decade of 3C technologies: detailed insights into nuclear organization. Genes Dev. 2016;30:1357–1382. doi: 10.1101/gad.281964.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, Hu M, Liu JS, Ren B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavahan WA, Drier Y, Liau BB, Gillespie SM, Venteicher AS, Stemmer-Rachamimov AO, Suva ML, Bernstein BE. Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature. 2016;529:110–114. doi: 10.1038/nature16490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudenberg G, Imakaev M, Lu C, Goloborodko A, Abdennur N, Mirny LA. Formation of Chromosomal Domains by Loop Extrusion. Cell Rep. 2016;15:2038–2049. doi: 10.1016/j.celrep.2016.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn EF, Megee PC, Yu HG, Mistrot C, Unal E, Koshland DE, DeRisi JL, Gerton JL. Genome-wide mapping of the cohesin complex in the yeast Saccharomyces cerevisiae. PLoS Biol. 2004;2:E259. doi: 10.1371/journal.pbio.0020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gostissa M, Yan CT, Bianco JM, Cogne M, Pinaud E, Alt FW. Long-range oncogenic activation of Igh-c-myc translocations by the Igh 3' regulatory region. Nature. 2009;462:803–807. doi: 10.1038/nature08633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullerova M, Proudfoot NJ. Cohesin complex promotes transcriptional termination between convergent genes in S. pombe. Cell. 2008;132:983–995. doi: 10.1016/j.cell.2008.02.040. [DOI] [PubMed] [Google Scholar]
- Hnisz D, Shrinivas K, Young RA, Chakraborty AK, Sharp PA. A Phase Separation Model for Transcriptional Control. Cell. 2017;169:13–23. doi: 10.1016/j.cell.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnisz D, Weintraub AS, Day DS, Valton AL, Bak RO, Li CH, Goldmann J, Lajoie BR, Fan ZP, Sigova AA, et al. Activation of proto-oncogenes by disruption of chromosome neighborhoods. Science. 2016;351:1454–1458. doi: 10.1126/science.aad9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C, Li L, Qin ZS, Corces VG. Gene density, transcription, and insulators contribute to the partition of the Drosophila genome into physical domains. Mol Cell. 2012;48:471–484. doi: 10.1016/j.molcel.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Itoh T, Mishra A, Katoh Y, Chan KL, Upcher W, Godlee C, Roig MB, Shirahige K, Nasmyth K. ATP hydrolysis is required for relocating cohesin from sites occupied by its Scc2/4 loading complex. Curr Biol. 2011;21:12–24. doi: 10.1016/j.cub.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Petela N, Kurze A, Chan KL, Chapard C, Nasmyth K. Biological chromodynamics: a general method for measuring protein occupancy across the genome by calibrating ChIP-seq. Nucleic Acids Res. 2015a;43:el32. doi: 10.1093/nar/gkv670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Zhang Y, Zhao L, Frock RL, Du Z, Meyers RM, Meng FL, Schatz DG, Alt FW. Chromosomal Loop Domains Direct the Recombination of Antigen Receptor Genes. Cell. 2015b;163:947–959. doi: 10.1016/j.cell.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, Ebmeier CC, Goossens J, Rahl PB, Levine SS, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–435. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanke M, Tahara E, Huis In't Veld PJ, Nishiyama T. Cohesin acetylation and Wapl-Pds5 oppositely regulate translocation of cohesin along DNA. EMBO J. 2016;35:2686–2698. doi: 10.15252/embj.201695756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer-Kwon K-R, Tang Z, Mathe E, Qian J, Sung M-H, Li G, Resch W, Baek S, Pruett N, Grøntved L, et al. Interactome maps of mouse gene regulatory domains reveal basic principles of transcriptional regulation. Cell. 2013;155:1507–1520. doi: 10.1016/j.cell.2013.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer-Kwon KR, Nimura K, Rao SSP, Xu J, Jung S, Pekowska A, Dose M, Stevens E, Mathe E, Dong P, et al. Myc Regulates Chromatin Decompaction and Nuclear Architecture during B Cell Activation. Mol Cell. 2017;67:566–578e510. doi: 10.1016/j.molcel.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk AL, Ansarah-Sobrinho C, Hakim O, Resch W, Tolarova H, Dubois W, Yamane A, Takizawa M, Klein I, Hager GL, et al. Mouse model of endemic Burkitt translocations reveals the long-range boundaries of Ig-mediated oncogene deregulation. Proc NatlAcad Sci USA. 2012;109:10972–10977. doi: 10.1073/pnas.1200106109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladurner R, Bhaskara V, Huis in't Veld PJ, Davidson IF, Kreidl E, Petzold G, Peters JM. Cohesin's ATPase activity couples cohesin loading onto DNA with Smc3 acetylation. Curr Biol. 2014;24:2228–2237. doi: 10.1016/j.cub.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengronne A, Katou Y, Mori S, Yokobayashi S, Kelly GP, Itoh T, Watanabe Y, Shirahige K, Uhlmann F. Cohesin relocation from sites of chromosomal loading to places of convergent transcription. Nature. 2004;430:573–578. doi: 10.1038/nature02742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, Amit I, Lajoie BR, Sabo PJ, Dorschner MO, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289–293. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupianez DG, Kraft K, Heinrich V, Krawitz P, Brancati F, Klopocki E, Horn D, Kayserili H, Opitz JM, Laxova R, et al. Disruptions of topological chromatin domains cause pathogenic rewiring of gene-enhancer interactions. Cell. 2015;161:1012–1025. doi: 10.1016/j.cell.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A, Adhya S. Demonstration of two operator elements in gal: in vitro repressor binding studies. Proc Natl Acad Sci U S A. 1984;81:6100–6104. doi: 10.1073/pnas.81.19.6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahashi H, Kwon KR, Resch W, Vian L, Dose M, Stavreva D, Hakim O, Pruett N, Nelson S, Yamane A, et al. A Genome-wide Map of CTCF Multivalency Redefines the CTCF Code. Cell Rep. 2013;3:1678–1689. doi: 10.1016/j.celrep.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K. Disseminating the genome: joining, resolving, and separating sister chromatids during mitosis and meiosis. Annu Rev Genet. 2001;35:673–745. doi: 10.1146/annurev.genet.35.102401.091334. [DOI] [PubMed] [Google Scholar]
- Natsume T, Kiyomitsu T, Saga Y, Kanemaki MT. Rapid Protein Depletion in Human Cells by Auxin-Inducible Degron Tagging with Short Homology Donors. Cell Rep. 2016;15:210–218. doi: 10.1016/j.celrep.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Nichols MH, Corces VG. A CTCF Code for 3D Genome Architecture. Cell. 2015;162:703–705. doi: 10.1016/j.cell.2015.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nora EP, Goloborodko A, Valton AL, Gibcus JH, Uebersohn A, Abdennur N, Dekker J, Mirny LA, Bruneau BG. Targeted Degradation of CTCF Decouples Local Insulation of Chromosome Domains from Genomic Compartmentalization. Cell. 2017;169:930–944 e922. doi: 10.1016/j.cell.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nora EP, Lajoie BR, Schulz EG, Giorgetti L, Okamoto I, Servant N, Piolot T, van Berkum NL, Meisig J, Sedat J, et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature. 2012;485:381–385. doi: 10.1038/nature11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SS, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, Sanborn AL, Machol I, Omer AD, Lander ES, et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159:1665–1680. doi: 10.1016/j.cell.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SSP, Huang SC, Glenn St Hilaire B, Engreitz JM, Perez EM, Kieffer-Kwon KR, Sanborn AL, Johnstone SE, Bascom GD, Bochkov ID, et al. Cohesin Loss Eliminates All Loop Domains. Cell. 2017;171:305–320 e324. doi: 10.1016/j.cell.2017.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JDP, Haarhuis JHI, Grimm JB, Rowland BD, Lavis LD, Nasmyth KA. Cohesin Can Remain Associated with Chromosomes during DNA Replication. Cell Rep. 2017;20:2749–2755. doi: 10.1016/j.celrep.2017.08.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JT, Turner D, Durand NC, Thorvaldsdottir H, Mesirov JP, Aiden EL. Juicebox.js Provides a Cloud-Based Visualization System for Hi-C Data. Cell Syst. 2018 doi: 10.1016/j.cels.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley MJ, Corces VG. The three-dimensional genome: principles and roles of long-distance interactions. Curr Opin Cell Biol. 2016;40:8–14. doi: 10.1016/j.ceb.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanborn AL, Rao SS, Huang SC, Durand NC, Huntley MH, Jewett AI, Bochkov ID, Chinnappan D, Cutkosky A, Li J, et al. Chromatin extrusion explains key features of loop and domain formation in wild-type and engineered genomes. Proc Natl Acad Sci U S A. 2015;112:E6456–6465. doi: 10.1073/pnas.1518552112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton T, Yaffe E, Kenigsberg E, Bantignies F, Leblanc B, Hoichman M, Parrinello H, Tanay A, Cavalli G. Three-dimensional folding and functional organization principles of the Drosophila genome. Cell. 2012;148:458–472. doi: 10.1016/j.cell.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Stigler J, Camdere GO, Koshland DE, Greene EC. Single-Molecule Imaging Reveals a Collapsed Conformational State for DNA-Bound Cohesin. Cell Rep. 2016;15:988–998. doi: 10.1016/j.celrep.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Luo OJ, Li X, Zheng M, Zhu JJ, Szalaj P, Trzaskoma P, Magalska A, Wlodarczyk J, Ruszczycki B, et al. CTCF-Mediated Human 3D Genome Architecture Reveals Chromatin Topology for Transcription. Cell. 2015;163:1611–1627. doi: 10.1016/j.cell.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terakawa T, Bisht S, Eeftens JM, Dekker C, Haering CH, Greene EC. The condensin complex is a mechanochemical motor that translocates along DNA. Science. 2017;358:672–676. doi: 10.1126/science.aan6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uuskula-Reimand L, Hou H, Samavarchi-Tehrani P, Rudan MV, Liang M, Medina-Rivera A, Mohammed H, Schmidt D, Schwalie P, Young EJ, et al. Topoisomerase II beta interacts with cohesin and CTCF at topological domain borders. Genome Biol. 2016;17:182. doi: 10.1186/s13059-016-1043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berkum NL, Dekker J. Determining spatial chromatin organization of large genomic regions using 5C technology. Methods Mol Biol. 2009;567:189–213. doi: 10.1007/978-1-60327-414-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Zan H, Pone EJ, Mai T, Casali P. Immunoglobulin class-switch DNA recombination: induction, targeting and beyond. Nat Rev Immunol. 2012;12:517–531. doi: 10.1038/nri3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Scatter plot shows a correlation between Nipbl and Rad21 signals genome wide. The datapoints are color coded based on CTCF signals. Each point represents the integrated value of ChIP-Seq signals at Rad21 peaks. (B) Western blot showing expression of Rad21 (migrating at ~100KDa) and Actin control in activated B cells that are untreated or treated with oligomycin. (C) Histograms display the global distribution of Rad21 at Nipbl+ loading and CTCF+ anchor sites in G0 (red) vs. activated B cells (red). (D) Cell cycle analysis (DAPI) of B cells activated for 30h in the presence of hydroxyurea (red histogram) or untreated (blue histogram). Right ChIP-Seq tracks show a representative example of PolII profiles in the presence (upper) or absence (lower) of transcription. (E) Cohesin “relative translocation” computed as the ratio of Rad21 or Smc3 ChIP-Seq signals at anchors/loading sites under the indicated conditions. (F) Box plot comparing ChIP-Seq signals for WT Smc3, or mutants carrying E-Q or K-A substitutions. p (WT vs. E-Q or K-A) = 2.2e-16. (G) FACS analysis of Rad21-GFP recovery in HCT-116 cohesin degron cells where replication, transcription, or ATP synthesis was inhibited. Data are represented as mean ± SEM.
(A) Examples of stripes (black arrowheads) in in situ Hi-C maps from human B lymphoblastoid cell lines (left), mouse ES cells (middle), or mouse CH12 B cells (right). (B) Computational approach for stripe detection. Stripes appear as streaks of pixels displaying enriched signal as compared to the local background (top left panel, black box). For each pixel separated by less than 3Mb (not shown), six local neighborhoods were defined which either included the pixel (vertical and horizontal zones) or were situated around it (Top, Bottom, Left and Right zones; top right panel). Raw signal in the vertical and horizontal zone was aggregated. Medians of the normalized signal in all the zones computed. For each pixel, four expected interaction strengths were obtained – given the Top, Bottom the Left and Right signal medians. Poisson statistics was used to assess whether the vertical or horizontal signal of the pixel was significantly higher than the local signal. Then stripes were identified and clustered as follows. For each pixel, four values of fold changes and four associated P-values were obtained from the step above. The 5’ and 3’ stripe identification (vertical and horizontal respectively) was separated at this stage. In the horizontal stripe identification (bottom left panel, stripes from the left-hand side of the domains), the horizontal to Top and the horizontal to Bottom comparisons were considered. Pixels with FC > 1.1 and p-value < 0.05 were retained and clustered to obtain stripes. Stripe calls were manually filtered using Juicebox visualization tool. In the final step, loop contacts were removed from consideration (bottom right panel). An analogous approach was used to identify 5’ stripes, whereby the vertical to Left and vertical to Right ratios and p-values were considered (not shown). (C) Comparison of CTCF signals at left (blue line) and right (red line) anchors of loop (left graph) or stripe (right graph) domains, as determined by ChIP-Seq from activated B cells. Data are represented as mean ± SEM. (D) Representative example of defective binding of ZF9–11 mutant to upstream (U)-containing core (C) sites. ChIP-Seq values are plotted as reads per kilobase per million (rpm). Right violin plots: Rad21 signals (ZF9-11/WT) at binding sites carrying various combinations of core (C), upstream (U) and downstream (D) motifs.
(A) Upper: APA analysis showing the cumulative Hi-C signals at loops where CTCF is either unchanged or >3 fold depleted in mutant cells. Lower: representative ChIA-PET example showing the loss of loops in ZF9-11 cells at loci where CTCF binding is reduced. (B) Example of loop domain and stripe loss when CTCF ZF9-11 fails to bind to loop anchors. (C) Stripe (Hi-C) signals associated with anchors that display defective CTCF binding in ZF9-11 cells (red line) or no defect (black line). (D) Box plot shows Nipbl signals at loop anchors (+20Kb). Anchors were classified as not associated or associated with a stripe, either at the left, right, or both boundaries (double). Data are represented as mean ± SEM. (E) Composite profile of Rad21 binding at loop domains stratified based on the overlap with double, right, and no stripes as classified in activated B cells. Each loop domain identified with HiCUPPS was divided into 100 equally sized intervals and the overlap with Rad21 assessed (extra 10 intervals were added to the start and end positions). Y-axis depicts the % of loop domains in each class. (F) Molecular dynamic simulation of Hi-C data (temperature = 1.75) where cohesin is loaded near the left CTCF anchor (upper left domain) or right anchor (lower right domain)
(A) Composite profile of H3K27Ac signals at loop domains stratified based on their overlap with stripes (double, left, right, or absent). Each loop domain identified with HiCUPPS was divided into 100 equally sized intervals and the overlap with H3K27Ac assessed (extra 10 intervals were added to the start and end positions). Y-axis depicts the % of loops in each class, with an overlap with a H3K27Ac peak. (B) Bar graph depicting the enrichment of SEs, active conventional enhancer, and poised enhancers within stripe domains as compared to randomly generated domains of equal size and genomic distance. Poised enhancers were defined as H3K4me1+H3K4me3-H3K27Ac-. (C) Venn diagram showing the overlap between stripe anchors in activated B cells and ES cells. (D) Box plot compares DNA demethylation between activated B cells and ES cells in cell-type specific stripes associated with SEs. Data are represented as mean ± SEM. (E) Hi-C contact matrices show stripes associated with the Foxp1 SE in ES cells (left map) and activated B cells (right map). CTCF and Nipbl ChIP-Seq tracks and the location of the SEs are shown below. CpG demethylation was calculated as activated B - ES cell Bis-Seq signals.
(A) Hi-C matrices comparing contacts at the Igh domain in ES cells and B cells. ChIP-Seq tracks show the distribution of H3K27Ac, Nipbl, Rad21, or CTCF at the two loci. (B) Gene expression (mRNA-Seq) comparison of CSRdriving genes (The 53 CSR genes were described in (Xu et al., 2012); correlation coefficient = 0.98. (C) Percentage of Igμ → Igα class switch recombination in SA+/+, SApartial, and SA−/− cells (in triplicate). Data are represented as mean ± SEM. (D) Hi-C2 analysis comparison between SApartial (upper) and wild type CH12 B cells (lower half).
(A) 4C-Seq profiles of Igh SE interactions in the plasmacytoma line 7134. The region shown encompasses the chromosome breakpoint surrounding of Igh and Myc translocated regions in wild-type (SA+/+) and superanchor deleted (SA−/−) cells. (B) ChromRNA-Seq profiles at the translocated region in WT and SA−/− 7134 plasmacytoma cells. ChromRNA-Seq reads on both plus and minus strand are shown (top and bottom strands respectively). (C) Examples of loops gained at promoters upon ATP depletion in activated B cells.