Abstract
Detecting mutations in single cells from cancer specimens is now a major area of translational research. In a recent article in this journal, Khalique et al validated an immunohistochemistry assay for ARID1A that reliably identifies loss of function mutations in single cells in tissue sections. This work exemplifies best practice for developing and orthogonally validating immunohistochemical assays to provide clearly interpretable mutational results with spatial context.
Keywords: Immunohistochemistry, Female reproductive system, Ovary, Mutation, Testing
The reputation of immunohistochemistry assays (IHC) as biomarkers for precision medicine is not the best. There is an impression in some quarters that interpreting different shades of brown is like reading tea leaves. Clinical IHC has been around for decades 1—so why do we have such a jaundiced view of its value for clinical biomarkers? Incomprehensibly, there is still wide use of low‐specificity antibodies and poorly standardized assays, resulting in variable biological and clinical signals. Over 20 000 publications using IHC are published each year and the majority should probably have never seen the light of day 2. By contrast, in a recent article in this journal Khalique and colleagues demonstrate what is needed to turn research‐grade IHC into a clinically useful assay for detecting mutations in ARID1A 3.
Their study rigorously validates three different ARID1A (AT‐rich interactive domain‐containing protein 1A; also called BAF250a) antibodies using an automated, standardized assay against the gold standard of next‐generation sequencing to detect ARID1A loss of function mutations. Staining from all three antibodies shows (almost) perfect agreement with very high concordance between loss of ARID1A expression and detection of predicted loss of function ARID1A mutations.
This finding is of immediate clinical relevance for diagnostic questions such as distinguishing clear cell and endometrioid histotypes of ovarian or endometrial carcinomas from histotypes in which ARID1A alterations do not occur 4, 5. While the prognostic value of ARID1A mutation is controversial, even contradictory, this well validated assay now enables large‐scale studies to definitively test prognostic utility 6. Looking forward, there is increasing clinical interest in the development of synthetic‐lethal treatments with loss of function of ARID1A mutations across a broad spectrum of tumors 7, 8, 9. The results from Khalique and colleagues now provide a robust biomarker for clinical trial inclusion and potentially a predictive biomarker for future therapies.
The history of ARID1A as a cancer‐relevant gene is relatively short. In 2010, two groups simultaneously identified recurrent ARID1A mutations in slightly less than half of endometriosis‐associated clear cell 10, 11 and endometrioid ovarian carcinomas 10. Since then, ARID1A mutations have been detected in many cancer types including endometrial endometrioid and clear cell carcinomas, urinary bladder carcinomas, Hodgkin lymphoma, gastroesophageal, colorectal and hepatobiliary carcinomas among others 12. ARID1A encodes one component of the SWI/SNF chromatin remodeling complex that facilitates target‐specific DNA binding and regulation of transcription by repositioning nucleosomes 6. Cancers with alterations in the SWI/SNF chromatin remodeling complex are not sensitive to conventional chemotherapy and have distinct clinical features 13, 14. Co‐inactivation of ARID1A and its paralog ARID1B give rise to particularly aggressive dedifferentiated endometrial and ovarian endometrioid carcinomas 15 and mutations in another SWI/SNF component SMARCA4 causes lethal small cell carcinoma of the ovary 16, 17, 18, 19. ARID1A‐targeted therapies are now in clinical development with trials focusing on cancers with loss of function ARID1A mutations (NCT02059265, NCT03297424; see https://clinicaltrials.gov/).
Although DNA sequencing is the gold standard for detecting ARID1A mutations, nonsequencing‐based assays still have important clinical utility. Firstly, next‐generation sequencing of ARID1A has been difficult owing to its large size and high GC content in exon 1. Secondly, the implementation of an IHC based test (once properly validated) has fewer barriers to implementation as the infrastructure required is inexpensive and has ISO accreditation in most pathology centers.
The origins of IHC can be traced back to the 1930s 20, 21 but wider clinical adoption had to wait until the 1980s, enabled by commercial‐scale production of monoclonal antibodies and other refinements allowing use of formalin‐fixed paraffin embedded tissue 1. Publications from the 1990s then showed that IHC could be used to identify mutant p53 22. However, it was not until very recently that an IHC assay could provide near‐perfect accuracy in predicting TP53 mutation status 23. Advancements have depended on 3 main factors: first, significantly improved sensitivity and specificity by using higher affinity rabbit monoclonal antibodies and newer polymer‐based detection systems with increased signal amplification. Second, robust standardization of experimental conditions from improved automated staining platforms. Third, improvements in interpretation by mandating use of normal cells as intrinsic controls for judging loss of protein expression 23. This has enabled sophisticated interpretation of abnormal p53 staining that maps the different classes of TP53 mutations to their functional consequences (Table 1).
Table 1.
Correlation of p53 immunohistochemical phenotype with TP53 genotype
| p53 staining pattern | Interpretation | TP53 mutation status |
|---|---|---|
| Nuclear staining in variable distribution and intensity | Wild type pattern | Wild type* |
| Diffuse strong overexpression in virtually all tumor cells | Mutant | Nonsynonymous/missense mutation |
| Complete absence with retained internal control | Mutant | Loss of function mutations including indels, stopgains and splicing mutations |
| Cytoplasmic (uncommon) | Mutant | Loss of function mutation disrupting nuclear localization domain |
with the exception of truncating or splicing TP53 mutations that may result in wild type IHC staining observed in 2–4% of tubo‐ovarian high‐grade serous carcinomas.
There are limitations to the ARID1A IHC assay from Khalique and colleagues that should be kept in mind. First, while most ARID1A mutations are loss of function mutations, there are a number of recurrent missense mutations that may impair ARID1A function, but do not appear to change protein expression. For a clinical trial, using only IHC could result in contamination of ARID1A‐competent trial populations. The extent to which IHC could miss deleterious ARID1A missense mutations is unknown and requires further study.
Second, ARID1A loss can be subclonal, indicating a later mutational event during tumor evolution. Temporal modeling of endometrial endometrioid carcinomas showed that only a portion of ARID1A mutations were truncal 24. Subclonal ARID1A mutations are common in mismatch repair deficient (MMR‐D) cancers because ARID1A has numerous short mononucleotide repeats that have a high chance of slippage errors (Figure 1) 25, 26. In such cases, convergent evolution of different ARID1A alterations resulting in phenotypically indistinguishable but mechanistically distinct loss of ARID1A protein can occur 24.
Figure 1.
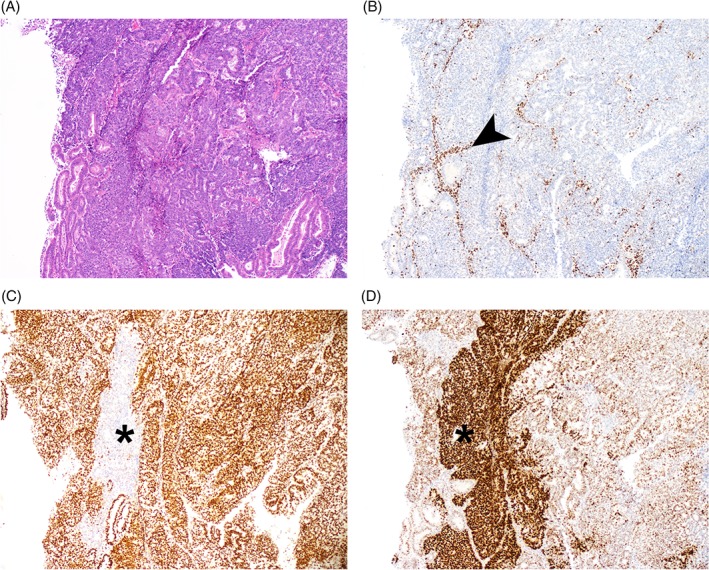
(A) H&E photomicrograph of endometrial endometrioid carcinoma, grade 3; (B) absence of MSH6 suggesting mismatch repair deficiency (additional MSH2 absence not shown). The arrow shows positive internal control stromal staining; (C) small focus of subclonal ARID1A loss (asterisk); (D) focus of p53 overexpression overlying but extending beyond the ARID1A focus (asterisk). Taken together these panels suggest a prototypical somatic mismatch repair deficiency resulting in subclonal TP53 missense alteration, and subsequent ARID1A loss of function mutation.
Subclonal loss may also be critical in identifying premalignant lesions. In the example of endometriosis, malignant transformation is rare and no biomarkers exist to distinguish “premalignant” endometriosis from the much more common chronic disease. A handful of studies have implicated subclonal loss of ARID1A immunoreactivity in endometriosis without co‐existing cancer (i.e. loss in only a subset of endometriosis glands and/or loss limited to contiguous epithelium within a gland) 27, 28, 29—although validation of these findings with orthogonal sequencing data is very limited 30. Now that we have high quality IHC tools, there is certainly sufficient justification for larger scale investigation of ARID1A loss (and mutation) in endometriosis, particularly if cohorts can be identified where progression to cancer has occurred. A clear understanding of how to interpret heterogeneous/subclonal ARID1A staining will undoubtedly accelerate such studies.
Finally, the mechanisms explaining total loss of ARID1A protein are still elusive. Unlike TP53, loss of heterozygosity at the ARID1A locus has not been widely reported, and at least for clear cell and endometrioid ovarian carcinomas, does not appear to be common 31, 32. In the cohort by Khalique and colleagues 8/45 (18%) cases harbored more than one ARID1A mutation and similar rates (6/27; 22%) have previously been reported 32. Systematic analyses are required to validate whether (or when) both alleles are affected and to rule out alternative possibilities, such as the presence of two co‐existing subclones carrying unique alterations.
Despite these caveats, the report from Khalique and colleagues suggests that ARID1A IHC can reliably detect loss of function mutations and is ready to be accepted as a clinical‐grade test. A categorical scoring system should now be agreed upon to distinguish between normal retained/present ARID1A expression and abnormal/absent expression, and whether this is complete or subclonal. As for p53 IHC, ARID1A IHC has the potential to achieve near‐single‐cell resolution for detecting mutations whilst also providing detailed information about the spatial context of mutated cells. IHC based prediction of ARID1A mutation now joins a small and exclusive group of well‐validated and clinically useful predictive IHC biomarkers.
Author contributions statement
MK, MSA and JDB conceptualized the commentary. MK wrote the initial draft, MSA and JDB contributed to subsequent versions and all authors approved the final version.
Invited commentary for Khalique et al. Optimised ARID1A immunohistochemistry is an accurate predictor of ARID1A mutational status in gynaecological cancer.
The authors declare no conflict of interest.
Reference
- 1. Matos LL, Trufelli DC, de Matos MG , et al. Immunohistochemistry as an important tool in biomarkers detection and clinical practice. Biomark Insights 2010; 5: 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ioannidis JP. Why most published research findings are false. PLoS Med 2005; 2: e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khalique S, Naidoo K, Attygalle AD , et al. Optimised ARID1A immunohistochemistry is an accurate predictor of ARID1A mutational status in gynaecological cancers. J Pathol Clin Res 2018; 4: 154–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Köbel M, Rahimi K, Rambau PF , et al. An immunohistochemical algorithm for ovarian carcinoma typing. Int J Gynecol Pathol 2016; 35: 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen W, Husain A, Nelson GS , et al. Immunohistochemical profiling of endometrial serous carcinoma. Int J Gynecol Pathol 2017; 36: 128–139. [DOI] [PubMed] [Google Scholar]
- 6. Wu RC, Wang TL, Shih IM. The emerging roles of ARID1A in tumor suppression. Cancer Biol Ther 2014; 15: 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Williamson CT, Miller R, Pemberton HN , et al. ATR inhibitors as a synthetic lethal therapy for tumors deficient in ARID1A. Nat Commun 2016; 7: 13837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller RE, Brough R, Bajrami I , et al. Synthetic lethal targeting of ARID1A mutant ovarian clear cell tumors with dasatinib. Mol Cancer Ther 2016; 15: 1472–1484. [DOI] [PubMed] [Google Scholar]
- 9. Bitler BG, Aird KM, Garipov A , et al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A‐mutated cancers. Nat Med 2015; 21: 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wiegand KC, Shah SP, OM Al‐Agha , et al. ARID1A mutations in endometriosis‐associated ovarian carcinomas. N Engl J Med 2010; 363: 1532–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones S, Wang TL, Shih IM , et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science 2010; 330: 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cerami E, Gao J, Dogrusoz U , et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012; 2: 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kadoch C, Hargreaves DC, Hodges C , et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat Genet 2013; 45: 592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McBride MJ, Kadoch C. Disruption of mammalian SWI/SNF and polycomb complexes in human sarcomas: mechanisms and therapeutic opportunities. J Pathol 2018; 244: 638–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Köbel M, Hoang LN, Tessier‐Cloutier B , et al. Undifferentiated endometrial carcinomas show frequent loss of core switch/sucrose nonfermentable complex proteins. Am J Surg Pathol 2018; 42: 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kupryjanczyk J, Dansonka‐Mieszkowska A, Moes‐Sosnowska J , et al. Ovarian small cell carcinoma of hypercalcemic type ‐ evidence of germline origin and SMARCA4 gene inactivation. a pilot study. Pol J Pathol 2013; 64: 238–246. [DOI] [PubMed] [Google Scholar]
- 17. Ramos P, Karnezis AN, Craig DW , et al. Small cell carcinoma of the ovary, hypercalcemic type, displays frequent inactivating germline and somatic mutations in SMARCA4. Nat Genet 2014; 46: 427–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jelinic P, Mueller JJ, Olvera N , et al. Recurrent SMARCA4 mutations in small cell carcinoma of the ovary. Nat Genet 2014; 46: 424–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Witkowski L, Carrot‐Zhang J, Albrecht S , et al. Germline and somatic SMARCA4 mutations characterize small cell carcinoma of the ovary, hypercalcemic type. Nat Genet 2014; 46: 438–443. [DOI] [PubMed] [Google Scholar]
- 20. Marrack J. Nature of antibodies. Nature 1934; 133: 292–293. [Google Scholar]
- 21. Coons AH, Creech HJ, Jones RN , et al. The demonstration of pneumococcal antigen in tissues by the use of fluorescent antibody. J Immunol 1942; 45: 159–170. [Google Scholar]
- 22. Iggo R, Gatter K, Bartek J , et al. Increased expression of mutant forms of p53 oncogene in primary lung cancer. Lancet 1990; 335: 675–679. [DOI] [PubMed] [Google Scholar]
- 23. Köbel M, Piskorz AM, Lee S , et al. Optimized p53 immunohistochemistry is an accurate predictor of TP53 mutation in ovarian carcinoma. J Pathol Clin Res 2016; 2: 247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gibson WJ, Hoivik EA, Halle MK , et al. The genomic landscape and evolution of endometrial carcinoma progression and abdominopelvic metastasis. Nat Genet 2016; 48: 848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ye J, Zhou Y, Weiser MR , et al. Immunohistochemical detection of ARID1A in colorectal carcinoma: loss of staining is associated with sporadic microsatellite unstable tumors with medullary histology and high TNM stage. Hum Pathol 2014; 45: 2430–2436. [DOI] [PubMed] [Google Scholar]
- 26. Allo G, Bernardini MQ, Wu RC , et al. ARID1A loss correlates with mismatch repair deficiency and intact p53 expression in high‐grade endometrial carcinomas. Mod Pathol 2014; 27: 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Samartzis EP, Samartzis N, Noske A , et al. Loss of ARID1A/BAF250a‐expression in endometriosis: a biomarker for risk of carcinogenic transformation? Mod Pathol 2012; 25: 885–892. [DOI] [PubMed] [Google Scholar]
- 28. Stamp JP, Gilks CB, Wesseling M , et al. BAF250a expression in atypical endometriosis and endometriosis‐associated ovarian cancer. Int J Gynecol Cancer 2016; 26: 825–832. [DOI] [PubMed] [Google Scholar]
- 29. Chene G, Ouellet V, Rahimi K , et al. The ARID1A pathway in ovarian clear cell and endometrioid carcinoma, contiguous endometriosis, and benign endometriosis. Int J Gynaecol Obstet 2015; 130: 27–30. [DOI] [PubMed] [Google Scholar]
- 30. Anglesio MS, Papadopoulos N, Ayhan A , et al. Cancer‐associated mutations in endometriosis without cancer. N Engl J Med 2017; 376: 1835–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anglesio MS, George J, Kulbe H , et al. IL6‐STAT3‐HIF signaling and therapeutic response to the angiogenesis inhibitor sunitinib in ovarian clear cell cancer. Clin Cancer Res 2011; 17: 2538–2548. [DOI] [PubMed] [Google Scholar]
- 32. Wang YK, Bashashati A, Anglesio MS , et al. Genomic consequences of aberrant DNA repair mechanisms stratify ovarian cancer histotypes. Nat Genet 2017; 49: 856–865. [DOI] [PubMed] [Google Scholar]


