Abstract
Background:
Risperidone is one of commonly utilized antipsychotic in clinical practice. Various metabolizing enzymes effect the plasma levels of risperidone and its active metabolite and thus its clinical efficacy. So, we attempted to evaluate the relationship between CYP2D6*10 (rs1065852) and CYP2D6*4 (rs3892097) gene polymorphism and the plasma concentration of risperidone and its metabolite in patients with schizophrenia.
Methodology:
It was a 12-week prospective study carried out in patients diagnosed with schizophrenia. The dose of risperidone was increased weekly by 1 mg and rating of psychopathology was done using Positive and Negative Syndrome Scale (PANSS). Quantification of plasma level of risperidone and 9-hydroxyrisperidone was carried out at week 6 and 12 of treatment. The *4 and *10 alleles of CYP2D6 were genotyped and their effect on metabolism of risperidone was assessed.
Results:
The number of CYP2D6*4 alleles affected the plasma levels of risperidone, 9-hydroxyrisperidone at 6 weeks of treatment but not at 12 weeks. On the other hand, the number of mutated alleles for CYP2D6*10 influenced the dose corrected plasma concentration of risperidone and active moiety at 12 weeks of treatment. The ratio of plasma concentration of risperidone and 9-hydroxyrisperidone was more than one in all study participants, thus, suggesting that they were poor metabolizers of risperidone.
Conclusion:
The polymorphism of CYP2D6*10 affects the steady state plasma concentration of risperidone in Indian patients with schizophrenia.
Keywords: 9-hydroxyrisperidone, CYP2D6, risperidone, schizophrenia
INTRODUCTION
Cytochrome P450 enzyme superfamily is involved in various oxidation/reduction reactions used in the metabolism of a range of endogenous and exogenous substrates. The vastly studied CYP2D6 variant is involved in metabolism of more than 40 drugs that include tricyclic antidepressants, antipsychotics, beta-blockers, anti-arrhythmics, anti-diabetics, and so on.[1] CYP2D6 enzyme exhibits high polymorphism consequent to different allelic combinations, and thus their frequencies vary substantially across the world populations.[2] Furthermore, depending on these combinations, individuals may display different metabolizing status: namely, poor metabolizers (PMs), intermediate metabolizers (IM), extensive metabolizers (EM), or ultra-rapid metabolizer (UM). Earlier studies carried out in healthy populations from India have shown that the prevalence of PM ranges from 0.6% to 3.5%.[1,3,4,5,6] In a South Indian study, the prevalence of CYP2D6 alleles in the descending order was CYP2D6*1, *2, *10, *4, and *5.[5] A similar pattern was observed in the Western Indian population.[7] However, an extensive genotype profile for different Indian population is not available.
Risperidone, a commonly used atypical antipsychotic, is mainly metabolized by the CYP2D6 enzyme. The resultant product, 9-hydroxyrisperidone (9-OHR), in turn, is mainly metabolized by CYP2D6 and in part by CYP3A4/A5 enzymes.[8] Both risperidone and 9-OHR are equipotent in their affinity to dopaminergic receptors and have similar pharmacological activity. The total plasma concentration of risperidone and 9-OHR is often called “active moiety,” and some researchers have suggested that the levels of active moiety are not influenced by CYP2D6 gene.[9] Previous pharmacogenetic studies have shown a significant presence of the CYP2D6*10 allele in Asian populations,[10] while it is rare in the Caucasians. Two of the earlier Japanese studies[11,12] and a Korean study[13] showed that the steady-state concentration of risperidone but not 9-OHR was influenced by CYP2D6*10 allele. However, in another Japanese study, Kakihara et al.[14] did not find any significant difference between plasma concentrations of risperidone, 9-OHR, or active moiety on the basis of presence or absence of CYP2D6*10 alleles. Similarly, two other studies[15,16] did not find any difference in the levels of active moiety of risperidone on the basis of the presence of CYP2D6 inactive allele CYP2D6*4. However, only a few pharmacogenetic studies have used multiple CYP2D6 alleles to classify their study subjects into PM, EM, or UM.[17,18,19]
Furthermore, it has been shown that mutations of CYP2D6 are less prevalent in some European countries while it is more prevalent in certain counties in the African and Asian continents which have large number of migrated populations.[7] Therefore, the results of pharmacogenetic studies conducted in one ethnic population cannot be generalized to others. A few earlier studies conducted in India have tried to evaluate the genotype profile of CYP2D6 and explored its association with susceptibility to tardive dyskinesia in chronic schizophrenia patients[20] and metabolism of venlafaxine in patients with depression[21] or association with Alzheimer's disease.[22] But no study about the effect of CYP2D6 polymorphism on antipsychotic drug metabolism is available.
Therefore, this study aimed at evaluation of the role of CYP2D6*10 (rs1065852) and *4 (rs3892097) gene polymorphism on plasma concentrations of risperidone and 9-OHR in North Indian patients with schizophrenia.
MATERIALS AND METHODS
This study, sponsored by the Indian Council of Medical Research, New Delhi, was carried out as per the national ethical guidelines for biomedical and health research involving human participants. This article is a part of a multicentric study that was undertaken from April 2011 to March 2014 at six centers across North India. The primary aim of this study was an assessment of the association of genotypic polymorphisms of dopamine receptors, serotonin receptors, and CYP 450 2D6 with schizophrenia. We also evaluated the association of the studied gene polymorphisms with response to risperidone in patients with schizophrenia in Indian setting (published elsewhere).[23] In addition, at Chandigarh center, we studied the effect of CYP2D6 gene polymorphism on plasma concentration of risperidone and 9-OHR.
A total of 443 patients who have schizophrenia were included in this study across different study centers. The patients included in this study were between the ages of 18 and 55 years and had experienced acute exacerbation of illness at the time of recruitment. Other inclusion criteria were the provision of consent by the patient and a family member who in turn monitored drug compliance too. Patients with additional Axis-I diagnosis including intellectual disability, patients with metabolic syndrome, those receiving long-acting antipsychotic agents, those with a comorbid severe medical/surgical illness, and those who refused to consent were excluded. In all, 24 patients were excluded due to lack of sufficient data or non-provision of a blood sample for genetic analysis, and 88 patients dropped out at various stages of the study. Another sample of 443 genetically related healthy controls was recruited, which comprised first-degree relatives of the patients, who consented to participate in the study, of either gender, between 15 and 70 years of age and also monitored the drug compliance of patients. The second control group comprised 150 genetically unrelated healthy controls. The control sample required exclusion of individuals who had any Axis-1 diagnosis, intellectual disability, substance use disorder other than tobacco abuse or dependence, and any severe medical or surgical illness.
During the study period, 1195 new patients were diagnosed to be suffering from schizophrenia, schizotypal disorder, or delusional disorders (F20- F29) at our center. Out of these, 109 consecutive patients diagnosed with schizophrenia according to ICD-10 DCR who met the inclusion and exclusion criteria were enrolled. Anticholinergic drug (trihexyphenidyl) and benzodiazepines (lorazepam or diazepam), for control of extrapyramidal symptoms and sleep disturbance, respectively, were the only other drugs that were allowed. Most of the benzodiazepines are metabolized by CYP3A4 and CYP2C19, while trihexyphenidyl is not a substrate for CYP2D6 enzyme. In addition, benzodiazepines and trihexyphenidyl do not induce/inhibit the CYP enzymes; thus, the metabolism of risperidone is not affected by their concurrent administration. Patients who were on oral antipsychotics and were non-responders to that antipsychotic were switched to risperidone. A washout period of 7 days was given before switching to risperidone, which in turn was increased by 1 mg/week, starting from day 1 of inclusion in the study to 12 mg at week 12. The dosage of risperidone was titrated depending on the achievement of response or development of adverse drug effects, and dosage more than 12 mg was not given. Additionally, the dose of risperidone was predominantly administered as a single dose or if required twice daily, and blood sampling was done after 12 h of the last dose. Plasma concentration of risperidone was first measured at 6 weeks of treatment because in most of the patients, adequate clinical response would have been achieved at this dose,[9] and again at 12 weeks so that adequate duration of trial and dose of risperidone would have been achieved by this time.
Clinical assessments
A proforma was devised for the recording of patient information that included personal details, clinical details, symptoms, socio-demographic details and relevant investigations. Positive and Negative Syndrome Scale (PANSS)[24] was used at baseline and subsequently at each week to monitor psychopathology.
Measurement of risperidone and 9-OHR in plasma by high-performance liquid chromatography
Four milliliter blood sample was collected in a heparinized vacutainer for measurement of plasma level of risperidone and 9-OHR at weeks 6 and 12. High-performance liquid chromatography as described earlier,[25] with minor modifications, was used for measurement of risperidone and 9-OHR. Chromatographic separations were carried out on a C-18 (3 μm × 100 mm × 4.6 mm ID). The mobile phase of potassium dihydrogen phosphate (0.05 M, pH 3.7 with 25% H3PO4):acetonitrile (70:30, v/v) was delivered at a flow rate of 1 mL/min. Triethylamine (0.3%) was added to suppress ionization of solutes. UV-visible detector with variable wavelength was used for detection. Concentration values were obtained using chromatography data system software.
DNA analysis for genotyping of CYP2D6
DNA isolation
Genomic DNA was isolated from whole blood samples using standard phenol/chloroform protocol. DNA samples were checked for both the quantity and the quality using spectrophotometer by measuring absorbance at 260 and 280 nm at a specific dilution. The ratio of OD260nm/280nm of isolated DNA more than 1.7 was used for genetic analysis. DNA fragmentation was checked by agarose gel electrophoresis.
Genetic analysis of CYP2D6 gene polymorphisms
Genetic analysis for CYP2D6 gene polymorphisms *4 and *10 was carried out using the technique of polymerase chain reaction (PCR)-based restriction fragment length polymorphism. Variants of CYP2D6 gene were amplified with appropriate primers using PCR. Amplification of DNA for each amplicon was checked by analyzing amplified products on an agarose gel of different concentrations depending on the size of PCR products. PCR products were incubated with different restriction enzymes at temperatures as recommended by the manufacturer's protocol. The loss or gain of restriction sites was detected using agarose gel (2%) electrophoresis or by polyacrylamide gel electrophoresis and by visualization under Gel Documentation System.
Statistical analysis
Statistical analyses were done using Statistical Package for Social Sciences (SPSS version 15; Chicago, IL, USA). Means and frequencies were calculated for continuous and categorical data, respectively, and comparison between groups was done by Chi-square test. Kolmogorov–Smirnov test was used to assess normal distribution of data. Student's t-test was used for comparison of two groups and analysis of variance (ANOVA) for comparison of more than two groups. Yates corrected Chi-square value or Fisher's exact value was used wherever applicable. For multiple comparisons in ANOVA, post hoc tests with Bonferroni correction were applied. A two-tailed P value of 0.05 or less was considered to be significant.
RESULTS
We had sociodemographic data and initial clinical data for all the study participants (N = 109). The patients who dropped out (N = 29) after initial enrollment were not considered for later analyses that required measurement of plasma levels of risperidone and 9-OHR. The sociodemographic and clinical details of the study participants are mentioned in Table 1. The majority of the participants were male (59.6%), were married (59.6%), were unemployed (73.4%), lived in a nuclear family setup (55%), had a family income of less than 5000 INR (57.8%), and hailed from a rural background (63.3%). Nearly a half had a formal education of 10 or more years (51.4%). The most common onset of illness was either acute or subacute (46.8%) followed by an insidious onset in nearly one-third of patients, and the illness largely followed a continuous course in two-third of patients. The mean duration of illness was 62.44 months [standard deviation (SD) =65.77], and slightly less than half (45%) of the patients had a family history of mental illness. The mean dose of risperidone administered at weeks 1, 6, and 12 was 2.90 (SD = 1.08; N = 109), 5.34 (SD = 1.73; N = 80), and 6.70 (SD = 1.60), respectively. The frequency distribution of dose of risperidone administered to the patients at week 1, 6 and 12 has been shown in Figure 1.
Table 1.
Socio-demographic and clinical details of study participants
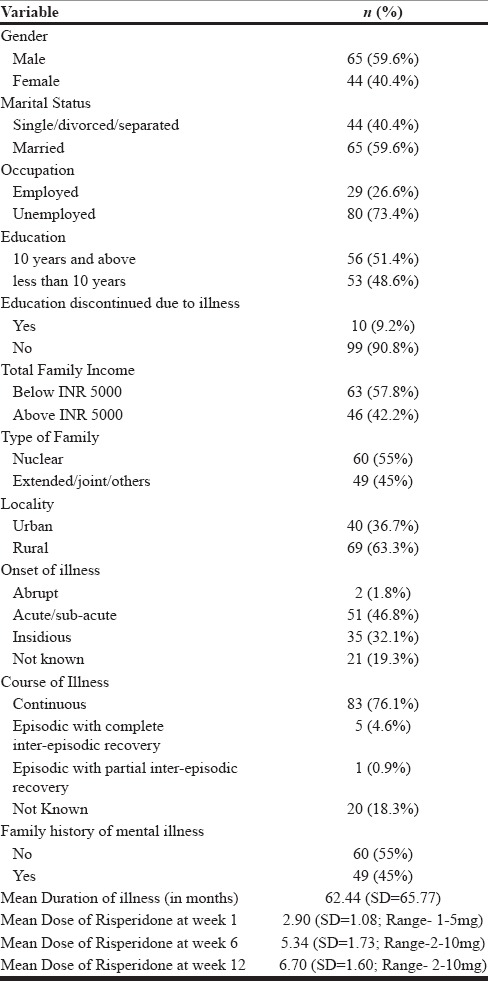
Figure 1.
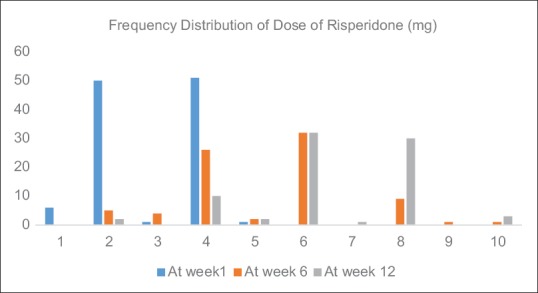
Frequency distribution of dose of risperidone administered to study participants at week 1 (N = 109), week 6 (N = 80; 29 drop-outs), and week 12 (N = 80; 29 drop-outs)
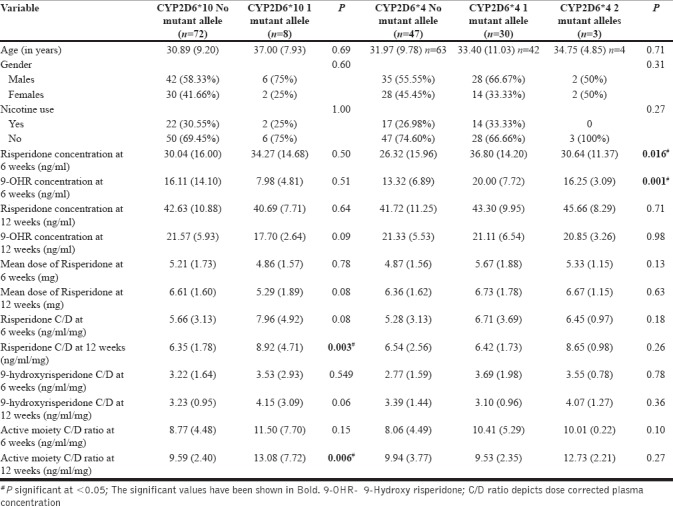
We analyzed two CYP2D6 genotypes, namely, CYP2D6*10 (rs1065852) and CYP2D6*4 (rs3892097), for their influence on the concentration of risperidone and 9-OHR. We found 47, 30, and 3 patients, respectively with no, one, and two mutated alleles of rs1065852 (CYP2D6*4), while 72 patients with schizophrenia had a wild variant and 8 patients had one mutated allele of rs3892097 (CYP2D6*10). No significant differences in gender distribution, age, or smoking status were found among the different genotype groups of CYP2D6*10 and *4 [Table 2]. The distribution of both the alleles in patients and genetically related and unrelated healthy controls is provided in Supplementary Table 1 (328KB, tif) .
Table 2.
Relationship between the CYP2D6*10 & 4 gene polymorphisms and plasma levels of risperidone and its metabolites
Distribution of CYP2D6*4 & *10 alleles in the study population
Relationship between the number of CYP2D6*10 and 4 alleles and plasma levels of risperidone, 9-OHR, and active moiety
The number of CYP2D6*4 alleles significantly affected the mean concentration of risperidone and 9-OHR at the end of 6 weeks of study, but this difference did not remain significant at the end of 12 weeks of treatment [Table 2]. Post hoc analysis (with Bonferroni correction) showed that pairwise comparison of plasma concentration of risperidone and 9-OHR at 6 weeks was significant between those without any mutation and with one mutant allele of CYP2D6*4 genotype (P = 0.02 and 0.001, respectively). But pairwise comparison for the groups with no mutant allele versus two mutant alleles and one mutant allele versus two mutant alleles of CYP2D6*4 did not reach significance on post hoc analysis. Similarly, the presence of mutation in CYP2D6*10 led to significantly higher levels of dose-corrected concentration of risperidone [calculated by plasma concentration of risperidone (ng/mL)/dose of risperidone (mg)] level at week 12 (P = 0.003) as well as that of active moiety at end of 12 weeks (P = 0.006). However, there was no significant difference between the dose of risperidone at 6 and 12 weeks of treatment among various groups depending on the presence of mutated alleles (See Supplementary Figures 1 (207.8KB, tif) –8 (205.5KB, tif) ). We also calculated the ratio of risperidone and 9-OHR as an index of CYP2D6 activity as suggested in a previous study.[26] This ratio was more than 1 at 6 and 12 weeks of treatment and showed no significant difference between patient groups based on the number of mutated alleles of CYP2D6*4 and *10 genotypes (data not shown). This suggests that most of the patients were PMs of risperidone.
The figure shows comparison of mean concentration of risperidone (95% Confidence Interval) according to the genotypic distribution of CYP2D6*4 at week 6 of treatment
The figure shows comparison of mean concentration of 9-hydroxyrisperidone (95% Confidence Interval) according to the genotypic distribution of CYP2D6*4 at week 6 of treatment
The figure shows comparison of mean concentration of risperidone (95% Confidence Interval) according to the genotypic distribution of CYP2D6*4 at week 12 of treatment
The figure shows comparison of mean concentration of 9-hydroxyrisperidone (95% Confidence Interval) according to the genotypic distribution of CYP2D6*4 at week 12 of treatment
The figure shows comparison of mean concentration of risperidone (95% Confidence Interval) according to the genotypic distribution of CYP2D6*10 at week 6 of treatment
The figure shows comparison of mean concentration of 9-hydroxyrisperidone (95% Confidence Interval) according to the genotypic distribution of CYP2D6*10 at week 6 of treatment
The figure shows comparison of mean concentration of risperidone (95% Confidence Interval) according to the genotypic distribution of CYP2D6*10 at week 12 of treatment
The figure shows comparison of mean concentration of 9-hydroxyrisperidone (95% Confidence Interval) according to the genotypic distribution of CYP2D6*10 at week 12 of treatment
Relationship between CYP2D6 polymorphism and response to treatment with risperidone
The outcome of treatment with risperidone was measured by reduction of PANSS score over the study period of 12 weeks. The participants who had a reduction of less than 25%, 25%–50%, and >50% from baseline to 6 and 12 weeks of the study were categorized as non-responders, partial responders, and responders, respectively. At the end of 6 weeks, 36 patients were non-responders, 43 were partial responders, 1 patient was a responder, and 29 had dropped out of treatment. However, at the end of 12 weeks of treatment, 9 were categorized as non-responders, 52 as partial responders, 19 as responders, and 29 as drop-outs. At the end of 6 weeks of treatment, significant difference for the mean dose of risperidone at 6 weeks (P = 0.015) was found between the groups (drop-outs excluded). Similarly, at the end of 12 weeks of treatment, significant difference was observed for the mean dose of risperidone prescribed at 6 weeks (P = 0.026) as well as at 12 weeks (P = 0.007) among responders, partial responders, and non-responders. However, no difference was found for plasma drug concentration and response to treatment (details in Kaur et al.).[23] Similarly, no difference in PANSS score at baseline, 6, and 12 weeks of treatment was noted for groups classified on the basis of presence or absence of mutant alleles for CYP2D6*4 and *10.
DISCUSSION
This study is the first Indian study that evaluated the effect of CYP2D6 polymorphism on plasma concentration of risperidone in patients with schizophrenia. In this study, CYP2D6*10 gene polymorphism affected the dose-corrected concentration of risperidone and active moiety at 12 weeks of treatment. Though CYP2D6*4 alleles too appeared to affect the concentration of risperidone and 9-OHR at the initial stage of treatment, the effect did not last at the end of 12 weeks.
CYP2D6 gene is highly polymorphic, with more than 100 different alleles described till date. CYP2D6*1 is considered to be wild type and exerts a normal functioning. However, many other alleles (CYP2D6*9, *10, *17, *41, etc.) are shown to have reduced function or an absent enzyme activity (CYP2D6*3, *4, *5, *6, *7, *11, *12, etc.).[27] CYP2D6*10 is one of the common gene polymorphism which has been investigated for its influence on metabolism of various drugs, including psychotropics. In one of the earliest study conducted in Japanese patients with schizophrenia,[28] significant differences of steady-state plasma concentration of risperidone and ratio of the concentration of risperidone to 9-OHR were observed among the groups with no, one, or two mutated alleles of CYP2D6 (*5 or *10) genotypes. Similarly, in a Chinese study[29] conducted in 118 patients with schizophrenia, it was shown that the ratio of risperidone/9-OHR was highest in patients with two alleles of CYP2D6*10 (0.42 ± 0.25) when compared with those with CYP2D6*10/*1 or *1/*1 alleles. Two other studies conducted in Japanese patients with schizophrenia reported that the number of CYP2D6*10 alleles significantly affect steady-state plasma levels of risperidone after 4 weeks of treatment but not that of 9-OHR and active moiety.[11,12] However, Yagihashi et al.[30] reported that CYP2D6*10/10 genotype was associated with higher steady-state levels of active moiety. In another Chinese study,[31] it was shown that significantly higher levels of risperidone and risperidone/9-OHR ratio were present in patients with two mutated alleles of CYP2D6*10 in comparison to those with one and no mutated allele. Our study results are partly in consonance to these studies because the number of CYP2D6*10 alleles did affect the steady-state plasma levels of risperidone and the active moiety but not that of 9-OHR. In addition, the differences were only significant at 12 weeks of treatment with risperidone.
Gene polymorphism coding for CYP2D6*3/*4/*5/*6 and so on are also commonly studied because they render the enzyme non-functional. Riedel et al.[16] assessed risperidone plasma levels, clinical response, and adverse effects in 51 patients who carried wild-type alleles for CYP2D6*4 and 8 patients with heterozygous alleles, but did not find any significant difference between response rates and plasma levels of active moiety. Jovanovic et al.[17] had reported significant impact of CYP2D6 polymorphism (*3, *4, *5, and *6) and plasma concentration of risperidone, 9-OHR, and active moiety, but the impact of *4 allele was not mentioned. Some other studies have used CYP2D6*4 in combination with other non-functioning alleles and correlated with plasma concentration of risperidone and its active metabolites[15,32] or did not find any patient carrying the allele in the study population.[19] Although we found significantly higher plasma concentration of risperidone and its active metabolite in patients with one or two mutant alleles of CYP2D6*4, the difference did not persist to a significant level at the end of 12 weeks. In addition, the presence of mutations in this gene in the index study is unlike a previous Chinese study which did not find any mutation of CYP2D6*4.[19] This might be explained in part by the fact that Han Chinese population is relatively a homogeneous population, whereas North Indian population has a mosaic pattern of genetic inheritance in view of Indo-Aryan descent and thus some of the genetic makeup may be similar to that of Europeans.
The ratio of plasma concentration of risperidone and 9-OHR in our study population was more than 1 at both stages of evaluation, thus implying that they were PMs. But it has been suggested that plasma concentration of the active moiety may be comparable in EMs and PMs.[33] Therefore, clinically, a genotyping of individuals may not help in prediction of response to treatment with risperidone. However, PMs of risperidone may be prone to develop adverse effects.
The differences between the findings of index study and the earlier studies may be attributed to methodological differences (utilization of multiple measures of phenotypic expression and long duration of study period but lesser numbers of genotypic variables in index study), sample size (smaller in most of earlier studies), and variation in the mutations of CYP2D6 genotypes across populations.
Strengths and limitations of the study
The strengths of index study include the following: the first Indian study to evaluate the effect of CYP2D6 polymorphism on plasma concentration of risperidone and its metabolite in patients with schizophrenia, prospective design of study with longest duration of observation (12 weeks) for a pharmacogenetic study, multiple evaluations of plasma drug concentration, and conduction of genetic analysis with stringent control of procedures. However, there are limitations too, like due to limited resources, we could not evaluate many other polymorphisms of CYP2D6 as well as other enzymes and transporter genes that impact the pharmacokinetics of risperidone. In addition, we could not do similar measurements at five other participating centers which could have provided us with a good sample size too. Additionally, the number of mutated alleles was small in comparison to that of the wild alleles, thus limiting the results to be conclusive. The compliance to medication was ensured by the caregiver, and hence the possibility of missing a dose, which could affect the plasma concentration of the drug, cannot be ruled out. Moreover, we did not measure the adverse effects, which in turn could have been corroborated with plasma concentration of risperidone and its metabolites.
CONCLUSION
In conclusion, this study confirms that CYP2D6*10 polymorphism affects the steady-state plasma concentration of risperidone and the active moiety in North Indian patients with schizophrenia. Also, CYP2D6*4 polymorphism has some impact on the plasma concentration of risperidone and its active metabolites during initial stages of treatment. All the study participants were PMs of risperidone. No association was found between plasma drug concentration and response to treatment. Further research in this field may focus on the evaluation of multiple gene polymorphisms of metabolic enzyme pathways with a larger sample size and inclusion of other Indian populations.
Financial support and sponsorship
This study was sponsored by Indian Council of Medical Research, New Delhi (Ref. No. 58/27/2008-BMS; Dated: 24-03-2011).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lamba V, Lamba JK, Dilawari JB, Kohli KK. Genetic polymorphism of CYP2D6 in North Indian subjects. Eur J Clin Pharmacol. 1998;54:787–91. doi: 10.1007/s002280050552. [DOI] [PubMed] [Google Scholar]
- 2.Llerena A, Naranjo ME, Rodrigues-Soares F, Penas-Lledo EM, Farina H, Tarazona-Santos E. Interethnic variability of CYP2D6 alleles and of predicted metabolic phenotypes across world populations. Expert Opin Drug Metab Toxicol. 2014;10:1569–83. doi: 10.1517/17425255.2014.964204. [DOI] [PubMed] [Google Scholar]
- 3.Buch S, Kotekar A, Kawle D, Bhisey R. Polymorphisms at CYP and GST gene loci: Prevalence in the Indian population. Eur J Clin Pharmacol. 2001;57:553–5. doi: 10.1007/s002280100337. [DOI] [PubMed] [Google Scholar]
- 4.Adithan C, Gerard N, Naveen AT, Koumaravelou K, Shashindran CH, Krishnamoorthy R. Genotype and allele frequency of CYP2D6 in Tamilian population. Eur J Clin Pharmacol. 2003;59:517–20. doi: 10.1007/s00228-003-0657-4. [DOI] [PubMed] [Google Scholar]
- 5.Naveen AT, Adithan C, Soya SS, Nathalie G, Krishnamoorhty R. CYP2D6 genetic polymorphism in South Indian Populations. Biol Pharm Bull. 2006;29:1655–8. doi: 10.1248/bpb.29.1655. [DOI] [PubMed] [Google Scholar]
- 6.Gogtay NJ, Mali NB, Iyer K, Kadam PP, Sridharan K, Shrimal D, et al. Evaluation of cytochrome P450 2D6 phenotyping in healthy adult Western Indians. Indian J Pharmacol. 2014;46:266–9. doi: 10.4103/0253-7613.132154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sistonen J, Fuselli S, Palo JU, Chauhan N, Padh H, Sajantila A. Pharmacogenetic variation at CYP2C9, CYP2C19, and CYP2D6 at global and microgeographic scales. Pharmacogenet Genomics. 2009;19:170–9. doi: 10.1097/FPC.0b013e32831ebb30. [DOI] [PubMed] [Google Scholar]
- 8.Huang ML, Van Peer A, Woestenborghs R, De Coster R, Heykants J, Jansen AA, et al. Pharmacokinetics of the novel antipsychotic agent risperidone and the prolactin response in healthy subjects. Clin Pharmacol Ther. 1993;54:257–68. doi: 10.1038/clpt.1993.146. [DOI] [PubMed] [Google Scholar]
- 9.de Leon J, Sandson NB, Cozza KL. A preliminary attempt to personalize risperidone dosing using drug-drug interactions and genetics: Part I. Psychosomatics. 2008;49:258–70. doi: 10.1176/appi.psy.49.3.258. [DOI] [PubMed] [Google Scholar]
- 10.Yoo HD, Lee SN, Kang HA, Cho HY, Lee IK, Lee YB. Influence of ABCB1 genetic polymorphisms on the pharmacokinetics of risperidone in healthy subjects with CYP2D6*10/10. Br J Pharmacol. 2011;164:433–43. doi: 10.1111/j.1476-5381.2011.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mihara K, Kondo T, Yasui-Furukori N, Suzuki A, Ishida M, Ono S, et al. Effects of various CYP2D6 genotypes on the steady-state plasma concentrations of risperidone and its active metabolite, 9-hydroxyrisperidone, in Japanese patients with schizophrenia. Ther Drug Monit. 2003;25:287–93. doi: 10.1097/00007691-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki Y, Fukui N, Tsuneyama N, Watanabe J, Ono S, Sugai T, et al. Effect of the cytochrome P450 2D6*10 allele on risperidone metabolism in Japanese psychiatric patients. Hum Psychopharmacol. 2012;27:43–6. doi: 10.1002/hup.1260. [DOI] [PubMed] [Google Scholar]
- 13.Roh HK, Kim CE, Chung WG, Park CS, Svensson JO, Bertilsson L. Risperidone metabolism in relation to CYP2D6*10 allele in Korean schizophrenic patients. Eur J Clin Pharmacol. 2000;57:671–75. doi: 10.1007/s002280100372. [DOI] [PubMed] [Google Scholar]
- 14.Kakihara S, Yoshimura R, Shinkai K, Matsumoto C, Goto M, Kaji K, et al. Prediction of response to risperidone treatment with respect to plasma concentrations of risperidone, catecholamine metabolites, and polymorphism of cytochrome P450 2D6. Int Clin Psychopharmacol. 2005;20:71–8. doi: 10.1097/00004850-200503000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Llerena A, Berecz R, Dorado P, Rubia ADL. QTc interval, CYP2D6 and CYP2C9 genotypes and risperidone plasma concentrations. J Psychopharmacol. 2004;18:189–93. doi: 10.1177/0269881104042618. [DOI] [PubMed] [Google Scholar]
- 16.Riedel M, Schwarz MJ, Strassnig M, Spellmann I, Muller-Arends A, Weber K, et al. Risperidone plasma levels, clinical response and side effects. Eur Arch Psychiatry Clin Neurosci. 2005;255:261–8. doi: 10.1007/s00406-004-0556-4. [DOI] [PubMed] [Google Scholar]
- 17.Jovanovic N, Bozina N, Lovric M, Medved V, Jakovljevic M, Peles AM. The role of CYP2D6 and ABCB1 pharmacogenetics in drug-naive patients with first-episode schizophrenia treated with risperidone. Eur J Clin Pharmacol. 2010;66:1109–17. doi: 10.1007/s00228-010-0850-1. [DOI] [PubMed] [Google Scholar]
- 18.Bartecek R, Jurica J, Zrustova J, Kasparek T, Pindurova E, Zourkova A. Relevance of CYP2D6 variability in first-episode schizophrenia patients treated with risperidone. Neuro Endocrinol Lett. 2012;33:236–44. [PubMed] [Google Scholar]
- 19.Wang ZH, Zhan YY, Li YX, Yang CC, Cai J, Dai DP, et al. Effects of 24 CYP2D6 variants found in the Chinese population on the metabolism of risperidone. Pharmacol. 2015;96:290–5. doi: 10.1159/000441007. [DOI] [PubMed] [Google Scholar]
- 20.Tiwari AK, Deshpande SN, Rao AR, Bhatia T, Lerer B, Nimgaonkar VL, et al. Genetic susceptibility to tardive dyskinesia in chronic schizophrenia subject: III. Lack of association of CYP3A4 and CYP2D6 gene polymorphism. Schizophr Res. 2005;75:21–6. doi: 10.1016/j.schres.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Kandasamy M, Srinivas P, Subramaniam K, Ravi S, John J, Shekar R, et al. Differential outcomes from metabolic ratios in the identification of CYP2D6 phenotypes – Focus on venlafaxine and O-desmethylvenlafaxine. Eur J Clin Pharmacol. 2010;66:879–87. doi: 10.1007/s00228-010-0829-y. [DOI] [PubMed] [Google Scholar]
- 22.Singh NK, Banerjee BD, Bala K, Basu M, Chhillar N. Polymorphism in cytochrome P 450 2D6, Glutathione S-Transferases Pi I genes and organochlorine pesticides in Alzheimer disease: A case-control study in North Indian population. J Geriatr Psychiatry Neurol. 2014;27:119–27. doi: 10.1177/0891988714522698. [DOI] [PubMed] [Google Scholar]
- 23.Kaur G, Gupta D, Chavan BS, Sinhmar V, Prasad R, Tripathi A. Identification of genetic correlates of response to risperidone: Findings of a multicentric schizophrenia study from India. Asian J Psychiatr. 2017;29:174–82. doi: 10.1016/j.ajp.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 24.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 25.Avenoso A, Facciola G, Salemi M, Spina E. Determination of risperidone and its major metabolite 9-hydroxyrisperidone in human plasma by reversed-phase liquid chromatography with ultraviolet detection. J Chromatogr B Biomed Sci Appl. 2000;746:173–81. doi: 10.1016/s0378-4347(00)00323-6. [DOI] [PubMed] [Google Scholar]
- 26.Spina E, de Leon J. Clinical applications of CYP genotyping in psychiatry. J Neural Transm. 2015;122:5–28. doi: 10.1007/s00702-014-1300-5. [DOI] [PubMed] [Google Scholar]
- 27.Dean L. Medical Genetics Summaries. In: Pratt V, McLeod H, Dean L, Malheiro A, Rubinstein W, editors. Risperidone therapy and CYP2D6 genotype. Bethesda, MD: 2017. [Google Scholar]
- 28.Furukori NY, Mihara K, Kondo T, Kubota T, Iga T, Takarada Y, et al. Effects of CYP2D6 genotypes on plasma concentrations of risperidone and enantiomers of 9-hydroxyrisperidone in Japanese patients with schizophrenia. J Clin Pharmac. 2003;43:122–7. doi: 10.1177/0091270002239819. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Yu L, Zhang AP, Fang C, Du J, Gu NF, et al. Serum prolactin levels, plasma risperidone levels, polymorphism of cytochrome P45- 2D6 and clinical response in patients with schizophrenia. J Psychopharmacol. 2007;21:837–42. doi: 10.1177/0269881107077357. [DOI] [PubMed] [Google Scholar]
- 30.Yagihashi T, Mizuno M, Chino B, Sato Y, Sakuma K, Takebayashi T, et al. Effects of the CYP2D6*10 alleles and co-medication with CYP2D6-dependent drugs on risperidone metabolism in patients with schizophrenia. Hum Psychopharmacol. 2009;24:301–8. doi: 10.1002/hup.1025. [DOI] [PubMed] [Google Scholar]
- 31.Xiang Q, Zhao X, Zhou Y, Duan JL, Cui YM. Effect of CYP2D6, CYP3A5, and MDR1 genetic polymorphism on the pharmacokinetics of Risperidone and its active moiety. J Clin Pharmacol. 2010;50:659–66. doi: 10.1177/0091270009347867. [DOI] [PubMed] [Google Scholar]
- 32.Almoguera B, Riverio-Alvarez R, Lopez-Castroman J, Dorado P, Vaquero-Lorenzo C, Fernandez-Piqueras J, et al. CYP2D6 poor metabolizer status might be associated with better response to risperidone treatment. Pharmacogenet Genomics. 2013;23:627–30. doi: 10.1097/FPC.0b013e3283659a94. [DOI] [PubMed] [Google Scholar]
- 33.Zhou SF. Polymorphism of human cytochrome P 450 2D6 and its clinical significance. Clin Pharmacokinet. 2009;48:761–804. doi: 10.2165/11318070-000000000-00000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of CYP2D6*4 & *10 alleles in the study population
The figure shows comparison of mean concentration of risperidone (95% Confidence Interval) according to the genotypic distribution of CYP2D6*4 at week 6 of treatment
The figure shows comparison of mean concentration of 9-hydroxyrisperidone (95% Confidence Interval) according to the genotypic distribution of CYP2D6*4 at week 6 of treatment
The figure shows comparison of mean concentration of risperidone (95% Confidence Interval) according to the genotypic distribution of CYP2D6*4 at week 12 of treatment
The figure shows comparison of mean concentration of 9-hydroxyrisperidone (95% Confidence Interval) according to the genotypic distribution of CYP2D6*4 at week 12 of treatment
The figure shows comparison of mean concentration of risperidone (95% Confidence Interval) according to the genotypic distribution of CYP2D6*10 at week 6 of treatment
The figure shows comparison of mean concentration of 9-hydroxyrisperidone (95% Confidence Interval) according to the genotypic distribution of CYP2D6*10 at week 6 of treatment
The figure shows comparison of mean concentration of risperidone (95% Confidence Interval) according to the genotypic distribution of CYP2D6*10 at week 12 of treatment
The figure shows comparison of mean concentration of 9-hydroxyrisperidone (95% Confidence Interval) according to the genotypic distribution of CYP2D6*10 at week 12 of treatment


