Abstract
Context:
Cognitive impairments are common among patients with alcohol dependence. It may involve frontal executive dysfunction, global cognitive impairments, or both. Motivation to quit alcohol involves recognition of alcohol use as a problem. This ability may be construed as a cognitive symptom.
Aims:
The aim is to study the frequency of cognitive dysfunction among patients with alcohol dependence and to study the association between cognitive dysfunction and the motivation to quit alcohol.
Materials and Methods:
Fifty-six adult males with alcohol dependence (International Classification of Diseases-10) who had completed a course of detoxification and who did not have active withdrawal symptoms or acute medical illnesses were recruited for this study. Their cognitive functions were tested using the Montreal Cognitive Assessment (MoCA) and Frontal Assessment Battery (FAB). Their motivation levels were assessed using the Stages of Change Readiness and Treatment Eagerness Scale. Clinical details were collected using a semi-structured pro forma.
Results:
Global cognitive impairment (MoCA < 26) was seen in 81% and frontal executive dysfunction (FAB < 12) in 16% of patients. Higher MoCA and FAB scores correlated with better education, while lower FAB scores correlated with higher age. The 14 patients (25%) with good motivation did not differ in age, education, years of dependence, or MoCA or FAB scores from poorly motivated patients. FAB scores, but not MoCA, were associated with poor motivation. All nine patients with FAB < 12 were poorly motivated to quit alcohol; likelihood score = 5.731, P = 0.017.
Conclusions:
Four-fifths of patients with alcohol dependence had global cognitive impairments after the detoxification period. One-sixth had frontal executive dysfunction. Cognitive functions were not significantly correlated with the duration of dependence. Presence of frontal executive dysfunction was associated with almost six times likelihood that the patient will be poorly motivated to quit alcohol.
Keywords: Alcohol-related disorders, cognitive dysfunction, frontal executive dysfunction, motivation
INTRODUCTION
Alcohol dependence is seen in about 6% of the population in Puducherry.[1] Some salient features of alcohol dependence are craving or a persistent urge to use alcohol, tolerance, or taking increasing amounts of alcohol over a period of time, experiencing a set of physical signs and symptoms when the use of alcohol is reduced or stopped (withdrawals), and an inability to control the use of alcohol. It is known that chronic and heavy use of alcohol (defined as more than 4 units [40 g of absolute alcohol] per day) can lead to a host of physical complications. Neurological complications of chronic and heavy alcohol use include peripheral neuropathy, myopathy, cerebellar degeneration, and cognitive impairment.[2] Cognitive impairment, as measured using the MoCA, has been detected in about 68% of patients with alcohol dependence.[3] A recently published study from Bengaluru found about 23% of patients with alcohol dependence having impairment in cognitive functions as defined by the Mini–Mental Status Examination score < 25.[4] In a study done at a tertiary care hospital in Nepal, it was found that about 34% of patients with alcohol dependence had frontal executive dysfunction and about 54% had memory disturbances.[5]
The cognitive impairment in alcohol dependence can be viewed under three broad categories: (i) cognitive deficits as a risk factor for alcohol use disorders,[6] (ii) cognitive processing as a target of cognitive therapies for alcohol use,[7] and (iii) cognitive impairments as a complication of chronic alcohol use.[8] These three aspects are not always mutually exclusive. For example, distortions in cognitive processing may increase the risk of alcohol use and progression to dependence. These distortions in cognitive processing may later interfere with the effectiveness of psychotherapy for alcohol dependence.[9]
Ihara et al.[8] have described three types of cognitive impairments among patients with alcohol dependence. While some patients have frontal executive dysfunction, others have frontal executive dysfunction along with amnesia. A third group of patients have global cognitive dysfunction due to alcohol use. Studies have suggested that cognitive dysfunction may show a gradual improvement over the first 1 year after the patient abstains from alcohol. Cognitive impairments are maximal in the initial 2 weeks after abstinence but show a gradual improvement thereafter.[10]
One of the important determinants of outcomes in alcohol use disorders is the motivation of the patient to quit alcohol. This motivation consists of the recognition of alcohol use as a problem followed by taking steps to quit alcohol and maintain abstinence. The inability to recognize alcohol use as a problem can itself be understood as a cognitive symptom. This concept is similar to anosognosia seen in neurological disorders such as stroke where the patient does not recognize that his/her limb is paralyzed.
We did the current study with the aims of assessing the cognitive functioning among patients with alcohol use disorders who have recently undergone detoxification and to correlate the cognitive deficits with the level of motivation to quit alcohol use. The objectives of the study were to (i) estimate the frequency of global cognitive impairment and frontal executive dysfunction among recently detoxified male patients with alcohol dependence syndrome and (ii) to study the association between cognitive functioning and the motivational state to quit alcohol use.
SUBJECTS AND METHODS
This study was conducted at the Deaddiction Clinic Services of the Department of Psychiatry of Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, in July–August 2017. Patients diagnosed with alcohol dependence syndrome (F10.20) as per the International Classification of Diseases-10 criteria were recruited. Male patients above the age of 18 years were included after completing a course of detoxification for at least 7 days. Those with persisting withdrawal symptoms at the time of assessment were excluded from the study. Patients with acute unstable medical illnesses or those with comorbid severe mental illnesses or other substance use disorders (except nicotine dependence) were also excluded from the study. We took written informed consent from the patients and their relatives.
The sample size was estimated using OpenEpi version 3.0.[11] The proportion of patients with cognitive impairment was estimated at 35%.[12] Using a 10% absolute precision and a confidence limit of 95%, we got a sample size of 56 out of the estimated 150 patients expected to seek treatment during the study period. The study began after obtaining clearance from the Institute Ethics Committee (Human Studies) of the hospital (Ref No: IEC/2017/0026).
Patients were interviewed regarding sociodemographic details and clinical features of alcohol use. Cognitive assessment was done using the MoCA,[13] and frontal executive functions were tested using the Frontal Assessment Battery (FAB).[14] The Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES) (version 8.0) was applied for the assessment of motivational state.[15] The scores on the SOCRATES were used to classify patients as having good or poor motivation by two authors (BB and KPP) independently using the decile scores guide given along with the SOCRATES tool. “Good motivation” included patients in preparation or action stage, whereas “poor motivation” was used for patients in precontemplation or contemplation stages. The scales were used in Tamil as already available (MoCA) or after translation and pilot validation (FAB and SOCRATES) after due permission from the authors of the original scale.
The data were analyzed using SPSS Statistics for Windows, version 17.0 (SPSS Inc., Chicago, Ill., USA). Descriptive statistics were used. Independent sample t-test and Mann–Whitney U-test were used to compare continuous variables having normal and nonnormal distribution, respectively.
RESULTS
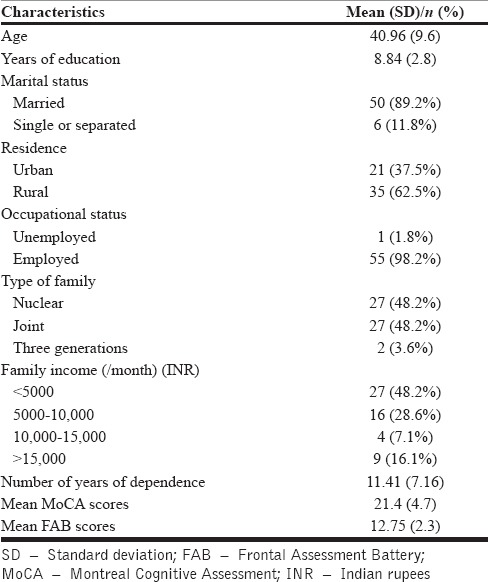
The clinical and demographic characteristics of the 56 men who participated in the study are summarized in Table 1. The mean age of patients was 40.96 years (standard deviation [SD] – 9.62). They had a mean duration of dependence of 11.41 years (SD – 7.16). Using a cutoff of 26 on the MoCA test, 45/56 patients (81%) had global cognitive impairment at the end of the detoxification period. However, on the FAB test, only 9/56 patients (16%) had frontal executive dysfunction.
Table 1.
Clinical and demographic characteristics of patients enrolled
At the time of assessment, 14 patients (25%) had good motivation to quit. Patients with good motivation and poor motivation did not differ significantly in terms of age (42.0 [8.2] vs. 40.6 [10.1], T = 0.462, P = 0.646), mean years of education (9.64 [2.9] vs. 8.57 [2.8], T = 1.240, P = 0.220), and duration of alcohol dependence (in years) (11.93 [6.9] vs. 11.24 [7.3], T = 0.310, P = 0.758). Although patients with poor motivation had lower total scores on MoCA test (global cognitive functions) (23.07 [3.8] vs. 20.86 [4.8], T = 1.547, P = 0.128) and FAB (frontal executive functions) (13.21 [1.4] vs. 12.60 [2.6], T = 1.123, P = 0.268), the difference was not statistically significant.
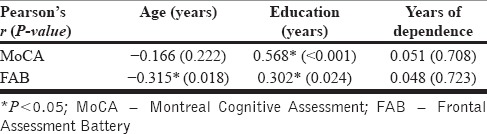
The cognitive functions, for both MoCA and FAB scores, were positively correlated with the number of years of education. However, only FAB scores showed a significant negative correlation with the age of the patient. Duration of alcohol dependence showed no significant correlation with the performance on either of the cognitive tests. The correlational analysis is depicted in Table 2.
Table 2.
Correlation between clinical features and cognitive functioning
We analyzed if the cognitive test scores correlated with the motivational state to quit alcohol. While the MoCA test scores were not associated with the motivational state of the patient [Table 3], we found that all the nine patients scoring below the cutoff score of 12 on the FAB had poor motivation to quit alcohol [Table 4]. However, the association was not statistically significant (Fisher's exact P = 0.09). The likelihood ratio for low FAB score being associated with poor motivation was 5.73 (P = 0.017).
Table 3.
Association between Montreal Cognitive Assessment scores and motivation
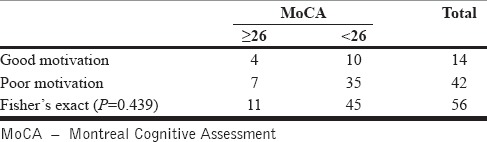
Table 4.
Association between Frontal Assessment Battery scores and motivation

Further, we explored the components of FAB that correlated with poor motivation. The six items are conceptualization, mental flexibility, programming, sensitivity to interference, inhibitory control, and environmental autonomy. Of these, scores in the mental flexibility domain were significantly lower among poorly motivated patients.
DISCUSSION
Cognitive impairments in alcohol dependence have not been widely studied. Previous studies have suggested that the cognitive deficits may depend on the duration of abstinence from alcohol and may steadily improve in the initial 1 year of sustained abstinence.[10] However, since sustained abstinence is largely dependent on the motivation levels of the patient to quit alcohol, in this study, we wanted to study the correlation between cognitive functions and motivation in recently detoxified patients with alcohol dependence. In addition, we thought that the motivation to quit itself involves recognition of alcohol dependence as a problem before the patient takes steps to quit alcohol. Such recognition of the problem constitutes insight which is conceivably a cognitive function.
We found that four-fifths of patients who have completed detoxification have a significant degree of cognitive impairment as defined by the MoCA score of < 26. Studies done in the United States, France, and Singapore have reported cognitive impairment in patients with substance use disorders (as assessed by the MoCA test) ranging from 38% to 73%.[3,12,16] However, this variation could be due to differences in the study setting, severity of dependence, and sample characteristics. This variation between countries indicates the need to standardize the MoCA test for use in different populations and derive age- and education-based norms for each population. Moreover, even in our study, we found a correlation between the performance on cognitive tests and the age of the patient (for FAB) and the number of years of education (for both MoCA and FAB scores). However, the cognitive tests did not correlate with the duration of dependence.
We found that one-sixth of patients displayed frontal executive dysfunction as defined by the FAB score of < 12. This finding is in contrast with results of a study from Nepal in which about 34% of patients had impairment of executive functions.[5]
While MoCA scores and FAB scores were not significantly different between the two groups of good and poor motivation patients, we found that all the nine patients who had FAB scores under 12 belonged to poorly motivated group. This finding suggests that frontal executive dysfunction may be a useful predictor of poor motivation among recently detoxified patients with alcohol dependence. However, this finding needs to be replicated in future studies with possibly larger sample sizes. Among the items of FAB, the mental flexibility task (item 2 of FAB) was particularly associated with poor motivation levels.
The study findings have implications in terms of management. Considering that cognitive impairment is associated with poor motivation, it might be imperative to assess cognitive impairment in patients with poor motivation. Further, assessing the cognitive impairment might be beneficial in planning cognitive remediation strategies for better patient-related outcomes.[17] Since the MoCA scores show a significant positive correlation with the education levels of patient, we recommend the use of a specific cognitive test for frontal lobe dysfunction in the form of FAB to predict poor motivation to quit among patients. FAB is less correlated with education status of the patient though it appears to be correlated with the age of the patient.
The strengths of this study include the use of standard scales for the assessment of global (MoCA) and frontal cognitive functions (FAB). Standard scale (SOCRATES) was used to assess motivation which was independently classified by two qualified psychiatrists with experience in addiction. The patients are representative of long-standing alcohol dependence of about 10 years' duration seeking treatment at a specialized addiction service.
The limitations of this study include lack of use of a standard measure of alcohol withdrawal signs at the time of intake. The sample size was adequate for the assessment of the frequency of cognitive impairment. However, it was underpowered (45%) for the assessment of the usefulness of FAB or MoCA in predicting poor motivational state. Hence, this part of the analysis needs to be replicated in future studies with larger sample sizes. We did not analyze the type or dose of benzodiazepine used or duration since discontinuation of the drugs. It could have had some influence on cognitive functions at the time of assessment. Other important confounders such as family history of substance use disorders, childhood behavioral problems, and severe traumatic brain injuries were not systematically screened for. As mentioned previously in the discussion, the lack of a control group and the lack of age- and education-based population norms for MoCA test are limitations in our study that need to be overcome in future studies. We also did not assess the severity of dependence or quantity of alcohol used by the patients. Moreover, future studies may also be designed to evaluate the longitudinal course of these deficits among patients after prolonged abstinence and track the possible changes in motivational levels with the improvement in cognition.
CONCLUSIONS
About four-fifths of patients with alcohol dependence have global cognitive dysfunction and about one-sixth have frontal executive dysfunction immediately after completion of detoxification treatment. While global cognitive dysfunction did not seem to predict motivational state, all patients with frontal executive dysfunction were poorly motivated. Frontal executive dysfunction may be a more robust predictor of poor motivation to quit alcohol.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sujiv A, Chinnakali P, Balajee K, Lakshminarayanan S, Kumar SG, Roy G, et al. Alcohol use and alcohol use disorder among male outpatients in a primary care setting in rural Puducherry. Ind Psychiatry J. 2015;24:135–9. doi: 10.4103/0972-6748.181711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noble JM, Weimer LH. Neurologic complications of alcoholism. Continuum (Minneap Minn) 2014;20:624–41. doi: 10.1212/01.CON.0000450970.99322.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alarcon R, Nalpas B, Pelletier S, Perney P. MoCA as a screening tool of neuropsychological deficits in alcohol-dependent patients. Alcohol Clin Exp Res. 2015;39:1042–8. doi: 10.1111/acer.12734. [DOI] [PubMed] [Google Scholar]
- 4.Gupta A, Murthy P, Rao S. Brief screening for cognitive impairment in addictive disorders. Indian J Psychiatry. 2018;60:S451–6. doi: 10.4103/psychiatry.IndianJPsychiatry_41_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adhikari S, Rana M, Shakya S, Ojha SP. Cognitive dysfunctions in patients with alcohol dependence syndrome in a tertiary hospital in Kathmandu. JNMA J Nepal Med Assoc. 2016;54:17–23. [PubMed] [Google Scholar]
- 6.Finn PR, Hall J. Cognitive ability and risk for alcoholism: Short-term memory capacity and intelligence moderate personality risk for alcohol problems. J Abnorm Psychol. 2004;113:569–81. doi: 10.1037/0021-843X.113.4.569. [DOI] [PubMed] [Google Scholar]
- 7.Rupp CI, Kemmler G, Kurz M, Hinterhuber H, Fleischhacker WW. Cognitive remediation therapy during treatment for alcohol dependence. J Stud Alcohol Drugs. 2012;73:625–34. doi: 10.15288/jsad.2012.73.625. [DOI] [PubMed] [Google Scholar]
- 8.Ihara H, Berrios GE, London M. Group and case study of the dysexecutive syndrome in alcoholism without amnesia. J Neurol Neurosurg Psychiatry. 2000;68:731–7. doi: 10.1136/jnnp.68.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Berre AP, Rauchs G, La Joie R, Segobin S, Mézenge F, Boudehent C, et al. Readiness to change and brain damage in patients with chronic alcoholism. Psychiatry Res. 2013;213:202–9. doi: 10.1016/j.pscychresns.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Fein G, Bachman L, Fisher S, Davenport L. Cognitive impairments in abstinent alcoholics. West J Med. 1990;152:531–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Dean AG, Sullivan KM, Soe MM. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version. www.OpenEpi.com. [Last updated on 2013 Apr 06]. Available from: http://www.openepi.com/Menu/OE_Menu.htm .
- 12.Copersino ML, Fals-Stewart W, Fitzmaurice G, Schretlen DJ, Sokoloff J, Weiss RD, et al. Rapid cognitive screening of patients with substance use disorders. Exp Clin Psychopharmacol. 2009;17:337–44. doi: 10.1037/a0017260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 14.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: A Frontal assessment battery at bedside. Neurology. 2000;55:1621–6. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 15.Miller W. Assessing drinker's motivations for change: The stage of change readiness and treatment eagerness scale (SOCRATES) Psychol Addict Behav. 1996;10:81–9. [Google Scholar]
- 16.Manning V, Gomez B, Guo S, Wong KE, Prysely AN, Chan ES. Screening for cognitive impairment in Asian substance-dependent patients: MMSE versus MoCA. Int Arch Addict Res Med. 2016;2(Suppl 2):19. [Google Scholar]
- 17.Bernardin F, Maheut-Bosser A, Paille F. Cognitive impairments in alcohol-dependent subjects. Front Psychiatry. 2014;5:78. doi: 10.3389/fpsyt.2014.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]


