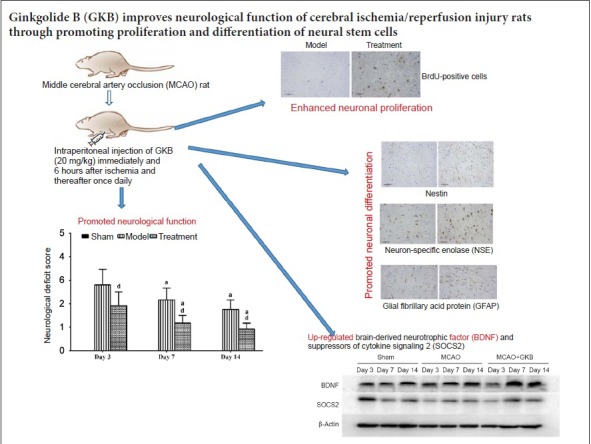
Keywords: nerve regeneration, brain-derived neurotrophic factor, epidermal growth factor, suppressor of cytokine signaling 2, neuron-specific enolase, glial fibrillary acid protein, nestin, bromodeoxyuridine, neurological function, middle cerebral artery occlusion, astrocytes, neural regeneration
Abstract
Neural stem cells have great potential for the development of novel therapies for nervous system diseases. However, the proliferation of endogenous neural stem cells following brain ischemia is insufficient for central nervous system self-repair. Ginkgolide B has a robust neuroprotective effect. In this study, we investigated the cell and molecular mechanisms underlying the neuroprotective effect of ginkgolide B on focal cerebral ischemia/reperfusion injury in vitro and in vivo. Neural stem cells were treated with 20, 40 and 60 mg/L ginkgolide B in vitro. Immunofluorescence staining was used to assess cellular expression of neuron-specific enolase, glial fibrillary acid protein and suppressor of cytokine signaling 2. After treatment with 40 and 60 mg/L ginkgolide B, cells were large, with long processes. Moreover, the proportions of neuron-specific enolase-, glial fibrillary acid protein- and suppressor of cytokine signaling 2-positive cells increased. A rat model of cerebral ischemia/reperfusion injury was established by middle cerebral artery occlusion. Six hours after ischemia, ginkgolide B (20 mg/kg) was intraperitoneally injected, once a day. Zea Longa’s method was used to assess neurological function. Immunohistochemistry was performed to evaluate the proportion of nestin-, neuron-specific enolase- and glial fibrillary acid protein-positive cells. Real-time quantitative polymerase chain reaction was used to measure mRNA expression of brain-derived neurotrophic factor and epidermal growth factor. Western blot assay was used to analyze the expression levels of brain-derived neurotrophic factor and suppressor of cytokine signaling 2. Ginkgolide B decreased the neurological deficit score, increased the proportion of nestin-, neuron-specific enolase- and glial fibrillary acid protein-positive cells, increased the mRNA expression of brain-derived neurotrophic factor and epidermal growth factor, and increased the expression levels of brain-derived neurotrophic factor and suppressor of cytokine signaling 2 in the ischemic penumbra. Together, the in vivo and in vitro findings suggest that ginkgolide B improves neurological function by promoting the proliferation and differentiation of neural stem cells in rats with cerebral ischemia/reperfusion injury.
Introduction
Neural stem cells (NSCs) are promising for the development of novel therapies for nervous system diseases. Their presence in the central nervous system indicates that it has the capacity for self-repair. Brain ischemia activates the proliferative potential of resident NSCs (Zhu et al., 2005; Ryu et al., 2016). Recent studies have shown that NSCs are present in the brains of adult humans and animals, and that following brain injury, the proliferation of endogenous NSCs is induced (Alagappan et al., 2009; Xie et al., 2016; Chen et al., 2017). However, the proliferation of endogenous NSCs following brain ischemia is insufficient for central nervous system self-repair. Thus, the use of exogenous drugs to induce self-repair by endogenous NSCs in the brain is a promising therapeutic strategy for promoting neurological recovery and reconstruction after stroke.
Neuronal development, axonal growth, neurotransmitter synthesis and apoptosis in the brain are regulated by multiple factors via signaling pathways. In the absence of exogenous drugs, only a small proportion of proliferative NSCs differentiate into mature neurons (Yu et al., 2016). Thus, inducing the proliferation of endogenous NSCs with exogenous growth factors should promote the migration of NSCs to the site of injury and their differentiation into functional neurons. Nerve growth factor and other growth factors, such as brain-derived neurotrophic factor (BDNF), promote the proliferation and differentiation of NSCs and the formation of protrusions in newly formed neurons (Ochi et al., 2016). Epidermal growth factor (EGF) regulates the proliferation, migration and differentiation of NSCs, and plays a critical role in the maintenance of central nervous system homeostasis (Huang et al., 2016). In addition, a variety of signaling pathways are involved in the regulation of neurite growth, including growth factor-mediated signaling pathways and suppressors of cytokine signaling (SOCS) (Barnat et al., 2016).
Traditional Chinese medicine has recently been found to enhance neural plasticity in the central nervous system (Maclennan et al., 2002; Wang et al., 2015). For centuries, extracts from leaves of the Ginkgo biloba tree have been used in China for the treatment of a variety of diseases (Gu et al., 2012). Ginkgolide B (GKB) is one of several major terpene lactone components that have been identified in Ginkgo biloba extracts (Qin et al., 2014), and it has a molecular weight of 424.4 Da (Cui et al., 2012). GKB is a potent platelet activating factor antagonist (Hu et al., 2011). A potentially important property of GKB is that it can pass through the brain-blood barrier, particularly following cerebral ischemia/reperfusion injury (Fang et al., 2010). A number of in vitro and in vivo studies have demonstrated that GKB has marked neuroprotective effects against ischemia-induced impairments (Maclennan et al., 2002; Xia and Fang, 2007; Hu et al., 2011; Gu et al., 2012; Qin et al., 2014). However, the mechanisms underlying the neuroprotective effects of GKB have yet to be clarified (Gu et al., 2012; Qin et al., 2014). The neuroprotective actions of GKB might be related to its anti-inflammatory effects, scavenging of oxygen free radicals, inhibition of thrombosis, and platelet activating factor antagonism (Hu et al., 2011; Gu et al., 2012; Qin et al., 2014).
Tang et al. (2011) found that GKB promotes the proliferation and functional activities of bone marrow-derived endothelial progenitor cells. We hypothesized that GKB might increase the proliferation and differentiation of NSCs in rats with ischemic/reperfusion injury. Therefore, in the present study, we investigate the effects of GKB on the proliferation and morphology of NSCs in rats with focal cerebral ischemia.
Materials and Methods
In vitro experiments
Cell culture
A NSC line was purchased by Cyagen Biological Technology Co., Ltd., China. For differentiation, NSCs were subcultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, New York, NY, USA) containing 20 ng/mL EGF and basic fibroblast growth factor (bFGF). Passage 2–3 neurospheres were harvested by centrifugation and seeded onto coverslips in 12-well plates pre-coated with 100 mg/L polylysine. Cells were randomly assigned to the following four groups: control, 20 mg/L GKB, 40 mg/L GKB and 60 mg/L GKB (6 wells per group). In the control group, cells were maintained in DMEM/F12 (Gibco) containing 10% fetal bovine serum. In the 20, 40 and 60 mg/L GKB groups, cells were maintained in DMEM/F12 containing 10% fetal bovine serum and treated with GKB (lyophilized powder, dissolved in 0.9% saline; China Pharmaceutical and Biological Products, China) at 20, 40 or 60 mg/L. Cells were grown at 37 °C in a humidified environment with 5% CO2. NSC morphology was observed at 3, 7 and 14 days.
Cell morphology
After cell culture, the coverslips were collected, and the morphology of the NSCs was observed under a phase contrast microscope (Changfang Optical Instrument Co., Shanghai, China). SPOT Advance software (Informer Technologies, Dominica, NY, USA) was used to measure the length of cell protrusions (3 days) and cell body area (14 days) as previously described (Mousavi and Doweidar, 2015).
Immunofluorescence
After the cells were cultured for 3, 7 and 14 days, the coverslips were collected, and the medium was carefully removed. The coverslips were rinsed three times with 0.01 M PBS (pH 7.3) and fixed in 4% paraformaldehyde in 0.1 M PBS (pH 7.3) at room temperature for 30 minutes. The residual paraformaldehyde was removed, and cells were washed three times in PBS. Cells were incubated with 10% goat serum in 0.01 M PBS (pH 7.3) at room temperature for 30 minutes. The medium was removed, and cells were incubated with primary antibodies (1:200; 100 μL), including mouse anti-neuron specific enolase (NSE) polyclonal (Abcam, Hong Kong, China), mouse anti-glial fibrillary acidic protein (GFAP) polyclonal (Santa Cruz Biotechnology, Dallas, TX, USA) and mouse anti-SOCS-2 polyclonal (Bioworld, Nanjing, China), at 4°C overnight. On day 2, after removal of the medium, cells were washed three times in PBS and incubated with biotinylated goat anti-mouse secondary antibody (1:200, 100 μL/well; Bioworld, Visalia, CA, USA) at room temperature for 2 hours. The cells were washed three times in PBS and incubated with avidin conjugated to Texas red (1:100, 100 μL/well; Thermo Fisher Scientific, Waltham, MA, USA) in the dark at room temperature for 2 hours. After washing in PBS, cells were mounted with glycerin buffer. Cells were then observed under a fluorescence microscope (Olympus, Tokyo, Japan), and positive cells were counted. There were five wells (coverslips) in each group, and five fields were randomly selected from each coverslip. The total number of cells and positive cells were independently counted, followed by calculation of the proportion of positive cells. NSE-positive cells (neurons) and GFAP-positive cells (astrocytes) were counted. After cell culture for 7 days, immunocytochemistry was performed for SOCS2 protein. Cells were observed using a fluorescence microscope (Olympus).
In vivo experiments
Animal model
This study was approved by the Ethics Committee of Zhejiang University of China (approval No. 2015-152), and all procedures were conducted according to the Experimental Animal Use and Welfare guidelines of Zhejiang University. A total of 150 male clean healthy Sprague-Dawley rats, weighing 250–280 g and aged 8 weeks, were purchased from the Zhejiang Academy of Medical Sciences of China (license No. SCXK2014-0001). The rats were randomly assigned to three groups: sham, middle cerebral artery occlusion (MCAO) and MCAO + GKB (n = 50 per group).
A cerebral ischemia/reperfusion model was established by MCAO (Tang et al., 2011). In brief, a 4-0 monofilament nylon thread was inserted into the right common carotid artery to obstruct the middle cerebral artery (MCA) for 90 minutes, and then the thread was withdrawn. In the sham group, the thread was inserted into the right common carotid artery without reaching the bifurcation between the MCA and anterior cerebral artery. The investigators who performed the procedures were blind to treatments.
GKB treatment
GKB at 20 mg/kg was intraperitoneally administered immediately and 6 hours after ischemia, and thereafter once daily. In the sham and MCAO groups, an equal volume of normal saline (2 mL) was intraperitoneally administered with the same schedule. In each group, rats received intraperitoneal injection of bromodeoxyuridine (BrdU) at 50 mg/kg three times (once every 4 hours) within 12 hours. Then, 4 hours after the last injection of BrdU, the rats were sacrificed for assessing the proliferation of NSCs.
At 3, 7 and 14 days after surgery, neurological function was evaluated (n = 5 per time point for each of the three groups). These rats were then sacrificed for immunohistochemistry, real-time quantitative polymerase chain reaction (RT-PCR) and western blot assay (n = 5 per group, for a total of 45 rats for each of the three assays).
Neurological function assessment
Neurological function was assessed using the Zea Longa method (Longa et al., 1989). The higher the neurological deficit score, the greater the impairment. Neurological function was evaluated 3, 7 and 14 days after surgery. To reduce variation caused by differences in impairment, rats with a score of 1–3 within 24 hours after surgery were used in the following experiments.
Immunohistochemistry
Immunohistochemistry was performed for nestin, NSE, GFAP and BrdU. After sacrifice, brains were collected, dehydrated in a graded ethanol series, and permeabilized in xylene. Brains from the ischemic penumbra were embedded in paraffin and cut into 4-μm sections, followed by drying at 37°C for 12–24 hours. The sections were stored at room temperature. Immunohistochemistry was performed using the SP immunohistochemical staining kit (Fuzhou Maixin Biotechnology Development Co., Ltd., Fuzhou, China) according to the manufacturer’s protocol to detect nestin (a neural stem cell marker; mouse polyclonal antibody, 1:200; Santa Cruz Biotechnology), NSE (a neuronal marker; mouse polyclonal antibody, 1:200; Abcam), GFAP (a marker for astrocytes; mouse polyclonal antibody, 1:200; Santa Cruz Biotechnology) and BrdU (mouse polyclonal antibody, 1:100; Santa Cruz Biotechnology). The slide was incubated with primary antibodies at 4°C overnight, followed by incubation with goat anti-mouse IgG (1:100; Bioworld) at 37°C for 20 minutes. The sections were observed under a light microscope (Shanghai Optical Instrument Factory, Shanghai, China). Six fields were randomly selected at the penumbra from each section at 400× magnification, and at least six sections were observed in each group. Positive cells were counted.
RT-PCR
Rats were sacrificed after anesthesia, and their brains were removed. Total RNA was extracted from the penumbra and reverse-transcribed into cDNA. The cDNA was used for quantitative RT-PCR (Ba et al., 2014) for BDNF (forward primer: 5′-TTG TGG TTT GTT GCC GTT GC-3′; reverse primer: 5′-TCC CCA TCC CCT AAG CCA GT-3′) and EGF (forward primer: 5′-CGC CGC AGA CTT ACC CAG A-3′; reverse primer: 5′-CGG AGA CGT ACC CTG TTT TGA C-3′). The thermocycling parameters were as follows: 50°C for 3 minutes, 95°C for 3 minutes; 45 cycles of 95°C for 10 seconds (denaturation), 59°C for 20 seconds (annealing) and 72°C for 20 seconds (extension); and 72°C for 10 minutes (final extension). Data were analyzed with BIO-RAD CFX Manager (Bio-Rad, Hercules, CA, USA) and output into PDF and EXCEL documents. Actin served as an internal reference. Relative expression of the target gene was determined as the ratio of expression of the target gene to that of β-actin.
Western blot assay
The brains were removed after sacrifice, and the penumbra was collected for protein extraction. Mouse polyclonal SOCS-2 (1:100; Bioworld), mouse polyclonal BDNF (1:200; Santa Cruz Biotechnology) and mouse polyclonal β-actin (1:200; Santa Cruz Biotechnology) were used as primary antibodies. Goat anti-rabbit IgG-horseradish peroxidase (1:200; Bioworld) and goat anti-mouse IgG-horseradish peroxidase (1:200; Bioworld) were used as secondary antibodies. The membrane was incubated with the primary antibody at 4°C overnight, followed by incubation with the secondary antibody at room temperature for 2 hours. The optical density of each band was measured with ImageJ software (NIH, Bethesda, MD, USA). β-Actin served as an internal reference. The level of the target protein was normalized to that of β-actin to obtain relative expression.
Statistical analysis
All data are presented as the mean ± SD and were analyzed using SPSS 22.0 software (IBM, Armonk, NY, USA). Differences in protrusion length and cell body area were evaluated by one-way analysis of variance (ANOVA). Independent t-test was used for testing the difference in percentage of positive NSCs between the control and 40 mg/L GKB groups. The effects of time and the three treatments were examined by two-way ANOVA. The two-way ANOVA model included interaction terms comprising time and group. If a significant interaction was identified, stratified analyses by time or group were then performed. Multiple comparisons were carried out with the Bonferroni correction method if a significant difference was revealed by one-way ANOVA and two-way ANOVA. A two-sided significance level of 0.05 was set.
Results
Effects of GKB on NSC proliferation and differentiation in vitro
Cell morphological changes
Twelve hours after differentiation was induced, most neurospheres in each group were adherent to the wall. Cells exhibited radial growth with the neurosphere at the center. The round cells became spindle-shaped and displayed some protrusions. After 3 days of differentiation, many cells migrated out of the neurosphere, and the processes were further lengthened. In the GKB groups, the growth of processes was rapid. A significant difference in the length of the processes was found 3 days after differentiation. Figure 1A–F show the cell morphologies at 3, 7 and 14 days after 40 mg/L GKB treatment. Compared with the 40 or 60 mg/L GKB group, process length was shorter in the control group and the 20 mg/L GKB group (both P < 0.05; Figure 1G). The mean cell body area of the NSCs was significantly smaller in the control and 20 mg/L GKB groups compared with the 40 and 60 mg/L GKB groups on day 14 (all P < 0.05; Figure 1H)
Figure 1.
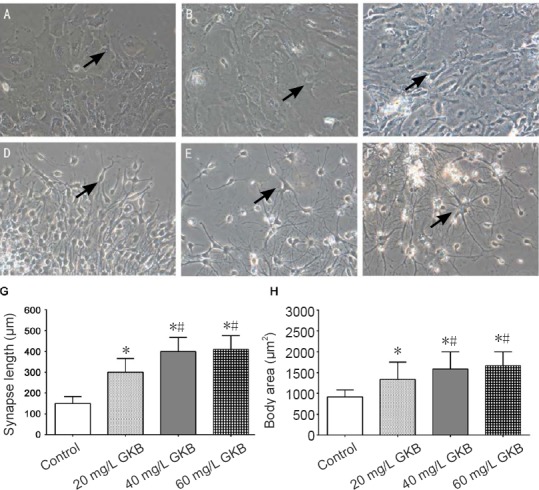
Effect of ginkgolide B (GKB) on the morphology of neural stem cells.
(A–F) Cell morphology of neural stem cells (arrows) at different time points (× 200). (A–C) Neural stem cells after induced differentiation for 3 (A), 7 (B) and 14 (C) days in the control group; (D–F) neural stem cells after induced differentiation for 3 (D), 7 (E) and 14 (F) days in the 40 mg/L GKB group. (G, H) The length of neural stem cell processes 3 days after differentiation. (G) Body area of neural stem cells 14 days after differentiation. (H) Data are presented as the mean ± SD and were tested with analysis of variance (n = 3 per group). *P < 0.05, vs. control group; #P < 0.05, vs. 20 mg/L GKB group.
Differentiation of NSCs
After 7 days of differentiation, the cells were fixed for immunofluorescence. Neuron-like cells (NSE-positive cells) had a large cell body, were round or oval, and displayed 1 or 2 long processes. Astrocytes (GFAP-positive cells) had some processes. The SOCS2-positive cells had a large cell body and became oval or spindle-shaped. The sizes of NSE-, GFAP- and SOCS2-positive cells were higher in the 40 mg/L group compared with the control group (all P < 0.05; Figure 2).
Figure 2.
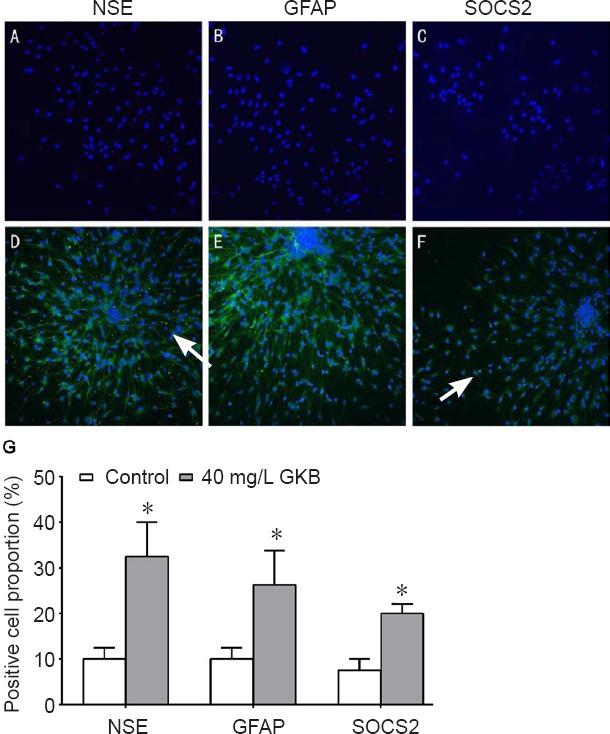
Effect of ginkgolide B (GKB) on differentiation of neural stem cells.
(A–F) Expression of neuron-specific enolase (NSE), glial fibrillary acid protein (GFAP) and suppressor of cytokine signaling 2 (SOCS2) in neural stem cells in the control group (A–C) and the 40 mg/L GKB group (D–F) after induced differentiation for 7 days. The blue color represents cell bodies, and the green color represents processes. Arrows indicate NSE-, GFAP- or SOCS2-positive cells. (G) Percentage of NSE-, GFAP- and SOCS2-positive cells after 7 days of induced differentiation. Data are presented as the mean ± SD (n = 3 per group; independent t-test). *P < 0.05, vs. control group.
Effects of GKB on NSC proliferation and differentiation in the brain of rats with cerebral ischemia/reperfusion injury
Neurological function
Three days after injury, neurological deficits were observed in rats, and the neurological scores continued to decline at 7 and 14 days. The neurological deficit score for rats in the sham group was 0. The score in the MCAO group decreased over time, and the score was significantly lower in rats given treatment compared with rats in the MCAO group from days 3 through 14 (all P < 0.05; Figure 3).
Figure 3.
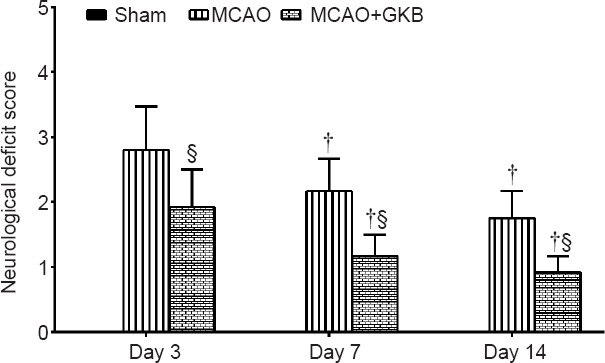
Effect of ginkgolide B (GKB) on neurological function in rats with cerebral ischemia/reperfusion injury.
Data are presented as the mean ± SD (n = 3 per group; independent t-test). †P < 0.05, vs. day 3; §P < 0.05, vs. middle cerebral artery occlusion (MCAO) group.
Nestin-positive cells
In the sham group, nestin-positive cells were observed in the ventricular ependyma and the subependymal zone. Nestin protein expression in the MCAO and MCAO + GKB groups was slightly increased on day 7, but then significantly reduced on day 14 (P = 0.013, vs. day 7; Figure 4A). Compared with the sham group, the proportion of nestin-positive cells was significantly higher in the MCAO + GKB group (P < 0.05; Figure 4B).
Figure 4.
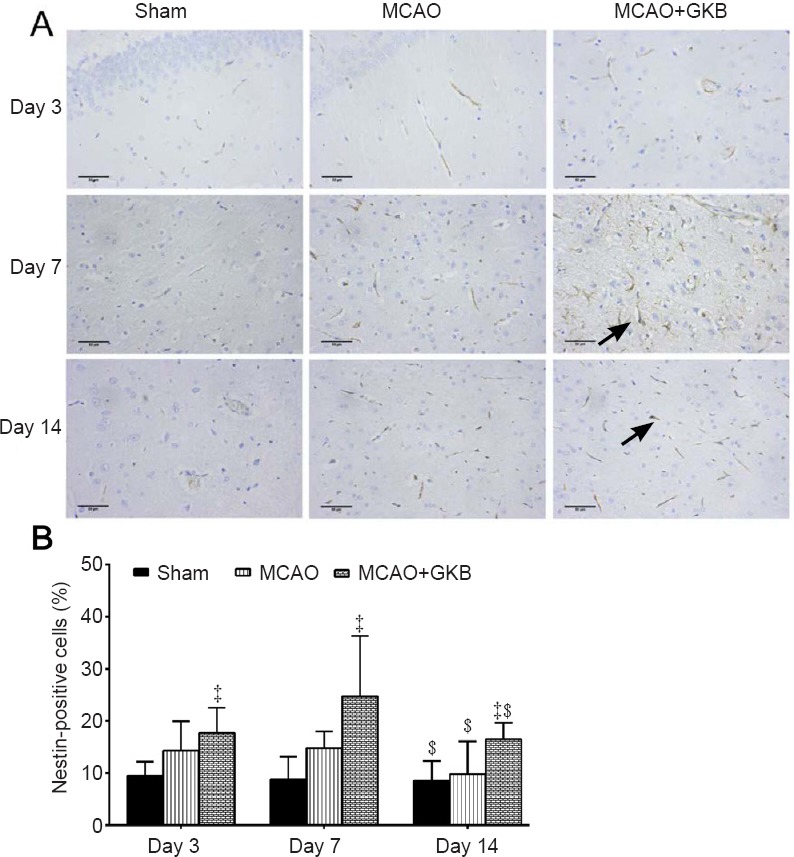
Effect of ginkgolide B (GKB) on nestin immunoreactivity in the penumbra of rats with cerebral ischemia/reperfusion injury.
(A) Nestin expression (arrows) in the brain of rats at 3, 7 and 14 days after middle cerebral artery occlusion (MCAO) (immunohistochemistry; scale bars: 50 μm). (B) Quantitative analysis of nestin immunoreactivity. Data are presented as the mean ± SD (n = 6 per group; two-way analysis of variance with Bonferroni correction for pairwise comparison). ‡P < 0.05, vs. sham group; $P < 0.05, vs. day 7.
NSE-positive cells
In the sham group, approximately 10% of the cells were positive for NSE at all three time points. In the model and MCAO + GKB groups, down-regulation of NSE protein was found at day 14 compared with day 3 or 7 (Figure 5A). In addition, although NSE expression was higher in the MCAO group than in the sham group at day 3, the proportions of NSE-positive cells in the two groups were comparable and significantly lower than those in the MCAO + GKB group after day 7 (all P ≤ 0.009; Figure 5B).
Figure 5.
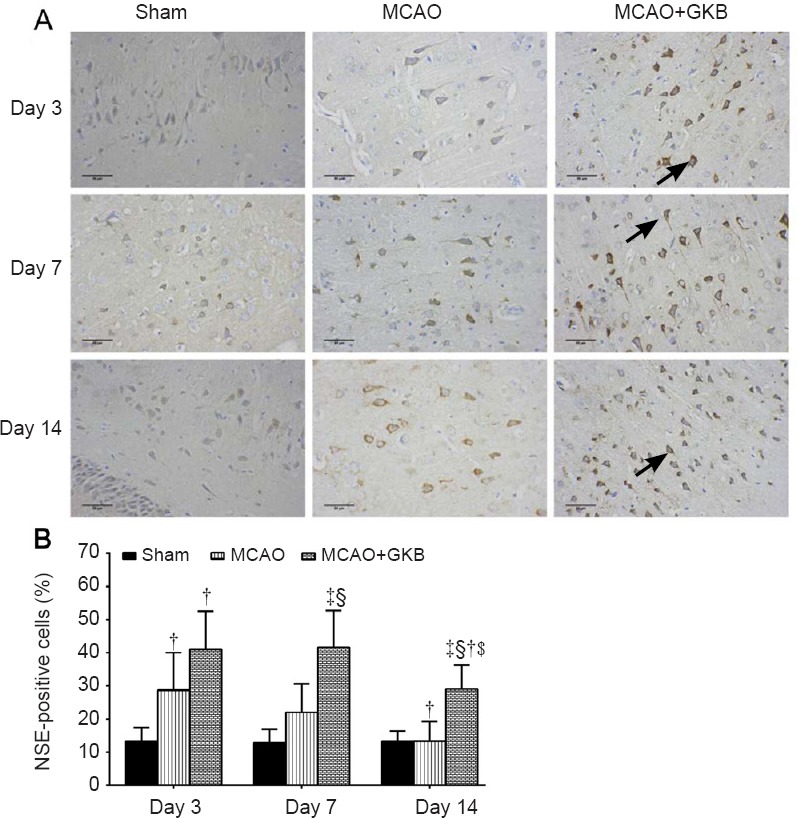
Effect of ginkgolide B (GKB) on neuron-specific enolase (NSE) immunoreactivity in the penumbra of rats with cerebral ischemia/reperfusion injury.
(A) NSE expression (arrows) in the brain of rats at 3, 7 and 14 days after reperfusion (immunohistochemistry; scale bars: 50 μm). (B) Quantitative analysis of NSE immunoreactivity. Data are presented as the mean ± SD (n = 6 per group; two-way analysis of variance with Bonferroni correction for pairwise comparison). ‡P < 0.05, vs. sham group; §P < 0.05, vs. middle cerebral artery occlusion (MCAO) group; †P < 0.05, vs. day 3; $P < 0.05, vs. day 7.
GFAP-positive cells
In the sham group, GFAP-positive cells were not observed in the various brain regions examined, including the cortex (Figure 6A). Throughout the whole observation period, the number of GFAP-positive cells was not significantly changed, except for an apparent decrease in the MCAO + GKB group on day 14 compared with day 7 (P = 0.002). The number of GFAP-positive cells was significantly higher in the MCAO + GKB group than in the sham or MCAO group (all P ≤ 0.015; Figure 6B).
Figure 6.
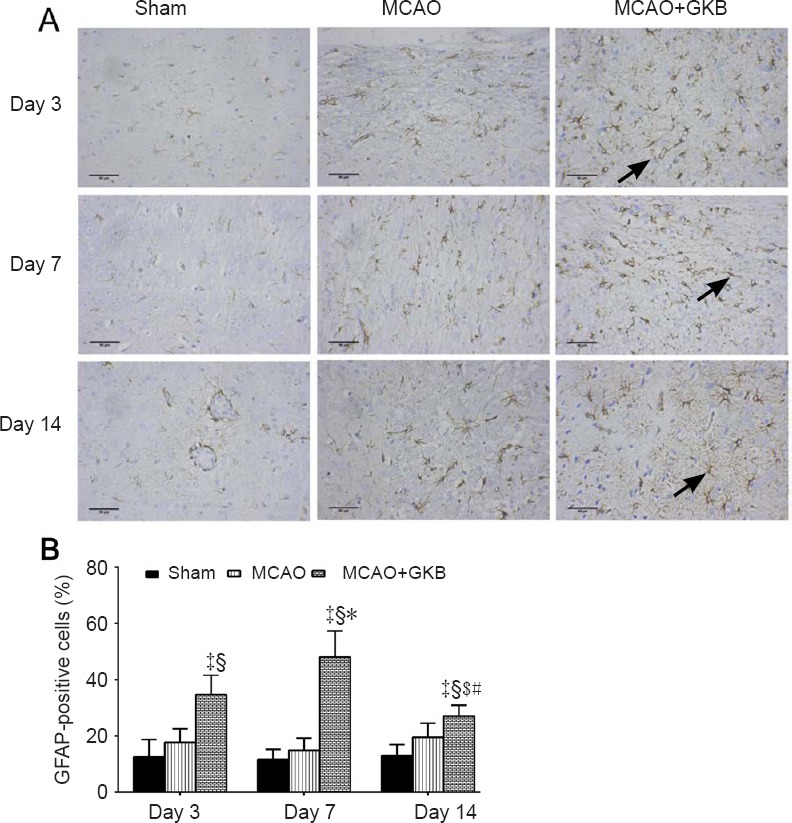
Effect of ginkgolide B (GKB) on glial fibrillary acid protein (GFAP) immunoreactivity in the penumbra of rats with cerebral ischemia/reperfusion injury
(A) GFAP expression (arrows) in the brain of rats at 3, 7 and 14 days after reperfusion (immunohistochemistry; scale bars: 50 μm). (B) Quantitative analysis of GFAP immunoreactivity. Data are presented as the mean ± SD (n = 6 per group; two-way analysis of variance with Bonferroni correction for pairwise comparison). ‡P < 0.05, vs. sham group; §P < 0.05, vs. middle cerebral artery occlusion (MCAO) group; $P < 0.05, vs. day 7; *P < 0.05, vs. day 3 within the same group; #P < 0.05, vs. day 7 within the same group.
BrdU-positive cells
In the sham group, BrdU-positive cells were only visible in the subgranular and subventricular zones of the dentate gyrus (Figure 7A). At 3 days after reperfusion, the number of BrdU-positive cells was reduced, and these cells were sparsely distributed. At 7 days after reperfusion, the number of BrdU-positive cells increased, and some dark-stained cells were aggregated. No significant temporal trend was seen for the BrdU-positive cells. A significant increase in BrdU-positive cells was observed in the MCAO + GKB group compared with the sham and MCAO groups at all time points (all P < 0.05; Figure 7B).
Figure 7.
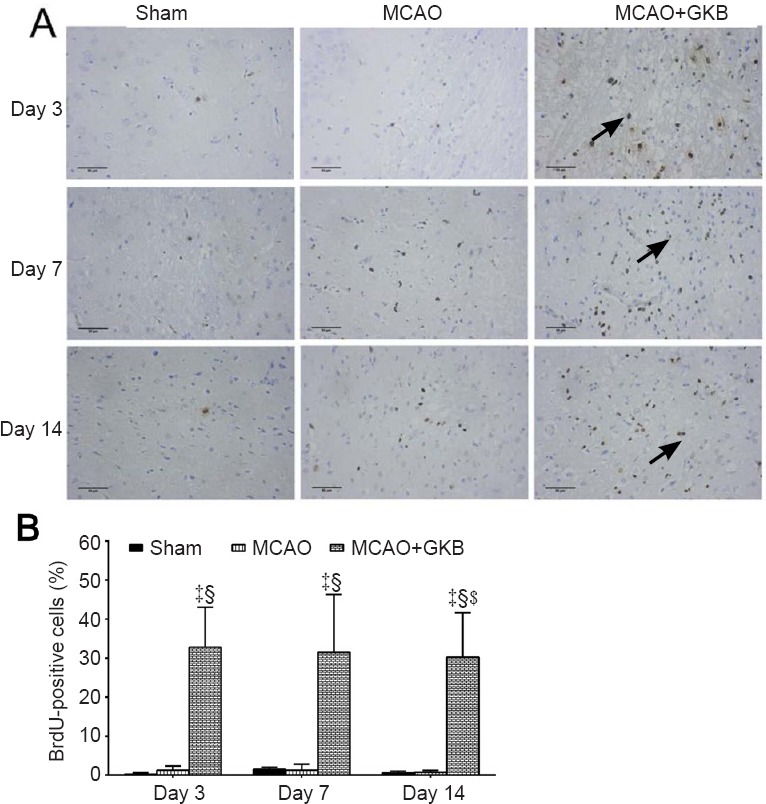
Effect of ginkgolide B (GKB) on bromodeoxyuridine (BrdU) immunoreactivity in rats with cerebral ischemia/reperfusion injury.
(A) BrdU-positive cells (arrows) in the brain of rats at 3, 7 and 14 days after reperfusion (immunohistochemistry; scale bars: 50 μm). (B) Quantitative analysis of BrdU immunoreactivity. Data are presented as the mean ± SD (n = 6 per group; two-way analysis of variance with Bonferroni correction for pairwise comparison). ‡P < 0.05, vs. sham group; §P < 0.05, vs. middle cerebral artery occlusion (MCAO) group.
BDNF and EGF mRNA expression
RT-PCR results are shown in Figure 8. Seven days after injury, mRNA expression of BDNF and EGF was highest in the MCAO + GKB group, followed by the MCAO group and the sham group.
Figure 8.
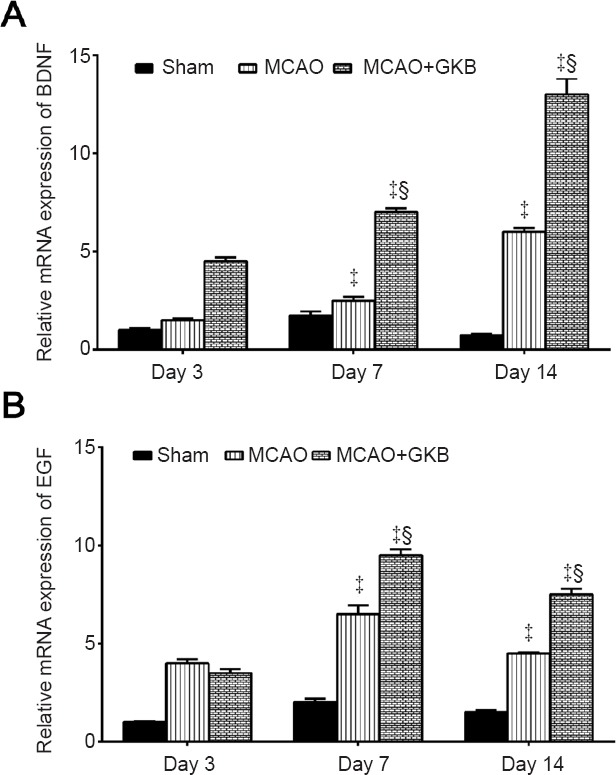
Effect of ginkgolide B (GKB) on brain-derived neurotrophic factor (BDNF) and epidermal growth factor (EGF) mRNA expression in rats with cerebral ischemia/reperfusion injury.
Relative expression of BDNF mRNA (A) and EGF mRNA (B) 3, 7 and 14 days after intervention. Data are presented as the mean ± SD (n = 3 per group; two-way analysis of variance with Bonferroni correction for pairwise comparison). ‡P < 0.05, vs. sham group; §P < 0.05, vs. middle cerebral artery occlusion (MCAO) group.
BDNF and SOCS2 protein expression
The protein bands were scanned, and their optical densities were determined and compared. Western blot assay results are shown in Figure 9. BDNF protein expression was not changed over time, and no difference was detected among the sham, MCAO, MCAO + GKB groups (Figure 9B). A similar result was obtained for SOCS2 protein expression, except downregulation was found in the MCAO and MCAO + GKB groups compared with the sham group at day 3 (all P < 0.05; Figure 9C).
Figure 9.
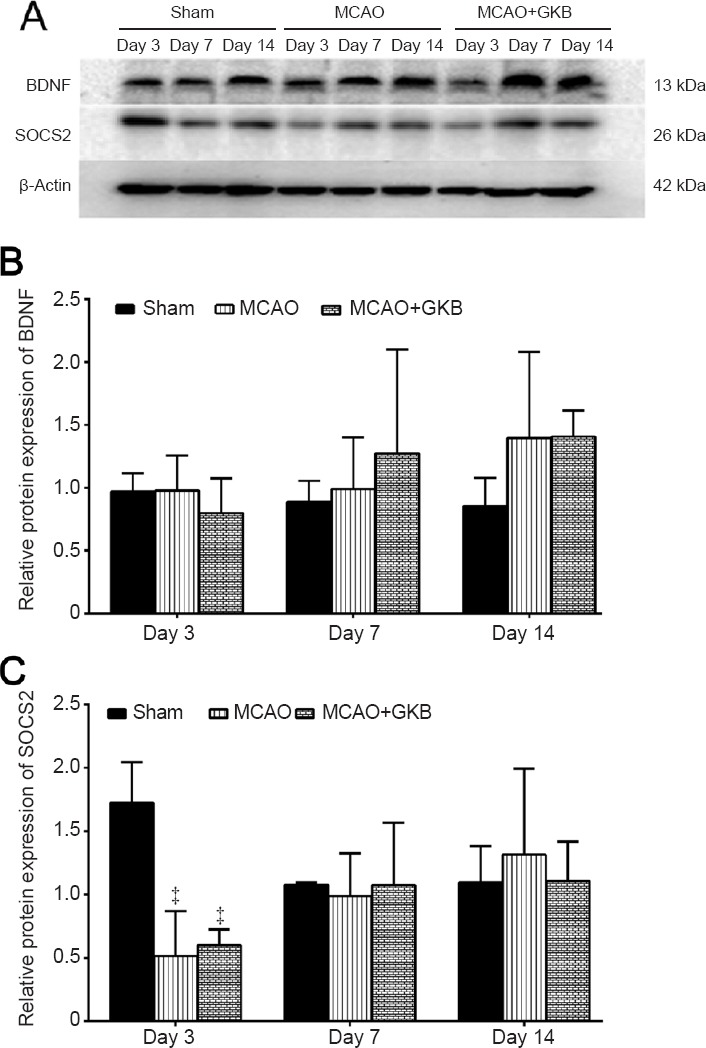
Effect of ginkgolide B (GKB) on brain-derived neurotrophic factor (BDNF) and suppressor of cytokine signaling 2 (SOCS2) protein expression in rats with cerebral ischemia/reperfusion injury after middle cerebral artery occlusion (MCAO).
(A) Expression of BDNF, SOCS2 and β-actin in the brain of rats at 3, 7 and 14 days after reperfusion (western blot assay). (B, C) Quantitative analysis of BDNF protein (B) and SOCS2 protein (C) expression. Relative protein expression was calculated as the ratio of the optical density of the target protein to that of β-actin. Data are presented as the mean ± SD (n = 3 per group; two-way analysis of variance with Bonferroni correction for pairwise comparison). ‡P < 0.05, vs. sham group.
Discussion
Our results indicate that GKB in an ischemia/reperfusion rat model increases the numbers of NSCs, neurons and astrocytes, and promotes the proliferation and self-renewal of NSCs. These effects of the herbal medicine might be related to the upregulation of EGF and SOCS2 expression, which serve to improve neurological function. These findings provide insight into the mechanisms underlying the neuroprotective effectiveness of GKB after ischemia/reperfusion. It should be noted that GKB can be absorbed by the gastrointestinal system and easily traverses the blood-brain barrier. The mean half-life of GKB in the free form is approximately 140 minutes (Mauri et al., 2001). GKB appears to have therapeutic potential for promoting the proliferation and differentiation of NSCs (Nabavi et al., 2015).
The adult central nervous system microenvironment is less than optimal for NSC survival and differentiation (Cai et al., 2015). To improve the microenvironment, researchers have co-transplanted NSCs with astrocytes and brain microvascular endothelial cells and found that this improved memory in a rat model of ischemic stroke (Cai et al., 2015). In a study to investigate the protective effects of NSCs, the cells were transplanted in a cerebral ischemia rat model and the results suggest that the mechanism underlying neuroprotection might involve the regulation of early inflammatory events (Watanabe et al., 2016). Our study showed that GKB increases the proliferation of NSCs and improves neurological function in the MCAO rat model, consistent with the results of these previous studies.
The mechanism underlying the GKB-induced differentiation of NSCs is poorly understood. In our study, NSE (a marker of neurons) and GFAP (a marker of astrocytes) were both expressed. GKB treatment significantly increased NSE and GFAP expression in the brains of rats with MCAO. This suggests that NSCs in the brain are pluripotent. Further experiments are needed to determine if GKB treatment enhances the differentiation of NSCs in the brains of rats with MCAO.
A number of factors may promote the growth of neurites, including growth factors, neurotrophic factors and neurite growth factors (Liu et al., 2016). SOCS has been shown to be involved in the regulation of neurite growth. In our study, SOCS2 was mainly expressed in the cell bodies of NSCs. After GKB treatment, SOCS2 expression increased significantly, as demonstrated by immunofluorescence and western blot assay. This was accompanied by the improvement of neurological function. This suggests that GKB upregulates SOCS2 expression, thereby inhibiting the JAK/STAT signaling pathway (Yang et al., 2012; Letellier and Haan, 2016) to block growth hormone and cytokine signaling. The upregulation of SOCS2 by GKB also likely promotes the binding of SOCS2 to and the activation of the EGF receptor, resulting in the differentiation of NSCs into neurons and rapid neurite growth.
We also found that nestin expression and BrdU levels were at a low level in the periphery of the ischemic region in the sham group. At 3, 7 and 14 days after reperfusion, cells positive for nestin or BrdU were seen in the periphery of the ischemic region. Nestin and BrdU levels peaked at 7 and 14 days after reperfusion in the MCAO group. In the MCAO + GKB group, the number of cells positive for Nestin or BrdU increased markedly. GKB treatment significantly increased the number of cells positive for NSE or GFAP, suggesting that the migratory NSCs had differentiated into neurons and glial cells.
A number of studies on the neuroprotective effects of GKB in ischemia have provided evidence for several different mechanisms of action. Gu et al. (2012) found that GKB inhixited NF-κB in an ischemia/reperfusion mouse model, perhaps accounting for the anti-inflammatory and anti-apoptotic effects of the herbal medicine. Qin et al. (2014) reported that in mice with ischemia/reperfusion injury, GKB prevented cathepsin-mediated cell death. Zhou et al. (2014) carried out an in vitro study to investigate the effect of hydrolyzed ginkgolides on cytochrome P-450s (CYPs), and found a marked increase in CYP34A mRNA expression. Wang et al. (2014) found that the neuroprotective effects of GKG in focal ischemia in rats might involve inhibition of stress-activated protein kinase/c-Jun N-terminal kinase activation, which would block the mitochondrial apoptotic pathway. Finally, Wu et al. (2015) found that GKB provides neuroprotection in ischemic injury by inhibiting the expression of the stress-related protein RTP801. Therefore, future studies should investigate whether these mechanisms are involved in the neuroprotective effects of GKB in the rodent model of ischemic stroke. It should be noted that we only investigated the short-term (at 3, 7 and 14 days after reperfusion) neuroprotective effects of GKB and that the intermediate and long-term effects remain to be elucidated. Thus, future studies should also examine the longer-term effects of the herbal medicine.
In conclusion, GKB promoted the proliferation and differentiation of NSCs in the rat brain following cerebral ischemia, thereby increasing the proportion of neurons to improve neurological outcome. GKB might accomplish these effects, at least in part, by enhancing expression of BDNF, EGF and SOCS2.
Footnotes
Conflicts of interest: The authors declare no competing financial interests.
Financial support: This work was supported by the National Natural science Foundation of China, No. 81073082 to JSZ. The funding body played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement: This study was approved by the Ethics Committee of Zhejiang University of China (approval No. 2015-152). The experimental procedure followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1985).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by the National Natural Science Foundation of China, No. 81073082 to JSZ.
(Copyedited by Patel B, Hindle A, Yu J, Li CH, Qiu Y, Song LP, Zhao M)
References
- 1.Alagappan D, Lazzarino DA, Felling RJ, Balan M, Kotenko SV, Levison SW. Brain injury expands the numbers of neural stem cells and progenitors in the SVZ by enhancing their responsiveness to EGF. ASN Neuro. 2009;1:e00009. doi: 10.1042/AN20090002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ba YY, Wang H, Ning XJ, Luo L, Li WS. Construction and identification of human glial cell-derived neurotrophic factor gene-modified Schwann cells from rhesus monkeys. Hum Gene Ther Methods. 2014;25:339–344. doi: 10.1089/hgtb.2014.119. [DOI] [PubMed] [Google Scholar]
- 3.Barnat M, Benassy MN, Vincensini L, Soares S, Fassier C, Propst F, Andrieux A, von Boxberg Y, Nothias F. The GSK3-MAP1B pathway controls neurite branching and microtubule dynamics. Mol Cell Neurosci. 2016;72:9–21. doi: 10.1016/j.mcn.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Cai Q, Chen Z, Song P, Wu L, Wang L, Deng G, Liu B, Chen Q. Co-transplantation of hippocampal neural stem cells and astrocytes and microvascular endothelial cells improve the memory in ischemic stroke rat. Int J Clin Exp Med. 2015;8:13109–13117. [PMC free article] [PubMed] [Google Scholar]
- 5.Chen T, Yu Y, Tang LJ, Kong L, Zhang CH, Chu HY, Yin LW, Ma HY. Neural stem cells over-expressing brain-derived neurotrophic factor promote neuronal survival and cytoskeletal protein expression in traumatic brain injury sites. Neural Regen Res. 2017;12:433–439. doi: 10.4103/1673-5374.202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui Y, Yi D, Bai X, Sun B, Zhao Y, Zhang Y. Ginkgolide B produced endophytic fungus (Fusarium oxysporum) isolated from Ginkgo biloba. Fitoterapia. 2012;83:913–920. doi: 10.1016/j.fitote.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Fang W, Deng Y, Li Y, Shang E, Fang F, Lv P, Bai L, Qi Y, Yan F, Mao L. Blood brain barrier permeability and therapeutic time window of Ginkgolide B in ischemia-reperfusion injury. Eur J Pharm Sci. 2010;39:8–14. doi: 10.1016/j.ejps.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Gu JH, Ge JB, Li M, Wu F, Zhang W, Qin ZH. Inhibition of NF-kappaB activation is associated with anti-inflammatory and anti-apoptotic effects of Ginkgolide B in a mouse model of cerebral ischemia/reperfusion injury. Eur J Pharm Sci. 2012;47:652–660. doi: 10.1016/j.ejps.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Hu YY, Huang M, Dong XQ, Xu QP, Yu WH, Zhang ZY. Ginkgolide B reduces neuronal cell apoptosis in the hemorrhagic rat brain: possible involvement of Toll-like receptor 4/nuclear factor-kappa B pathway. J Ethnopharmacol. 2011;137:1462–1468. doi: 10.1016/j.jep.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 10.Huang F, Wu Y, Wang H, Chang J, Ma G, Yin Z. Effect of controlled release of brain-derived neurotrophic factor and neurotrophin-3 from collagen gel on neural stem cells. Neuroreport. 2016;27:116–123. doi: 10.1097/WNR.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 11.Letellier E, Haan S. SOCS2: physiological and pathological functions. Front Biosci (Elite Ed) 2016;8:189–204. doi: 10.2741/E760. [DOI] [PubMed] [Google Scholar]
- 12.Liu D, Liu Z, Liu H, Li H, Pan X, Li Z. Brain-derived neurotrophic factor promotes vesicular glutamate transporter 3 expression and neurite outgrowth of dorsal root ganglion neurons through the activation of the transcription factors Etv4 and Etv5. Brain Res Bull. 2016;121:215–226. doi: 10.1016/j.brainresbull.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 14.Maclennan KM, Darlington CL, Smith PF. The CNS effects of Ginkgo biloba extracts and ginkgolide B. Prog Neurobiol. 2002;67:235–257. doi: 10.1016/s0301-0082(02)00015-1. [DOI] [PubMed] [Google Scholar]
- 15.Mauri P, Simonetti P, Gardana C, Minoggio M, Morazzoni P, Bombardelli E, Pietta P. Liquid chromatography/atmospheric pressure chemical ionization mass spectrometry of terpene lactones in plasma of volunteers dosed with Ginkgo biloba L. extracts. Rapid Commun Mass Spectrom. 2001;15:929–934. doi: 10.1002/rcm.316. [DOI] [PubMed] [Google Scholar]
- 16.Mousavi SJ, Doweidar MH. Three-dimensional numerical model of cell morphology during migration in multi-signaling substrates. PLoS One. 2015;10:e0122094. doi: 10.1371/journal.pone.0122094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nabavi SM, Habtemariam S, Daglia M, Braidy N, Loizzo MR, Tundis R, Nabavi SF. Neuroprotective effects of Ginkgolide B against ischemic stroke: a review of current literature. Curr Top Med Chem. 2015;15:2222–2232. doi: 10.2174/1568026615666150610142647. [DOI] [PubMed] [Google Scholar]
- 18.Ochi T, Nakatomi H, Ito A, Imai H, Okabe S, Saito N. Temporal changes in the response of SVZ neural stem cells to intraventricular administration of growth factors. Brain Res. 2016;1636:118–129. doi: 10.1016/j.brainres.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 19.Qin XF, Lu XJ, Ge JB, Xu HZ, Qin HD, Xu F. Ginkgolide B prevents cathepsin-mediated cell death following cerebral ischemia/reperfusion injury. Neuroreport. 2014;25:267–273. doi: 10.1097/WNR.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 20.Ryu S, Lee SH, Kim SU, Yoon BW. Human neural stem cells promote proliferation of endogenous neural stem cells and enhance angiogenesis in ischemic rat brain. Neural Regen Res. 2016;11:298–304. doi: 10.4103/1673-5374.177739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Y, Huang B, Sun L, Peng X, Chen X, Zou X. Ginkgolide B promotes proliferation and functional activities of bone marrow-derived endothelial progenitor cells: involvement of Akt/eNOS and MAPK/p38 signaling pathways. Eur Cell Mater. 2011;21:459–469. doi: 10.22203/ecm.v021a34. [DOI] [PubMed] [Google Scholar]
- 22.Wang NQ, Wang LY, Zhao HP, Liu P, Wang RL, Song JX, Gao L, Ji XM, Luo YM. Luoyutong treatment promotes functional recovery and neuronal plasticity after cerebral ischemia-reperfusion injury in rats. Evid Based Complement Alternat Med. 2015;2015:369021. doi: 10.1155/2015/369021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Jiang CM, Wan HY, Wu JL, Quan WQ, Wu KY, Li D. Neuroprotection against permanent focal cerebral ischemia by ginkgolides A and B is associated with obstruction of the mitochondrial apoptotic pathway via inhibition of c-Jun N-terminal kinase in rats. J Neurosci Res. 2014;92:232–242. doi: 10.1002/jnr.23306. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe T, Nagai A, Sheikh AM, Mitaki S, Wakabayashi K, Kim SU, Kobayashi S, Yamaguchi S. A human neural stem cell line provides neuroprotection and improves neurological performance by early intervention of neuroinflammatory system. Brain Res. 2016;1631:194–203. doi: 10.1016/j.brainres.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 25.Wu X, Su J, Chen L, Ma B, Gu X, Zhu L. Ginkgolide B protects neurons from ischemic injury by inhibiting the expression of RTP801. Cell Mol Neurobiol. 2015;35:943–952. doi: 10.1007/s10571-015-0189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia SH, Fang DC. Pharmacological action and mechanisms of ginkgolide B. Chin Med J (Engl) 2007;120:922–928. [PubMed] [Google Scholar]
- 27.Xie Q, Wang F, Zhou GP, Zhang H, Ma JX. Comparative analysis of the proliferation and differentiation of neural stem cells in the hippocampal dentate gyrus of rats with different ages. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:5426–5431. [Google Scholar]
- 28.Yang HL, Sun C, Sun C, Qi RL. Effect of suppressor of cytokine signaling 2 (SOCS2) on fat metabolism induced by growth hormone (GH) in porcine primary adipocyte. Mol Biol Rep. 2012;39:9113–9122. doi: 10.1007/s11033-012-1783-9. [DOI] [PubMed] [Google Scholar]
- 29.Yu JH, Seo JH, Lee JY, Lee MY, Cho SR. Induction of neurorestoration from endogenous stem cells. Cell Transplant. 2016;25:863–882. doi: 10.3727/096368916X690511. [DOI] [PubMed] [Google Scholar]
- 30.Zhou XW, Ma Z, Geng T, Wang ZZ, Ding G, Yu-an B, Xiao W. Evaluation of in vitro inhibition and induction of cytochrome P450 activities by hydrolyzed ginkgolides. J Ethnopharmacol. 2014;158(Pt A):132–139. doi: 10.1016/j.jep.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 31.Zhu LL, Wu LY, Yew DT, Fan M. Effects of hypoxia on the proliferation and differentiation of NSCs. Mol Neurobiol. 2005;31:231–242. doi: 10.1385/MN:31:1-3:231. [DOI] [PubMed] [Google Scholar]


