Keywords: nerve regeneration, acute spinal cord injury, decompression, Governor Vessel electroacupuncture, platelet-activating factor, apoptosis, methylprednisolone, caspase family, upper cervical spine, animal model, Basso, Beattie and Bresnahan locomotor scale, neural regeneration
Abstract
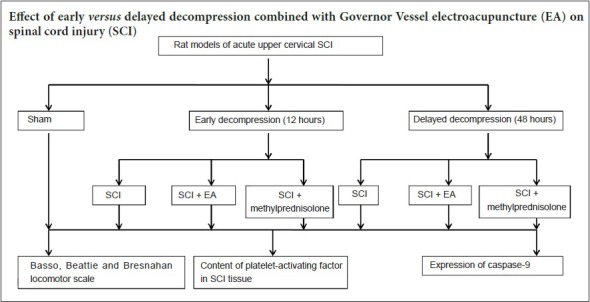
Decompression is the major therapeutic strategy for acute spinal cord injury, but there is some debate about the time window for decompression following spinal cord injury. An important goal and challenge in the treatment of spinal cord injury is inhibiting or reversing secondary injury. Governor Vessel electroacupuncture can improve symptoms of spinal cord injury by inhibiting cell apoptosis and improving the microenvironment of the injured spinal cord. In this study, Governor Vessel electroacupuncture combined with decompression at different time points was used to treat acute spinal cord injury. The rat models were established by inserting a balloon catheter into the atlanto-occipital space. The upper cervical spinal cord was compressed for 12 or 48 hours prior to decompression. Electroacupuncture was conducted at the acupoints Dazhui (GV14) and Baihui (GV 20) (2 Hz, 15 minutes) once a day for 14 consecutive days. Compared with decompression alone, hind limb motor function recovery was superior after decompression for 12 and 48 hours combined with electroacupuncture. However, the recovery of motor function was not significantly different at 14 days after treatment in rats receiving decompression for 12 hours. Platelet-activating factor levels and caspase-9 protein expression were significantly reduced in rats receiving electroacupuncture compared with decompression alone. These findings indicate that compared with decompression alone, Governor Vessel electroacupuncture combined with delayed decompression (48 hours) is more effective in the treatment of upper cervical spinal cord injury. Governor Vessel electroacupuncture combined with early decompression (12 hours) can accelerate the recovery of nerve movement in rats with upper cervical spinal cord injury. Nevertheless, further studies are necessary to confirm whether it is possible to obtain additional benefit compared with early decompression alone.
Introduction
Traumatic spinal cord injury (SCI) occurs in 3.6 to 195.4 patients per million worldwide (Jazayeri et al., 2015). Many cases of SCI result in tetraplegia and although this complication leads to tremendous personal loss and societal costs, treatments for SCI are not standardized and are highly variable.
The pathophysiology of SCI involves the primary injury and the subsequent secondary injury, which results from a progressive local cascade of tissue destruction, including the change in the microenvironment of the injured spinal cord (ischemia of the microcirculation, edema, inflammation, glutamatergic excitotoxicity) and apoptosis. The change in the microenvironment is the pathological basis of the functional deficits after SCI. The increased concentration of platelet-activating factor in the blood and tissue is an important factor that promotes the changes in the microenvironment (Faden et al., 1992; Wang et al., 2016). The long-term neurological deficits after SCI may be due in part to widespread apoptosis of the neurons and oligodendroglia in distant regions (Crowe et al., 1997; Emery et al., 1998). Many studies have shown that an important apoptotic pathway that mediates apoptosis after SCI is the family of caspases, including caspase-3 and caspase-9 (Springer et al., 1999; Nakagawa et al., 2000; Dong et al., 2015).
Decompression has been proven to be effective (Rabinowitz et al., 2008; Fehlings et al., 2012; Jones et al., 2012; Wilson et al., 2012). The focus of debate is on outlining the optimal timing of decompression for patients with acute SCI. Many scholars agree that early decompression (< 24 hours) leads to a clinical improvement in neurological status, but delayed decompression (> 24 hours) for acute SCI has not resulted in optimal outcomes in neurological status (Wilson et al., 2012; Dahdaleh et al., 2013). SCI cannot be cured by decompression, so performing decompression along with effective adjunctive therapies is an appropriate approach to enhance the treatment of acute SCI.
Adjunctive therapies are a key factor in continuously promoting optimal treatment of acute SCI. The adjunctive therapies, such as corticosteroids (Fehlings et al., 2014; Schroeder et al., 2014) and neuroprotectant agents (Wilson et al., 2013; Grossman et al., 2014), have some protective effect on the spinal cord and nerve roots, but the overall effects are not ideal.
Regarding traditional Chinese medicine, the spine has a close relationship with the Governor Vessels. SCI is regarded as a stasis in the meridian of the Governor Vessels. The cardiovascular complications and the change in hemorheology after SCI are evidence of the relationship between SCI and stasis in the Governor Vessel (Berlly et al., 2007; Furlan et al., 2008). Also, Governor Vessel electroacupuncture (EA) has been proven to prevent secondary damage and to improve neuroprotective effects after SCI (Liu et al., 2011; Juarez Becerril et al., 2015; Wei et al., 2017). A recent meta-analysis showed that combining acupuncture with other therapies had a higher cure rate and effectiveness than acupuncture alone (Deng et al., 2017). Thus, Governor Vessel EA may be an effective adjunctive therapy for SCI.
The upper cervical spine is adjacent to the medulla oblongata, so when the cervical spine is injured, it can affect breathing and be life-threatening. So far, no mature upper cervical SCI animal model has been developed. Thus, there have been fewer studies of upper cervical SCI (Sharifalhoseini et al., 2017).
To identify effective treatment strategies for acute upper SCI, we attempted to combine decompression with EA to treat acute upper SCI. Given the different effects of early and delayed decompression, we investigated the effect of EA combined with early or delayed decompression in rats with acute upper cervical SCI.
Materials and Methods
Animals
A total of 42 female Wistar rats aged 6 months and weighing 280 ± 20 g were obtained from the Laboratory Animal Center of the Academy of Military Medical Sciences in Beijing of China (animal license No. SCXK (Jun) 2017-0004). The rats were housed in individual cages at 23 ± 2°C, and allowed free access to food and water. The rats (n = 42) were equally and randomly divided into seven groups (n = 6 per group): sham, 12-hour SCI, 12-hour EA (SCI + EA), 12-hour methylprednisolone (MP) (SCI + MP), 48-hour SCI, 48-hour EA (SCI + EA), and 48-hour MP (SCI + MP). All experiments were approved by the Institutional Animal Care and Use Committee of the China-Japan Friendship Hospital of China (No. 170102).
Establishment of SCI models
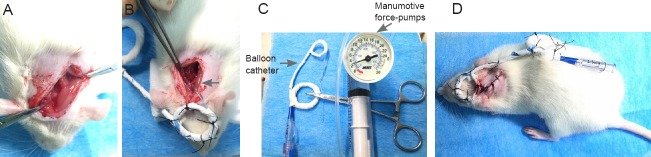
The establishment of an acute upper cervical SCI animal model was based on a previous study of ours (Tan et al., 2016). All rats were anesthetized with pentobarbital (Sigma-Aldrich, St. Louis, MO, USA). The external occipital protuberance was cut longitudinally to expose the atlanto-axial space and the atlanto-occipital space on the right side (Figure 1A). After removal of soft tissue and ligaments above the atlanto-axial space and the atlanto-occipital space, a balloon catheter (SPL25012X, 2.5 mm × 12.0 mm; Medtronic, Inc., Minneapolis, MN, USA) was inserted into the atlanto-occipital space, and the top of a balloon catheter was pulled out from the atlanto-occipital space (Figure 1B) by a balloon catheter compression system (Figure 1C). Finally, the balloon catheter was fixed on the back and head of the rats (Figure 1D). When the rat models were successfully generated 24 hours later, the sham group was non-pressurized; in the other groups, the end of the balloon catheters was connected with manumotive force-pumps (30 atm, Medtronic, Inc.). Iohexol (General Electric Pharmaceutical Co., Ltd., Shanghai, China) was injected into the balloon catheter continuously until the pressure reached 3 bar (1 bar = 100 kPa). Half of the rats maintained the compression for 48 hours and the other half maintained the compression for 12 hours. The balloon catheters were then decompressed and removed. During the model development, all rats were kept in separate cages with free access to food and water at 25 ± 3°C. The standard of model evaluation was spasmodic oscillation of the tail, unilateral limb and body retraction, and unilateral or bilateral paralysis. If there were rat deaths or serious complications during the experiment, we would fill in the rats and make sure that each group had six rats.
Figure 1.
Establishment of a rat model of acute upper cervical spinal cord injury.
(A) Exposure of the atlanto-occipital and atlanto-axial space; (B) insertion of balloon catheter (arrow) into the space; (C) balloon catheter compression system; (D) completion of the model.
EA and decompression
The rats in the sham group, the 12-hour SCI group, and the 48-hour SCI group were not handled again. The rats in the 12-hour EA (SCI + EA) group and the 48-hour EA (SCI + EA) groups were given the EA treatment 2 hours after decompression. The stainless steel acupuncture needles (0.3 mm in diameter, Jiajian Medical Equipment Co., Ltd., Wuxi, China) were inserted at the acupoints Dazhui (GV14; between the seventh cervical vertebra and the first thoracic vertebra at the middle of the back) and Baihui (GV 20; the center of the parietal bone) to a depth of 3–4 mm (Figure 2). The EA treatment (Hans-200A, Jisheng Medical Technology Co., Ltd., Nanjing, China) was administered at 2 Hz for 15 minutes each day for 14 consecutive days. The current intensity ranged from 0.6 mA to 1.0 mA. The 12-hour and 48-hour MP groups received 30 mg/kg MP (Pfizer Pharmaceuticals Ltd., New York, NY, USA) via the tail vein within 1 hour after decompression, and then at a dose of 5.4 mg/kg per hour once every four hours; all doses were given within 24 hours. All rats were sacrificed after 14 days of treatment. Afterwards, the damaged spinal cord segments were extracted.
Figure 2.
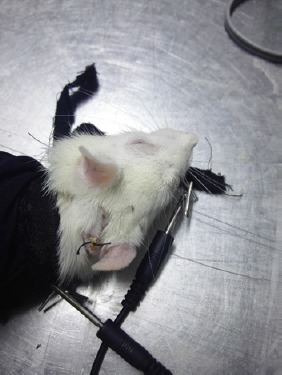
Treatment by Governor Vessel electroacupuncture.
Hind limb locomotor function score
Evaluation of motor ability was graded using a 0-21 point scoring system called the Basso, Beattie, and Bresnahan locomotor scale (BBB) (Bhimani et al., 2017), which assesses a combination of rat hind limb movements, trunk position, stability, stepping, coordination, paw placement, toe clearance, and tail position. The score was recorded at 0 and 24 hours, as well as at 3, 7, and 14 days after decompression independently by two outside researchers, and the results were averaged.
Enzyme linked immunosorbent assay (ELISA) for platelet-activating factor concentration in the damaged spinal cords
The platelet-activating factor concentration of damaged spinal cords was measured using a rat ELISA kit (Huamei Biological Engineering Co., Ltd., Wuhan, China). Optical density (OD) values were measured at 450 nm. Platelet-activating factor concentration was calculated using OD values in each group.
Western blot assay for the expression of caspase-9
After caspase-9 protein extraction, protein quantification, and the adjustment of protein concentration, the proteins were subjected to electrophoresis (Mini P-4 electrophoresis chamber; Kaiyuan Xinrui Instrument Co., Ltd., Beijing, China) and then transferred on the membrane. The membrane was incubated with primary GAPDH mouse monoclonal antibodies (ImmunoWay Biotechnology, Newark, DE, USA) for 10 minutes at 4°C overnight, and with secondary IgG/FITC (H + L) horseradish peroxidase antibodies (1:10,000; Kangwei Century Biotechnology Co., Ltd., Beijing, China), and IgG/TRITC (H + L) horseradish peroxidase antibodies (1:10,000; Kangwei Century Biotechnology Co., Ltd.), with oscillation for 40 minutes at room temperature. Reaction products were visualized using ECL Gel Pro analysis software (Image J, National Institutes of health, Bethesda, MD, USA) to quantify the amount of protein. OD values were normalized to those of β-actin (1:400; Sigma, St. Louis, MO, USA).
Statistical analysis
The data were recorded as the mean ± SD and analyzed using SPSS 20.0 software (IBM, Armonk, NY, USA). The BBB scores among the groups were compared using repeated measures analysis of variance, while the other data were analyzed using one-way analysis of variance followed by the least significant difference post hoc test. A P value of < 0.05 was considered statistically significant.
Results
Effects of decompression combined with EA on hind limb locomotor function in rats with acute upper cervical SCI
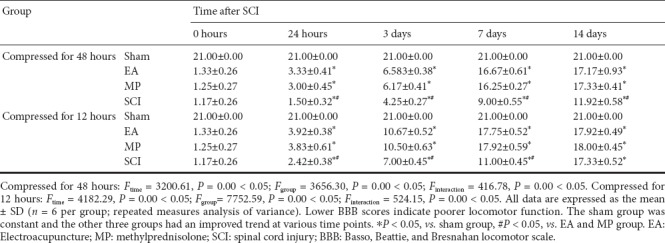
BBB scores were significantly lower in the SCI groups compared with the sham group. There was no significant difference in BBB scores among the SCI groups at 0 hours after decompression (P > 0.05). For the groups compressed for 12 hours, there were no significant differences in BBB scores at any time point between the 12-hour EA and 12-hour MP groups. BBB scores were lower in the 12-hour SCI group than in the 12-hour EA and 12-hour MP groups at 24 hours, 3 days, and 7 days (P < 0.05). Also, BBB scores were similar at 0 and 48 hours between the 12-hour EA and MP groups (P > 0.05). For the groups compressed for 48 hours, there were no significant differences in BBB scores at any time point between the 48-hour EA and MP groups. BBB scores at all time points were lower in the 48-hour SCI group than in the 48-hour EA and MP groups (P < 0.05; Table 1).
Table 1.
BBB scores of the groups compressed for 12 and 48 hours
Effects of decompression combined with EA on platelet-activating factor contents in injured tissues of rats with acute upper cervical SCI
ELISA results showed that the trends for changes in platelet-activating factor among groups were similar. The platelet-activating factor contents for the groups were as follows: sham group < EA group < MP group < SCI group (P < 0.05; Figure 3).
Figure 3.
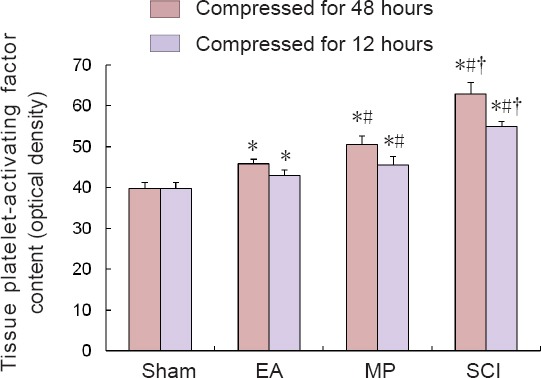
Comparison of tissue platelet-activating factor content in the groups compressed for 12 and 48 hours.
All data are expressed as the mean ± SD (n = 6 per group). *P < 0.05, vs. sham group; #P < 0.05, vs. EA group; †P < 0.05, vs. MP group (one-way analysis of variance followed by the least significant difference post hoc test). EA: Electroacupuncture; MP: methylprednisolone; SCI: spinal cord injury.
Effects of decompression combined with EA on caspase-9 protein expression in injured spinal cord tissues of rats with acute upper cervical SCI
Western blot assay results showed that the trends for changes in caspace-9 expression among groups were similar. The caspase-9 protein expression in the groups were as follows: sham group < EA group < MP group < SCI group (P < 0.05; Figure 4).
Figure 4.
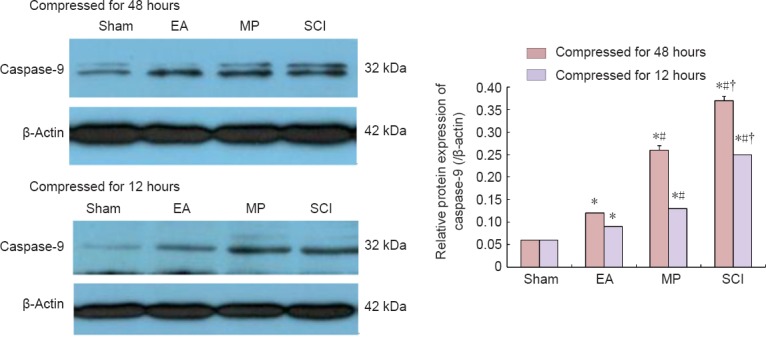
Comparison of caspase-9 protein expression in the groups compressed for 12 and 48 hours (western blot assay).
The caspase-9 expression was lower in the 12-hour and the 48-hour EA groups than in the 12-hour MP and SCI groups, and the 48-hour MP and SCI groups. The caspase-9 expression was lower in the 12-hour and the 48-hour MP groups than in the 12-hour and the 48-hour SCI groups. All data are expressed as the mean ± SD (n = 6 per group). *P < 0.05, vs. sham group; #P < 0.05, vs. EA group; †P < 0.05, vs. MP group (one-way analysis of variance followed by the least significant difference post hoc test). EA: Electroacupuncture; MP: methylprednisolone; SCI: spinal cord injury.
Discussion
Early decompression has been proven to be more effective in neural functional recovery than delayed decompression following acute SCI. Regarding the pathophysiology, early decompression can more effectively improve neurological function after SCI by inhibiting the expression of tumor necrosis factor α compared with delayed decompression (Xie et al., 2015). Additionally, delayed decompression can exacerbate reperfusion injury and is associated with ongoing enhanced levels of cytokine expression, microglial activation, and astrogliosis (Vidal et al., 2017). Decompression can only remove the insult above the spinal cord. The pathological lesion cannot be completely cured, and thus adjuvant treatment is necessary. In light of the different therapeutic effects of early and delayed decompression, different adjuvant treatments are necessary.
In the traditional Chinese medicine approach, SCI is thought to be strongly associated with stasis of the Governor Vessels. The disturbance of the microcirculation after SCI is an important link between neurological injury, cardiovascular complications, and the change in hemodynamics after SCI (Sezer et al., 2015), which are consistent with stasis of the Governor Vessels in traditional Chinese medicine. Governor Vessel EA can be used at the lesion site to facilitate the recovery of nerve function. This study investigated the effect of early and delayed decompression combined with EA after acute SCI from the standpoint of recovering neural function, improving spinal microenvironment, and inhibiting apoptosis.
In this study, in the groups compressed for 12 hours, BBB scores showed that the recovery of neural function was fast in the EA and MP groups. However, the final recovery of the three groups was similar. For the groups compressed for 48 hours, BBB scores showed that the recovery of neural function was fast and the final recovery was good in the EA and MP groups; moreover, the final recovery was similar among the three groups. Therefore, for decompression at 12 hours, EA and MP treatment can promote the recovery of motor function, but may not make a difference in final functional recovery. For decompression at 48 hours, EA and MP treatment can promote the recovery of motor function and improve the final functional recovery.
Platelet-activating factor is a soluble phospholipid metabolite that can cause the platelet to be activated, released, and aggregated. Platelet-activating factor has been proven to be an important pathological factor for secondary damage after SCI. Once SCI occurs, the platelet-activating factor content will rise rapidly in the SCI tissue, and prompt microvascular thrombosis, vascular endothelial cell damage, increased permeability of blood vessel walls, and blood-spinal cord barrier disruption, thereby altering the microenvironment of the injured spinal cord (Lindsberg et al., 1990; Faden et al., 1992; Xiao et al., 1996). Platelet-activating factor can promote platelet activation by strengthening the expression of adhesion molecule on the surface of platelets. The activation of platelets can combine and activate leukocytes, releasing a large number of oxygen free radicals, interleukins, and tumor necrosis factor-α, with inflammatory infiltration occurring around the injured spinal cord tissue (Guo et al., 2005), In this study, for the groups compressed for 12 and 48 hours, the change was similar; in other words, platelet-activating factor content was decreased in the EA group compared with the MP and SCI groups. These results confirm that EA can strongly improve the microenvironment of the injured spinal cord, and the EA group had a better long-term outcome than the MP and SCI groups.
Previous studies have found that the death of the spinal cord neurons after SCI occurs mainly via apoptosis (Springer et al., 1999; Zhang et al., 2012). Permanent or long-term loss of neural function caused by SCI is associated with spinal cord edema, degeneration, necrosis, and apoptosis of neurons and oligodendrocytes. SCI-induced apoptotic cell death of neurons and oligodendrocytes has been shown to cause progressive degeneration of the spinal cord, leading to permanent functional deficits (Moon et al., 2012). The key link in cell apoptosis is the cleavage of related cysteine protease proteins (Park et al., 2012). Among them, caspase-9 is an initiator of the caspase apoptotic protease. When caspase-9 is activated, it can activate the downstream protease cascade, including caspase-3, which can act on the poly (ADP-ribose) polymerase and lead to apoptosis, finally causing cell death (Eldadah et al., 2000). Therefore, caspase-9 is an important mediator of cell apoptosis. Inactivating caspase-9 can inhibit apoptosis and promote the recovery of nerve function. In the present study, for the groups compressed for 12 and 48 hours, there were similar changes; in other words, there was greater inactivation of caspase-9 in the EA group than in the MP and SCI groups. This result proves that EA can more strongly promote the recovery of neural function and may lead to a better long-term outcome than observed in the MP and SCI groups.
Our results showed that the platelet-activating factor contents of the 12-hour EA group were lower than in the 12-hour SCI group, and the caspase-9 expression of the 12-hour EA group was lower than in the 12-hour SCI group. Thus, why were there no significant differences in BBB scores at 14 days between the 12-hour EA and the 12-hour SCI groups? The authors believe that the reasons are as follows. First, we can conclude that the EA group had a faster recovery than the 12-hour SCI group, which was the effect of EA, but the short duration of compression and the strong ability of the rats to recover may have been the primary reasons for this. Second, spinal cord decompression may have some beneficial effects that have not yet been identified. For example, over time, the levels of substance P, a potential anti-inflammatory modulator used to treat injury-induced inflammatory central nervous system disorders (Jiang et al., 2012), constantly change after decompression in SCI patients (Da et al., 2017), which may influence recovery. Third, acute SCI is an extremely complex physiological process, so we cannot exclude factors that we have not studied yet that may influence recovery.
Our study focused on acute upper cervical SCI. Given the high mortality and instability of the previous upper cervical SCI animal models, therapeutic and mechanistic studies of acute upper cervical SCI have been limited, and our model solved the problem of high mortality and instability and enriched this academic field. Early and late decompression leads to different outcomes following acute upper cervical SCI. We chose two time points to intervene with Governor Vessel EA, which made the study systematic and integrated. Meanwhile, the syndrome differentiation and treatment theory of traditional Chinese medicine was reflected.
The limitation of this study was primarily the small sample size, which may have increased error bias and influenced the results. Besides, the mechanism of secondary injury includes not only changes in the microenvironment of the spinal cord and apoptosis, but also the axonal regeneration barrier (Tran, et al., 2018). Our follow-up studies will assess different factors associated with acute upper cervical SCI.
In summary, EA was effective for SCI, and delayed decompression combined with EA was a more effective treatment than decompression alone. Early decompression combined with EA promoted the recovery of motor function more effectively than decompression alone. However, whether it has a favorable final effect requires additional study.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
Financial support: This study was supported by a grant from the Capital Characteristic Clinical Application Research Project of Beijing Municipal Science and Technology Plan of China, No. Z16110000516009. The funding body played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement: All experiments were approved by the Institutional Animal Care and Use Committee of China-Japan Friendship Hospital of China (No. 170102). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the Capital Characteristic Clinical Application Research Projects of Beijing Municipal Science and Technology Plan of China, No. Z16110000516009.
(Copyedited by Mittwede P, Hindle A, Wang J, Li CH, Qiu Y, Song LP, Zhao M)
References
- 1.Berlly M, Shem K. Respiratory management during the first five days after spinal cord injury. J Spinal Cord Med. 2007;30:309–318. doi: 10.1080/10790268.2007.11753946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhimani AD, Kheirkhah P, Arnone GD, Nahhas CR, Kumar P, Wonais M, Hidrogo H, Aguilar E, Spalinski D, Gopalka M, Roth S, Mehta AI. Functional gait analysis in a spinal contusion rat model. Neurosci Biobehav Rev. 2017;83:540–546. doi: 10.1016/j.neubiorev.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nature Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- 4.Da SJ, Evangelista BG, Rag V, Oliveira ME, Chacur M. Early and late behavioral changes in sciatic nerve injury may be modulated by nerve growth factor and substance P in rats: a chronic constriction injury long-term evaluation. J Biol Regul Homeost Agents. 2017;31:309–319. [PubMed] [Google Scholar]
- 5.Dahdaleh NS, Lawton CD, El Ahmadieh TY, Nixon AT, El Tecle NE, Oh S, Fessler RG, Smith ZA. Evidence-based management of central cord syndrome. Neurosurg Focus. 2013;35:E6. doi: 10.3171/2013.3.FOCUS13101. [DOI] [PubMed] [Google Scholar]
- 6.Deng YZ, Xu LG, Chen L, Zhou D, Liu Y. Effectiveness of acupuncture in the management of cervical spondylosis: a meta-analysis. J Biol Regul Homeost Agents. 2017;31:1017–1022. [PubMed] [Google Scholar]
- 7.Dong Y, Miao L, Long H, Ding H. Neuroprotective effects and impact on caspase-12 expression of tauroursodeoxycholic acid after acute spinal cord injury in rats. Int J Clin Exp Pathol. 2015;8:15871. [PMC free article] [PubMed] [Google Scholar]
- 8.Eldadah BA, Faden A. Caspase pathways, neuronal apoptosis, and CNS injury. J Neurotrauma. 2000;17:811–829. doi: 10.1089/neu.2000.17.811. [DOI] [PubMed] [Google Scholar]
- 9.Emery E, Aldana P, Bunge MB, Puckett W, Srinivasan A, Keane RW, Bethea J, Levi AD. Apoptosis after traumatic human spinal cord injury. J Neurosurg. 1998;89:911–920. doi: 10.3171/jns.1998.89.6.0911. [DOI] [PubMed] [Google Scholar]
- 10.Faden AI, Halt P. Platelet-activating factor reduces spinal cord blood flow and causes behavioral deficits after intrathecal administration in rats through a specific receptor mechanism. J Pharmacol Exp Ther. 1992;261:1064–1070. [PubMed] [Google Scholar]
- 11.Fehlings MG, Vaccaro A, Wilson JR, Singh A, W Cadotte D, Harrop JS, Aarabi B, Shaffrey C, Dvorak M, Fisher C, Arnold P, Massicotte EM, Lewis S, Rampersaud R. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the surgical timing in acute spinal cord injury study (STASCIS) PLoS One. 2012;7:e32037. doi: 10.1371/journal.pone.0032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fehlings MG, Wilson JR, Cho N. Methylprednisolone for the treatment of acute spinal cord injury counterpoint. Neurosurgery. 2014;61:36–42. doi: 10.1227/NEU.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 13.Furlan JC, Fehlings MG. Cardiovascular complications after acute spinal cord injury: pathophysiology, diagnosis, and management. Neurosurg Focus. 2008;25:E13. doi: 10.3171/FOC.2008.25.11.E13. [DOI] [PubMed] [Google Scholar]
- 14.Grossman RG, Fehlings MG, Frankowski RF, Burau KD, Chow DS, Tator C, Teng A, Toups EG, Harrop JS, Aarabi B, Shaffrey CI, Johnson MM, Harkema SJ, Boakye M, Guest JD, Wilson JR. A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J Neurotrauma. 2014;31:239–255. doi: 10.1089/neu.2013.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo NJ, Chang YL, Xiao DJ, Huang P. Activation of platelet-neutrophil mediated by platelet-activating factor. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2005;13:447–451. [PubMed] [Google Scholar]
- 16.Jazayeri SB, Beygi S, Shokraneh F, Hagen EM, Rahimi-Movaghar V. Incidence of traumatic spinal cord injury worldwide: a systematic review. Eur Spine J. 2015;24:905–918. doi: 10.1007/s00586-014-3424-6. [DOI] [PubMed] [Google Scholar]
- 17.Jiang MH, Chung E, Chi GF, Ahn W, Lim JE, Hong HS, Kim DW, Choi H, Km J, Son Y. Substance P induces M2-type macrophages after spinal cord injury. Neuroreport. 2012;23:786–792. doi: 10.1097/WNR.0b013e3283572206. [DOI] [PubMed] [Google Scholar]
- 18.Jones CF, Newell RS, Lee JH, Cripton PA, Kwon BK. The pressure distribution of cerebral spinal fluid responds to residual compression and decompression in an animal model of acute spinal cord injury. Spine. 2012;37:1422–1431. doi: 10.1097/BRS.0b013e31826ba7cd. [DOI] [PubMed] [Google Scholar]
- 19.Juarez Becerril O, Salgado Ceballos H, Anguiano Solis C, Alvarado Sanchez B, Lopez Hernandez ME, Diaz Ruiz A, Torres Castillo S. Electro-acupuncture at GV.4 improves functional recovery in paralyzed rats after a traumatic spinal cord injury. Acupunct Electrother Res. 2015;40:355–369. [PubMed] [Google Scholar]
- 20.Lindsberg PJ, Yue TL, Frerichs KU, Hallenbeck JM, Feuerstein G. Evidence for platelet-activating factor as a novel mediator in experimental stroke in rabbits. Stroke. 1990;21:1452–1457. doi: 10.1161/01.str.21.10.1452. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Ding Y, Zeng YS. A new combined therapeutic strategy of governor vessel electro-acupuncture and adult stem cell transplantation promotes the recovery of injured spinal cord. Curr Med Chem. 2011;18:5165–5171. doi: 10.2174/092986711797636144. [DOI] [PubMed] [Google Scholar]
- 22.Moon YJ, Lee JY, Oh MS, Pak YK, Park KS, Oh TH, Yune TY. Inhibition of inflammation and oxidative stress by Angelica dahuricae radix extract decreases apoptotic cell death and improves functional recovery after spinal cord injury. J Neurosci Res. 2012;90:243–256. doi: 10.1002/jnr.22734. [DOI] [PubMed] [Google Scholar]
- 23.NakagawaT, Zhu H, Morishima N. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 24.Park HH. Structural features of caspase-activating complexes. Int J Mol Sci. 2012;13:4807–4818. doi: 10.3390/ijms13044807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabinowitz RS, Eck JC, Harper CM, Jr, Larson DR, Jimenez MA, Parisi JE, Friedman J, AYaszemski MJ, Currier BL. Urgent surgical decompression compared to methylprednisolone for the treatment of acute spinal cord injury: a randomized prospective study in beagle dogs. Spine. 2008;33:2260–2268. doi: 10.1097/BRS.0b013e31818786db. [DOI] [PubMed] [Google Scholar]
- 26.Schroeder GD, Kwon BK, Eck JC, Savage JW, Hsu WK, Patel AA. Survey of cervical spine research society members on the use of high-dose steroids for acute spinal cord injuries. Spine. 2014;39:971–977. doi: 10.1097/BRS.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 27.Sezer N, Akkuş S, Uğurlu FG. Chronic complications of spinal cord injury. World J Orthop. 2015;6:24–33. doi: 10.5312/wjo.v6.i1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharif-Alhoseini M, Khormali M, Rezaei M, Safdarian M, Hajighadery A, Khalatbari MM, Safdarian M, Meknatkhah S, Rezvan M, Chalangari M, Derakhshan P, Rahimi-Movaghar V. Animal models of spinal cord injury: a systematic review. Spinal Cord. 2017;55:714–721. doi: 10.1038/sc.2016.187. [DOI] [PubMed] [Google Scholar]
- 29.Springer JE, Azbill RD, Knapp PE. Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nat Med. 1999;5:943–946. doi: 10.1038/11387. [DOI] [PubMed] [Google Scholar]
- 30.Tan MS, Qi YN, Jiang LH, Liu CY, Wang YL, Wang W, Hao QY, Yi P, Yang F, Tang XS. Governor-vessel-blockade-type upper cervical spinal cord injury rat model created using Foley’s tube. Zhongyi Zhenggu. 2016;28:1–5. [Google Scholar]
- 31.Tran AP, Warren PM, Silver J. The biology of regeneration failure and success after spinal cord injury. Physiol Rev. 2018;98:881–917. doi: 10.1152/physrev.00017.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vidal PM, Karadimas SK, Ulndreaj A, Laliberte AM, Tetreault L, Forner S, Wang J, Foltz WD, Fehlings MG. Delayed decompression exacerbates ischemia-reperfusion injury in cervical compressive myelopathy. JCI Insight. 2017;2:92512. doi: 10.1172/jci.insight.92512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Gao Z, Zhang Y, Feng SQ, Liu Y, Shields LBE, Zhao YZ, Zhu Q, Gozal D, Shields CB, Cai J. Attenuated reactive gliosis and enhanced functional recovery following spinal cord injury in null mutant mice of platelet-activating factor receptor. Mol Neurobiol. 2016;53:3448–3461. doi: 10.1007/s12035-015-9263-6. [DOI] [PubMed] [Google Scholar]
- 34.Wei Z, Wang Y, Zhao W, Schachner M. Electro-acupuncture modulates L1 adhesion molecule expression after mouse spinal cord injury. Zhongguo Jiehe Yixue Zazhi. 2017;45:37–52. doi: 10.1142/S0192415X17500045. [DOI] [PubMed] [Google Scholar]
- 35.Wilson JR, Forgione N, Fehlings MG. Emerging therapies for acute traumatic spinal cord injury. CMAJ. 2013;185:485–492. doi: 10.1503/cmaj.121206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson JR, Singh A, Craven C, Verrier MC, Drew B, Ahn H, Ford M, Fehlings MG. Early versus late surgery for traumatic spinal cord injury: the results of a prospective Canadian cohort study. Spinal Cord. 2012;50:840–843. doi: 10.1038/sc.2012.59. [DOI] [PubMed] [Google Scholar]
- 37.Xiao J, Zhao D, Jia L. Effect of platelet activating factor receptor at spinal cord neurocyte membrane on secondary damage after spinal cord injury. Zhonghua Yi Xue Za Zhi. 1996;76:120–123. [PubMed] [Google Scholar]
- 38.Xie JB, Zhang, Quan-hui, Xu ZJ. Inhibition of inflammatory cytokines after early decompression may mediate recovery of neurological function in rats with spinal cord injury. Neural Regen Res. 2015;10:219–224. doi: 10.4103/1673-5374.152374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang N, Yin Y, Xu SJ, Wu YP, Chen WS. Inflammation apoptosis in spinal cord injury. Indian J Med Res. 2012;135:287–296. [PMC free article] [PubMed] [Google Scholar]