Mouse and non-human primate models of neurodegenerative disease: The prevalence of age-related neurodegenerative diseases continues to increase with ever increasing aging population over the age of 60. Although the difficulties associated with neurodegenerative diseases present an urgent global issue, there is no effective treatment for these conditions. To develop therapeutic methods and therapeutic agents for neurodegenerative diseases, model animals that simulate the human disease pathology are eagerly anticipated. There have been significant advancement in embryonic stem cell and genetic engineering in mice, and various transgenic models of neurodegenerative diseases provided great contribution to our understanding of basic disease mechanisms and the development of potential therapeutic molecules for neurodegenerative diseases (Rockenstein et al., 2007; Giuliani et al., 2017). However, differences between humans and rodents in the structure and physiological functions of the brain have resulted in difficulty in reproducing the selective vulnerability of specific neurons or circuits in mouse and rat models (Chan, 2013). Non-human primates, on the other hand, more closely share genetic, physiological, and morphological similarities with humans and can provide a better test system for drug and biomarker discovery for various psychological disorders and neurological diseases. Despite their value, non-human primates are not widely used due to limited availability of the animals, requiring a large breeding space, specialized breeders and veterans, which increases the cost of the study, not to mention the ethical issues.
Among non-human primates, the common marmoset (Callithrix jacchus) is a small, non-endangered new world primate that is native to Brazil. Adult marmosets have an average height of 20–30 cm, weigh 350–450 g, and have advantages related to their small size. In comparison with macaque species, the relatively small size of marmosets translates to lower caging and feeding costs and reduced floor space requirements. Additionally, they can be handled relatively easily by one researcher. Marmosets also offer many advantages in studies of reproductive technology compared to other laboratory primates, such as a shorter gestation period, a faster sexual maturation, and higher fecundity, permitting the rapid establishment of gene-modified model lines. In fact, various basic research tools for marmosets have been developed recently, including transgenic (Sasaki et al., 2009) and genome-editing techniques (Sato et al., 2016) and elucidation of the entire genome sequence (Marmoset Genome Sequencing and Analysis Consortium, 2014). The natural advantages of the marmoset as a model of human systems in combination with its advantages as an experimental animal have led to a surge in interest among neuroscience researchers. In 2016, marmosets have been adopted for Brain/MINDS (Brain Mapping by Integrated Neurotechnologies for Disease Studies), a national brain project started in Japan with a goal to develop marmoset as a model animal for neuroscience. The project aims to: (1) build a multiscale marmoset brain map, (2) develop new technologies for researchers, and (3) create transgenic lines for modeling brain diseases.
In this context, we focused on polyglutamine disease, a generic term for nine neurodegenerative diseases, including Huntington’s disease and various spinocerebellar ataxias, in an attempt to create an improved animal model of a human neurological disease. The abnormal elongation of the CAG repeat sequence encoding glutamine within the causative gene has revealed a common onset molecular mechanism that induces protein misfolding and aggregation, leading to neurodegeneration. Our first transgenic marmoset model for spinocerebellar ataxia type 3 (SCA3), also known as Machado-Joseph disease, used the cytomegalovirus (CMV) promoter to induce transgene expression and progressive neurological symptoms, including motor impairment (Tomioka et al., 2017b). Pathological examination revealed neurodegeneration and intranuclear polyglutamine protein inclusions accompanied by gliosis, all of which recapitulate the neuropathological features of patients with polyglutamine disease. Consistent with the neuronal loss in the cerebellum, non-invasive brain magnetic resonance imaging of a living symptomatic marmoset showed cerebellar atrophy. However, the model had a high fetal death rate, widespread juvenile disease onset, and rapid disease progression, which are unusual in humans with SCA3. Moreover, F1 offspring were difficult to obtain by natural mating. It appears that, although ubiquitously expressed promoters induce strong transgene expression, they also lead to irrelevant symptoms and abnormalities in fetal development. Therefore, controlling transgene expression is one of the most important challenges in the development of more sophisticated animal models of human disease.
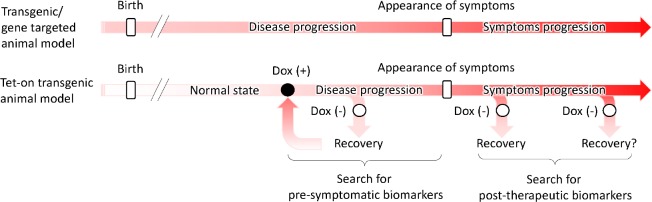
Advantages of model animals carrying the tetracycline-inducible transgene expression (Tet-On) system for neurodegenerative disease research: The Tet-On system has yielded new information about neurodegeneration. Tet-On system is based on two constructs: the first contains the gene of interest under the control of the tetracycline response element (TRE), and the second contains the transactivator [the tetracycline transactivator protein (tTA) or recombinant tTA (rtTA)] under the control of a constitutive or tissue-specific promoter (Chtarto et al., 2003). In mice, the transactivator and TRE strains are crossed to produce transgene-inducible transgenic animals. The main advantage of this system is its ability to turn transgene expression on and off by adding tetracycline or its derivate doxycycline, thereby enabling us to study the possibilities of proceeding and preventing disease progression. A transgenic mouse model of Huntington’s disease using this system showed neuronal inclusions, characteristic neuropathology, and progressive motor dysfunction (Yamamoto et al., 2000). Blocking the transgene expression led to the disappearance of inclusions and the amelioration of the behavioral phenotype, suggesting that neurodegenerative disease is reversible. A SCA3 mouse model using this system also revealed that reducing the production of pathogenic ataxin-3 may be a promising approach for treating Machado–Joseph disease (Boy et al., 2009). Albeit yielding interesting results in mice, Tet-On system is difficult to apply to large animals with greater longevity due to a long breeding cycle and a large number of animals required for the studies. Thus, a single construct carrying both elements of the Tet-On system has been established and has demonstrated the feasibility of the use of this system in larger animals (Jin et al., 2014). Recently, we successfully generated a transgenic marmoset line carrying the mutant human ataxin-3 gene controlled by the Tet-On system in a single vector (Tomioka et al., 2017a). The founder Tet-On transgenic marmosets showed inducible transgene expression with oral doxycycline treatment. Compared with the classic SCA3 model marmosets with the CMV promoter, fetal death in the early gestation period was reduced, and no juvenile disease onset was observed in these Tet-On transgenic marmosets. Moreover, F1 transgenic marmosets were obtained by natural mating. Although induced disease onset experiments with doxycycline administration are future challenges in the F1 or later generations, this Tet-On transgenic marmoset model could provide pre-symptomatic and post-therapeutic data similar to those in humans, which can help develop pre-symptomatic and post-therapeutic biomarkers of neurodegenerative diseases (Figure 1).
Figure 1.
Scheme of neurodegenerative disease progression in classic and tetracycline-inducible transgene expression (Tet-On) transgenic animal models.
In classic transgenic animal model, the transgene is constitutively expressed from conception. In our Tet-On transgenic marmosets, however, the transgenes are induced by the oral administration of doxycycline, allowing us to study the cellular dynamics at the disease onset as well as pre-symptomatic biomarkers. Our Tet-On transgenic marmosets also offer the ability to turn transgene expression off by removing doxycycline, thereby allowing us to study the post-therapeutic states and biomarkers.
Perspectives on the usage of Tet-On transgenic marmosets for developing pre-symptomatic and post-therapeutic biomarkers: Because neurodegenerative diseases are slowly progressive, patients are often not aware of the disease in the early stages. Consequently, sensitive biomarkers for predicting disease onset are essential to developing therapies for treating neurodegenerative diseases. Generally, gene-targeted animals, such as knock-in/out animals, are moderately suitable models of genetic disease because they simulate some aspects of the disease pathology. Classic transgenic models have limitations in that the molecular pathology begins during gestation, which may not be the case in humans. Because of the constitutive expression of the transgene, some symptoms are the result of brain development abnormalities that have little to do with the actual disease in question. Indeed, our previous classic transgenic models using the CMV promoter (Tomioka et al., 2017b) suffered from the same limitations. In our Tet-On transgenic marmosets, however, the transgenes are induced by the oral administration of doxycycline, allowing us to study the cellular dynamics at the disease onset as well as pre-symptomatic biomarkers. Because non-human primates share physiological similarities with humans, there are great expectations for the development of biomarkers close to those of humans by periodic omics analysis of RNA, proteins, and metabolites in blood or spinal fluid after oral administration of doxycycline.
Although various model animals have proved to be important tools for screening potential therapeutic molecules and genetic modifiers of disease, there is an urgent need for sensitive post-therapeutic biomarkers to evaluate therapeutic efficacy in clinical trials and to monitor the responses of patients to new therapies. A conditional mouse model using the Tet system was generated to test whether disease symptoms can be reversed by switching off expression of mutant ATXN3 (Boy et al., 2009). The fact that phenotypic and pathological features of disease were reversed in a conditional mouse model of Machado–Joseph disease by switching off the transgene indicates that a state in which the transgene is stopped after disease onset simulates a post-therapeutic state. Our Tet-On transgenic marmosets offer the ability to turn transgene expression off by removing doxycycline, thereby allowing us to study the post-therapeutic states and biomarkers. Technical advancements in such an inducible transgene expression system in non-human primates will help delineate disease progression and recovery, and will provide a great tool for the development of novel biomarkers and drug discovery with better translation to human subjects.
Footnotes
Copyright transfer agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Willian Orlando Castillo, Universidade de Sao Paulo, Brazil.
References
- 1.Boy J, Schmidt T, Wolburg H, Mack A, Nuber S, Bottcher M, Schmitt I, Holzmann C, Zimmermann F, Servadio A, Riess O. Reversibility of symptoms in a conditional mouse model of spinocerebellar ataxia type 3. Hum Mol Genet. 2009;18:4282–4295. doi: 10.1093/hmg/ddp381. [DOI] [PubMed] [Google Scholar]
- 2.Chan AW. Progress and prospects for genetic modification of nonhuman primate models in biomedical research. ILAR J. 2013;54:211–223. doi: 10.1093/ilar/ilt035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chtarto A, Bender HU, Hanemann CO, Kemp T, Lehtonen E, Levivier M, Brotchi J, Velu T, Tenenbaum L. Tetracycline-inducible transgene expression mediated by a single AAV vector. Gene Ther. 2003;10:84–94. doi: 10.1038/sj.gt.3301838. [DOI] [PubMed] [Google Scholar]
- 4.Giuliani D, Ottani A, Neri L, Zaffe D, Grieco P, Jochem J, Cavallini GM, Catania A, Guarini S. Multiple beneficial effects of melanocortin MC4 receptor agonists in experimental neurodegenerative disorders: Therapeutic perspectives. Prog Neurobiol. 2017;148:40–56. doi: 10.1016/j.pneurobio.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Jin YX, Jeon Y, Lee SH, Kwon MS, Kim T, Cui XS, Hyun SH, Kim NH. Production of pigs expressing a transgene under the control of a tetracycline-inducible system. PLoS One. 2014;9:e86146. doi: 10.1371/journal.pone.0086146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marmoset Genome Sequencing and Analysis Consortium. The common marmoset genome provides insight into primate biology and evolution. Nat Genet. 2014;46:850–857. doi: 10.1038/ng.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rockenstein E, Crews L, Masliah E. Transgenic animal models of neurodegenerative diseases and their application to treatment development. Adv Drug Deliv Rev. 2007;59:1093–1102. doi: 10.1016/j.addr.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, Tomioka I, Sotomaru Y, Hirakawa R, Eto T, Shiozawa S, Maeda T, Ito M, Ito R, Kito C, Yagihashi C, Kawai K, Miyoshi H, Tanioka Y, Tamaoki N, et al. Generation of transgenic non-human primates with germline transmission. Nature. 2009;459:523–527. doi: 10.1038/nature08090. [DOI] [PubMed] [Google Scholar]
- 9.Sato K, Oiwa R, Kumita W, Henry R, Sakuma T, Ito R, Nozu R, Inoue T, Katano I, Sato K, Okahara N, Okahara J, Shimizu Y, Yamamoto M, Hanazawa K, Kawakami T, Kametani Y, Suzuki R, Takahashi T, Weinstein EJ, et al. Generation of a nonhuman primate model of severe combined immunodeficiency using highly efficient genome editing. Cell Stem Cell. 2016;19:127–138. doi: 10.1016/j.stem.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Tomioka I, Nogami N, Nakatani T, Owari K, Fujita N, Motohashi H, Takayama O, Takae K, Nagai Y, Seki K. Generation of transgenic marmosets using a tetracyclin-inducible transgene expression system as a neurodegenerative disease model. Biol Reprod. 2017a;97:772–780. doi: 10.1093/biolre/iox129. [DOI] [PubMed] [Google Scholar]
- 11.Tomioka I, Ishibashi H, Minakawa EN, Motohashi HH, Takayama O, Saito Y, Popiel HA, Puentes S, Owari K, Nakatani T, Nogami N, Yamamoto K, Noguchi S, Yonekawa T, Tanaka Y, Fujita N, Suzuki H, Kikuchi H, Aizawa S, Nagano S, et al. Transgenic monkey model of the polyglutamine diseases recapitulating progressive neurological symptoms. 2017b doi: 10.1523/ENEURO.0250-16.2017. eNeuro 4:ENEURO. 0250-0216.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington’s disease. Cell. 2000;101:57–66. doi: 10.1016/S0092-8674(00)80623-6. [DOI] [PubMed] [Google Scholar]