Keywords: nerve regeneration, stress, depression, protein kinase C ε, aldehyde dehydrogenase 2, 4-hydroxy-2-nonenal, apoptosis, hippocampus, prefrontal cortex, myocardium, neural regeneration
Abstract
Chronic stress is strongly associated with the occurrence and development of depression and cardiovascular disease. Stress can induce altered mitochondrial function and activation of apoptosis in the cardio-cerebral system. However, it is unknown whether the protein kinase C ε (PKCε)-aldehyde dehydrogenase 2 (ALDH2) pathway is altered under chronic stress, and this study sought to address this question. A rat model of depression was established using a chronic unpredictable mild stress (CUMS) protocol. After experiencing CUMS for 4 weeks, the sucrose preference test and the forced swim test verified depressive-like behaviors. Enzyme linked immunosorbent assays showed that ALDH2 activity was decreased in the rat hippocampus and prefrontal cortex, but was not altered in the myocardium. Western blot assays demonstrated reduced levels of ALDH2 and PKCε, but increased levels of 4-hydroxy-2-nonenal (4HNE) adducts. Caspase-3 expression did not obviously alter, but active forms of caspase-3 were increased in the hippocampus and prefrontal cortex. In the myocardium, expression of ALDH2, PKCε and 4HNE adducts did not remarkably alter; while caspase-3 expression was reduced and the active forms of caspase-3 were upregulated. Pearson’s correlation test demonstrated that expression of 4HNE adducts was positively correlated with levels of the active forms of caspase-3 in the hippocampus and prefrontal cortex, but not in the myocardium. In conclusion, chronic stress can damage the PKCε-ALDH2 signaling pathway in the hippocampus and prefrontal cortex, but not in the myocardium. Moreover, 4HNE is associated with active forms of caspase-3 in the hippocampus and prefrontal cortex.
Introduction
Stressful life situations lead to an increased likelihood of depression and cardiovascular diseases. A large sample survey revealed that 70–80% of patients with depression experienced major stress events, and chronic stress is significantly associated with depression prognosis and treatment resistance (Lethbridge and Allen, 2008). Permanent stress was calculated to give a 2.17 higher risk of suffering a myocardial infarction (Rosengren et al., 2004). Although a considerable amount of evidence supports the association between stress, depression, and cardiovascular diseases, the underlying mechanism has remained elusive. However, accumulating evidence suggests that mitochondrial dysfunction causing oxidative stress and dysregulation of apoptosis might be involved. In the clinic, mitochondrial DNA deletions have been recorded in patients with depression or myocardial infarction (Gardner et al., 2003; Wang et al., 2015).
Mitochondrial aldehyde dehydrogenase 2 (ALDH2) plays a crucial role in sustaining the normal function of mitochondria. ALDH2 is a tetrameric enzyme, and it is highly expressed in organs that need a large amount of adenosine triphosphate, including the heart and brain (Endo et al., 2009). The activity of ALDH2 can be bi-directionally regulated. Endogenous protein kinase C-epsilon (PKCε) can enhance the activity of ALDH2 (Budas et al., 2010), and the exogenous agent, daidzin, can inhibit its activity (Lowe et al., 2008). ALDH2 is capable of detoxifying reactive aldehydes, generated from reactive oxygen species-dependent lipid peroxidation. Reactive aldehydes are also named “toxic second messengers”. Reactive aldehydes can extend the damage caused by reactive oxygen species, whose toxicity is often limited to the generation site (Carletto et al., 2000). 4-Hydroxy-2-nonenal (4HNE) is a representative active aldehyde and is considered to be a marker of oxidative stress and cell damage (Shoeb et al., 2014). 4-HNE possesses carbonyl functional groups that can covalently bind to histidine, lysine, and cysteine amino acids of proteins via Schiff base and/or Michael adducts (Cebak et al., 2017). Subsequent conformational changes in protein structure impair cell function. A constitutive basal level of 4HNE is necessary for normal cellular function. However, excessive accumulation of 4HNE can be harmful and can induce the activation of caspase-3 and apoptosis (Singhal et al., 2015).
The PKCε-ALDH2 pathway might be involved in the etiology of depression and cardiovascular diseases. Clinical data shows that the serum levels of active aldehydes are increased in patients with depression. Abnormally high levels of active aldehydes are not only associated with the severity of depressive symptoms, but are also related to the impairment of working memory and declarative memory (Talarowska et al., 2012). ALDH2 has been proposed as a key mediator against myocardial ischemia and reperfusion injury (Chen et al., 2008). Furthermore, an inactive form of the ALDH2 gene is linked to an increased risk of myocardial infarction (Jo et al., 2007).
In animal studies, a chronic unpredictable mild stress (CUMS) model is widely used to study the role of stress in depression and cardiovascular diseases. CUMS induces depressive-like behaviors and also causes abnormal heart function in rats (Grippo and Johnson, 2009). Therefore, we used this model to explore the following questions: (i) Whether ALDH2, as well as PKCε and 4HNE, is perturbed in the hippocampus, prefrontal cortex, and/or myocardium under chronic stress. (ii) Whether there is a correlation between 4HNE accumulation and caspase-3 activation in these tissues under chronic stress.
Materials and Methods
Animals
Experiments were conducted on fourteen male specific-pathogen-free Sprague-Dawley rats (200 ± 20 g, aged 6–8 weeks), provided by the Experimental Animal Center of the Hunan Provincial People’s Hospital [license No. SYXK (Xiang) 2015-0013]. All rats were maintained under standard laboratory conditions (12-hour light/dark cycle, temperature 22 ± 1°C, relative humidity 55 ± 10%, and food and water ad libitum except for rats under deprivation conditions). The rats were habituated for seven days before the experiment. The rats were randomly divided into control and CUMS groups (n = 7 per group). The rats were individually housed (cage size: 47.5 cm × 35 cm × 20 cm). The control rats were undisturbed except for necessary procedures such as cage cleaning, and the CUMS rats were subjected to the CUMS protocol. The animal experiments were performed with the approval of the Animal Ethics Committee of Zhongshan Affiliated Hospital of Sun Yat-sen University of China (archives No. Z5-2014-08).
CUMS model establishment
The CUMS procedure followed a previous method with minor modification (Zheng et al., 2010). The CUMS procedure lasted for 4 weeks: food deprivation for 24 hours and water deprivation for 24 hours; 45° cage tilting for 24 hours; damp bedding and group housing for 24 hours; restraint for 4 hours in an empty water bottle (Wahaha, Hangzhou, China); 20 minutes of noise; 1 minute of tail clamping; and day-night reversal (12 hours/12 hours). Rats suffered one of these stressors each day. However, the same stressor was not implemented on two consecutive days.
Sucrose preference test
The sucrose preference test was performed as described previously (Rygula et al., 2005) at one-week intervals throughout the experiment (starting from baseline). During the test, all rats were provided a free choice for 24 hours between two bottles, one containing tap water and the other 0.8% sucrose solution. To avoid possible spatial bias in drinking, the side (left and right) of the two bottles was switched after 12 hours. The bottles were weighed to measure the consumption of water and sucrose solution. The sucrose preference was determined as a percentage of the consumed sucrose solution relative to the total liquid consumption. The rats were not deprived of food or water.
Forced swim test
The forced swim test was performed according to a classic method with minor modifications (Porsolt et al., 1978) and was performed 24 hours after the final sucrose preference test. The forced swim test contained two trials, during which the rats were individually forced to swim in a self-made cylinder (50 cm high, 21 cm in diameter,) filled with water (25 ± 1°C) to a height of 30 cm. The rats were forced to swim for 15 minutes in the first trial, and for 5 minutes in the subsequent trial 24 hours later. In the second trial, the duration of immobility was measured by two observers who were blind to the group assignment of animals. A rat was considered as immobile when it was floating motionless or only making the movements necessary to maintain its head just above the water surface.
Sample preparation
Twenty-four hours after the final behavioral test, all the rats were anesthetized with a peritoneal injection of 10% chloral hydrate (0.3 mL/100 g). Prefrontal cortex, hippocampus, and heart samples were dissected and thoroughly washed with cold physiological saline and then immediately frozen in liquid nitrogen. Samples were stored at −80°C until analysis.
Enzyme linked immunosorbent assay (ELISA)
The activity of ALDH2 in the hippocampus, prefrontal cortex, and myocardium was determined in duplicate using enzyme immunoassay kits (Abcam, Cambridge, UK). Rat-specific antibodies in the kits were used to immunocapture ALDH2 within the wells of the microplate. The activity of ALDH2 was measured through the generation of NADH in the following ALDH2-catalyzed reaction: acetaldehyde + NAD+ → acetic acid + NADH. The NADH was coupled to the 1:1 reduction of a reporter dye to yield a yellow colored product whose concentration could be determined by the increase in absorbance at 450 nm (dye molar extinction coefficient −37,000 M−1cm−1). The ALDH2 activity was calculated as the change in absorbance per minute per amount of sample loaded into the well.
Western blot assay
Total protein was prepared from 100 mg of tissue (hippocampus, prefrontal cortex, and myocardium). The protein concentration was determined using the Bradford method (Kruger, 1994). Samples were loaded onto a precast 12% SDS-PAGE gel with 50 µg protein in each lane. Proteins in the gels were transferred to a polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA) and then blocked for one hour in 5% non-fat dried milk in Tris buffered saline-Tween (25 mM Tris, 150 mM NaCl, pH 7.5, 0.05% Tween-20). Antibodies at concentrations listed below were used overnight at 4°C: ALDH2 (rabbit anti-rat; 1:500; SCBT, Santa Cruz, CA, USA), PKCε (rabbit anti-rat; 1:500; Proteintech, Chicago, IL, USA), 4HNE (goat anti-rat; 1:3000; Millipore), caspase-3 (rabbit anti-rat; 1:1000; CST, Boston, MA, USA), and β-actin (mouse anti-rat; 1:4000; Proteintech). Membranes were subsequently probed with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:3000; Proteintech) for 45 minutes at room temperature. Finally, film signals were scanned and quantified using Image-Pro Plus 6.0 (Media Cybernetics, Baltimore, MD, USA). Signals were normalized to β-actin as an internal standard.
Statistical analysis
Data, expressed as the mean ± SEM, were analyzed using SPSS version 13.0 software (SPSS, Chicago, IL, USA). Differences between groups were determined by two-tailed unpaired Student’s t-test or Welch’s t-test. Correlation analysis was performed using the Pearson correlation test. P < 0.05 was considered statistically significant.
Results
CUMS rats exhibited depressive-like behaviors
The CUMS group had decreased sucrose preference versus the control group after the second week (week 2: P < 0.05; week 3: P < 0.05; week 4: P < 0.01). In the forced swim test, the CUMS group showed a longer immobility time compared with the control group (P < 0.01; Figure 1).
Figure 1.
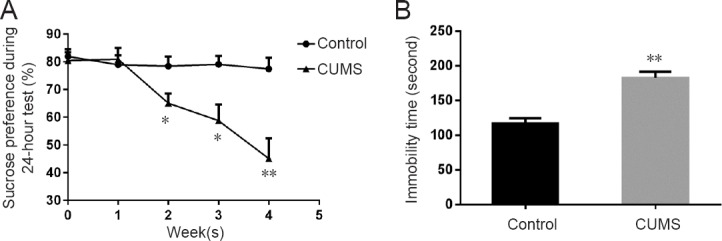
Depressive-like behaviors in CUMS rats.
(A) Sucrose preference test in control and CUMS rats. (B) Forced swim test in control and CUMS rats. Data are expressed as the mean ± SEM (n = 7), and were analyzed by the two-tailed unpaired Student’s t-test. *P < 0.05, **P < 0.01, vs. control group. CUMS: Chronic unpredictable mild stress.
ALDH2 activities were decreased in the hippocampus and prefrontal cortex, but not the myocardium of CUMS rats
After 4 weeks of CUMS, ALDH2 activity was significantly decreased in the CUMS group compared with the control group in the hippocampus (P < 0.05) and prefrontal cortex (P < 0.01), but not in the myocardium (P > 0.05; Figure 2).
Figure 2.
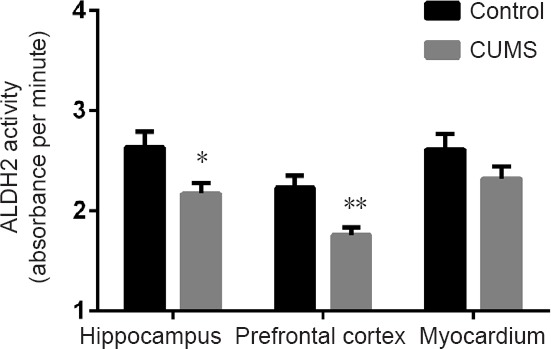
ALDH2 activity in the hippocampus, prefrontal cortex, and myocardium of CUMS rats.
Data are expressed as the mean ± SEM (n = 7 rats; two-tailed unpaired Student’s t-test; each sample was determined in duplicate). *P < 0.05, **P < 0.01, vs. control group. CUMS: Chronic unpredictable mild stress; ALDH2: aldehyde dehydrogenase 2.
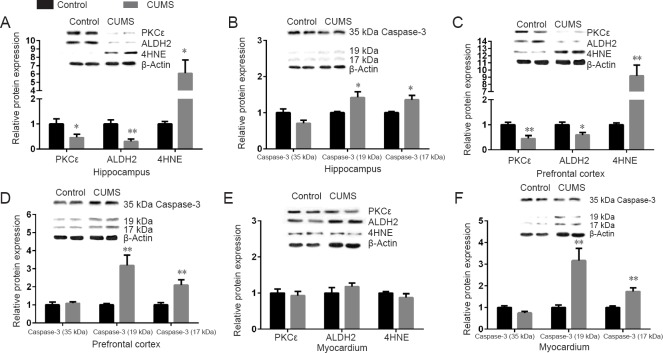
Expression of PKCε, ALDH2, 4HNE adducts and caspase-3 in the hippocampus and prefrontal cortex of CUMS rats
The protein levels of PKCε (P < 0.05) and ALDH2 (P < 0.01) were significantly decreased in the hippocampus of the CUMS group compared with the control group. As a result, levels of 4HNE adducts (P < 0.05, Welch’s correction) were significantly increased in the CUMS group (Figure 3A). There was no significant difference in levels of caspase-3 (35 kDa) between groups (P > 0.05); however, the active forms of caspase-3 (P < 0.05, Welch’s correction) were significantly increased in the CUMS group (Figure 3B).
Figure 3.
Relative protein levels of PKCε/ALDH2/4HNE adducts/caspase-3 (35 kDa)/caspase-3 (19 kDa)/caspase-3(17 kDa) in the hippocampus, prefrontal cortex, and myocardium of CUMS rats.
The relative protein expression is expressed as the optical density ratio of a target protein to β-actin. Data are expressed as the mean ± SEM (n = 7 rats; each sample was tested once), and were analyzed by two-tailed unpaired Student’s t-test (Welch’s correction was used for 4HNE in the hippocampus and prefrontal cortex and for active forms of caspase-3 in the hippocampus). *P < 0.05, **P < 0.01, vs. control group. CUMS: Chronic unpredictable mild stress; PKCε: protein kinase C ε; ALDH2: aldehyde dehydrogenase 2; 4HNE: 4-hydroxy-2-nonenal.
Similar results were found in the prefrontal cortex. The levels of PKCε (P < 0.01) and ALDH2 (P < 0.05) were significantly decreased in the CUMS group compared with the control group. As a result, levels of 4HNE adducts (P < 0.01, Welch’s correction) were significantly increased in the CUMS group compared with the control group (Figure 3C). Caspase-3 (35 kDa) levels were not significantly different between groups (P > 0.05); however, the active forms of caspase-3 (P < 0.01) were significantly increased in the CUMS group compared with the control group (Figure 3D).
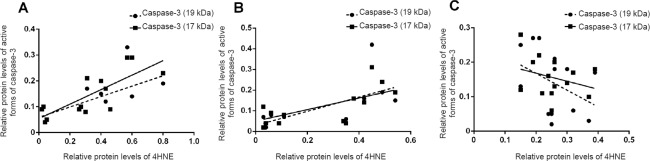
Pearson’s correlation test showed that 4HNE adducts were positively correlated with caspase-3 (19 kDa) (r = 0.675, P < 0.01, hippocampus; r = 0.657, P < 0.05, prefrontal cortex) and caspase-3 (17 kDa) (r = 0.793, P < 0.01, hippocampus; r = 0.659, P < 0.05, prefrontal cortex) (Figure 4A, B).
Figure 4.
Pearson’s correlation tests between 4HNE adducts and active forms of caspase-3.
(A) In the hippocampus, 4HNE adducts were positively correlated with caspase-3 (19 kDa) (r = 0.675, P < 0.01) and caspase-3 (17 kDa) (r = 0.793, P < 0.01). (B) In the prefrontal cortex, 4HNE adducts were positively correlated with caspase-3 (19 kDa) (r = 0.657, P < 0.05) and caspase-3 (17 kDa) (r = 0.659, P < 0.05). (C) In the myocardium, 4HNE adducts were not correlated with caspase-3 (19 kDa) (r = –0.389, P > 0.05) or caspase-3 (17 kDa) (r = –0.290, P > 0.05). 4HNE: 4-Hydroxy-2-nonenal.
Expression of PKCε, ALDH2, 4HNE adducts and caspase-3 in the myocardium of CUMS rats
In the myocardium, there were no significant differences in the levels of PKCε, ALDH2 and 4HNE adducts (P > 0.05) between the CUMS and control groups (Figure 3E). However, levels of caspase-3 (35 kDa), caspase-3 (19 kDa), and caspase-3 (17 kDa) were significantly different. Caspase-3 (35 kDa) levels were decreased in the CUMS group, while the active forms of caspase-3 were upregulated in the CUMS group (P < 0.01; Figure 3F). Pearson’s correlation test showed that 4HNE adducts were not correlated with caspase-3 (19 kDa) (r = –0.389, P > 0.05) or caspase-3 (17 kDa) (r = –0.290, P > 0.05; Figure 4C).
Discussion
In this study, CUMS rats, which showed a depressive-like state, had decreased expression and activity of ALDH2, decreased levels of PKCε and an accumulation of 4HNE adducts in the hippocampus and prefrontal cortex. However, these indicators were unchanged in the myocardium. Moreover, chronic stress increased the levels of the active forms of caspase-3 in these tissues. A significantly positive correlation was found between 4HNE adducts and active forms of caspase-3 in the hippocampus and prefrontal cortex, but not in the myocardium. These data indicated that PKCε-ALDH2 pathway perturbation and the elevated active forms of caspase-3 might participate in stress-related disorders in the brain and heart.
The CUMS model is a classic model that resembles depression in humans. The sucrose preference test reflects an anhedonia-like change of behavior, a core depressive symptom (Sigwalt et al., 2011). Decreased preference for sucrose, representing a reduction in responsiveness to rewards, is considered as anhedonia. Furthermore, the immobility time of the forced swim test is considered to be “behavioral despair” as observed in depression (Porsolt et al., 1978). Consistent with previous findings (Grippo et al., 2003; Sun et al., 2016), we found that CUMS reduced sucrose preference and prolonged immobility time in the forced swim test, indicating a depressive-like state of rats.
The hippocampus is part of the limbic system and plays important roles in learning, memory, behavior, and emotion. The hippocampus represents one of the most vulnerable regions to stressors in the brain (McGregor et al., 2003). The prefrontal cortex is involved in personality expression, planning complex cognitive behavior, moderating social behavior, and decision making (Yang and Raine, 2009). Both these brain regions are important in depression, and have morphological and functional alterations in patients with depression, such as hippocampal atrophy (Jiang et al., 2013), and altered metabolism in the prefrontal cortex (Galeotti and Ghelardini, 2012). In this study, the expression of PKCε was reduced in the hippocampus and prefrontal cortex. Consistent with this study, one post-mortem study found that PKCε was decreased in the prefrontal cortex in persons with depression (Shelton et al., 2009). In addition, a genome-wide study found an association between the gene encoding PKCε and suicide attempts in patients with depression (Perlis et al., 2010). In the central nervous system, PKCε is highly expressed in presynaptic nerve fibers, and PKCε plays a role in synapse formation, neurite outgrowth and neurotransmitter release (Shirai et al., 2008). Furthermore, PKCε can stimulate the expression of brain-derived neurotrophic factor, an important biomarker involved in the pathogenic mechanism of depression (Sun et al., 2008; Duan et al., 2016).
In this study, we found decreased expression and activity of ALDH2 and subsequent accumulation of 4HNE adducts in the hippocampus and prefrontal cortex in the CUMS group. As the brain is rich in unsaturated fatty acids and contains a high level of oxygen within the lipid bilayer, these features make the brain susceptible to the generation of active aldehydes. The impaired protein function caused by active aldehydes could contribute to overall cellular disorders in the brain (Petersen and Doorn, 2004), such as decreased production of monoaminergic neurotransmitters (Beckman and Koppenol, 1996). All these changes might contribute to the development of depression. The over-accumulation of 4HNE adducts in the CUMS rats also suggests that the activation of ALDH2 may have potential as a novel therapeutic strategy for depression. An in vitro study showed that upregulation of the ALDH2 gene could relieve 4HNE-induced death of neurons by reducing the levels of reactive oxygen species and caspase-3 protein in cultured hippocampal neurons (Bai and Mei, 2011). Recently, an in vivo study found that the ALDH2 activator, Alda-1, could markedly attenuate the depressive-like behaviors in prenatally stressed rats (Stachowicz et al., 2016).
It has been proposed that apoptosis is a contributing factor to the decrease in hippocampal volume and loss of neuronal function and viability seen in depressive disorders (Bachis et al., 2008). We found that CUMS upregulated the active forms of caspase-3, a generally accepted apoptosis executor, in the hippocampus and prefrontal cortex. 4HNE itself can induce apoptosis in neurons (Kruman et al., 1997). In this experiment, we found a significant positive correlation between 4HNE adducts and the active forms of caspase-3. However, in addition to its well-known role in apoptosis, limited caspase-3 activation can be essential for synaptic plasticity in neurons (Snigdha et al., 2012). Thus, we cannot assume that the increased levels of active forms of caspase-3 indicate activation of apoptosis. However, chronic stress can increase apoptosis in these brain regions (Kubera et al., 2011).
The mechanisms that underlie the relationships between stress and cardiovascular diseases are complex, and include dysfunction of the neuroendocrine system, activation of pro-inflammatory cytokines, and dysregulation of the autonomic heart system (Grippo and Johnson, 2009). In the myocardium, the levels of active forms of caspase-3 were increased in the CUMS rats compared with the control rats, but there was no statistical difference in levels of PKCε, ALDH2, and 4HNE adducts. In addition, there was no correlation between 4HNE adducts and the active forms of caspase-3. These results indicate that the myocardium is relatively more resistant to oxidative stress than the brain, and the elevated active forms of caspase-3 induced by stress might be independent of the PKCε-ALDH2 pathway in the myocardium.
The major limitations of this study are that we only used the CUMS model and we did not test apoptosis directly. Despite for these limitations, the conclusion still can be made that chronic stress compromises the ability of ALDH2 to detoxify 4HNE in the hippocampus and prefrontal cortex, but not in the myocardium. Furthermore, chronic stress elevates levels of the active forms of caspase-3 in these tissues.
Footnotes
Conflicts of interest: None declared.
Financial support: This study was supported by the Medical Research Fund of Guangdong Province of China, No. B2014449; and a grant from the Science and Technology Project of Zhongshan City of China, No. 2014A1FC137. The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, or in the decision to submit the paper for publication.
Institutional review board statement: The animal experiments were carried out with the approval of the Animal Ethics Committee of Zhongshan Affiliated Hospital of Sun Yat-sen University (archives No. Z5-2014-08).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Wenrui Qu, Indiana University School of Medicine, USA.
Funding: This study was supported by the Medical Research Fund of Guangdong Province of China, No. B2014449; a grant from the Science and Technology Project of Zhongshan City of China, No. 2014A1FC137.
(Copyedited by Yu J, Li CH, Qiu Y, Song LP, Zhao M)
References
- 1.Bachis A, Cruz MI, Nosheny RL, Mocchetti I. Chronic unpredictable stress promotes neuronal apoptosis in the cerebral cortex. Neurosci Lett. 2008;442:104–108. doi: 10.1016/j.neulet.2008.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai J, Mei Y. Overexpression of aldehyde dehydrogenase-2 attenuates neurotoxicity induced by 4-hydroxynonenal in cultured primary hippocampal neurons. Neurotox Res. 2011;19:412–422. doi: 10.1007/s12640-010-9183-1. [DOI] [PubMed] [Google Scholar]
- 3.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 4.Budas GR, Churchill EN, Disatnik MH, Sun L, Mochly-Rosen D. Mitochondrial import of PKCepsilon is mediated by HSP90: a role in cardioprotection from ischaemia and reperfusion injury. Cardiovasc Res. 2010;88:83–92. doi: 10.1093/cvr/cvq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carletto C, Nicolay JF, Courbebaisse Y. Oxidative stress and cutaneous ageing: the ‘toxic second messengers’ concept and an interesting family of products, ‘pseudodipeptides’. Int J Cosmet Sci. 2000;22:361–370. doi: 10.1046/j.1467-2494.2000.00025.x. [DOI] [PubMed] [Google Scholar]
- 6.Cebak JE, Singh IN, Hill RL, Wang JA, Hall ED. Phenelzine protects brain mitochondrial function in vitro and in vivo following traumatic brain injury by scavenging the reactive carbonyls 4-hydroxynonenal and acrolein leading to cortical histological neuroprotection. J Neurotrauma. 2017;34:1302–1317. doi: 10.1089/neu.2016.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan DM, Tu Y, Liu P, Jiao S. Antidepressant effect of electroacupuncture regulates signal targeting in the brain and increases brain-derived neurotrophic factor levels. Neural Regen Res. 2016;11:1595–1602. doi: 10.4103/1673-5374.193238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endo J, Sano M, Katayama T, Hishiki T, Shinmura K, Morizane S, Matsuhashi T, Katsumata Y, Zhang Y, Ito H, Nagahata Y, Marchitti S, Nishimaki K, Wolf AM, Nakanishi H, Hattori F, Vasiliou V, Adachi T, Ohsawa I, Taguchi R, et al. Metabolic remodeling induced by mitochondrial aldehyde stress stimulates tolerance to oxidative stress in the heart. Circ Res. 2009;105:1118–1127. doi: 10.1161/CIRCRESAHA.109.206607. [DOI] [PubMed] [Google Scholar]
- 10.Galeotti N, Ghelardini C. Selective modulation of the PKCvarepsilon/p38MAP kinase signalling pathway for the antidepressant-like activity of amitriptyline. Neuropharmacology. 2012;62:289–296. doi: 10.1016/j.neuropharm.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Gardner A, Johansson A, Wibom R, Nennesmo I, von Dobeln U, Hagenfeldt L, Hallstrom T. Alterations of mitochondrial function and correlations with personality traits in selected major depressive disorder patients. J Affect Disord. 2003;76:55–68. doi: 10.1016/s0165-0327(02)00067-8. [DOI] [PubMed] [Google Scholar]
- 12.Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress. 2009;12:1–21. doi: 10.1080/10253890802046281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grippo AJ, Beltz TG, Johnson AK. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiol Behav. 2003;78:703–710. doi: 10.1016/s0031-9384(03)00050-7. [DOI] [PubMed] [Google Scholar]
- 14.Jiang P, Zhang WY, Li HD, Cai HL, Liu YP, Chen LY. Stress and vitamin D: altered vitamin D metabolism in both the hippocampus and myocardium of chronic unpredictable mild stress exposed rats. Psychoneuroendocrinology. 2013;38:2091–2098. doi: 10.1016/j.psyneuen.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 15.Jo SA, Kim EK, Park MH, Han C, Park HY, Jang Y, Song BJ, Jo I. A Glu487Lys polymorphism in the gene for mitochondrial aldehyde dehydrogenase 2 is associated with myocardial infarction in elderly Korean men. Clin Chim Acta. 2007;382:43–47. doi: 10.1016/j.cca.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Kruger NJ. The Bradford method for protein quantitation. Methods Mol Biol. 1994;32:9–15. doi: 10.1385/0-89603-268-X:9. [DOI] [PubMed] [Google Scholar]
- 17.Kruman I, Bruce-Keller AJ, Bredesen D, Waeg G, Mattson MP. Evidence that 4-hydroxynonenal mediates oxidative stress-induced neuronal apoptosis. J Neurosci. 1997;17:5089–5100. doi: 10.1523/JNEUROSCI.17-13-05089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubera M, Obuchowicz E, Goehler L, Brzeszcz J, Maes M. In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:744–759. doi: 10.1016/j.pnpbp.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 19.Lethbridge R, Allen NB. Mood induced cognitive and emotional reactivity, life stress, and the prediction of depressive relapse. Behav Res Ther. 2008;46:1142–1150. doi: 10.1016/j.brat.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Lowe ED, Gao GY, Johnson LN, Keung WM. Structure of daidzin, a naturally occurring anti-alcohol-addiction agent, in complex with human mitochondrial aldehyde dehydrogenase. J Med Chem. 2008;51:4482–4487. doi: 10.1021/jm800488j. [DOI] [PubMed] [Google Scholar]
- 21.McGregor IS, Gurtman CG, Morley KC, Clemens KJ, Blokland A, Li KM, Cornish JL, Hunt GE. Increased anxiety and “depressive” symptoms months after MDMA (“ecstasy”) in rats: drug-induced hyperthermia does not predict long-term outcomes. Psychopharmacology (Berl) 2003;168:465–474. doi: 10.1007/s00213-003-1452-8. [DOI] [PubMed] [Google Scholar]
- 22.Perlis RH, Huang J, Purcell S, Fava M, Rush AJ, Sullivan PF, Hamilton SP, McMahon FJ, Schulze TG, Potash JB, Zandi PP, Willour VL, Penninx BW, Boomsma DI, Vogelzangs N, Middeldorp CM, Rietschel M, Nothen M, Cichon S, Gurling H, et al. Genome-wide association study of suicide attempts in mood disorder patients. Am J Psychiatry. 2010;167:1499–1507. doi: 10.1176/appi.ajp.2010.10040541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen DR, Doorn JA. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic Biol Med. 2004;37:937–945. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 25.Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi-amorn C, Sato H, Yusuf S INTERHEART investigators. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- 26.Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Shelton RC, Hal Manier D, Lewis DA. Protein kinases A and C in post-mortem prefrontal cortex from persons with major depression and normal controls. Int J Neuropsychopharmacol. 2009;12:1223–1232. doi: 10.1017/S1461145709000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shirai Y, Adachi N, Saito N. Protein kinase Cepsilon: function in neurons. FEBS J. 2008;275:3988–3994. doi: 10.1111/j.1742-4658.2008.06556.x. [DOI] [PubMed] [Google Scholar]
- 29.Shoeb M, Ansari NH, Srivastava SK, Ramana KV. 4-Hydroxynonenal in the pathogenesis and progression of human diseases. Curr Med Chem. 2014;21:230–237. doi: 10.2174/09298673113209990181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sigwalt AR, Budde H, Helmich I, Glaser V, Ghisoni K, Lanza S, Cadore EL, Lhullier FL, de Bem AF, Hohl A, de Matos FJ, de Oliveira PA, Prediger RD, Guglielmo LG, Latini A. Molecular aspects involved in swimming exercise training reducing anhedonia in a rat model of depression. Neuroscience. 2011;192:661–674. doi: 10.1016/j.neuroscience.2011.05.075. [DOI] [PubMed] [Google Scholar]
- 31.Singhal SS, Singh SP, Singhal P, Horne D, Singhal J, Awasthi S. Antioxidant role of glutathione S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated signaling. Toxicol Appl Pharmacol. 2015;289:361–370. doi: 10.1016/j.taap.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snigdha S, Smith ED, Prieto GA, Cotman CW. Caspase-3 activation as a bifurcation point between plasticity and cell death. Neurosci Bull. 2012;28:14–24. doi: 10.1007/s12264-012-1057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stachowicz A, Glombik K, Olszanecki R, Basta-Kaim A, Suski M, Lason W, Korbut R. The impact of mitochondrial aldehyde dehydrogenase (ALDH2) activation by Alda-1 on the behavioral and biochemical disturbances in animal model of depression. Brain Behav Immun. 2016;51:144–153. doi: 10.1016/j.bbi.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Sun J, Wang F, Hong G, Pang M, Xu H, Li H, Tian F, Fang R, Yao Y, Liu J. Antidepressant-like effects of sodium butyrate and its possible mechanisms of action in mice exposed to chronic unpredictable mild stress. Neurosci Lett. 2016;618:159–166. doi: 10.1016/j.neulet.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Sun MK, Hongpaisan J, Nelson TJ, Alkon DL. Poststroke neuronal rescue and synaptogenesis mediated in vivo by protein kinase C in adult brains. Proc Natl Acad Sci U S A. 2008;105:13620–13625. doi: 10.1073/pnas.0805952105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Talarowska M, Galecki P, Maes M, Gardner A, Chamielec M, Orzechowska A, Bobinska K, Kowalczyk E. Malondialdehyde plasma concentration correlates with declarative and working memory in patients with recurrent depressive disorder. Mol Biol Rep. 2012;39:5359–5366. doi: 10.1007/s11033-011-1335-8. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Xie L, Zhang Q, Cai X, Tang Y, Wang L, Hang T, Liu J, Gong J. Plasma nuclear and mitochondrial DNA levels in acute myocardial infarction patients. Coron Artery Dis. 2015;26:296–300. doi: 10.1097/MCA.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Y, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: a meta-analysis. Psychiatry Res. 2009;174:81–88. doi: 10.1016/j.pscychresns.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng S, Yu M, Lu X, Huo T, Ge L, Yang J, Wu C, Li F. Urinary metabonomic study on biochemical changes in chronic unpredictable mild stress model of depression. Clin Chim Acta. 2010;411:204–209. doi: 10.1016/j.cca.2009.11.003. [DOI] [PubMed] [Google Scholar]