Keywords: nerve regeneration, puerarin, Radix Puerariae, traditional Chinese medicine, Trpv1, Trpa1, dorsal root ganglion, peripheral nerve injury, neuropathic pain, mechanical allodynia, thermal hyperalgesia, neural regeneration
Abstract
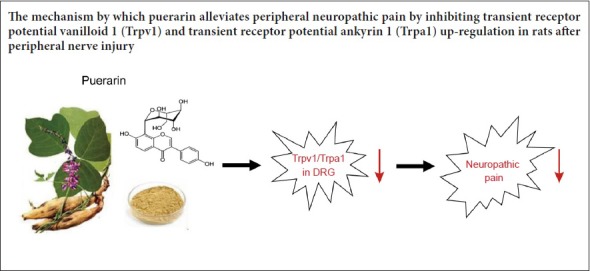
Puerarin is a major active ingredient of the traditional Chinese plant medicine, Radix Puerariae, and commonly used in the treatment of myocardial and cerebral ischemia. However, the effects of puerarin on neuropathic pain are still unclear. In this study, a neuropathic pain animal model was created by partial sciatic nerve ligation. Puerarin (30 or 60 mg/kg) was intraperitoneally injected once a day for 7 days. Mechanical allodynia and thermal hyperalgesia were examined at 1 day after model establishment. Mechanical threshold and paw withdrawal latency markedly increased in a dose-dependent manner in puerarin-treated rats, especially at 7 days after model establishment. At 7 days after model establishment, quantitative real-time reverse transcriptase-polymerase chain reaction results showed that puerarin administration reversed mRNA expression of transient receptor potential vanilloid 1 (Trpv1) and transient receptor potential ankyrin 1 (Trpa1) in a dose-dependent manner in dorsal root ganglion neurons after peripheral nerve injury. These results suggest that puerarin dose-dependently ameliorates neuropathic pain by suppressing Trpv1 and Trpa1 up-regulation in dorsal root ganglion of neuropathic pain rats.
Introduction
Neuropathic pain is caused by injury or disease of the somatosensory nervous system, which ultimately causes an increased response to painful stimuli (hyperalgesia), unpleasant and abnormal sensation (dysesthesia), and pain in response to a stimulus that does not normally provoke pain (allodynia) (Finnerup et al., 2016; Kumar et al., 2017). Neuropathic pain is a major public health problem as it has a considerable impact on quality of life for patients (Elzahaf et al., 2016; Burke et al., 2017). Therefore, development of novel effective analgesics for neuropathic pain relief and treatment is warranted.
Transient receptor potential (TRP) ion channels constitute a major class of calcium-permeable, non-selective cation channels (Montell and Rubin, 1989; Gonzalez-Ramirez et al., 2017). TRP channels are characterized by various activation mechanisms (Nilius, 2007; Balemans et al., 2017). Some of the TRP ion channel superfamily are reportedly involved in nociception and thermosensation, in both the peripheral and central nervous system (Caterina et al., 1997; Moore et al., 2018). TRP vanilloid 1 (Trpv1) is activated by multiple mechanisms, such as noxious heat (> 43°C), protons, and pungent chemicals (e.g., capsaicin) (Caterina et al., 1997; Caterina and Julius, 2001). TRP ankyrin 1 (Trpa1) is expressed in sensory neurons, which are involved in the pain pathway (Naziroglu and Braidy, 2017). Trpa1 functions as a sensor of environmental and endogenous chemical irritants such as acrolein, 4-hydroxynonena, and allyl isothiocyanate (Cho et al., 2012). Trpv1 and Trpa1 play a crucial role in nociceptive transmission under pathological forms of pain (Lappin et al., 2006; Spicarova and Palecek, 2008; Chen et al., 2009). Moreover, spinal synaptic plasticity accounts for the transition from acute to chronic pain, in which Trpv1 and Trpa1 play critical roles presynaptically and postsynaptically (Choi et al., 2016). Trpv1 and Trpa1 expression in nociceptors is altered in different models of neuropathy under pathological conditions (Biggs et al., 2007; Kim et al., 2008).
Natural plant products are traditional Chinese medicines for the therapy of chronic comorbidities (e.g., diabetes, cancer, and pain) (Yuan et al., 2015; Hager et al., 2016; Tsai et al., 2017). Among the compounds examined, puerarin was isolated from Radix Puerariae (Zhou et al., 2014). Since then, the pharmacological properties of puerarin have been widely investigated. Puerarin is commonly used in the treatment of myocardial and cerebral ischemia (Tao et al., 2017; Xiao et al., 2018). Puerarin also serves as a potent antioxidant and anti-inflammatory agent (Kim et al., 2010; Zhao et al., 2016). Moreover, puerarin alleviates diabetic neuropathic pain by regulating P2X3 receptor expression in dorsal root ganglion (DRG) neurons (Xu et al., 2012). Puerarin plays a neuroprotective effect on acute spinal cord injury (Zhang et al., 2016). Altogether, these studies suggest a potential role of puerarin in relief of pain originating from the central or peripheral nervous system.
The putative impact of puerarin on neuropathic pain and the underlying mechanisms remain unclear. Consequently, we hypothesized that puerarin may ameliorate neuropathic pain via Trpv1 or Trpa1. Here, we investigated the analgesic role and possible mechanisms of puerarin on neuropathic pain using animal behavioral tests and molecular biological methods. Thus, our aim in the present study was to examine the effect of puerarin on neuropathic pain.
Materials and Methods
Animals
Twenty-eight male Sprague-Dawley rats weighing 200–220 g were purchased from the Experimental Animal Center of Wuhan University of China (SYXK (E) 2014-0013). Rats were housed with 2–3 rats/cage under a standard 12-hour light/dark cycle at 23 ± 1°C, and allowed free access to water and chow. All rats were acclimated to the experimental circumstances for a week before experiments. All experimental procedures were performed in accordance with the guidelines of the International Association for the Study of Pain, and approved by the Institutional Animal Care and Use Committee of Renmin Hospital of Wuhan University of China (WDRMSPF/SQ-26).
Surgery
All surgeries were performed with the rats under deep anesthesia induced by intraperitoneal injection of sodium pentobarbital (50 mg/kg). To create the neuropathic pain model, partial sciatic nerve ligation (pSNL) was performed as described previously (Shir and Seltzer, 1991). In brief, a tight ligation of approximately one-third to one-half the diameter of the right sciatic nerve (ipsilateral) was performed using a 6-0 silk suture, as described previously (Seltzer et al., 1990). In sham-operated rats, the nerve was exposed without ligation. Behavioral tests were performed to confirm the success of pSNL.
Drug administration and experimental design
Puerarin was dissolved in 10% dimethyl sulfoxide diluted with 0.9% saline. Stock solution was filtered through a 0.22 μm membrane before use. Rats were randomly divided into four groups (n = 7): sham group, pSNL group, pSNL + puerarin 1 group (30 mg/kg, intraperitoneally), and pSNL + puerarin 2 group (60 mg/kg, intraperitoneally). Rats received intraperitoneal injection of puerarin daily for 7 consecutive days, while sham and pSNL rats received the same volume of dimethyl sulfoxide in saline.
Nociceptive tests
Mechanical allodynia
All behavioral tests were performed during the day, before and after pSNL every day. Rats were habituated to the testing apparatus for at least 30 minutes before behavioral testing. Von Frey filaments were applied with increasing force (from 0.4 to 60.0 g) until the paw withdrawal threshold was detected (Wang et al., 2011). Briefly, von Frey hairs were pressed vertically onto the hind plantar surface for approximately 4–5 seconds. Each filament was used ten times, with a 5-minute interval between different forces. The minimal force that caused a lifting or licking response at least five-times was considered the paw withdrawal threshold.
Thermal hyperalgesia
Heat sensitivity was examined using a Hargreaves radiant heat apparatus (Ugo Basile, Milan, Italy). Rats were acclimated in a plastic cage for 30 minutes before testing. The cage was then placed on a glass plate above the plantar test apparatus and a movable noxious heat source placed directly under the plantar surface of the hind paw (Cherng et al., 2014). When activated, the apparatus applied a continuous infrared heat stimulus to the plantar surface, which elicits a distinctive paw withdrawal reflex and stops an automated timer (based on infrared reflection). Each rat was measured three-times separated by a 10-minute interval. The average value was used as the response latency.
Quantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR)
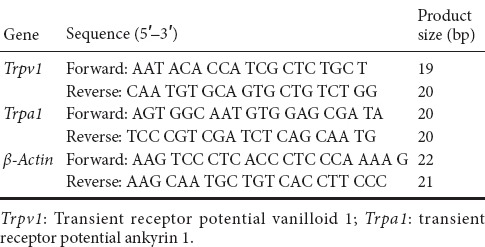
Rats were sacrificed after pSNL. Naive rats were used as controls. Lumbar 4, 5, and 6 DRGs were collected both ipsilaterally and contralaterally, with respect to the injury side. DRGs from all three levels were pooled from each side and placed into tubes with Trizol reagent (Sigma-Aldrich, St. Louis, MO, USA). RNA isolation was followed by chloroform extraction and isopropanol precipitation. Extracted RNA was dissolved in 20 μL RNase-free water and stored at −80°C. cDNA was synthesized using the iScript™ cDNA Synthesis kit (Biorad, Hercules, CA, USA). Copy number of rat genes was determined by RT-PCR using SYBR Green MASTER (Invitrogen, Waltham, MA, USA), following the manufacturer’s protocol. Data were collected during each extension phase of PCR and analyzed using ABI-7700 SDS software (Applied Biosystems, Foster City, CA, USA). RNA levels were normalized to β-actin levels, and calculated by delta-delta threshold cycle (ΔΔCT). Primers were used according to previous studies (Xue et al., 2007; Pan et al., 2014; Pohoczky et al., 2016). Primer sequences are shown in Table 1.
Table 1.
Sequences of primers for quantitative real-time reverse transcriptase-polymerase chain reaction
Statistical analysis
Data are presented as mean ± SEM. Statistical analysis was performed using repeated measures analysis of variance followed by the least significant difference post hoc test or one-way analysis of variance followed by Bonferroni correction using Origin 10.0 software (OriginLab Corporation, Northampton, MA, USA). Only two-tailed P values less than 0.05 were considered statistically significant.
Results
Establishment of a rat model of neuropathic pain
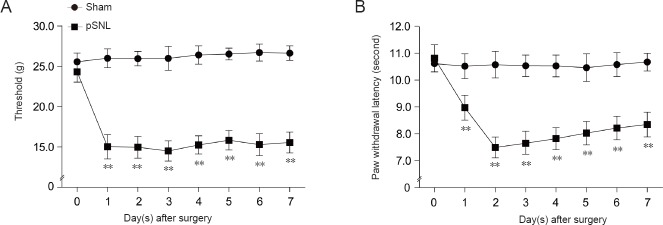
After surgery, mechanical threshold and paw withdrawal latency was tested in rats from sham and pSNL groups. pSNL model rats showed mechanical sensitivity at 1 day after pSNL surgery (P < 0.01; Figure 1A). Simultaneously, significant thermal hyperalgesia to noxious heat stimulation was observed in pSNL model rats (P < 0.01; Figure 1B). These results suggest that the pSNL model has been successfully established in rats.
Figure 1.
Mechanical and thermal tests in rats with peripheral nerve injury.
Mechanical threshold (A) and paw withdrawal latency (B) in response to thermal stimulation in rats after pSNL. **P < 0.01, vs. sham group at the same time point (mean ± SEM, n = 7; repeated measures analysis of variance followed by least significant difference post hoc test). pSNL: Partial sciatic nerve ligation.
Puerarin ameliorates mechanical allodynia in neuropathic pain rats
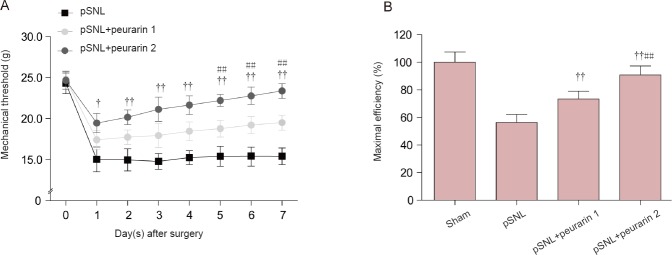
Next, we investigated the analgesic effect of puerarin by intraperitoneal administration in rats with peripheral nerve injury. Our results show that the mechanical threshold (ipsilateral) was significantly increased in both the pSNL + puerarin 1 and pSNL + puerarin 2 groups compared with the pSNL group (P < 0.05 or P < 0.01; Figure 2A). Moreover, in rats at 7 days after pSNL, maximal efficiency and ratio of response size of mechanical allodynia after 7-day puerarin treatment compared with no puerarin treatment showed that puerarin dose-dependently relieved neuropathic pain (P < 0.01; Figure 2B). These results show that puerarin dose-dependently ameliorates mechanical allodynia in rats after peripheral nerve injury.
Figure 2.
Analgesic role of puerarin on mechanical allodynia in rats with peripheral nerve injury.
(A) Mechanical allodynia in rats treated with or without puerarin (intraperitoneally, 30 or 60 mg/kg) after pSNL. (B) Maximal efficiency and ratio of response size of mechanical allodynia in rats at 7 days after pSNL and after 7-day puerarin treatment compared with no puerarin treatment. †P < 0.05, ††P < 0.01, vs. pSNL group; ##P < 0.01, vs. pSNL + puerarin 1 group at the same time point (mean ± SEM, n = 7). Statistical analysis was performed with repeated measures analysis of variance followed by least significant difference post hoc test (A) or one-way analysis of variance followed by Bonferroni correction (B). pSNL + puerarin 1 group and pSNL + puerarin 2 group: each rat was administrated 30 and 60 mg/kg (intraperitoneally) for 7 days after surgery. pSNL: Partial sciatic nerve ligation.
Puerarin mitigates thermal hyperalgesia in neuropathic pain rats
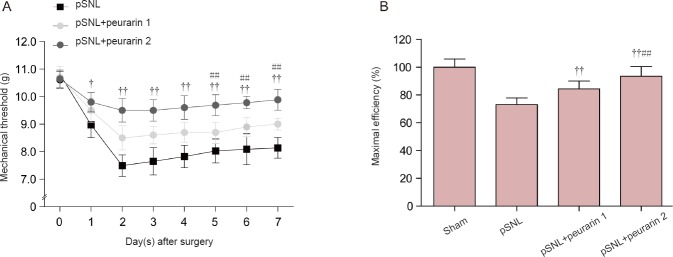
Paw withdrawal latency upon thermal stimulation was examined in rats. Puerarin also reversed thermal hyperalgesia after only one-day of injection in pSNL model rats (P < 0.05 or P < 0.01; Figure 3A). Moreover, in rats at 7 days after pSNL, maximal efficiency and ratio of response size of thermal hyperalgesia after 7-day puerarin treatment compared with no puerarin treatment also showed that puerarin dose-dependently relieved neuropathic pain (P < 0.05 or P < 0.01; Figure 3B). This indicates that puerarin reduces thermal pain. Altogether, our data suggest that puerarin plays a notable analgesic role in neuropathic pain.
Figure 3.
Analgesic effect of puerarin on thermal hyperalgesia in rats with peripheral nerve injury.
(A) Thermal hyperalgesia in rats treated with or without puerarin (intraperitoneally, 30 or 60 mg/kg) after pSNL. (B) Maximal efficiency (%) and ratio of response size of thermal hyperalgesia in rats at 7 days after pSNL and after 7-days puerarin treatment compared with no puerarin treatment. †P < 0.05, ††P < 0.01, vs. pSNL group (mean ± SEM, n = 7; repeated measures analysis of variance followed by least significant difference post hoc test); ##P < 0.01, vs. pSNL + puerarin 1 group at the same time point (mean ± SEM, n = 7). Statistical analysis was performed with repeated measures analysis of variance followed by least significant difference post hoc test (A) or one-way analysis of variance followed by Bonferroni correction (B). pSNL: Partial sciatic nerve ligation.
Puerarin suppresses increased Trpv1 and Trpa1 mRNA expression in DRG of neuropathic pain rats
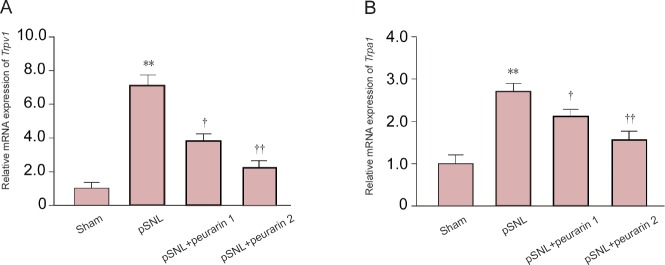
Quantitative RT-PCR was performed to investigate the potential mechanism involving puerarin. We examined well-known cation channels that are expressed in nociceptive neurons. Both Trpv1 and Trpa1 mRNA expression levels were significantly increased in DRG of neuropathic pain model rats after injury (P < 0.01; Figure 4A and B). In contrast, throughout 7 days of puerarin administration, Trpv1 and Trpa1 mRNA expression was significantly reversed in DRG of pSNL model rats (P < 0.05 or P < 0.01; Figure 4A and B). These results show that puerarin relieves neuropathic pain by inhibiting Trpv1 and Trpa1 up-regulation in DRG of neuropathic pain rats.
Figure 4.
mRNA expression levels of Trpv1 and Trpa1 in dorsal root ganglion of pSNL model rats after puerarin treatment.
Trpv1 (A) and Trpa1 (B) mRNA expression levels relative to levels in the sham group in the dorsal root ganglion of pSNL model rats after puerarin treatment for 7 days. **P < 0.01, vs. sham group. †P < 0.01; ††P < 0.01, vs. pSNL group (mean ± SEM, n = 7; one-way analysis of variance followed by Bonferroni correction). pSNL: Partial sciatic nerve ligation; Trpv1: transient receptor potential vanilloid 1; Trpa1: transient receptor potential ankyrin 1.
Discussion
Neuropathic pain is characterized by hyperalgesia, allodynia, altered sensation, and spontaneous pain. Once a peripheral nerve is damaged, a series of pathophysiological events are induced, including changes in perineuronal homeostasis, neuronal hyperexcitability, alterations in gene expression and the immune response (Hanani, 2012; Ji et al., 2016). Meanwhile, subsequent events in central pain processing pathways consolidate and exaggerate the steady-state pain condition (Kuner, 2010; Kumar et al., 2017). Neuropathic pain is challenging to manage, and current treatment strategies often lack efficacy or have severe side-effects in most patients (Artemiadis and Zis, 2018). In the present study, we show that puerarin isolated from Radix Puerariae ameliorates neuropathic pain. Puerarin injection also reversed Trpv1 and Trpa1 mRNA up-regulation in the DRG of rats with peripheral nerve injury. These results suggest that puerarin may play a promising analgesic role by preventing up-regulation of Trpv1 and Trpa1 in the DRG of neuropathic pain animals.
Trpv1 and Trpa1 are well-known channels involved in nociception and thermosensation, both in the peripheral and central nervous system (Caterina et al., 1997). Trpv1 and Trpa1 play a crucial role in nociceptive transmission under pathological forms of pain (Lappin et al., 2006; Spicarova and Palecek, 2008; Chen et al., 2009). Moreover, it is clear that spinal synaptic plasticity accounts for the transition from acute to chronic pain, in which Trpv1 and Trpa1 play critical roles presynaptically and postsynaptically (Yuan and Burrell, 2010; Jensen and Edwards, 2012; Choi et al., 2016). Trpv1 and Trpa1 expression in nociceptors is altered under pathological conditions in different models of neuropathy (Biggs et al., 2007; Kim et al., 2008). Here, our results show that consecutive puerarin administration ameliorates mechanical allodynia and thermal hyperalgesia in rats with peripheral nerve injury. Further, cumulative puerarin markedly recovered mechanical threshold and paw withdrawal latency (in response to thermal stimulation) in a dose-dependent manner. Altogether, these results suggest that puerarin may be an effective anti-nociceptive agent. To further confirm the analgesic role of puerarin in neuropathic pain, it is necessary to examine its effect in different neuropathic pain models.
Mechanisms of the analgesic role of puerarin in neuropathic pain are still not clear. Puerarin can alleviate diabetic neuropathic pain by regulating expression of purinoceptor 3 (P2X3) in DRG neurons (Li et al., 2011; Xu et al., 2012; Liu et al., 2014b). Moreover, puerarin alleviates neuropathic pain by inhibiting neuroinflammation in the spinal cord (Liu et al., 2014a). Puerarin also exerts a neuroprotective effect in acute spinal cord injury rats by decreasing neuronal loss, inhibiting glial cell activation, and alleviating inflammation in the injured spinal cord. In addition, downregulated phosphoinositide 3-kinase and phospho-Akt protein expression was restored by puerarin in acute spinal cord injury rats (Zhang et al., 2016). Our present observations demonstrate that the natural plant product, puerarin, largely prevents up-regulation of Trpv1 and Trpa1 mRNA in DRG neurons of neuropathic pain rats in a dose-dependent manner. These results suggest that the analgesic role of puerarin may act by suppressing Trpv1 and Trpa1 up-regulation. Furthermore, puerarin modulates expression of protein kinase C (Zhu et al., 2008; Tang et al., 2014), which acts upstream of Trpv1 and Trpa1 (Tominaga, 2010; Mandadi et al., 2011; Ozdemir et al., 2016; Simoes et al., 2016). Therefore, it is possible that puerarin prevents Trpv1 and Trpa1 up-regulation through protein kinase C modulation. Further studies are warranted in the future.
In conclusion, cumulative puerarin (30 and 60 mg/kg) administration (intraperitoneally) mitigated allodynia and hyperalgesia induced by pSNL. Thus, in the current study, we provide novel evidence suggesting that Trpv1 and Trpa1 in DRG contribute to the analgesic role of puerarin in neuropathic pain. Taken together, puerarin plays an important analgesic role in neuropathic pain and might serve as a potential compound for clinical treatment of neuropathic pain.
Additional file (5.8KB, pdf) : Open peer review report 1.
Open peer review report 1
Footnotes
Conflicts of interest: None declared.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81671891. The funding body played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement: This study was approved by the Institutional Animal Care and Use Committee of Renmin Hospital of Wuhan University of China (approval No. WDRMSPF/SQ-26). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Tufan Mert, Department of Biophysics, School of Medicine, University of Cukurova, Turkey.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81671891.
(Copyedited by James R, Stow A, Wang J, Li CH, Qiu Y, Song LP, Zhao M)
References
- 1.Artemiadis AK, Zis P. Neuropathic pain in acute and subacute neuropathies: a systematic review. Pain Physician. 2018;21:111–120. [PubMed] [Google Scholar]
- 2.Balemans D, Boeckxstaens GE, Talavera K, Wouters MM. Transient receptor potential ion channel function in sensory transduction and cellular signaling cascades underlying visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2017;312:G635–648. doi: 10.1152/ajpgi.00401.2016. [DOI] [PubMed] [Google Scholar]
- 3.Biggs JE, Yates JM, Loescher AR, Clayton NM, Boissonade FM, Robinson PP. Changes in vanilloid receptor 1 (Trpv1) expression following lingual nerve injury. Eur J Pain. 2007;11:192–201. doi: 10.1016/j.ejpain.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Burke D, Fullen BM, Stokes D, Lennon O. Neuropathic pain prevalence following spinal cord injury: A systematic review and meta-analysis. Eur J Pain. 2017;21:29–44. doi: 10.1002/ejp.905. [DOI] [PubMed] [Google Scholar]
- 5.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 6.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Willcockson HH, Valtschanoff JG. Influence of the vanilloid receptor Trpv1 on the activation of spinal cord glia in mouse models of pain. Exp Neurol. 2009;220:383–390. doi: 10.1016/j.expneurol.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherng CH, Lee KC, Chien CC, Chou KY, Cheng YC, Hsin ST, Lee SO, Shen CH, Tsai RY, Wong CS. Baicalin ameliorates neuropathic pain by suppressing HDAC1 expression in the spinal cord of spinal nerve ligation rats. J Formos Med Assoc. 2014;113:513–520. doi: 10.1016/j.jfma.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Cho JH, Jeong MY, Choi IS, Lee HJ, Jang IS. Trpa1-like channels enhance glycinergic transmission in medullary dorsal horn neurons. J Neurochem. 2012;122:691–701. doi: 10.1111/j.1471-4159.2012.07817.x. [DOI] [PubMed] [Google Scholar]
- 10.Choi SI, Lim JY, Yoo S, Kim H, Hwang SW. Emerging role of spinal cord Trpv1 in pain exacerbation. Neural Plast. 2016;2016:5954890. doi: 10.1155/2016/5954890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elzahaf RA, Johnson MI, Tashani OA. The epidemiology of chronic pain in Libya: a cross-sectional telephone survey. BMC Public Health. 2016;16:776. doi: 10.1186/s12889-016-3349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DL, Bouhassira D, Cruccu G, Freeman R, Hansson P, Nurmikko T, Raja SN, Rice AS, Serra J, Smith BH, Treede RD, Jensen TS. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157:1599–1606. doi: 10.1097/j.pain.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Ramirez R, Chen Y, Liedtke WB, Morales-Lazaro SL . Neurobiology of TRP Channels (nd, Emir TLR, eds) Boca Raton (FL): Florida, CRC Press, USA; 2017. TRP Channels and Pain; pp. 125–147. [PubMed] [Google Scholar]
- 14.Hager S, Dai J, Fischer V, Luthke F, Staudinger A. East meets west: synergy through diversity. Forsch Komplementmed 23 Suppl. 2016;2:3–7. doi: 10.1159/000444766. [DOI] [PubMed] [Google Scholar]
- 15.Hanani M. Intercellular communication in sensory ganglia by purinergic receptors and gap junctions: implications for chronic pain. Brain Res. 2012;1487:183–191. doi: 10.1016/j.brainres.2012.03.070. [DOI] [PubMed] [Google Scholar]
- 16.Jensen T, Edwards JG. Calcineurin is required for Trpv1-induced long-term depression of hippocampal interneurons. Neurosci Lett. 2012;510:82–87. doi: 10.1016/j.neulet.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354:572–577. doi: 10.1126/science.aaf8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HY, Park CK, Cho IH, Jung SJ, Kim JS, Oh SB. Differential changes in Trpv1 expression after trigeminal sensory nerve injury. J Pain. 2008;9:280–288. doi: 10.1016/j.jpain.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Kim KM, Jung DH, Jang DS, Kim YS, Kim JM, Kim HN, Surh YJ, Kim JS. Puerarin suppresses AGEs-induced inflammation in mouse mesangial cells: a possible pathway through the induction of heme oxygenase-1 expression. Toxicol Appl Pharmacol. 2010;244:106–113. doi: 10.1016/j.taap.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Kaur H, Singh A. Neuropathic pain models caused by damage to central or peripheral nervous system. Pharmacol Rep. 2017;70:206–216. doi: 10.1016/j.pharep.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Kuner R. Central mechanisms of pathological pain. Nat Med. 2010;16:1258–1266. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- 22.Lappin SC, Randall AD, Gunthorpe MJ, Morisset V. Trpv1 antagonist, SB-366791, inhibits glutamatergic synaptic transmission in rat spinal dorsal horn following peripheral inflammation. Eur J Pharmacol. 2006;540:73–81. doi: 10.1016/j.ejphar.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Zhang J, Gao Y, Yang Y, Xu C, Li G, Guo G, Liu S, Xie J, Liang S. Puerarin alleviates burn-related procedural pain mediated by P2X(3) receptors. Purinergic Signal. 2011;7:489–497. doi: 10.1007/s11302-011-9248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu M, Liao K, Yu C, Li X, Liu S, Yang S. Puerarin alleviates neuropathic pain by inhibiting neuroinflammation in spinal cord. Mediators Inflamm. 2014a;2014:485927. doi: 10.1155/2014/485927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S, Zhang C, Shi Q, Li G, Song M, Gao Y, Xu C, Xu H, Fan B, Yu S, Zheng C, Zhu Q, Wu B, Peng L, Xiong H, Wu Q, Liang S. Puerarin blocks the signaling transmission mediated by P2X3 in SG and DRG to relieve myocardial ischemic damage. Brain Res Bull. 2014b;101:57–63. doi: 10.1016/j.brainresbull.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Mandadi S, Armati PJ, Roufogalis BD. Protein kinase C modulation of thermo-sensitive transient receptor potential channels: Implications for pain signaling. J Nat Sci Biol Med. 2011;2:13–25. doi: 10.4103/0976-9668.82311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- 28.Moore C, Gupta R, Jordt SE, Chen Y, Liedtke WB. Regulation of pain and Itch by TRP channels. Neurosci Bull. 2018;34:120–142. doi: 10.1007/s12264-017-0200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naziroglu M, Braidy N. Thermo-sensitive TRP channels: novel targets for treating chemotherapy-induced peripheral pain. Front Physiol. 2017;8:1040. doi: 10.3389/fphys.2017.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nilius B. Transient receptor potential (TRP) cation channels: rewarding unique proteins. Bull Mem Acad R Med Belg. 2007;162:244–253. [PubMed] [Google Scholar]
- 31.Ozdemir US, Naziroglu M, Senol N, Ghazizadeh V. Hypericum perforatum attenuates spinal cord injury-induced oxidative stress and apoptosis in the dorsal root ganglion of rats: involvement of TRPM2 and Trpv1 channels. Mol Neurobiol. 2016;53:3540–3551. doi: 10.1007/s12035-015-9292-1. [DOI] [PubMed] [Google Scholar]
- 32.Pan Y, Wang Y, Zhao Y, Peng K, Li W, Wang Y, Zhang J, Zhou S, Liu Q, Li X, Cai L, Liang G. Inhibition of JNK phosphorylation by a novel curcumin analog prevents high glucose-induced inflammation and apoptosis in cardiomyocytes and the development of diabetic cardiomyopathy. Diabetes. 2014;63:3497–3511. doi: 10.2337/db13-1577. [DOI] [PubMed] [Google Scholar]
- 33.Pohoczky K, Kun J, Szalontai B, Szoke E, Saghy E, Payrits M, Kajtar B, Kovacs K, Kornyei JL, Garai J, Garami A, Perkecz A, Czegledi L, Helyes Z. Estrogen-dependent up-regulation of Trpa1 and Trpv1 receptor proteins in the rat endometrium. J Mol Endocrinol. 2016;56:135–149. doi: 10.1530/JME-15-0184. [DOI] [PubMed] [Google Scholar]
- 34.Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- 35.Shir Y, Seltzer Z. Effects of sympathectomy in a model of causalgiform pain produced by partial sciatic nerve injury in rats. Pain. 1991;45:309–320. doi: 10.1016/0304-3959(91)90056-4. [DOI] [PubMed] [Google Scholar]
- 36.Simoes RR, Dos Santos Coelho I, do Espirito Santo CC, Morel AF, Zanchet EM, Santos AR. Oral treatment with methanolic extract of the root bark of Condalia buxifolia Reissek alleviates acute pain and inflammation in mice: Potential interactions with PGE2, Trpv1/ASIC and PKA signaling pathways. J Ethnopharmacol. 2016;185:319–326. doi: 10.1016/j.jep.2016.03.050. [DOI] [PubMed] [Google Scholar]
- 37.Spicarova D, Palecek J. The role of spinal cord vanilloid (Trpv1) receptors in pain modulation. Physiol Res 57 Suppl. 2008;3:S69–77. doi: 10.33549/physiolres.931601. [DOI] [PubMed] [Google Scholar]
- 38.Tang L, Liu D, Yi X, Xu T, Liu Y, Luo Y, Yin D, He M. The protective effects of puerarin in cardiomyocytes from anoxia/reoxygenation injury are mediated by PKCepsilon. Cell Biochem Funct. 2014;32:378–386. doi: 10.1002/cbf.3026. [DOI] [PubMed] [Google Scholar]
- 39.Tao J, Cui Y, Duan Y, Zhang N, Wang C, Zhang F. Puerarin attenuates locomotor and cognitive deficits as well as hippocampal neuronal injury through the PI3K/Akt1/GSK-3beta signaling pathway in an in vivo model of cerebral ischemia. Oncotarget. 2017;8:106283–106295. doi: 10.18632/oncotarget.22290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tominaga M. Activation and regulation of nociceptive transient receptor potential (TRP) channels, Trpv1 and Trpa1. Yakugaku Zasshi. 2010;130:289–294. doi: 10.1248/yakushi.130.289. [DOI] [PubMed] [Google Scholar]
- 41.Tsai FJ, Ho TJ, Cheng CF, Liu X, Tsang H, Lin TH, Liao CC, Huang SM, Li JP, Lin CW, Lin JG, Lin JC, Lin CC, Liang WM, Lin YJ. Effect of Chinese herbal medicine on stroke patients with type 2 diabetes. J Ethnopharmacol. 2017;200:31–44. doi: 10.1016/j.jep.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Goffer Y, Xu D, Tukey DS, Shamir DB, Eberle SE, Zou AH, Blanck TJ, Ziff EB. A single subanesthetic dose of ketamine relieves depression-like behaviors induced by neuropathic pain in rats. Anesthesiology. 2011;115:812–821. doi: 10.1097/ALN.0b013e31822f16ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao Y, Huang J, Xu J, Zeng L, Tian J, Lou Y, Liu Y, Hu B, Tong F, Shen R. Targeted delivery of puerarin/glycyrrhetinic acid-PEG-PBLA complex attenuated liver ischemia/reperfusion injury via modulating Toll-like receptor 4/nuclear factor-kappaB pathway. Ther Deliv. 2018;9:245–255. doi: 10.4155/tde-2017-0106. [DOI] [PubMed] [Google Scholar]
- 44.Xu C, Xu W, Xu H, Xiong W, Gao Y, Li G, Liu S, Xie J, Tu G, Peng H, Qiu S, Liang S. Role of puerarin in the signalling of neuropathic pain mediated by P2X3 receptor of dorsal root ganglion neurons. Brain Res Bull. 2012;87:37–43. doi: 10.1016/j.brainresbull.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Xue Q, Jong B, Chen T, Schumacher MA. Transcription of rat Trpv1 utilizes a dual promoter system that is positively regulated by nerve growth factor. J Neurochem. 2007;101:212–222. doi: 10.1111/j.1471-4159.2006.04363.x. [DOI] [PubMed] [Google Scholar]
- 46.Yuan QL, Guo TM, Liu L, Sun F, Zhang YG. Traditional Chinese medicine for neck pain and low back pain: a systematic review and meta-analysis. PLoS One. 2015;10:e0117146. doi: 10.1371/journal.pone.0117146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan S, Burrell BD. Endocannabinoid-dependent LTD in a nociceptive synapse requires activation of a presynaptic TRPV-like receptor. J Neurophysiol. 2010;104:2766–2777. doi: 10.1152/jn.00491.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang D, Ma G, Hou M, Zhang T, Chen L, Zhao C. The neuroprotective effect of puerarin in acute spinal cord injury rats. Cell Physiol Biochem. 2016;39:1152–1164. doi: 10.1159/000447822. [DOI] [PubMed] [Google Scholar]
- 49.Zhao L, Wang Y, Liu J, Wang K, Guo X, Ji B, Wu W, Zhou F. Protective effects of genistein and puerarin against chronic alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms. J Agric Food Chem. 2016;64:7291–7297. doi: 10.1021/acs.jafc.6b02907. [DOI] [PubMed] [Google Scholar]
- 50.Zhou YX, Zhang H, Peng C. Puerarin: a review of pharmacological effects. Phytother Res. 2014;28:961–975. doi: 10.1002/ptr.5083. [DOI] [PubMed] [Google Scholar]
- 51.Zhu Z, Xu Y, Zou H, Zhang Z, Ni W, Chen S. Effects of puerarin on pulmonary vascular remodeling and protein kinase C-alpha in chronic cigarette smoke exposure smoke-exposed rats. J Huazhong Univ Sci Technolog Med Sci. 2008;28:27–32. doi: 10.1007/s11596-008-0107-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Open peer review report 1