Nerve guidance channels are limited by lack of topographical guidance: Treatment of sizeable nerve gaps remains problematic following peripheral nerve injury. Functional outcomes are good when neurorrhaphy, or direct end-to-end suture repair, is possible. The problem arises when there is significant segmental loss, which can occur following trauma as well as oncological procedures. In such scenarios, it is often not possible to appose severed nerve ends without causing significant tension. The current gold standard for management is to utilize autologous nerve grafts, commonly obtained from the sural nerve, to bridge these defects. This inevitably results in loss of cutaneous sensation over the lower limb, and the risk of donor site morbidities including infection and scarring. Suitable donor nerves remain finite in supply, and are often not ideally matched with recipient sites in terms of calibre and length.
Nerve guidance channels have been designed to address these limitations, with proximal and distal nerve stumps telescoped and sutured to the ends of the artificial conduit during operative repair. Design objectives of nerve guidance channels have evolved over time with the emergence of new materials (Gaudin et al., 2016). Silicone represents a first-generation channel utilized to restore continuity and to prevent fibrous ingrowth from surrounding tissues. In being non-resorbable, silicone tubes frequently had to be removed as they caused extrinsic compression, offsetting their usefulness despite promising functional recovery. Thus, second-generation conduits shifted towards usage of biodegradable materials. These include commercially available products composed of collagen (Neuragen, Neuroflex, NeuroMatrix), polyglycolic acid (Neurotube), polylactide-caprolactone (Neurolac) and polyvinylalcohol-based hydrogel (SaluTunnel). It is essential that the next generation of guidance channels can facilitate repair across larger nerve gaps, with 2 cm representing a critical threshold beyond which the performance of artificial conduits remains fair. The present generation of nerve guidance channels are lacking in microstructure to provide physical guidance of the regenerative process. Provision of nanotopography within the channel lumen serves to minimize aberrant sprouting, and potentially enhance regeneration along the intended axis.
Electrospinning is a means of generating aligned nanofibers to mimic the nerve microstructure and thus guide axonal growth and cellular migration within nerve guidance channels. Here, we emphasise how determination of appropriate physical and biological properties of the nanofiber scaffold can optimize neural regeneration, and in doing so, contribute towards design of a new generation of nerve guidance channels.
Optimizing physical properties of the nanofiber scaffold for neural regeneration: Electrospinning is a common and versatile technique for manufacturing uniaxial nanofibers intended for use as a scaffold for neural repair. A number of biocompatible materials have been utilized alone and in combination to generate aligned nanofibers, including poly-L-lactic acid (PLLA), polycaprolactone (PCL), poly(lactic-co-glycolic acid) (PLGA), poly{(lactic acid)-co-[(glycolic acid)-alt-(L-lysine)]} (PLGL), polycaprolactone (PCL), collagen, carbon, and chitosan. In the context of nerve guidance channel design it is important that nanofibers support axonal attachment and maximize outgrowth parallel to the main fiber axis. Equally important are their effects on Schwann cell growth and migration. In response to peripheral nerve injury, Schwann cells proliferate, upregulate neurotrophic factors, phagocytose inhibitory myelin debris and form aligned columns known as Bands of Büngner to guide axonal regeneration, thus forming a critical cellular component of the endogenous repair response (Jessen and Mirsky, 2016).
Control of nanofiber diameter is a means towards optimizing axonal outgrowth and Schwann cell migration (Wang et al., 2010). Rat dorsal root ganglia cultured upon PLLA fibers demonstrated maximal axonal outgrowth upon fibers of intermediate diameter (760 nm) whilst Schwann cell migration was highest upon large fibers (1325 nm). The lowest displacement in both axonal outgrowth and Schwann cell migration was observed upon fibers of small diameter (293 nm), where neurites also exhibited an increased tendency to grow perpendicular to the main fiber axis. It is known that reduction of nanofiber diameter limits cell adhesion due to an inability for focal adhesion cues to be recognized (Huang et al., 2015). Furthermore, fiber dimensions had differential effects on neurite outgrowth and Schwann cell migration, leading to pioneer neurites extending beyond the Schwann cell front as guided by the nanofibers. Separately, it has been shown that undesired neurite outgrowth perpendicular to the main fiber axis is increased when fibers of the same diameter are deposited at a higher density (Xie et al., 2014). The strength of adherence between neurites and the nanofiber surface in comparison to the underlying substrata are crucial determinants in the directionality of axonal sprouting, and can be adjusted by coating with extracellular matrix components such as laminin (Xie et al., 2014). These physical parameters need to be purposely determined in the generation of nanofiber scaffolds towards achieving intended regenerative effects.
Crosslinkers can be utilized to modify the biological interface between nanofibers and neural cell types. We recently demonstrated that treatment with genipin, a biocompatible cross-linker extracted from the fruit of Gardenia jasminoides, enhanced the intra-fiber mechanical and regenerative properties of electrospun chitosan nanofibers (Lau et al., 2017). Whilst not affecting fiber diameter, an increase in genipin treatment concentration resulted in proportional elevation in nanofiber stiffness. Genipin treatment preserved nanofiber integrity which otherwise lead to swelling and degradation resulting in loss of surface topography. Dorsal root ganglion (DRG) neurites were able to adhere to genipin-treated nanofibers without additional coating of potentially immunogenic peptides. Strikingly, axonal outgrowth from neurons of dorsal root ganglia cultured upon genipin-treated fibers doubled the length of those in untreated controls. This was consistent with the prior finding that axonal regrowth from injured nerves in the peripheral nervous system, but not central nervous system, was affected by stiffness of the underlying substrate (Koch et al., 2012). Crosslinking therefore provides for tunable control of the properties of nanofibers towards application in nerve guidance channel design.
Surface modification and biological functionalization of the nanofiber scaffold: Cell attachment upon the native nanofiber surface is often limited by hydrophobicity. Surface modification by means of plasma treatment allows for addition of hydrophilic chemical groups in order to facilitate cell adhesion. Coating of appropriate extracellular matrix components upon the nanofiber surface is another strategy to promote growth cone development and neurite pathfinding. Laminin in particular allows for robust cell attachment and interactions via integrins. Neurites demonstrate a clear preference for guided growth along the axis of laminin-coated nanofibers, resultant in a concentration-dependent increase in axonal displacement and reduction in perpendicular outgrowth (Xie et al., 2014). Schwann cells similarly favour laminin-coated substrata and demonstrate increased proliferation. Immunogenicity and early biodegradability of laminin are potential barriers to in vivo application and incorporation of shortened motifs such as RGD and IKVAV to enhance attachment and neurite outgrowth respectively (Sun et al., 2016) have been utilized as an alternative.
Ligands with desired biological properties can be incorporated into the nanofiber scaffold to enhance neural regeneration. Here, the scaffold functions as delivery system for immobilization and controlled release of relevant cues to allow for localized enhancement of the regenerative response. An example of this is in the grafting of neurotrophins such as GDNF to the scaffold by means of microencapsulation. In combination with topography provided via aligned PCL nanofibers, GDNF-grafted nanofibers had an enhanced capacity to promote neurite outgrowth (Mohtaram et al., 2015). In targeting the crucial Schwann cell response to injury (Jessen and Mirsky, 2016), neuregulin-1 has been conjugated to PCL fibers (Tonazzini et al., 2017) and these functionalized fibers showed improved capacity to provide for Schwann cell colonization of the directional scaffold.
Advances in electrospinning techniques have allowed for adjustability in the shape of extruded nanofibers, imparting secondary surface characteristics. The presence of longitudinal grooves along nanofibers increased total surface area, which had the biological impact of enhancing cell adhesion and proliferation (Huang et al., 2015). Upon incorporation into nerve guidance channels, grooved nanofibers enhanced peripheral nerve regeneration in comparison to channels containing control nanofibers with a smooth surface.
Utilizing nanofiber scaffolds to maintain and direct transplanted cell populations: Another limitation of nerve guidance channels as compared to nerve grafts originates from the lack of supporting cells. Seeding of immunocompatible cell types into the channel lumen is a means of providing trophic and functional support, with Schwann cells being a leading cell candidate. Exogenous cells must be available for transplantation within a defined window period, as prolonged denervation leads to irreversible functional deficits. Towards this end, our group has been able to rapidly generate functional Schwann cells from human bone marrow stromal cells, allowing for a robust autologous cell source that spares the need to sacrifice a peripheral nerve for cell harvest (Cai et al., 2017). It is essential that nanofibers support the attachment, proliferation and migration of seeded cells, and that the chosen biomaterial and breakdown products have minimal cytotoxicity. A further consideration when seeding stem/progenitor cells is in the effect that nanofiber properties have on cellular differentiation. Nanofiber alignment, diameter, and surface properties all have bearing on neural precursor differentiation towards the Schwann cell lineage (Xue et al., 2017). In this context, the nanofiber scaffold must be considered a key aspect of the microenvronment that influences differentiation and maintenance of phenotype. Nanofiber scaffolds pre-seeded with stem/progenitor cells may even be cultured in vitro prior to transplantation to direct differentiation. In order to approach the performance of nerve grafts, augmentation by transplantation of exogenous cell types contained within the intraluminal scaffold will become a necessity (Figure 1). Towards this end, expeditous generation of autologous bone marrow-derived Schwann cells together with optimized genipin-treated uniaxial nanofibers to bridge the injured peripheral nerve illustrates combined biological and mechanical approaches towards addressing the present deficiencies of commercially available conduits.
Figure 1.
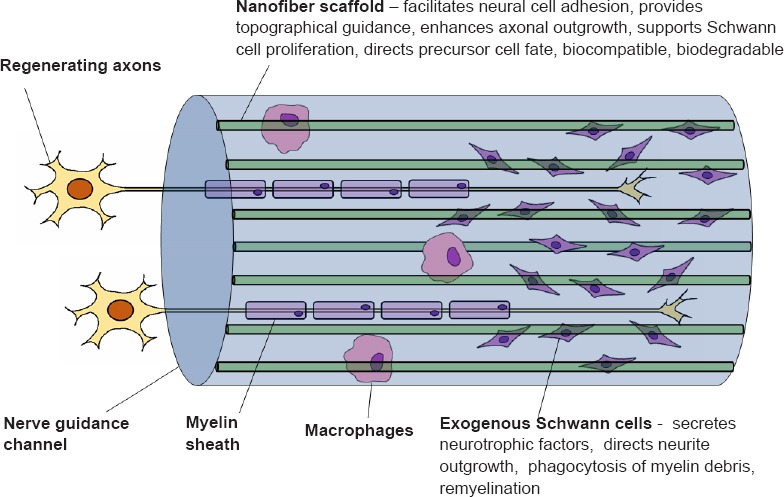
Nerve guidance channels utilizing optimized nanofiber scaffolds in conjunction with exogenous Schwann cells.
Conclusion: These advances highlight the interdependency between physical and cellular processes when considering biomaterials for nerve guidance channel design. Conceptually, the nanofiber scaffold must be considered a biologically active component that has the capacity to expedite the regenerative process. In considering appropriate biomaterials, it is imperative that results are validated in animal models that allow for histological and functional assessment of regeneration, while ensuring biocompatibility and biodegradability.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
References
- 1.Cai S, Tsui YP, Tam KW, Shea GK, Chang RS, Ao Q, Shum DK, Chan YS. Directed differentiation of human bone marrow stromal cells to fate-committed schwann cells. Stem Cell Reports. 2017;9:1097–1108. doi: 10.1016/j.stemcr.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaudin R, Knipfer C, Henningsen A. Approaches to peripheral nerve repair: generations of biomaterial conduits yielding to replacing autologous nerve grafts in craniomaxillofacial surgery. Biomed Res Int. 2016;2016:3856262. doi: 10.1155/2016/3856262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Ouyang Y, Niu H, He N, Ke Q, Jin X, Li D, Fang J, Liu W, Fan C, Lin T. Nerve guidance conduits from aligned nanofibers: improvement of nerve regeneration through longitudinal nanogrooves on a fiber surface. ACS Appl Mater Interfaces. 2015;7:7189–7196. doi: 10.1021/am509227t. [DOI] [PubMed] [Google Scholar]
- 4.Jessen KR, Mirsky R. The repair Schwann cell and its function in regenerating nerves. J Physiol. 2016;594:3521–3531. doi: 10.1113/JP270874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koch D, Rosoff WJ, Jiang J, Geller HM, Urbach JS. Strength in the periphery: growth cone biomechanics and substrate rigidity response in peripheral and central nervous system neurons. Biophys J. 2012;102:452–460. doi: 10.1016/j.bpj.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lau YT, Kwok LF, Tam KW, Chan YS, Shum DK, Shea GK. Genipin-treated chitosan nanofibers as a novel scaffold for nerve guidance channel design. Colloids Surf B Biointerfaces. 2017;162:126–134. doi: 10.1016/j.colsurfb.2017.11.061. [DOI] [PubMed] [Google Scholar]
- 7.Mohtaram NK, Ko J, Agbay A, Rattray D, Neill PO, Rajwani A, Vasandani R, Thu HL, Jun MBG, Willerth SM. Development of a glial cell-derived neurotrophic factor-releasing artificial dura for neural tissue engineering applications. J Mater Chem B. 2015;3:7974–7985. doi: 10.1039/c5tb00871a. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y, Li W, Wu X, Zhang N, Zhang Y, Ouyang S, Song X, Fang X, Seeram R, Xue W, He L, Wu W. Functional self-assembling peptide nanofiber hydrogels designed for nerve degeneration. ACS Appl Mater Interfaces. 2016;8:2348–2359. doi: 10.1021/acsami.5b11473. [DOI] [PubMed] [Google Scholar]
- 9.Tonazzini I, Moffa M, Pisignano D, Cecchini M. Neuregulin 1 functionalization of organic fibers for Schwann cell guidance. Nanotechnology. 2017;28:155303. doi: 10.1088/1361-6528/aa6316. [DOI] [PubMed] [Google Scholar]
- 10.Wang HB, Mullins ME, Cregg JM, McCarthy CW, Gilbert RJ. Varying the diameter of aligned electrospun fibers alters neurite outgrowth and Schwann cell migration. Acta Biomater. 2010;6:2970–2978. doi: 10.1016/j.actbio.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Xie J, Liu W, MacEwan MR, Bridgman PC, Xia Y. Neurite outgrowth on electrospun nanofibers with uniaxial alignment: the effects of fiber density, surface coating, and supporting substrate. ACS Nano. 2014;8:1878–1885. doi: 10.1021/nn406363j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xue J, Yang J, O’Connor DM, Zhu C. Differentiation of bone marrow stem cells into schwann cells for the promotion of neurite outgrowth on electrospun fibers. ACS Appl Mater Interfaces. 2017;9:12299–12310. doi: 10.1021/acsami.7b00882. [DOI] [PMC free article] [PubMed] [Google Scholar]


