Keywords: nerve regeneration, fractional anisotropy, T2*, rotenone, 6-hydroxydopamine, Parkinson's disease, magnetic resonance imaging, diffusion tensor imaging, dopaminergic neurons, neural regeneration
Abstract
Rotenone and 6-hydroxydopamine are two drugs commonly used to generate Parkinson’s disease animal models. They not only achieve degenerative changes of dopaminergic neurons in the substantia nigra, but also satisfy the requirements for iron deposition. However, few studies have compared the characteristics of these two models by magnetic resonance imaging. In this study, rat models of Parkinson’s disease were generated by injection of 3 μg rotenone or 10 μg 6-hydroxydopamine into the right substantia nigra. At 1, 2, 4, and 6 weeks after injection, coronal whole-brain T2-weighted imaging, transverse whole-brain T2-weighted imaging, and coronal diffusion tensor weighted imaging were conducted to measure fractional anisotropy and T2* values at the injury site. The fractional anisotropy value on the right side of the substantia nigra was remarkably lower at 6 weeks than at other time points in the rotenone group. In the 6-hydroxydopamine group, the fractional anisotropy value was decreased, but T2* values were increased on the right side of the substantia nigra at 1 week. Our findings confirm that the 6-hydroxydopamine-induced model is suitable for studying dopaminergic neurons over short periods, while the rotenone-induced model may be appropriate for studying the pathological and physiological processes of Parkinson’s disease over long periods.
Introduction
Parkinson’s disease (PD) is an age-related neurodegenerative disorder with a prevalence ranging from 41 per 100,000 people in the fourth decade of life to over 1900 per 100,000 people over 80 years of age (Cacabelos, 2017). The clinical symptoms of PD may not appear until more than half of the dopaminergic neurons in the substantia nigra are lost, highlighting the importance for identifying the disease at an early stage to implement appropriate therapeutic regimens (Caslake et al., 2008; Xing et al., 2016). However, the accurate diagnosis of early-stage PD in clinical practice remains a primary challenge (Long et al., 2012; Gazewood et al., 2013; Li et al., 2015).
Diffusion tensor imaging (DTI)-derived parameters, including fractional anisotropy (FA) values and iron-sensitive parameters such as T2*, may provide valuable information for early diagnosis of PD. One study found that FA values on both sides of the substantia nigra pars compacta, particularly those on the cranial side, were significantly decreased in PD patients (Chen et al., 2014). T2* parameters, which correlate with iron content, are feasible to measure in daily clinical practice. However, only a limited number of studies have characterized these parameters and concurrent central nervous system diseases typically seen in patients has led to inaccurate findings. In addition, PD patients may display poor compliance, and the frequent absence of pathological data in clinical trials has compromised the validity of study results (Blackwell et al., 2003). Therefore, a non-invasive method is required to effectively and efficiently reveal pathological modifications in the early stages of PD.
The establishment of animal models of PD has enabled the pathogenesis, early diagnosis, and treatment of PD to be evaluated. Many quantitative magnetic resonance imaging (MRI) studies investigating the diagnosis of PD have been conducted using animal models (Boska et al., 2007). Despite promising results from these models, variability in study results has hindered the widespread application of MRI techniques. This variability may result from differences in study design, magnetic fields, image resolution, tissue pre- and post-processing protocols, and statistical analysis. Differences in modeling methods may also be an important factor, which may affect the reproducibility of study findings. Rotenone- and 6-hydroxydopamine (6-OHDA)-induced rat models are two commonly used PD models; however, limited information is available regarding variations in common quantitative parameters, such as FA and T2* values. Therefore, the objective of this study was to measure FA and T2* values at different time points and to compare FA and T2* values between rotenone- and 6-OHDA-induced rat PD models.
Materials and Methods
Animals
Thirty-six male specific-pathogen-free Sprague-Dawley rats (average weight: 250 g, average age: 2 months) were provided by Beijing HFK Bioscience Co., China [approval No. SCXK (Jing) 2009-0004]. All rats were randomly divided into rotenone (n = 12), 6-OHDA (n = 12), rotenone-control (n = 6) and 6-OHDA-control (n = 6) groups. This study was approved by the Animal Ethics Committee and the Animal Care of Qinhuangdao Municipal No. 1 Hospital in China (No. 20140018).
Establishment of rotenone- and 6-OHDA-induced PD rat models
The rats were anesthetized with 0.6 mL 0.3% pentobarbital (Solarbio, Beijing, China) via intraperitoneal injection. The 12 rats in the rotenone group were administered 3 µg rotenone (R8875-1G; Sigma, St. Louis, MO, USA) dissolved in 2 µL dimethyl sulfoxide, through intracranial injection. The 12 rats in the 6-OHDA group received 10 μg 6-OHDA (H4381; Sigma) dissolved in 0.9% saline supplemented with 0.1% ascorbic acid (2 μL). The rotenone-control group received DMSO (2 μL). The 6-OHDA-control group received physiological saline (2 μL). The injection point was fixed in the right substantia nigra using stereotaxic coordinates (P: 4.8 mm, R: 1.9 mm, I: 8.5 mm) (Figure 1).
Figure 1.
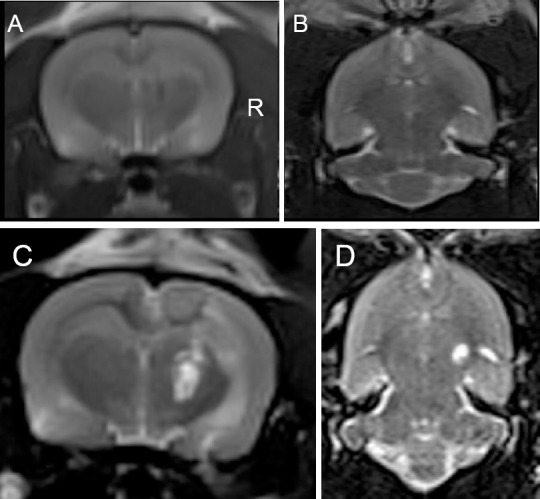
Magnetic resonance images of Parkinson’s disease rat models induced by rotenone or 6-hydroxydopamine.
(A, B) Coronal slice (A) and transverse (B) images of rotenone- and 6-hydroxydopamine-control groups (injection of 0.9% saline and dimethyl sulfoxide in the right substantia nigra), respectively; (C, D) Coronal slice (C) and transverse (D) images of the right substantia nigra at 1 week after rotenone or 6-hydroxydopamine injection: the hyperintense signal indicates treatment agent, rotenone or 6-hydroxydopamine. R: Right substantia nigra.
Magnetic resonance image acquisition and post-processing
All rats were anesthetized before MRI with 0.6 mL pentobarbital via intraperitoneal injection. The rats were placed on temperature-controlled animal beds. Each rat underwent MRI using a 3.0 Tesla MR scanner (MAGNETOM Verio; Siemens, Erlangen, Germany) with a 4-channel, 50-mm diameter phased array animal coil (part number: 10-F04885) at 1, 2, 4, and 6 weeks after rotenone or 6-OHDA injection. The imaging protocol included coronal whole-brain T2-weighted imaging (cT2WI), transverse whole-brain T2-weighted imaging, and coronal diffusion tensor weighted imaging (Figure 1).
Magnetic resonance parameters were as follows: coronal T2WI turbo spin-echo (repetition time/echo time: 3000/113 ms, flip angle: 150°, field of view: 74 mm × 74 mm, average: 14, voxel size: 0.3 mm × 0.3 mm × 2.0 mm, slice thickness: 2.0 mm, number of slices: 10) and transverse T2-weighted turbo spin-echo (repetition time/echo time: 4000/111 ms, flip angle: 150°, average: 10, field of view: 70 mm × 70 mm, voxel size: 0.4 mm × 0.3 mm × 2.0 mm, number of slices: 12). With the same slice orientation and position as cT2WI, single-shot spin-echo planar imaging was used in DTI and the settings were as follows: repetition time/echo time: 1800/110 ms, field of view: 74 mm × 74 mm, voxel size: 1.5 mm × 1.0 mm × 2.0 mm, sensitivity encoding factor 2, 15 nonlinear directions, b-value 0 and 1000 s/mm2. Gradient echo was used in fast low angle shot MRI, and the settings were as follows: repetition time/echo time: 4000/15/30/45/60 ms, field of view: 65 mm × 65 mm, voxel size: 1.0 mm × 1.0 mm × 2.5 mm.
Image analysis was performed using prototype software on a Siemens Verio 3.0T MR Leonardo 3682 workstation. FA and T2* maps of mouse brains were viewed, and each slice was compared using coronal T2WI. A radiologist with 10 years of experience in neural MRI placed the 0.30–0.60 cm2 circular regions of interest in the center of the substantia nigra.
Statistical analysis
Repeated measures analysis of variance was used to test differences in FA and T2* values among different time points in bilateral rotenone- and 6-OHDA lesions of the substantia nigra, and post hoc analysis was performed using a Bonferroni post hoc test. Statistical comparisons were performed using SPSS 16.0 (SPSS, Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
Results
Variation of FA and T2* values in the rotenone-induced PD rat model
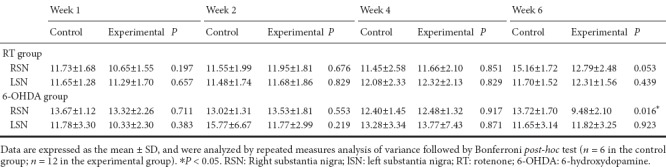
FA values on the right side of the substantia nigra at 6 weeks were significantly lower than those at other weeks (at 6 weeks, 0.220 ± 0.020 in the rotenone-control group vs. 0.178 ± 0.029 in the rotenone group; P = 0.007) (Table 1). No significant differences in T2* values were observed in the substantia nigra between the control and experimental groups or among the different time points (Table 2).
Table 1.
Comparison of mean fractional anisotropy values between RT- and 6-OHDA-induced Parkinson's disease and control rats
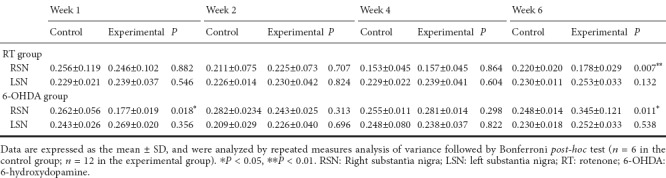
Table 2.
Comparison of mean T2* values between RT- and 6-OHDA-induced Parkinson's disease and control rats
Variation of FA and T2* values in the 6-OHDA-induced PD rat model
FA values on the right side of the substantia nigra were significantly lower at 1 week (0.256 ± 0.119 in the 6-OHDA-control group vs. 0.177 ± 0.019 in the 6-OHDA group; P = 0.018); but significantly higher at 6 weeks (0.220 ± 0.020 in the 6-OHDA-control group vs. 0.345 ± 0.121 in the 6-OHDA group; P = 0.011) compared with the other weeks (Table 1). T2* values in the 6-OHDA group were significantly higher at 6 weeks compared with values at other weeks (Table 2).
Discussion
6-OHDA- and rotenone-induced rat models are commonly used to study PD. 6-OHDA, a hydroxylated analog of the neurotransmitter dopamine, can selectively destroy catecholaminergic neurons. The specificity of this neurotoxin results from its preferential uptake by dopamine and norepinephrine transporters, with the underlying toxicity involving oxidative stress and the inhibition of mitochondrial respiration (Schober, 2004). Previous studies have revealed a marked decrease in the number of TH-immunoreactive neuronal cell bodies in the ipsilateral substantia nigra after unilaterally injecting 6-OHDA into the striatum, which persists for 10 months (Ichitani et al., 1994). This model is similar to human PD in both biochemistry and pathology, except for the absence of Lewy bodies. Rotenone is widely used as an insecticide and pesticide. It has high liposolubility and readily traverses the brain-blood barrier into brain tissues. Rotenone impairs oxidative phosphorylation in mitochondria and inhibits the formation of microtubules from tubulin. The chronic administration of rotenone to rodents produces a progressive model of PD associated with α-synuclein upregulation and Lewy-like body accumulation (Xiong et al., 2012).
Compared with traditional MRI techniques, functional MRI such as DTI and T2* mapping can provide vital information for diagnosing PD. DTI was established to visualize and quantify the movement of water molecules within brain tissues, providing a map of the integrity of the highly developed neuronal architecture. FA is the directional measurement of diffusivity, and FA values range from 0 to 1. High FA values reflect isotropic movement of water molecules along intact fiber tracts. Reduced FA values reflect decreased isotropic movement of water molecules, which may correspond to microstructural damage such as that observed in PD (Deng et al., 2013). Iron deposition induces local magnetic field fluctuation and non-fluctuation, which decreases the homogeneity of the magnetic field and increases related relaxation rates (transverse signal decay). Thus, some studies have suggested that quantitative MRI relaxometry for T2, T2*, or R2*, is a useful tool for assessing the progression of neurodegeneration in PD. It may also be a powerful tool for testing the efficiency of specific iron chelators (Ulla et al., 2013). Our study demonstrated different variation trends in FA and T2* values, which may indirectly reflect different in vivo pathological features in the substantia nigra of the PD model rats. We also observed pathological similarities in the substantia nigra between the two PD models. There was obvious apoptosis in tyrosine hydroxylase-positive cells on the right side of the substantia nigra after rotenone or 6-OHDA injection, indicating successful establishment of the models. The area where cell density was most reduced was near the injection point. Another similarity was that compared to the control group, quantitative analysis revealed that both rotenone- and 6-OHDA-induced models demonstrated decreased FA values on the right side of the substantia nigra, which is similar to the results of a previous study evaluating the degeneration of the substantia nigra pars compacta using DTI parameters (Boska et al., 2007).
This study highlights our differences between the rotenone and 6-OHDA models. First, pathogenic mechanisms. Rotenone is a highly lipophilic and potent irreversible mitochondrial complex I inhibitor. Its toxic metabolite is selectively transported by the dopamine transporter, resulting in PD (Friedrich et al., 1994). An absence in overall toxicity and the selectivity of brain tissue impairment shows that the pathology in the rotenone model is adequate and can be used to study the effects of the dopaminergic neuronal degeneration in PD (Sherer et al., 2003; Voitenko and Nikonenko, 2015). In addition, dopaminergic neuron loss continues after the use of rotenone (Chen et al., 2010). 6-OHDA shares structural similarities with dopamine and norepinephrine and exhibits a high affinity for several catecholaminergic plasma membrane transporters, such as the dopamine and norepinephrine transporters (Bové et al., 2005). Consequently, 6-OHDA can enter both dopaminergic and noradrenergic neurons to inflict damage to the catecholaminergic pathways of the central and peripheral nervous systems. 6-OHDA destroys catecholaminergic structures through the combined effect of reactive oxygen species and quinines and causes nigrostriatal depletion and gliosis, resulting in the development of PD symptoms (Carvey et al., 2005). Second, differences in FA values at different time points: FA values in the 6-OHDA group on the right side of the substantia nigra were significantly decreased at 1 week. However, in the rotenone group, FA values on the right side of the substantia nigra at 6 weeks were significantly lower compared with those on the left side of the substantia nigra or those in the control group. This may be because 6-OHDA can cause acute injury of the substantia nigra after the initial injection, including neuronal loss, glial proliferation, and demyelination of white matter fiber tracts, which may lead to decreased FA values on the right side of the substantia nigra at 1 week after injection (Dauer and Przedborski, 2003). The period over which dopaminergic neurons were damaged was long, and the lowest FA value was demonstrated at a later time point (6 weeks). Third, differences in FA value trends. Over time, FA values on the right of the substantia nigra in the rotenone group decreased, while those in the 6-OHDA group increased. The decreasing trend in the rotenone group indicated gradual demyelination of dopaminergic neurons, while increased FA values at 6 weeks in the 6-OHDA group may be caused by: (1) 6-OHDA injection causing initial neuronal loss, glial proliferation and demyelination, leading to an increase in water diffusion and an increase in FA parameter values. (2) Activation of microglial cells induced by the neural inflammatory reaction, which is in accordance with the study by Rosas et al. (2006). The neural inflammatory reaction has been confirmed as an important factor in the pathological processes of PD (Rosas et al., 2006; McGeer and McGeer, 2008). Fourth, differences in T2* value trends: Numerous human and animal studies have demonstrated that iron metabolism disorders may affect the neurodegeneration of dopamine neurons and glial proliferation in the substantia nigra (Wang et al., 2013). Although iron is required for normal neuronal metabolism, excess levels can contribute to the formation of free radicals, leading to lipid peroxidation and neurotoxicity (Sánchez Campos et al., 2015). In the study by Sánchez Campos et al., iron levels were indirectly detected by modifying the relaxation time of ferritin and hemosiderin interacting with nearby hydrogen nuclei. In the T2* technique, quantitative analysis of the substantia nigra indicated no significant differences in T2* values with time or between control and experimental rotenone groups. However, T2* values in the 6-OHDA group at 6 weeks were significantly higher than those at the other weeks. We have previously found that 6-OHDA, which is a neurotoxic drug, can influence ferritin content in the substantia nigra, which shortens proton relaxation times, particularly for T2 and T2*, an effect termed susceptibility-induced relaxation (Haacke et al., 2005; Brass et al., 2006). This phenomenon indicated that at an early stage, the amount of iron was insufficient to distinguish between the controls and the two model groups on T2* images, but with disease development, differences were observed.
However, the rotenone-induced model of this study is inconsistent with considerable iron deposition appearing within 4–8 weeks, as described by Song et al. (2004). The possible reasons for this include different injection methods and the influence of the tissue water environment. Song et al. (2004) investigated dopaminergic neuronal degeneration in animals subjected to systemic rotenone treatment via subcutaneous injection, while we performed stereotaxic intracranial injection. The effects of rotenone toxicity have been reported to be widespread, and the subcutaneous administration of this toxin does not provide the neuropathological and behavioral basis for a relevant and reliable PD model (Lapointe et al., 2004). The influence of the tissue water environment, accompanied by neuronal loss in damaged regions, reveals that the increase in local water content may led to increased T2 values, which may compensate for the decreased T2 values caused by iron (Alonso-Ortiz et al., 2015).
Despite the positive results obtained in this study, certain limitations must be recognized. It was difficult to obtain high-quality immunohistochemical results (tyrosine hydroxylase staining), so we did not perform quantitative immunohistochemistry. We intend to perform histology in future studies, which will help determine the molecular mechanism of PD pathogenesis and inform therapeutic strategy.
In conclusion, FA values in the rotenone group were remarkably increased compared with the rotenone-control group. The FA value was lowest at 6 weeks in the rotenone group. However, the FA value was lowest at 1 week in the 6-OHDA group. T2* values were significantly different in the 6-OHDA group at 6 weeks, while no significant difference in T2* values was observed in the rotenone group. Collectively, our findings reveal that the 6-OHDA-induced model may be suitable for studying dopaminergic neurons over short time periods, while the rotenone-induced model may be appropriate for studying the pathological and physiological processes of PD over long periods.
Additional file (47.7KB, pdf) : Open peer review report 1.
Open peer review report 1
Acknowledgments:
We thank Dr. Hong-Bin Han from Beijing Key Laboratory of Magnetic Resonance Imaging Device and Technique for his generous technical help.
Footnotes
Conflicts of interest: Qing-Lei Shi is a scientific clinical specialist of Siemens Ltd., China. There are no other conflicts of interest to declare.
Financial support: This study was supported by a grant from the Qinhuangdao Science-Technology Support Projects of China, No. 201402B036; a grant from the Science and Technology Project of Hebei Province of China, No. 1427777118D. The conception, design, execution, and analysis of experiments, as well as the preparation of and decision to publish this manuscript, were made independent of any funding organization.
Institutional review board statement: The experiments and conditions were in accordance with the international ethical statutes and law for the protection of animals and were also approved by the Animal Ethics Committee of Qinhuangdao Municipal No. 1 Hospital of China (approval No. 20140018).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Xavier d’Anglemont de Tassigny, Hospital Universitario Virgen del Rocio, Spain.
Funding: This study was supported by a grant from the Qinhuangdao Science-Technology Support Project of China, No. 201402B036; a grant from the Science and Technology Project of Hebei Province of China, No. 1427777118D.
(Copyedited by Yu J, Li CH, Qiu Y, Song LP, Zhao M)
References
- 1.Alonso-Ortiz E, Levesque IR, Pike GB. MRI-based myelin water imaging: A technical review. Magn Reson Med. 2015;73:70–81. doi: 10.1002/mrm.25198. [DOI] [PubMed] [Google Scholar]
- 2.Blackwell KT, Czubayko U, Plenz D. Quantitative estimate of synaptic inputs to striatal neurons during up and down states in vitro. J Neurosci. 2003;23:9123–9132. doi: 10.1523/JNEUROSCI.23-27-09123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boska MD, Hasan KM, Kibuule D, Banerjee R, McIntyre E, Nelson JA, Hahn T, Gendelman HE, Mosley RL. Quantitative diffusion tensor imaging detects dopaminergic neuronal degeneration in a murine model of Parkinson’s disease. Neurobiol Dis. 2007;26:590–596. doi: 10.1016/j.nbd.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bové J, Prou D, Perier C, Przedborski S. Toxin-induced models of Parkinson’s disease. NeuroRx. 2005;2:484–494. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brass SD, Chen NK, Mulkern RV, Bakshi R. Magnetic resonance imaging of iron deposition in neurological disorders. Top Magn Reson Imaging. 2006;17:31–40. doi: 10.1097/01.rmr.0000245459.82782.e4. [DOI] [PubMed] [Google Scholar]
- 6.Cacabelos R. Parkinson’s disease: from pathogenesis to pharmacogenomics. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carvey PM, Zhao CH, Hendey B, Lum H, Trachtenberg J, Desai BS, Snyder J, Zhu YG, Ling ZD. 6-Hydroxydopamine-induced alterations in blood-brain barrier permeability. Eur J Neurosci. 2005;22:1158–1168. doi: 10.1111/j.1460-9568.2005.04281.x. [DOI] [PubMed] [Google Scholar]
- 8.Caslake R, Moore JN, Gordon JC, Harris CE, Counsell C. Changes in diagnosis with follow-up in an incident cohort of patients with parkinsonism. J Neurol Neurosurg Psychiatry. 2008;79:1202–1207. doi: 10.1136/jnnp.2008.144501. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Zhang N, Li C, Song YW, Yu SY. Parallel relationship between microglial activation and substantia nigra damage in a rotenone-induced Parkinson’s disease rat model. Neural Regen Res. 2010;5:245–250. [Google Scholar]
- 10.Chen YS, Fang Y, Shi WZ, Liu DF, Wu SF, Du D, Liu LX. A comparative study of FA and T2* values of the substantia nigra pars compacta for diagnosis of early Parkinson’s disease. Fangshe Xue Shijian. 2014;29:155–158. [Google Scholar]
- 11.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 12.Deng B, Zhang Y, Wang L, Peng K, Han L, Nie K, Yang H, Zhang L, Wang J. Diffusion tensor imaging reveals white matter changes associated with cognitive status in patients with Parkinson’s disease. Am J Alzheimers Dis Other Demen. 2013;28:154–164. doi: 10.1177/1533317512470207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedrich T, van Heek P, Leif H, Ohnishi T, Forche E, Kunze B, Jansen R, Trowitzsch-Kienast W, Hofle G, Reichenbach H, et al. Two binding sites of inhibitors in NADH: ubiquinone oxidoreductase (complex I).Relationship of one site with the ubiquinone-binding site of bacterial glucose:ubiquinone oxidoreductase. Eur J Biochem. 1994;219:691–698. doi: 10.1111/j.1432-1033.1994.tb19985.x. [DOI] [PubMed] [Google Scholar]
- 14.Gazewood JD, Richards DR, Clebak K. Parkinson disease: an update. Am Fam Physician. 2013;87:267–273. [PubMed] [Google Scholar]
- 15.Haacke EM, Cheng NY, House MJ, Liu Q, Neelavalli J, Ogg RJ, Khan A, Ayaz M, Kirsch W, Obenaus A. Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging. 2005;23:1–25. doi: 10.1016/j.mri.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Ichitani Y, Okamura H, Nakahara D, Nagatsu I, Ibata Y. Biochemical and immunocytochemical changes induced by intrastriatal 6-hydroxydopamine injection in the rat nigrostriatal dopamine neuron system: evidence for cell death in the substantia nigra. Exp Neurol. 1994;130:269–278. doi: 10.1006/exnr.1994.1205. [DOI] [PubMed] [Google Scholar]
- 17.Lapointe N, St-Hilaire M, Martinoli MG, Blanchet J, Gould P, Rouillard C, Cicchetti F. Rotenone induces non-specific central nervous system and systemic toxicity. FASEB J. 2004;18:717–719. doi: 10.1096/fj.03-0677fje. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Ji X, Mao C, Chen Y, Guo S, Li S, Liu C. A clinical study of changes in retina and visual field in patients with early Parkinson’s disease. Zhonghua Nei Ke Za Zhi. 2015;54:521–524. [PubMed] [Google Scholar]
- 19.Long D, Wang J, Xuan M, Gu Q, Xu X, Kong D, Zhang M. Automatic classification of early Parkinson’s disease with multi-modal MR imaging. PLoS One. 2012;7:e47714. doi: 10.1371/journal.pone.0047714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGeer PL, McGeer EG. Glial reactions in Parkinson’s disease. Mov Disord. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- 21.Rosas HD, Tuch DS, Hevelone ND, Zaleta AK, Vangel M, Hersch SM, Salat DH. Diffusion tensor imaging in presymptomatic and early Huntington’s disease: Selective white matter pathology and its relationship to clinical measures. Mov Disord. 2006;21:1317–1325. doi: 10.1002/mds.20979. [DOI] [PubMed] [Google Scholar]
- 22.Sánchez Campos S, Rodríguez Diez G, Oresti GM, Salvador GA. Dopaminergic neurons respond to iron-induced oxidative stress by modulating lipid acylation and deacylation cycles. PLoS One. 2015;10:e0130726. doi: 10.1371/journal.pone.0130726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schober A. Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res. 2004;318:215–224. doi: 10.1007/s00441-004-0938-y. [DOI] [PubMed] [Google Scholar]
- 24.Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol. 2003;179:9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- 25.Song SK, Kim JH, Lin SJ, Brendza RP, Holtzman DM. Diffusion tensor imaging detects age-dependent white matter changes in a transgenic mouse model with amyloid deposition. Neurobiol Dis. 2004;15:640–647. doi: 10.1016/j.nbd.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Ulla M, Bonny JM, Ouchchane L, Rieu I, Claise B, Durif F. Is R2* a new MRI biomarker for the progression of Parkinson’s disease?. A longitudinal follow-up. PLoS One. 2013;8:e57904. doi: 10.1371/journal.pone.0057904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voitenko LP, Nikonenko AG. Modification of experimental rotenone model of Parkinson’s disease. Fiziol Zh. 2015;61:83–90. doi: 10.15407/fz61.01.083. [DOI] [PubMed] [Google Scholar]
- 28.Wang C, Fan G, Xu K, Wang S. Quantitative assessment of iron deposition in the midbrain using 3D-enhanced T2 star weighted angiography (ESWAN): a preliminary cross-sectional study of 20 Parkinson’s disease patients. Magn Reson Imaging. 2013;31:1068–1073. doi: 10.1016/j.mri.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Xing HX, Jiang JK, Qin YY, Wang YM. Minocycline affects the expression of glial cell derived neurotrophic factor family in a rat model of Parkinson’s disease. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:4020–4028. [Google Scholar]
- 30.Xiong N, Long X, Xiong J, Jia M, Chen C, Huang J, Ghoorah D, Kong X, Lin Z, Wang T. Mitochondrial complex I inhibitor rotenone-induced toxicity and its potential mechanisms in Parkinson’s disease models. Crit Rev Toxicol. 2012;42:613–632. doi: 10.3109/10408444.2012.680431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Open peer review report 1


