Abstract
Objectives—
To evaluate the frequency with which gestational weight gain and estimated fetal weight do not track across gestation and to assess the risk of small-for-gestational-age (SGA) and large-for-gestational-age (LGA) birth weight as a function of tracking.
Methods—
This study included a pregnancy cohort (2009–2013) of 2438 women from 4 racial/ethnic groups in the United States. We calculated race- and trimester-specific gestational weight gain and estimated fetal weight z scores. The prevalence of how often gestational weight gain and estimated fetal weight did not or did directly track was examined by grouping z scores into measure-specific categories (< ‒ 1 SD, ‒ 1 to + 1 SD, and >1 SD) and then examining 2-measure combinations. Trimester-specific relative risks for SGA and LGA births were estimated with a gestational weight gain and estimated fetal weight z score interaction. We estimated coefficients for selected gestational weight gain and estimated fetal weight values (−1 SD, 0 SD, and +1 SD) compared with the referent of 0 SD for both measures. Small and large for gestational age were calculated as birth weight below the 10th and at or above the 90th percentiles, respectively.
Results—
Gestational weight gain and estimated fetal weight were within 1 SD 55.5%, 51.5%, and 48.2% of the time in the first, second, and third trimesters, respectively. There was no significant interaction between gestational weight gain and estimated fetal weight on the risk of SGA in the first and second trimesters (interaction term P=.48; P=.79). In the third trimester, there was a significant interaction (P = .002), resulting in a 71% (95% confidence interval, 1.45–2.02) increased risk of SGA when estimated fetal weight was low and gestational weight gain was high. These relationships were similar for the risk of LGA.
Conclusions—
Deviations in either measure, even in the presence of average gestational weight gain or estimated fetal weight, still suggest an increased risk of SGA and LGA.
Keywords: birth weight, estimated fetal weight, gestational weight gain, obstetrics
Birth weight is an indicator of neonatal morbidity, mortality, and long-term chronic disease, with weights at the extremes (small-for-gestational-age [SGA] and large-for-gestational-age [LGA], typically defined as <10th percentile and ≥90th percentile for gestational age [GA], respectively), associated with increased risk.1–3 Gestational weight gain is also known to be associated with birth weight, for which low gestational weight gain is associated with smaller estimated fetal weights4 and an increased risk of SGA,5 whereas high gestational weight gain is associated with larger estimated fetal weights4,6 and an increased risk of LGA5
When gestational weight gain and fetal growth do not directly track across gestation, such as when a mother gains excessively, but fetal growth is not proportionally high, it is unknown whether there is an increased risk for adverse birth weight outcomes. If there is an association, it is unclear at what point during pregnancy the divergence may be most important (eg, early, mid, or late gestation). Our objectives were to evaluate how frequently gestational weight gain and estimated fetal growth did not directly track across gestation and assess whether the risk of SGA and LGA was associated with this tracking.
Materials and Methods
National Institute of Child Health and Human Development Fetal Growth Studies
The Singletons study was a prospective cohort with longitudinal data collection that enrolled pregnant women from 4 racial/ethnic groups between 2009 and 2013 in the United States.7 Women between the ages of 18 and 40 years with a viable singleton pregnancy and planning to deliver at participating hospitals were recruited from 12 sites. Low-risk healthy women with spontaneous singleton pregnancies before 14 weeks’ gestation were selected for inclusion into nonobese (body mass index [BMI], 19.0–29.9 kg/m2; n = 2334) and obese (BMI, 30.0–45.0 kg/m2; n = 468) cohorts. Exclusion criteria were similar between cohorts and included chronic hypertension or high blood pressure under medical supervision (obese women if requiring ≥2 medications), pregestational diabetes, chronic renal disease under medical supervision, autoimmune disease, psychiatric disorders, cancer, and human immunodeficiency virus/ AIDS.7 Additional exclusion criteria for the nonobese cohort included smoking, illicit drug use, infertility treatment, asthma, thyroid disease, hematologic disorders, epilepsy, current eating disorders, previous pregnancy complications, and adverse birth outcomes. We limited our analysis to nonanomalous live births with complete birth weight data (n = 2438).
After enrollment, women were randomized into 1 of 4 follow-up schedules to capture weekly representation of data.7 Gestational age dating was confirmed by ultrasound assessment (8 weeks 0 days to 13 weeks 6 days) to ensure consistency with the self-reported last menstrual period. Research nurses conducted in-person interviews to obtain demographic characteristics (self-reported race, income, student status, education, parity, and age) and reproductive and pregnancy histories. At each of the 5 follow-up visits, fetal ultrasound measurements of standard fetal biometric parameters were taken by using standard operating procedures and identical equipment (Voluson E8; GE Healthcare, Milwaukee, WI). Fetal ultrasound measurements of femur length, head circumference, and abdominal circumference were used to compute the estimated fetal weight by using the formula of Hadlock et al8 [log10(weight) = 1.326 – 0.00326 × abdominal circumference × femur length + 0.0107 × head circumference + 0.0438 × abdominal circumference + 0.158 × femur length].
At enrollment and each research visit, women’s weights were measured without shoes or excessive clothing on a beam balance or digital scale. Antenatal weights were also abstracted from medical charts. To improve the precision of estimates by increasing the number of measurements per woman, we evaluated the quality of abstracted weights to see whether measurements from both sources could be combined. To ensure the quality of abstracted weights, we calculated the rate of change between each weight and the next closest measurement, regardless of the source, to examine for large implausible gains/losses. Chart-abstracted and measured weights occurring on the same day were within a mean (SD) of 0.04 (0.95) kg, indicating the consistency across sources. Both sources of maternal weight were used in the analysis. Gestational weight gain was calculated as the difference between the maternal weight (measured or abstracted) and self-reported prepregnancy weight. The maternal reported prepregnancy weight and measured height were used to calculate the prepregnancy BMI (kilograms per square meter). After delivery, certified staff abstracted maternal and neonatal information from medical records. The birth weight at delivery was used to calculate SGA below the 10th percentile and LGA at or above the 90th percentile based on sex-specific birth weight references.9 Institutional Review Board approval (09-CH-N152) was obtained by the intramural Institutional Review Board at the National Institutes of Health for the National Institute of Child Health and Human Development, all participating clinical institutions, and the data and imaging coordinating centers in December 2009, and women gave informed consent before enrollment.
Statistical Analysis
We separately calculated the mean change in maternal gestational weight gain and estimated fetal weight in the first trimester (GA < 14 weeks), second trimester (GA 14-<28 weeks), and third trimester (GA 28 weeks-delivery). To assess and compare gestational weight gain and estimated fetal weight on the same scale, we standardized the values by calculating population based trimester- and race-specific z scores [z = (x - μ)/σ], where x is the observed gestational weight gain or estimated fetal weight value; μ is a mean; and σ is a standard deviation. The prevalence of how often gestational weight gain and estimated fetal weight did not directly track (discordant) or did directly track (concordant) was examined by categorizing gestational weight gain and estimated fetal weight z scores into categories (<−1 SD, −1 to + 1 SD, and >1 SD) and then estimating the prevalence of all possible combinations of the gestational weight gain and estimated fetal weight z scores in each trimester.
Poisson regression with a robust error variance was used to calculate trimester-specific relative risks (RRs) for SGA and LGA We included a multiplicative interaction term between gestational weight gain and estimated fetal weight z scores (continuous by continuous) in all models to assess the combined effect of gestational weight gain and estimated fetal weight on the risk of SGA and LGA. After model estimation, we used linear combinations to calculate coefficients and 95% confidence intervals (CIs) for selected gestational weight gain and estimated fetal weight values (−1 SD, 0 SD, and +1 SD) compared with the referent of 0 SD for both gestational weight gain and estimated fetal weight. For example, we assessed the risk of SGA when gestational weight gain was high (+1 SD) and estimated fetal weight was low (−1 SD) compared with average gestational weight gain (0 SD) and average estimated fetal weight (0 SD). We repeated these linear combinations for the risk of LGA. In adjusted models, we a priori included the following covariates: maternal age, prepregnancy BMI, maternal height, parity, insurance, race, student status, and education. We tested for effect modification by the prepregnancy BMI (tested both as continuous and categorical variables based on the World Health Organization) and by preterm birth in fully adjusted models by including 3-way interaction terms with gestational weight gain and estimated fetal weight (likelihood ratio test conducted at the .05 significance level). Analyses were conducted with SAS version 9.4 software (SAS Institute Inc, Cary, NC)10 and Stata version 13.0 software (StataCorp, College Station, TX).11
Results
Most of the women in the study population were 20 to 39 years of age, married, and educated beyond high school (Table 1). The average gestational weight gain increased from a mean (SD) of 0.26 (1.12) kg in the first trimester to 3.70 (2.23) kg in the second trimester and 7.0 (2.85) kg in the third trimester. The average trimester-specific gain in estimated fetal weight also increased from the first to second and third trimesters by a mean (SD) of 68 (14) to 395 (142) and 1748 (432) g, respectively.
Table 1.
Demographic Characteristics of the National Institute of Child Health and Human Development Fetal Growth Studies Singletons Population (n = 2438)
| Characteristic | n (%) |
|---|---|
| Maternal age, y | |
| <20 | 136 (5.6) |
| 20–29 | 1265 (51.9) |
| 30–39 | 1016 (41.6) |
| 40–44 | 21 (0.9) |
| Insurance | |
| Other | 884 (36.3) |
| Private or managed | 1554 (63.7) |
| Race/ethnicity | |
| Non-Hispanic white | 682 (28.0) |
| Non-Hispanic black | 658 (27.0) |
| Hispanic | 703 (28.8) |
| Asian and Pacific Islander | 395 (16.2) |
| Marital status | |
| Never married | 535 (22.0) |
| Married | 1825 (74.9) |
| Divorced/widowed | 76 (3.1) |
| Education | |
| ≤High school Diploma | 721 (29.6) |
| Some college or associate degree | 724 (29.7) |
| Bachelor’s or advanced degree | 993 (40.7) |
| Income, $ | |
| <39,000 | 793 (37.4) |
| 40,000–74,900 | 469 (22.1) |
| ≥75,000–99,900 | 859 (40.5) |
| Parity | |
| 0 | 1144 (46.9) |
| 1 | 833 (34.2) |
| ≥2 | 461 (18.9) |
| Prepregnancy BMI | |
| Normal weight | 1372 (56.3) |
| Overweight | 648 (26.6) |
| Obese | 418 (17.2) |
Table 2 shows the proportion of women with gestational weight gain and estimated fetal weight that were concordant and discordant in each trimester. Gestational weight gain and estimated fetal weight were concordant (ie, were within 1 SD of each other) 55.5%, 51.5%, and 48.2% of the time in the first, second, and third trimesters, respectively. The prevalence of discordance for the most extreme categories (high gestational weight gain and low estimated fetal weight or vice versa) was rare (between 1% and 3.4%) at all time points.
Table 2.
Prevalence of Tracking and Nontracking in Gestational Weight Gain and Estimated Fetal Weight by Trimester
| Trimester 1 (0−<14 wk) |
Trimester 2 (14−<28 wk) |
Trimester 3 (28 wk-Delivery) |
||
|---|---|---|---|---|
| Gestational Weight Gaina |
Estimated Fetal Weighta |
(n = 2060), n (%) |
(n = 2324), n (%) |
(n = 2372), n (%) |
| Tracking | ||||
| −1 to + 1 SD | −1 to + 1 SD | 1061 (51.5) | 1051 (45.2) | 952 (40.1) |
| <−1 SD | <−1 SD | 48 (2.3) | 75 (3.2) | 86 (3.6) |
| > +1 SD | > +1 SD | 34 (1.7) | 72 (3.1) | 107 (4.5) |
| Sum of tracking, % | 55.5 | 51.5 | 48.2 | |
| Nontracking | ||||
| <−1 SD | −1 to +1 SD | 123 (5.9) | 378 (16.3) | 394 (16.6) |
| >1 SD | −1 to +1 SD | 111 (5.4) | 287 (12.4) | 275 (11.6) |
| −1 to +1 SD | <−1 SD | 381 (18.5) | 173 (7.4) | 153 (6.5) |
| −1 to +1 SD | >1 SD | 241 (11.7) | 186 (8.0) | 287 (12.1) |
| >1 SD | <−1 SD | 39 (1.9) | 41 (1.8) | 37 (1.6) |
| <−1 SD | >1 SD | 22 (1.1) | 61 (2.6) | 81 (3.4) |
| Sum of nontracking, % | 44.5 | 48.5 | 51.8 | |
Values were standardized by race- and trimester-specific means and standard deviations.
Figure 1 illustrates the risk of SGA (Figure 1A) and LGA (Figure 1B) based on the linear combinations of gestational weight gain and estimated fetal weight at selected values. In the first and second trimesters, there was no multiplicative interaction between gestational weight gain and estimated fetal weight on the risk of SGA, (interaction term P = .48; P = .79, respectively) or LGA (interaction term P = .77; P = .18, respectively). However, we observed an increased risk of SGA and LGA when either gestational weight gain or estimated fetal weight alone deviated below or above average (0 SD). For example, in the second trimester with a low gestational weight gain (−1 SD) and average estimated fetal weight (0 SD), there was a 24% increased risk of SGA (RR, 1.24; 95% CI, 1.11–1.37). A similar 27% increased risk of SGA was observed with a low estimated fetal weight (−1 SD) and average gestational weight gain (0 SD; RR, 1.27; 95% CI, 1.08–1.46; Figure 1A). These relationships were similar for the risk of LGA. There was an increased risk of LGA when gestational weight gain alone was high (+1 SD; RR, 1.19; 95% CI, 1.09–1.30) or estimated fetal weight alone was high (RR, 1.22; 95% CI, 1.09–1.36; Figure 1B).
Figure 1.
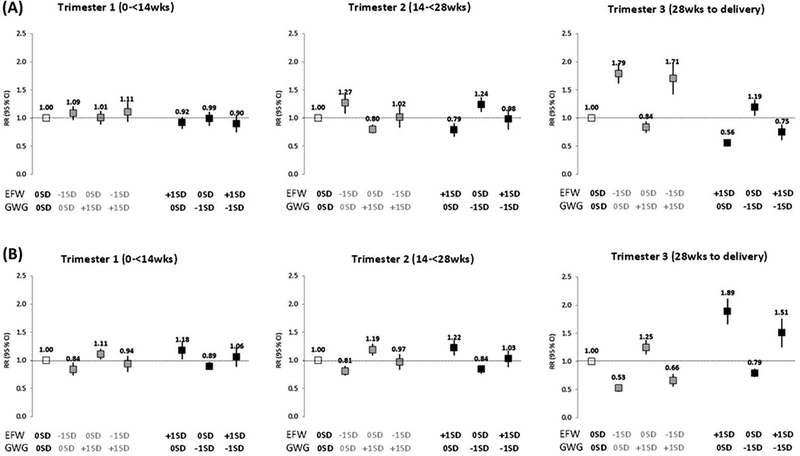
Risk of SGA (A) and LGA (B) by linear combinations of estimated fetal weight (EFW) and gestational weight gain (GWG) based on modified Poisson models. Estimates were adjusted for maternal age, prepregnancy BMI (calculated from measured height and self-reported pre-gravid weight at enrollment), maternal height (measured at enrollment), parity, insurance, race, student status, and education.
In the third trimester, there was a significant interaction between gestational weight gain and estimated fetal weight on the risk of SGA (interaction term P = .002). The risk of SGA remained increased when estimated fetal weight was low (−1 SD) even when gestational weight gain was high (+1 SD; RR, 1.71; 95% CI, 1.45–2.02). A high estimated fetal weight indicated a decreased risk of SGA, despite low gestational weight gain (RR, 0.75; 95% CI, 0.57–0.87; Figure 1A, trimester 3). These relationships were similar for LGA, although the interaction was not significant in the third trimester (P = .794).
Discussion
Main Findings
In a large, racially diverse, prospective US cohort study, gestational weight gain and estimated fetal weight were concordant between 48% and 55% of the time, depending on the trimester. Notably, when the two measures did not follow the same pattern across gestation and one measure suggested average gains/growth but the other measure did not, there was an increased risk of SGA or LGA. For example, in instances when the estimated fetal weight was average, there was an increased risk of SGA if gestational weight gain was low (−1 SD). Our findings suggest that estimated fetal weight and gestational weight gain each provide valuable insight into the risk of adverse birth weight outcomes.
Strengths and Limitations
This study may be limited in generalizability to low-risk women because of the inclusion criteria of the cohort. Our findings cannot imply that temporal changes in gestational weight gain and estimated fetal weight are causally related to adverse birth weight outcomes. Instead, we have presented clinically relevant cross sections of time. This study was strengthened by the repeated measurements of antenatal weight gain and detailed assessment of longitudinal fetal growth. An additional strength of this study was the racial/ethnic diversity of the study population. We were able to incorporate racial differences in gestational weight gain and estimated fetal weight7 through z score standardizations.
Interpretation
Gestational weight gain is an important predictor of size at birth, and it reflects the combined placental, maternal (eg, tissue expansion and fluid accretion), and fetal changes during pregnancy.12 A large body of evidence supports an association between total and trimester-specific gestational weight gain and the risk of SGA and LGA.5 Consistent with previous literature, we observed an association between second- and third-trimester gestational weight gain and an increased risk of SGA and LGA.13–16 This study expanded on existing knowledge by assessing the combined effect of gestational weight gain and estimated fetal weight on the risk of SGA and LGA, particularly when these two measures were discordant.
Although there was not a statistical interaction between gestational weight gain and estimated fetal weight in the first or second trimester, there remained an increased risk of SGA based on the independent effect of both gestational weight gain and estimated fetal weight. For example, when gestational weight gain was low in the presence of an average estimated fetal weight, there was still an increased risk of SGA, indicating the importance of the association between gestational weight gain and fetal growth. In the third trimester, the estimated fetal weight was more strongly associated with SGA and LGA than was gestational weight gain. These findings are consistent with the literature reporting that second-trimester gestational weight gain has a stronger association with adverse birth weight outcomes than that in the first or third trimester.13,14,16 Although the finding that estimated fetal weight is more strongly associated than gestational weight gain with birth weight outcomes is somewhat clinically intuitive, there has been little evidence on how a clinician should interpret the risk of birth weight outcomes when gestational weight gain and estimated fetal weight indicate contradictory clinical messages. There has been a large body of evidence supporting the use of gestational weight gain monitoring to identify pregnant women at risk of a poor birth weight outcome. However, these studies did not take into account how low or high gestational weight gain, which would suggest an increased risk of adverse birth weight outcomes, should be interpreted in the presence of an adequate estimated fetal weight, which would suggest no risk of adverse birth weight outcomes. To our knowledge, this work was the first study to provide insight into the gestational timing when each gestational weight gain and estimated fetal weight may suggest a risk to clinicians about the potential birth outcome.
Conclusions
Our findings suggest that both tracking and nontracking between gestational weight gain and estimated fetal weight are relatively common, and even in the presence of an average measure, low or high gestational weight gain and estimated fetal weight can still suggest an increased risk.
Acknowledgments
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, and included American Recovery and Reinvestment Act funding (contract numbers HHSN275200800013C, HHSN275200800002I, HHSN27500006,HHSN275200800003IC, HHSN2752008 00014C, HHSN275200800012C, HHSN 275200800028C, and HHSN275201000 009C).
Abbreviations
- BMI
body mass index
- CI
confidence interval
- GA
gestational age
- LGA
large-for-gestational age
- RR
relative risk
- SGA
small-for-gestational-age
References
- 1.Larkin JC, Speer PD, Simhan HN. A customized standard of large size for gestational age to predict intrapartum morbidity. Am J Obstet Gynecol 2011; 204:499.e1–499.e10. [DOI] [PubMed] [Google Scholar]
- 2.Gardosi J, Clausson B, Francis A. The value of customised centiles in assessing perinatal mortality risk associated with parity and maternal size. BJOG 2009; 116:1356–1363. [DOI] [PubMed] [Google Scholar]
- 3.Tenhola S, Martikainen A, Rahiala E, Herrgard E, Halonen P, Voutilainen R. Serum lipid concentrations and growth characteristics in 12-year-old children born small for gestational age. Pediatr Res 2000; 48:623–628. [DOI] [PubMed] [Google Scholar]
- 4.Galjaard S, Pexsters A, Devlieger R, et al. The influence of weight gain patterns in pregnancy on fetal growth using cluster analysis in an obese and nonobese population. Obesity (Silver Spring) 2013; 21:1416–1422. [DOI] [PubMed] [Google Scholar]
- 5.Rasmussen K, Yaktine A Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: Institute of Medicine and National Research Council of the National Academies; 2009. [PubMed] [Google Scholar]
- 6.Hinkle SN, Johns AM, Albert PS, Kim S, Grantz KL. Longitudinal changes in gestational weight gain and the association with intrauterine fetal growth. Eur J Obstet Gynecol Reprod Biol 2015; 190:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buck Louis GM, Grewal J, Albert PS, et al. Racial/ethnic standards for fetal growth: the NICHD Fetal Growth Studies. Am J Obstet Gynecol 2015; 213:449.e1–449.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology 1991; 181:129–133. [DOI] [PubMed] [Google Scholar]
- 9.Duryea EL, Hawkins JS, McIntire DD, Casey BM, Leveno KJ. A revised birth weight reference for the United States. Obstet Gynecol 2014; 124:16–22. [DOI] [PubMed] [Google Scholar]
- 10.SAS Institute Inc; The SAS System for Windows: Release 9.4. Cary, NC: SAS Institute Inc; 2013. [Google Scholar]
- 11.StataCorp. Stata Statistical Software: Release 13 College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 12.Pitkin RM. Nutritional support in obstetrics and gynecology. Clin Obstet Gynecol 1976; 19:489–513. [DOI] [PubMed] [Google Scholar]
- 13.Margerison-Zilko CE, Shrimali BP, Eskenazi B, Lahiff M, Lindquist AR, Abrams BF. Trimester of maternal gestational weight gain and offspring body weight at birth and age five. Matern Child Health J 2012; 16:1215–1223. [DOI] [PubMed] [Google Scholar]
- 14.Abrams B, Selvin S. Maternal weight gain pattern and birth weight. Obstet Gynecol 1995; 86:163–169. [DOI] [PubMed] [Google Scholar]
- 15.Sridhar SB, Xu F, Hedderson MM. Trimester-specific gestational weight gain and infant size for gestational age. PLoS One 2016; 11: e0159500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drehmer M, Duncan BB, Kac G, Schmidt MI. Association of second and third trimester weight gain in pregnancy with maternal and fetal outcomes. PLoS One 2013; 8:e54704. [DOI] [PMC free article] [PubMed] [Google Scholar]


