Abstract
Prinz et al. 2004 demonstrated that virtually indistinguishable network activity can arise from widely different sets of underlying membrane and synaptic parameters, and thus likely arise from different cellular and network mechanisms. This now broadly accepted principle guides research into individual variation in neuronal and synaptic properties, and into their homeostatic regulation.
Keywords: Homeostasis, Neuronal Models, Conductance Parameter Sets, Network Stability
Main text
With the close of the 20th century during the Decade of the Brain, experimental neuroscientists were gathering ever more extensive data sets on brain neurons and networks, while computing power and data storage were becoming ever more powerful and affordable. In parallel, theoretical neuroscience was undergoing a renaissance as ever more physicists, engineers, and mathematicians swelled their ranks.
A nascent area of research centered on how stability in neuronal and network activity could be achieved, given the myriad intrinsic membrane currents (channels) as well as the pattern and properties of synaptic connections that must be brought into alignment to assure this stability1–4. Activity-dependent and -independent processes were uncovered that promised future mechanistic insight into how such stability was achieved3–5. Computational models played a major role in visualizing how these mechanisms might play out1,2. These models led to multiple solutions to achieving stability. The laboratory of Eve Marder working in the crustacean stomatogastric nervous system (STN) contributed many of these early experimental and theoretical studies of homeostatic regulation of neuronal and network activity1–4.
A parallel development was the burgeoning work on biophysically-realistic neuronal and network models, which used as their bases experimental data on membrane and synaptic currents (conductance parameters). Hand-tuning such models to the data, however, was a nightmare. Automated search algorithms to fine tune the models’ parameters seemed, in principle, to have some promise, but in practice rarely succeeded. And to compound the problem, the physiological data on which these models were built varied, and widely so; first the physiological properties varied (perhaps not surprisingly) when collected under slightly different recording conditions; but more strikingly, they varied across experimental repetitions (i.e., across different animals) even when effort was made to carefully control the experimental settings. The standard approach to the latter issue – as in many paradigms across biology – had been to average parameters over repetitions, with the hope of obtaining some meaningful ‘mean value’ and while basically ignoring more fundamental questions on the source and meaning of the apparently inherent variability.
With the dawn of the 21st century, seminal modeling work from the Marder lab questioned the reliability of the averaging strategy. In fact, the analyses showed, the average value of a parameter might not be even present in the set of biologically ‘allowable’ values that permit functional neuronal activity6. This paper along with early explorations of the parameter space in the same system set the stage for an assault on determining which parameters were commensurate with functional activity7. First presented as an alternative to hand-tuning cellular models of neurons, Prinz, Billimora and Marder 20038 constructed a brute-force parameter database of an STN-inspired neuron (involving maximal conductances of 8 different membrane currents/channels, and consisting of 1.7 million parameter sets or model instances), to map the full range of parameters that permitted functional activity of various types – from bursting to silence. The analyses uncovered an inconvenient truth: in each case, several model instances, often up to thousands of them (depending on the stringency of the selection criteria for the activity type), with diverse underlying membrane parameters, could provide the desired activity. The authors were now in position to expand their analyses from a single neuron, to the circuit level. The stage was set for Prinz, Bucher and Marder 20049.
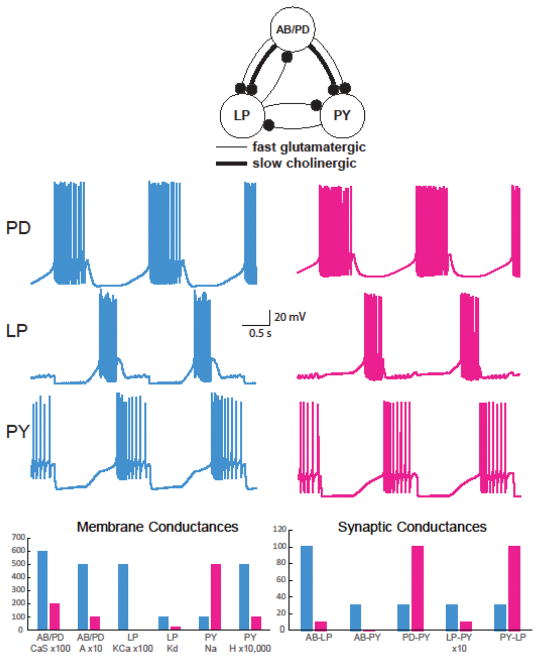
In this ground-breaking paper, the authors put forward a simplified model of the pyloric subnetwork of the STN; this sub-network drives rhythmic constrictions of the foregut and is continuously active in the isolated STN (Fig. 1). The network model consisted of three neuron types: PD/AB, LP and PY. PD/AB neurons (2 PDs and 1 AB) are tightly electrically-coupled, and constitute a rhythmically bursting core of the network that drives continuous pattern generation. The dynamics of the PD and AB synapses differ, and were considered separately in the model, but the neuronal properties are generally similar and these neurons were therefore lumped into a single bursting AB/PD model neuron. The LP neuron and PY neurons (there are 5–8 of them but they were represented as one in the model) do not burst, but have post-inhibitory rebound properties. The previous database of neuron models8 was used as a source of diverse examples of each type of neuron (5 AB/PD, 5 LP, and 6 PY instances) chosen from the available pools to be markedly different in their underlying membrane parameters, but which hewed closely to physiological determined activity characteristics. As an empirical backdrop, the investigators had a physiological database of nearly 100 STN preparations in which they had measured the characteristics of the pyloric motor pattern; this pattern is consistently triphasic, with rhythmic burst in an AB/PD → LP→ PY sequence, and it shows a range of cycle periods, burst durations, phase, and spike frequency. Importantly, the AB/PD neuron model instances used in the simulations showed a range of burst periods.
Figure 1.
Different instances of the Pyloric Model Generate Very Similar Activity Patterns using Widely Differing Cellular and Synaptic Properties. Top: schematic of a simplified version of the modeled pyloric circuit (black balls: inhibitory synapses). The PD and AB neurons are electrically coupled (not shown). Middle: Voltage traces from two pyloric network model instances (cyan and magenta). Bottom: Selected membrane and synaptic conductances of the network model instances (cyan and magenta) shown above. Data replotted and adapted from [9]9.
The authors then constructed all possible combinations of AB/PD, LP, and PY model instances into networks, and varied the strength (i.e., maximal conductance) of each of the 7 synaptic connections in the network over large ranges, resulting in some 20 million model instances of the network. Searching the database for pyloric-like activity (i.e., where the basic triphasic rhythm is preserved) yielded a remarkable 4 million network instances with incredibly diverse synaptic parameters! Moreover, this resulting set spanned all neuron instances that had been examined. The authors then sliced and diced this population of model instances into more stringently defined activity categories, constrained by the physiological database, for instance, by identifying pyloric rhythms that stringently conform to the physiological database in 15 salient characteristics, or even subsets of this pool that display a period falling into a specific range. The long-term impact of these analyses is epitomized in a simple figure (Fig. 1). Here we see very similar pyloric network activity in two network instances with vastly different properties of the component neurons and vastly different synaptic conductances. Indeed, incredibly diverse synaptic parameters and the whole gamut of neuron instances were represented amongst pyloric rhythms. Dicing the set of model instances further into specific burst period segments did restrict the AB/PD cell instances – confirming the role of AB/PD is setting period in the network – but did not materially restrict the other cells’ instances or the synaptic parameters (in most cases). In sum, the analyses demonstrated that virtually indistinguishable network activity can arise from widely disparate sets of underlying membrane and synaptic parameters and thus likely different cellular and network mechanisms. There really is more than one way to skin a cat (or drive the gut).
The broader implications of these findings are notable and they resonated immediately with neuroscientists and have especially driven work in small networks and more widely in systems neuroscience. Shortly after the Prinz et al. study, work in Marder’s lab and in a number of other labs was directed towards examining more closely the sources of variability across experimental preparations. By measuring ionic conductances and mRNA expression of ion channel genes in neurons/networks, these investigators aimed to decipher the underlying variability in cellular and synaptic parameters 10–12. It is now a broadly accepted principle in neuroscience that such parameters often vary widely among individuals, despite similar functional neuronal and network activity. This exploration of biophysical variability has served as impetus for a growing interest in individual variation in network activity and behavior, as neuroscientist have begun to explore how ‘similar’ is ‘similar network activity’13. The database approach of Prinz et al. led to the concept of ensemble modeling, where a number of equivalent model instances are analyzed to assess how network functions. The concepts of individual variation and ensemble modeling are now embedded in the fabric of the NIH Brain Initiative. In addition, these advances had implications in the related area of homeostatic regulation. Here, the developments helped change the focus from the neuron as a monitor of network activity that simply helps calibrate overall activity levels, to a more refined assessment of circuit performance. These studies further supported the notions that not all (indeed if any) parameters need to be genetically specified and that correlations in conductance parameters, rather than just individual and independent parameters, would emerge as the target of homeostatic regulation14. The hunt for such correlations is now one of the key areas of investigation in trying to understand the fundamental principles homeostatic regulation, a property crucial to the remarkable ability of neural circuits to cope with environmental changes, perturbation and injury15.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.LeMasson G, et al. Activity-dependent regulation of conductances in model neurons. Science. 1993;259:1915–1917. doi: 10.1126/science.8456317. [DOI] [PubMed] [Google Scholar]
- 2.Marder E, Prinz AA. Modeling stability in neuron and network function: the role of activity in homeostasis. Bioessays. 2002;24:1145–1154. doi: 10.1002/bies.10185. [DOI] [PubMed] [Google Scholar]
- 3.Turrigiano GG, et al. Selective regulation of current densities underlies spontaneous changes in the activity of cultured neurons. J Neurosci. 1995;15:3640–3652. doi: 10.1523/JNEUROSCI.15-05-03640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golowasch J, et al. Activity-dependent regulation of potassium currents in an identified neuron of the stomatogastric ganglion of the crab Cancer borealis. J Neurosci. 1999;19:RC33. doi: 10.1523/JNEUROSCI.19-20-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacLean JN, et al. Activity-independent homeostasis in rhythmically active neurons. Neuron. 2003;37:109–120. doi: 10.1016/s0896-6273(02)01104-2. [DOI] [PubMed] [Google Scholar]
- 6.Golowasch J, et al. Failure of averaging in the construction of a conductance-based neuron model. J Neurophysiol. 2002;87:1129–1131. doi: 10.1152/jn.00412.2001. [DOI] [PubMed] [Google Scholar]
- 7.Goldman MS, et al. Global structure, robustness, and modulation of neuronal models. J Neurosci. 2001;21:5229–5238. doi: 10.1523/JNEUROSCI.21-14-05229.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prinz AA, et al. Alternative to hand-tuning conductance-based models: construction and analysis of databases of model neurons. J Neurophysiol. 2003;90:3998–4015. doi: 10.1152/jn.00641.2003. [DOI] [PubMed] [Google Scholar]
- 9.Prinz AA, et al. Similar network activity from disparate circuit parameters. Nat Neurosci. 2004;7:1345–1352. doi: 10.1038/nn1352. [DOI] [PubMed] [Google Scholar]
- 10.Goaillard JM. Functional consequences of animal-to-animal variation in circuit parameters. Nat Neurosci. 2009;12:1424–1430. doi: 10.1038/nn.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulz DJ, et al. Quantitative expression profiling of identified neurons reveals cell-specific constraints on highly variable levels of gene expression. Proc Natl Acad Sci U S A. 2007;104:3187–13191. doi: 10.1073/pnas.0705827104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amendola J, et al. Ca2+/cAMP-sensitive covariation of I(A) and I(H) voltage dependences tunes rebound firing in dopaminergic neurons. J Neurosci. 2012;32:2166–2181. doi: 10.1523/JNEUROSCI.5297-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wenning A, et al. Output variability across animals and levels in a motor system. Elife. 2018;7 doi: 10.7554/eLife.31123. pii: e31123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Leary T, et al. Correlations in ion channel expression emerge from homeostatic tuning rules. Proc Natl Acad Sci U S A. 2013;110:2645–2654. doi: 10.1073/pnas.1309966110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz DJ, Lane BJ. Homeostatic plasticity of excitability in crustacean central pattern generator networks. Curr Opin Neurobiol. 2017;43:7–14. doi: 10.1016/j.conb.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]