Abstract
Minor changes (~0.1 m/s) in human gait speed are predictive of various measures of decline and can be used to identify at-risk individuals prior to further decline. These associations are possible due to an abundance of human clinical research. However, age-related gait changes are not well defined in rodents, even though rodents are used as the primary pre-clinical model for many disease states as well as aging research. Our study investigated the usefulness of a novel automated system, the CatWalk™ XT, to measure age-related differences in gait. Furthermore, age-related functional declines have been associated with decreases in the reduced to oxidized glutathione ratio leading to a pro-oxidizing cellular shift. Therefore the secondary aim of this study was to determine whether chronic glutathione deficiency led to exacerbated age-associated impairments. Groups of male and female wild-type (gclm+/+) and knock-out (gclm-/-) mice aged 4, 10 and 17 months were tested on the CatWalk and gait measurements recorded. Similar age-related declines in all measures of gait were observed in both males and females, and chronic glutathione depletion was associated with some delays in age-related declines, which were further exacerbated. In conclusion, the CatWalk is a useful tool to assess gait changes with age, and further studies will be required to identify the potential compensating mechanisms underlying the effects observed with the chronic glutathione depletion.
Keywords: Aging, glutathione deficiency, gait, speed, catwalk
Aging is associated with a decline in overall motor function across various species, including rodents and humans [1-4]. Interestingly, human gait speed can be utilized to stratify risk of decline in cognitive function, mobility, loss of independence, increased fall risk, institutionalization, as well as overall mortality risk [5]. This association of gait speed with many dimensions of aging has been found consistently in humans, where minor changes (~0.1 m/s) in gait speed were predictive of various measures of decline and can be used to identify at-risk individuals prior to further decline [6]. While human gait research is abundant, age-related gait changes in rodents are not well defined even though they are used as the primary pre-clinical model for many disease states and aging research [7].
Although rodent gait changes as a result of aging are not as well characterized as changes in human gait, research on other aspects of motor function in rodents has shown age-related decreases in spontaneous movement speed [2, 3, 8], physical activity [9, 10], as well as balance and motor coordination [2]. Importantly, both human and mouse functional motor decline follow similar patterns, where motor function decline occurs earlier in life than other more debilitating outcomes, highlighting the importance of motor function as both a clinical and preclinical measure [9, 11]. Indirect measures of gait speed through open field [12] or balance beam testing [13] hinted at age-related slowing of gait speed, but with methodological limitations as gait analysis was not the primary measure and no other gait variables were recorded. Direct studies of rodent gait via treadmill analysis at a set speed have shown age-related changes, however studies in mice and humans have demonstrated that treadmill and free gait parameters are not equivalent [14, 15]. Furthermore, most gait analyses in rodents have focused on changes across the first 12 months of life, which equates to adult growth along with aging. This presents an opportunity to determine quantitative relationships between gait changes and age across the lifespan of free-running rodents. A relatively novel way to measure gait is to use the CatWalk™ XT, an automated system using a sophisticated software allowing rodents to walk in a low-stress environment allowing gait analyses in a low-stress environment. Thus far, the CatWalk has primarily been validated in modeled disease states including traumatic brain injury, stroke, spinal cord injury, Parkinson’s disease, osteoarthritis, and ataxia [16-20]. However, to date there have been no studies on the effect of age in mice on the qualitative and quantitative gait outcomes using this apparatus.
Additionally, the redox stress theory of aging postulates that while a moderate level of reactive oxygen species (ROS) is biologically useful for cell signaling, an overabundance of ROS can lead to accumulation of oxidative damage and a pro-oxidizing shift in reduction-oxidation (redox) state preceding cellular dysfunction [21, 22]. This pro-oxidant shift in aging is primarily measured via changes in the ratio of reduced to oxidized glutathione (GSH:GSSG), where aging leads to a decline in the GSH:GSSG ratio indicating less redox potential [23]. Although several redox couples interact with GSH to maintain overall cellular redox state, the glutathione redox couple (GSH:GSSG) is the most abundant, and as such modulation of redox status can be achieved by altering levels of GSH [24]. Synthesis of GSH occurs in two subsequent enzymatic reactions, formation of y-glutamylcysteine (y-GC) from glutamate and cysteine via glutamate cysteine ligase followed by conversion of y-GC to GSH via GSH synthetase [25]. The synthesis of GSH is rate-limited by the glutamate cysteine ligase (gcl) enzyme, a heterodimer consisting of a catalytic (gclc) and modifier (gclm) subunit [25]. While gclc contains all catalytic capacity, gclm increases the Vmax and the affinity for glutamate, and decreases feedback inhibition from GSH [26]. While homozygous knockout of gclc is embryonic lethal, global knockout of the gcl modifier subunit (gclm-/-) in mice leads to a 70-90% decrease in GSH levels across various tissues, including liver, brain, kidney, and lung [27]. Adult gclm-/- mice present with chronically decreased levels of GSH, are more susceptible to oxidative insults [28], and have a more pro-oxidative redox cellular environment [27]. These changes in gclm-/- mice could lead to an early aging phenotype if a more oxidative redox state is in fact a determinant factor in aging.
Accordingly, the objectives of this study were to validate the Catwalk™ XT in assessment of age-related gait changes and determine if chronic glutathione depletion exacerbated these gait variations. To achieve the goals, male and female gclm-/- and gclm+/+ mice were tested on the Catwalk at 3 different target ages (4, 10 and 17 months). The operational hypothesis was that gait measures would decline with advanced age and that impairments would occur earlier in glutathione depleted mice.
MATERIALS AND METHODS
Animals
Procedures were approved by the Institutional Animal Care and Use Committee at the University of North Texas Health Science Center at Fort Worth, and adhered to NIH guidelines. The mice heterozygous for gclm were generated on a C57BL/6J (B6.129) background, acquired from Dr. Terence Kavanaugh, rederived and backcrossed at least 7 generations into C57BL/6 mice by Jackson Laboratories. Once in the UNT Health Science Center vivarium, triads of gclm+/- were mated to obtain wild-type (gclm+/+) and knock-out (gclm-/-) littermates. From the in-house breeding colony, male and female mice were housed and aged in groups of 2-4, separated according to sex and genotype, in standard polycarbonate cages (28 x 17 x 12.5 cm) with corncob bedding and ad libitum access to water and standard rodent chow (LabDiet® R&M 5LG6 5S84; catalog number: 1813505 from TestDiet, Richmond, IN), and were maintained at ambient temperature (23 ± 1° C), under a 12-h light/dark cycle starting at 0600. At the target ages of 4, 10, and 17 months for this study, squads of mice from the aging colony were used for gait analyses (Table 1) in a cross-sectional design.
Table 1.
Number of animals per group according to genotype and age
| Males | Females | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age (months) | 4 | 10 | 17 | 4 | 10 | 17 | 4 | 10 | 17 |
| Gclm+/+ | 15 | 15 | 15 | 13 | 15 | 9 | 28 | 30 | 24 |
| Gclm-/- | 15 | 15 | 8 | 14 | 12 | 12 | 29 | 27 | 20 |
Quantitative gait analyses
Gait measures were determined using the CatWalk™ XT system (Noldus Information Technology, The Netherlands). The CatWalk is an apparatus used for semi-automated objective rodent gait analysis via video recording. Importantly, this system allows for the animals to voluntarily move at preferred speeds in a similar fashion to clinical gait testing in humans. The device consists of (i) a 130 cm long hardened glass platform with an adjustable alleyway to limit movements to straight lines, (ii) a red overhead light, (iii) a green LED light attached to the glass platform, and (iv) a high speed color camera mounted below the platform. The green LED light attached to the apparatus emits light into the glass plate, and this light is only refracted wherever rodent paws contact the glass, allowing the high speed digital camera to capture precise rodent paw placement in real time. The overhead red light creates contrast for recording of the body outline. The visual data is digitized and transferred to an attached computer where the CatWalk™ XT software can be used for semi-automated labeling and analysis of static and dynamic gait kinematics via distance, time, and intensity differences between paw prints. Gait data can then be exported for data storage and subsequent analyses.
The alley was adjusted to be 8 cm wide, and the walkway for data recoding was defined at 8 cm x 32 cm which allowed 4 full step cycles in the center of the alley. Visual scaling was calibrated prior to each use. On testing days, animals are transferred from their home cages into a polycarbonate carrier and brought into the pitch black room to acclimate for 10 minutes prior to testing. A single animal was placed onto the platform and allowed to cross the defined walkway up to 20 times. Each crossing of the platform is called a run and non-compliant runs were defined as more than 60% speed variation within a run or longer than 5 seconds. From the pool of compliant runs, only runs with less than 10% speed variation between runs were used for further analyses. The mice were not given a formal training to the apparatus, and if they stopped mid-run and/or required auditory stimulation to move, that run was not included in the final analyses as it did not reach our a-priori set criterion for compliant runs. We selected five variables that are similar in humans and relevant to human aging (Table 2), including gait speed, base of support, stride length, swing speed, and step cycle duration. All measures besides gait speed were analyzed separately for front and hind paws.
Table 2.
Gait variable definitions
| Gait speed | Rate of body movement in cm/s |
|---|---|
| Base of support | Width between the two front or two hind paws in cm |
| Stride Length | Distance between subsequent placements of the same paw for the two front or two hind paws in cm |
| Swing Speed | Rate of movement of a paw during the swing phase for the two front or two hind paws in cm/s |
| Step Cycle Duration | Time to go through both the stand and swing phases for the two front or two hind paws in s |
Statistical Analyses
A three-way analysis of covariance (ANCOVA) was run with Sex, Genotype and Age as between-group factors and Body Weight as the covariate to ensure that body weights were not responsible for driving the main effects. Body weights and the various gait measures were compared using three-way analyses of variance (ANOVA) with Sex, Genotype and Age as between-group factors. Following significance of either a main effect or interaction, individual comparisons between different Sex, Genotype, or Age were performed using a single degree-of-freedom F test involving the error term from the overall ANOVA. The individual relationships between various gait measures with gait speed were determined via Pearson’s or logarithmic correlations. The α level was set at 0.05 for all analyses. The software used for the analyses was Systat 13 (Systat Software Inc., San Jose, CA, USA).
RESULTS
Body Weight
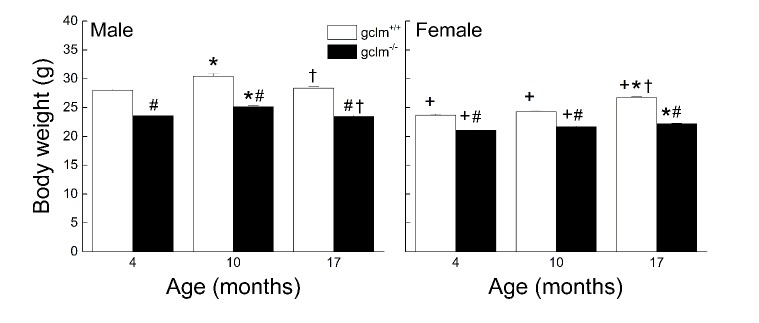
The effects of sex, age and genotype on the body weights are presented in Fig. 1. Overall, the males weighed more than the females regardless of genotype (13%, 17% and 5% difference in young, adult, and old respectively). The gclm-/- weighed less than the gclm+/+ regardless of age (11-17% difference). The weight difference was more pronounced between 4 and 10 months in males, and between 10 and 17 months in females. A three-way ANOVA yielded a significant interaction between Sex, Age and Genotype supporting these observations (p = 0.015).
Figure 1. Effects of age, sex and genotype on body weights (g) in young (4 month), adult (10 month), and old (17 month) gclm+/+ and gclm-/- mice.
Each value represents the mean + SEM. + p<0.05 compared to age and genotype-matched males; *p < 0.05 compared to genotype-matched young; †p<0.05 adult compared to genotype-matched old; #p < 0.05 compared to age-matched gclm+/+.
Gait Speed
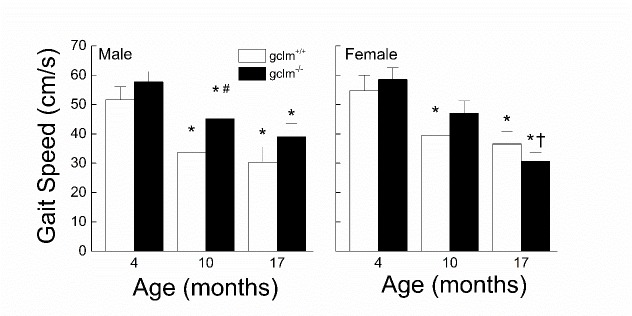
The effects of sex, age and genotype on the gait speed are presented in Fig. 2. There was no major difference in speed between males and females at any age. While the gait speed in both the gclm+/+ and gclm-/- mice declined with age (gclm+/+: 41% in males, 33% in females; gclm-/-: 32% in males, 48% in females), the rate of decline was different between the two genotypes. In the gclm+/+ mice, the majority of the age-related decline in speed occurred between 4 and 10 months (35% for males, 28% for females), whereas in the gclm-/- mice the decline was more gradual between 4 and 10 months and between 10 and 17 months. However, while males and females gclm-/- declined similarly between 4 and 10 months (~21%), the decrease in speed between 10 and 17 months was more than double in the females (35%) than in the males (14%). A three-way ANOVA revealed significant main effects of Age (p < 0.001) and Genotype (p = 0.032), but there was no main effect of Sex or interactions between Sex, Age and Genotype (all ps ≧ 0.162).
Figure 2. Effects of sex, age and genotype on gait speed (cm/s) in young (4 month), adult (10 month), and old (17 month) gclm+/+ and gclm-/- mice.
Each value represents the mean + SEM. *p < 0.05 compared to genotype-matched young; †p<0.05 adult compared to genotype-matched old; #p < 0.05 compared to age-matched gclm+/+.
Base of Support
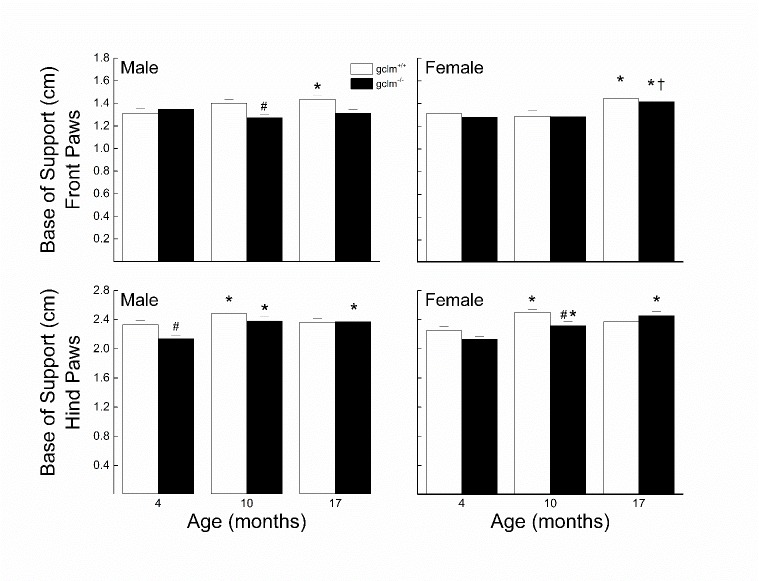
The effects of sex, age and genotype on the front and hind paw base of support are presented in Fig. 3. In males, the front and hind paws base of support widened by 10% in gclm+/+, which occurred mostly between 4 and 10 months. The hind paws base of support for the gclm-/- was widened by 11% by 10 months along with a narrowing of 6% for the front paws base of support. Additionally, the base of support was narrower by 9% in the gclm-/- at 10 and 17 months for the front paws and by 8% at 4 months for the hind paws. In gclm+/+ females, there was a widening of the base of support for the front paws by 10% which occurred between 10 and 17 months, while the 11% widening of the base of support for the hind paws occurred between 4 and 10 months, followed by a small narrowing of 5% between 10 and 17 months. The widening of the front paws base of support for the gclm-/- was similar to that of the gclm+/+, while there was a gradual widening of the hind paws base of support for the gclm-/-. The hind paws base of support was about 6% narrower in the gclm-/- than the gclm+/+ at 4 and 10 months. For the front paws base of support, a three-way ANOVA revealed a significant main effect of Age (p = 0.01), but there was no main effect of Genotype (p = 0.084) or any interaction between Sex, Age and Genotype (all ps≧ 0.224). For the hind paws base of support, a three-way ANOVA revealed significant main effects of Age (p < 0.001) and Genotype (p = 0.011) and interaction of Age x Genotype (p = 0.028).
Figure 3. Effects of sex, age and genotype on width of the front and hind paw base of support (cm) in young (4 month), adult (10 month), and old (17 month) gclm+/+ and gclm-/- mice.
Each value represents the mean + SEM. *p < 0.05 compared to genotype-matched young; †p<0.05 adult compared to genotype-matched old; #p < 0.05 compared to age-matched gclm+/+.
Stride Length
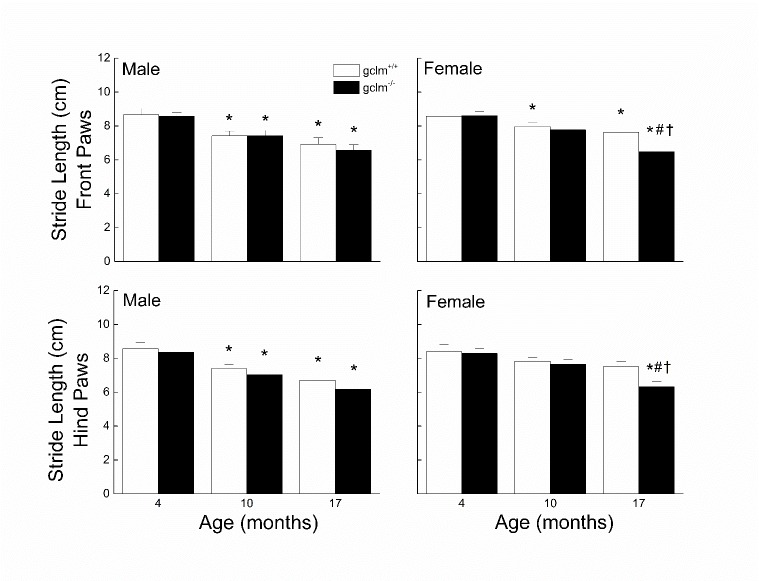
The effects of sex, age and genotype on the front and hind paw stride length are presented in Fig. 4. In males, the front and hind paws stride length in both gclm+/+ and gclm-/- mice decreased by 14% at 10 months, and further decreased by another 7-12% at 17 months. There was no difference between the gclm+/+ and gclm-/- mice. In females, the gclm+/+ mice exhibited a gradual decrease in stride length reaching 10% for both front and hind paws. While the shortening of the stride was similar in both genotypes between 4 and 10 months, it was 4 times higher between 10 and 17 months in the gclm-/- compared to the gclm+/+. At 17 months, the female gclm-/- exhibited stride length for either paws 15% shorter than that of the age-matched gclm+/+. For the front paws, a three-way ANOVA revealed a significant main effect of Age (p < 0.001) but there was no main effect of Genotype (p = 0.125) or Sex (p = 0.201) or any interactions between Sex, Age and Genotype (all ps ≧ 0.265). For the hind paws, a three-way ANOVA revealed significant main effects of Age (p < 0.001) and Genotype (p = 0.02), but there was no main effect of Sex (p = 0.112) or interaction of Sex, Age and Genotype (all ps ≧ 0.237).
Figure 4. Effects of sex, age and genotype on the front and hind paw stride length (cm) in young (4 month), adult (10 month), and old (17 month) gclm+/+ and gclm-/- mice.
Each value represents the mean + SEM. *p < 0.05 compared to genotype-matched young; †p<0.05 adult compared to genotype-matched old; #p < 0.05 compared to age-matched gclm+/+.
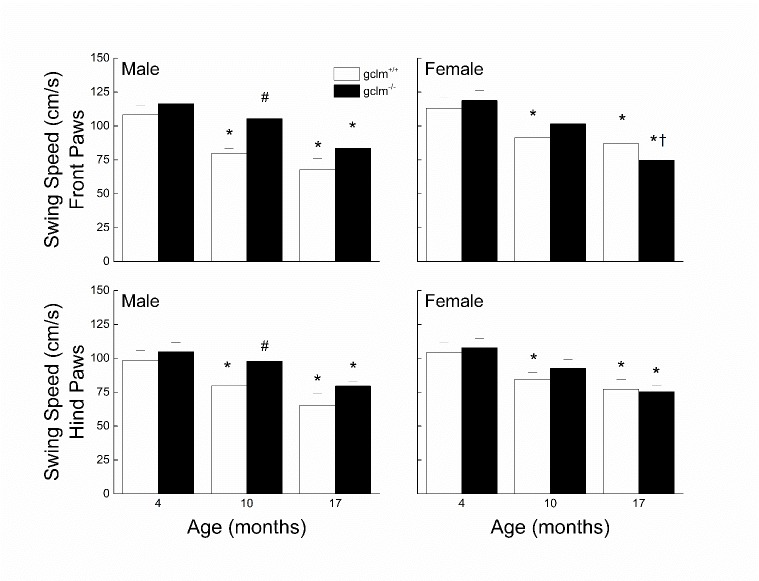
Swing Speed
The effects of sex, age and genotype on the front and hind paw swing speed are presented in Fig. 5. In males, the swing speed for both hind and front paws declined by ~36% in glcm+/+ and by 26% in gclm-/-. While the decline was gradual in the gclm+/+, most of the decrease occurred between 10 and 17 months in the gclm-/-. The swim speed of the gclm-/- was higher than that of the gclm+/+ at 10 (front paws: 32%; hind paws: 22%) and 17 months (~22% for both front and hind paws), though it was only statistically significant at 10 months. In females, the swing speed for both hind and front paws declined by ~25% in glcm+/+ and by ~34% in gclm-/-. Contrary to the males, the decline was gradual in the gclm-/- while most of the decrease occurred between 4 and 10 months in the gclm+/+. For both front and hind paws, three-way ANOVAs revealed significant main effects of Age (all ps < 0.001) and Genotype (all ps ≤ 0.035), however there was no main effect of Sex (all ps > 0.305) or interaction of Sex, Age and Genotype (All ps ≥ 0.064).
Figure 5. Effects of sex, age and genotype on the front and hind paw swing speed (cm/s) in young (4 month), adult (10 month), and old (17 month) gclm+/+ and gclm-/- mice.
Each value represents the mean + SEM. *p < 0.05 compared to genotype-matched young; †p<0.05 adult compared to genotype-matched old; #p < 0.05 compared to age-matched gclm+/+.
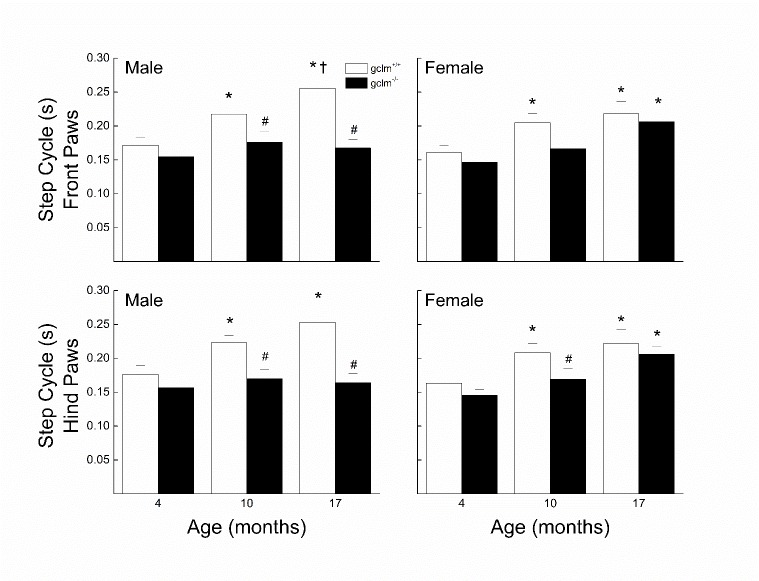
Step Cycle Duration
The effects of sex, age and genotype on the front and hind paw step cycle duration are presented in Fig. 6. In males, step cycle duration for both front and hind paws increased with age in the gclm+/+ by ~ 45% while it remained largely unaffected in the gclm-/-. The step cycle duration of the gclm-/- was similar to that of the gclm+/+ at 4 months, but was 20% and 34% lower at 10 and 17 months, respectively. In females, both genotypes exhibited an increase in step cycle duration for both front and hind paws by 36-40%. The observed deficit was fairly gradual in the gclm-/- but occurred mostly between 4 and 10 months in the gclm+/+. At 10 months, the gclm-/- had a step cycle duration 18% shorter than that of the gclm+/+. Three-way ANOVAs for front and hind paws step cycle duration revealed significant main effects of Age (all ps < 0.001) and Genotype (all ps < 0.001), but there was no interaction of Sex, Age and Genotype (all ps ≥ 0.19) even though Sex x Genotype approached significance (p = 0.064 (front paws), p= 0.088 (hind paws)).
Figure 6. Effects of sex, age and genotype on the front and hind paw step cycle duration (s) in young (4 month), adult (10 month), and old (17 month) gclm+/+ and gclm-/- mice.
Each value represents the mean + SEM. *p < 0.05 compared to genotype-matched young; †p<0.05 adult compared to genotype-matched old; #p < 0.05 compared to age-matched gclm+/+.
Relationship of gait kinematics to gait speed
The relationships of gait speed with front and hind base of support, stride length, step cycle duration, and swing speed are presented in Fig. 7 (since there were no differences between males and females, these analyses use the combined dataset). Front paw and hind paw stride length and swing speed increased as a function of gait speed. Pearson’s correlation revealed significant relationships with gait speed for front paw (r = 0.86, p < 0.01) and hind paw stride length (r = 0.83, p < 0.01), as well as for front paw (r = 0.95, p < 0.01) and hind paw (r = 0.95, p < 0.01) swing speed. Front paw and hind paw base of support decreased as a function of gait speed. Pearson’s correlations revealed significant linear relationships of both front paw (r = -0.31, p < 0.01) and hind paw (r = -0.35, p < 0.01) base of support with gait speed. Front paw and hind paw step cycle duration decreased as a curvilinear function of gait speed. Polynomial regression revealed significant curvilinear relationships of both front paw (r2 = 0.88, p < 0.01) and hind paw (r2 = 0.87, p < 0.01) step cycle duration with gait speed.
Figure 7. Relationship of speed with base of support, stride length, step cycle duration, and swing speed in the front and hind paws of young (4 month), adult (10 month), and old (17 month) gclm+/+ and gclm-/- male and female mice.
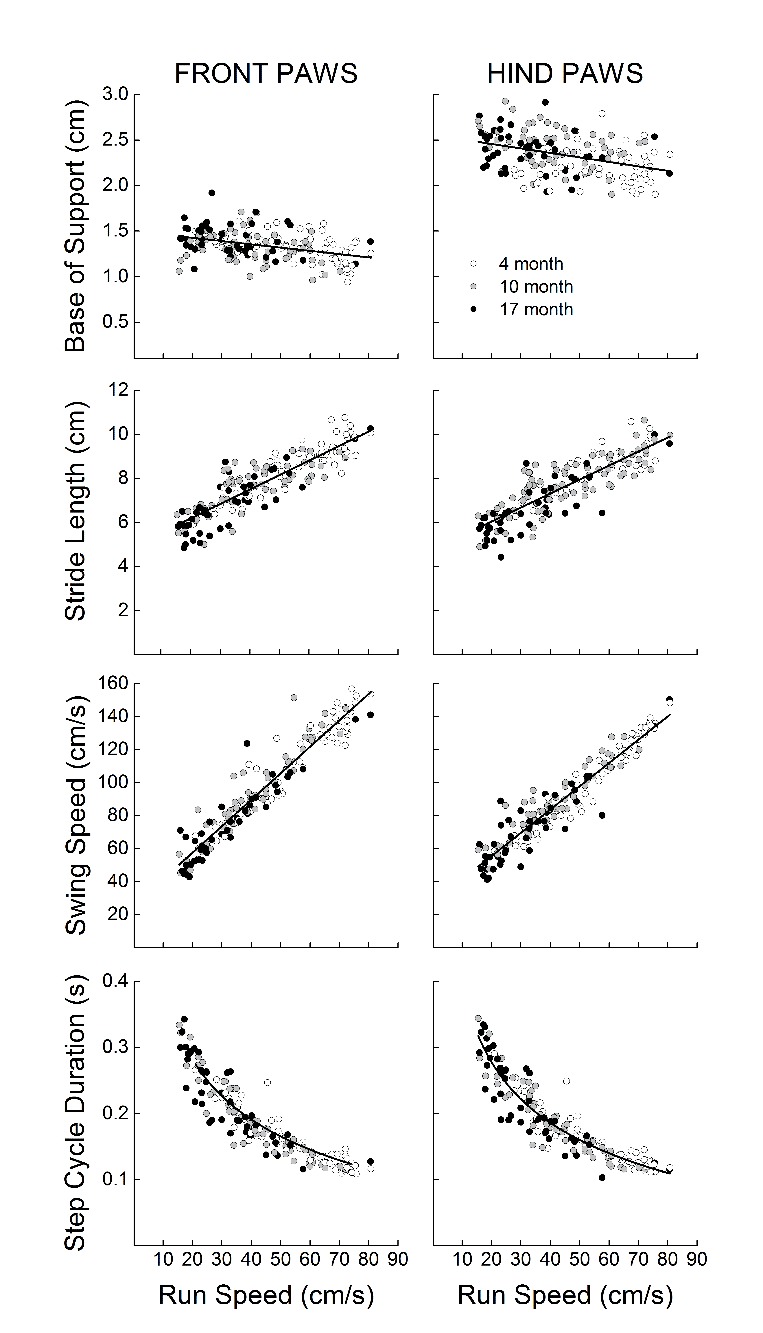
Each value represents a single animal.
DISCUSSION
The main findings of this study were that (1) aging is associated with a slowing of gait speed, swing speed, and step cycle duration, a widening of base of support, and a shortening of the stride length, (2) glutathione deficiency delayed some of these age-related declines, with the delays being more pronounced in males, and (3) a strong relationship between all the gait variables and gait speed exists.
While CatWalk measured gait changes have been used to determine or predict function in rodent disease models such as spinal cord and brain injury, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, and arthritic damage [16-20], there remains a lack of research on quantitative gait changes as a result of aging alone. The CatWalk is a novel tool in preclinical aging research, allowing for robust quantitative measurement of rodent gait and analysis of changes across the lifespan. Most studies of aged mice have utilized measures of gait such as gait speed and function in the open field [12] or treadmill-based systems that require animals to move at set speeds [29]. While these techniques have been previously published, each has its own limitations, such as manual scoring of weight distribution, gait coordination, or stepping in the open field which leads to substantial inter and intra-observer variability, set speed of the treadmill which artificially mandates a gait speed rather than allowing the animals to move at their preferred gait speed, and ink smearing for painted foot print analysis which makes quantification difficult [16], and all lack gait kinematics analyses.
While treadmill-based gait analyses objectively quantify the same variables as the CatWalk, gait results are not equivalent between free movement and treadmill-based systems as there are known differences in gait mechanics used for the respective apparatus [14, 15]. Furthermore, Wooley et al. have demonstrated that the primary factor affecting gait results on the treadmill in mice is body size [4]. They later performed a predictive regression analysis based on body size (weight) for animals aged 1 - 12 months, however at 18 months animals did not fit into this trend, showing changes opposite the expected outcomes due to a decrease in body size. These results indicate that changes observed at 18 months are likely not dependent on body size changes and rather hint at age-specific gait changes [29]. Therefore at a set speed, which is the procedure for treadmill-based measurements, larger mice will have changes in various gait measures as they physically have longer legs and wider bodies. To determine whether body weight was a contributing factor to the age-related changes in gait in our study, we ran an ANCOVA on each measure with body weight as the covariate and all age effects remained indicating that body weight was not a confounding factor in our analyses of gait (all ps > 0.1 for age effect). Overall, the CatWalk method allows for freely-moving animals to move at their preferred speed, objectively measures many gait variables within individual runs, allows for instantaneous data analysis through digitization, thereby improving preclinical measurements of gait.
Age-related deficits in movement speed, stride length, base of support, step cycle duration and swing speed were observed at both 10 and 17 months. The gradual slowing and alteration in kinematic gait variables in the aged mice is similar to that observed with aging in humans, primarily a decline in gait speed, shortening of stride length, and a wider base of support [30]. These strategies that the elderly use are to reduce fall risk by limiting vertical center of mass displacement and increasing dynamic stability, leading to an overall less destabilizing gait pattern [31-34]. Static and dynamic balance is dependent upon the vestibular, visual and somatosensory systems as well as overall strength, and these systems have been shown to decline with age in both humans [35-37] and rodents [2, 38-41]. As a result of these changes with age, both rodents and humans employ similar adaptive strategies in gait speed and kinematics. The similarities in age-related declines in balance and gait adaptation between the two species strengthen the adequacy of the use of rodents for pre-clinical assessments of gait during aging.
In addition to the novel use of the CatWalk™ XT to examine age-related gait changes in mice, we wanted to examine potential effects of redox impairment on gait measurements. Of note, gclm-/- mice exhibited age-related decline across most variables, but the age-related impairments seemed to be delayed compared to the gclm+/+ mice. However, the results of the ANCOVA determined that body weight was a confounding factor in the genotype differences we observed, as including weight as a covariate did not yield any significant main effects of Genotype. It is noteworthy that in the gclm-/- mice step cycle duration was largely unaffected by age and swing speed declines were delayed after 10 months. While our data confirmed previous studies done in 3-5 months old mice that reported no declines in motor function of gclm-/- mice [42, 43], literature evidence of effects in the old mice is non-existent. Previous studies have demonstrated that increased oxidative damage in the cerebellum with age was correlated with a worsening of motor function and balance [44]. Furthermore, acute GSH deficiency leads to impaired motor function and balance in young rodents, worsens motor neuron decline in disease models, and disrupts redox signaling in young and old rodents [45-47]. Based on the literature and our hypothesis, we expected that chronic GSH deficiency across the lifespan would lead to an accelerated pattern of functional declines, including gait measures, however, our results do not support our original hypothesis as the gclm-/- mice exhibited age-related declines similar to gclm+/+. It is possible that the endogenous antioxidant defense or beneficial redox signaling pathways have compensated for the large deficiency in GSH and concurrent shift in redox state, and further research is warranted to pursue this hypothesis.
Sex has been implicated as a variable of interest due to potential sexual dimorphism in gait mechanics [4, 29]. Our results do not support sex differences in gait, as none of our variables had main effects or interactions with sex (all p values > 0.112), which remained even once body weights were used as a covariate. Several other studies that examined both sexes have also reported no sex differences in CatWalk-tested mice [48] or ink-tested freely-moving mice [49]. Other studies have simply combined sexes without explicit statistical justification [50]. Alternatively, sex differences in some gait measures were observed in studies using treadmill-based systems [4, 29], and could be related to differences in body size at a set speed. Additionally, sex-dependent responses in motor and gait decline in transgenic mouse models of ALS have been observed [29, 51], suggesting a differential response to both redox state and disease. There are few studies examining age-related functional decline that report the use of both sexes, but those that do have combined sexes or noted a lack of sexual dimorphism in these aged but otherwise healthy animals [52, 53], although one group examining overall frailty rather than motor function reported sex differences in their very old cohort (28 months) [54]. Including both sexes when collecting mouse data across many aspects of functional testing is generally lacking, and warrants further research to determine if both sexes age differentially in motor function and gait.
Previous studies have determined that more than 90% of the 162 gait variables measured via the CatWalk are dependent on speed [55], which we confirmed for our variables through correlation and regression testing (Fig. 7). In the context of a disease model at a set age, it is important to not confound test results that are dependent on the disease state with changes simply due to speed variance between animals. In our case, we were primarily interested in the age-related slowing of gait, and as such we did not seek to control for speed between animals [55]. By allowing speed variation we were able to capture the natural age-related slowing across the lifespan, which would otherwise be lost in a treadmill-based system. Importantly, the gait signature in relation to speed is similar between mice and humans, and hind paw gait measures have been used to compare gait dysfunction between mice and humans [56]. Additionally, the normal and maximal walking speeds both decline with age in humans [57], and this gait speed decline has previously been correlated with a shorter life expectancy [6].
Conclusion
The associative nature of gait speed with overall health that is found in aging humans could be found to a similar extent in rodents, allowing for an additional functional outcome measure in preclinical aging studies or similar predictions of mortality, disability, or disease used in humans to be used in future mice models. Our study determined that the CatWalk is a useful tool in aging research, allowing for robust quantitative measurement of rodent gait, and analysis of changes across the lifespan. Overall, mice appear to undergo similar changes to humans, namely a “slowing” of gait with concurrent changes in gait measures. Interestingly, chronic glutathione deficiency had no detrimental effects but rather partially and mildly delayed some age-related deficits. These results warrant further examination to determine if alternative redox compensation is occurring, but the current data appears to not support the redox stress theory of aging.
Acknowledgements
We would like to thank Dr. Kavanaugh for the gclm+/- mice that we used to begin our breeding colony. This work was supported by the National Institutes of Health/National Institute on Aging (P01 AG027956; P01 AG022550; T32 AG020494).
Contributor Information
J. Thomas Mock, Department of Pharmacology & Neuroscience, Center for Neuroscience Discovery, Institute for Healthy Aging, University of North Texas Health Science Center at Fort Worth, Fort Worth, TX, 76107 USA..
Sherilynn G Knight, Department of Pharmacology & Neuroscience, Center for Neuroscience Discovery, Institute for Healthy Aging, University of North Texas Health Science Center at Fort Worth, Fort Worth, TX, 76107 USA..
Philip H Vann, Department of Pharmacology & Neuroscience, Center for Neuroscience Discovery, Institute for Healthy Aging, University of North Texas Health Science Center at Fort Worth, Fort Worth, TX, 76107 USA..
Jessica M Wong, Department of Pharmacology & Neuroscience, Center for Neuroscience Discovery, Institute for Healthy Aging, University of North Texas Health Science Center at Fort Worth, Fort Worth, TX, 76107 USA..
Delaney L Davis, Department of Pharmacology & Neuroscience, Center for Neuroscience Discovery, Institute for Healthy Aging, University of North Texas Health Science Center at Fort Worth, Fort Worth, TX, 76107 USA..
Michael J Forster, Department of Pharmacology & Neuroscience, Center for Neuroscience Discovery, Institute for Healthy Aging, University of North Texas Health Science Center at Fort Worth, Fort Worth, TX, 76107 USA..
Nathalie Sumien, Department of Pharmacology & Neuroscience, Center for Neuroscience Discovery, Institute for Healthy Aging, University of North Texas Health Science Center at Fort Worth, Fort Worth, TX, 76107 USA..
References
- [1].Rosso AL, Studenski SA, Chen WG, Aizenstein HJ, Alexander NB, Bennett DA, et al. (2013). Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci, 68: 1379-1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sumien N, Sims MN, Taylor HJ, Forster MJ (2006). Profiling psychomotor and cognitive aging in four-way cross mice. Age (Dordr), 28: 265-282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hebert MA, Gerhardt GA (1998). Normal and drug-induced locomotor behavior in aging: comparison to evoked DA release and tissue content in fischer 344 rats. Brain Res, 797: 42-54 [DOI] [PubMed] [Google Scholar]
- [4].Wooley CM, Xing S, Burgess RW, Cox GA, Seburn KL (2009). Age, experience and genetic background influence treadmill walking in mice. Physiol Behav, 96: 350-361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, et al. (2009). Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. The journal of nutrition, health & aging, 13: 881-889 [DOI] [PubMed] [Google Scholar]
- [6].Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. (2011). Gait speed and survival in older adults. JAMA : the journal of the American Medical Association, 305: 50-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Denayer T, Stöhr T, Van Roy M (2014). Animal models in translational medicine: Validation and prediction. New Horizons in Translational Medicine, 2: 5-11 [Google Scholar]
- [8].Skalicky M, Bubna-Littitz H, Viidik A (1996). Influence of physical exercise on aging rats: I. Life-long exercise preserves patterns of spontaneous activity. Mech Ageing Dev, 87: 127-139 [DOI] [PubMed] [Google Scholar]
- [9].Ingram DK (2000). Age-related decline in physical activity: generalization to nonhumans. Medicine and science in sports and exercise, 32: 1623-1629 [DOI] [PubMed] [Google Scholar]
- [10].Hamrick MW, Ding KH, Pennington C, Chao YJ, Wu YD, Howard B, et al. (2006). Age-related loss of muscle mass and bone strength in mice is associated with a decline in physical activity and serum leptin. Bone, 39: 845-853 [DOI] [PubMed] [Google Scholar]
- [11].Hamrick MW, Ding K-H, Pennington C, Chao YJ, Wu Y-D, Howard B, et al. (2006). Age-related loss of muscle mass and bone strength in mice is associated with a decline in physical activity and serum leptin. Bone, 39: 845-853 [DOI] [PubMed] [Google Scholar]
- [12].Justice JN, Carter CS, Beck HJ, Gioscia-Ryan RA, McQueen M, Enoka RM, et al. (2014). Battery of behavioral tests in mice that models age-associated changes in human motor function. Age (Dordr), 36: 583-592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fahlstrom A, Yu Q, Ulfhake B (2011). Behavioral changes in aging female C57BL/6 mice. Neurobiology of aging, 32: 1868-1880 [DOI] [PubMed] [Google Scholar]
- [14].Herbin M, Hackert R, Gasc JP, Renous S (2007). Gait parameters of treadmill versus overground locomotion in mouse. Behavioural brain research, 181: 173-179 [DOI] [PubMed] [Google Scholar]
- [15].Elliott BC, Blanksby BA (1976). A cinematographic analysis of overground and treadmill running by males and females. Med Sci Sports, 8: 84-87 [PubMed] [Google Scholar]
- [16].Hamers FP, Koopmans GC, Joosten EA (2006). CatWalk-assisted gait analysis in the assessment of spinal cord injury. J Neurotrauma, 23: 537-548 [DOI] [PubMed] [Google Scholar]
- [17].Neumann M, Wang Y, Kim S, Hong SM, Jeng L, Bilgen M, et al. (2009). Assessing gait impairment following experimental traumatic brain injury in mice. Journal of neuroscience methods, 176: 34-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vandeputte C, Taymans JM, Casteels C, Coun F, Ni Y, Van Laere K, et al. (2010). Automated quantitative gait analysis in animal models of movement disorders. BMC Neurosci, 11: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gerber YN, Sabourin JC, Rabano M, Vivanco M, Perrin FE (2012). Early functional deficit and microglial disturbances in a mouse model of amyotrophic lateral sclerosis. PloS one, 7: e36000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Parvathy SS, Masocha W (2013). Gait analysis of C57BL/6 mice with complete Freund’s adjuvant-induced arthritis using the CatWalk system. BMC musculoskeletal disorders, 14: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sohal RS, Orr WC (2012). The redox stress hypothesis of aging. Free Radic Biol Med, 52: 539-555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H (2007). Trends in oxidative aging theories. Free Radic Biol Med, 43: 477-503 [DOI] [PubMed] [Google Scholar]
- [23].Rebrin I, Sohal RS (2008). Pro-oxidant shift in glutathione redox state during aging. Advanced drug delivery reviews, 60: 1545-1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schafer FQ, Buettner GR (2001). Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med, 30: 1191-1212 [DOI] [PubMed] [Google Scholar]
- [25].Lu SC (2013). Glutathione synthesis. Biochimica et biophysica acta, 1830: 3143-3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Franklin CC, Backos DS, Mohar I, White CC, Forman HJ, Kavanagh TJ (2009). Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med, 30: 86-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McConnachie LA, Mohar I, Hudson FN, Ware CB, Ladiges WC, Fernandez C, et al. (2007). Glutamate cysteine ligase modifier subunit deficiency and gender as determinants of acetaminophen-induced hepatotoxicity in mice. Toxicol Sci, 99: 628-636 [DOI] [PubMed] [Google Scholar]
- [28].Yang Y, Dieter MZ, Chen Y, Shertzer HG, Nebert DW, Dalton TP (2002). Initial characterization of the glutamate-cysteine ligase modifier subunit Gclm(-/-) knockout mouse. Novel model system for a severely compromised oxidative stress response. J Biol Chem, 277: 49446-49452 [DOI] [PubMed] [Google Scholar]
- [29].Wooley CM, Sher RB, Kale A, Frankel WN, Cox GA, Seburn KL (2005). Gait analysis detects early changes in transgenic SOD1(G93A) mice. Muscle & nerve, 32: 43-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Samson MM, Crowe A, de Vreede PL, Dessens JAG, Duursma SA, Verhaar HJJ (2001). Differences in gait parameters at a preferred walking speed in healthy subjects due to age, height and body weight. Aging clinical and experimental research, 13: 16-21 [DOI] [PubMed] [Google Scholar]
- [31].Maki BE (1997). Gait changes in older adults: predictors of falls or indicators of fear. Journal of the American Geriatrics Society, 45: 313-320 [DOI] [PubMed] [Google Scholar]
- [32].Orendurff MS, Segal AD, Klute GK, Berge JS, Rohr ES, Kadel NJ (2004). The effect of walking speed on center of mass displacement. Journal of rehabilitation research and development, 41: 829-834 [DOI] [PubMed] [Google Scholar]
- [33].England SA, Granata KP (2007). The influence of gait speed on local dynamic stability of walking. Gait & posture, 25: 172-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Winter DA, Patla AE, Frank JS, Walt SE (1990). Biomechanical walking pattern changes in the fit and healthy elderly. Phys Ther, 70: 340-347 [DOI] [PubMed] [Google Scholar]
- [35].Manchester D, Woollacott M, Zederbauer-Hylton N, Marin O (1989). Visual, vestibular and somatosensory contributions to balance control in the older adult. Journal of gerontology, 44: M118-127 [DOI] [PubMed] [Google Scholar]
- [36].Cabeza R, Anderson ND, Locantore JK, McIntosh AR (2002). Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage, 17: 1394-1402 [DOI] [PubMed] [Google Scholar]
- [37].Fukagawa NK, Wolfson L, Judge J, Whipple R, King M (1995). Strength Is a Major Factor in Balance, Gait, and the Occurrence of Falls. The Journals of Gerontology: Series A, 50A: 64-67 [DOI] [PubMed] [Google Scholar]
- [38].Sturnieks DL, George R, Lord SR (2008). Balance disorders in the elderly. Neurophysiol Clin, 38: 467-478 [DOI] [PubMed] [Google Scholar]
- [39].Shiga A, Nakagawa T, Nakayama M, Endo T, Iguchi F, Kim TS, et al. (2005). Aging effects on vestibulo-ocular responses in C57BL/6 mice: comparison with alteration in auditory function. Audiology & neuro-otology, 10: 97-104 [DOI] [PubMed] [Google Scholar]
- [40].Shaffer SW, Harrison AL (2007). Aging of the somatosensory system: a translational perspective. Phys Ther, 87: 193-207 [DOI] [PubMed] [Google Scholar]
- [41].Gutierrez-Castellanos N, Winkelman BH, Tolosa-Rodriguez L, De Gruijl JR, De Zeeuw CI (2013). Impact of aging on long-term ocular reflex adaptation. Neurobiology of aging, 34: 2784-2792 [DOI] [PubMed] [Google Scholar]
- [42].Cole TB, Giordano G, Co AL, Mohar I, Kavanagh TJ, Costa LG (2011). Behavioral Characterization of GCLM-Knockout Mice, a Model for Enhanced Susceptibility to Oxidative Stress. Journal of toxicology, 2011: 157687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chen Y, Curran CP, Nebert DW, Patel KV, Williams MT, Vorhees CV (2012). Effect of chronic glutathione deficiency on the behavioral phenotype of Gclm-/- knockout mice. Neurotoxicology and teratology, 34: 450-457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS (1996). Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci U S A, 93: 4765-4769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Diaz-Hung ML, Blanco L, Pavon N, Leon R, Estupinan B, Orta E, et al. (2014). Sensory-motor performance after acute glutathione depletion by L-buthionine sulfoximine injection into substantia nigra pars compacta. Behavioural brain research, 271: 286-293 [DOI] [PubMed] [Google Scholar]
- [46].Chi L, Ke Y, Luo C, Gozal D, Liu R (2007). Depletion of reduced glutathione enhances motor neuron degeneration in vitro and in vivo. Neuroscience, 144: 991-1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Morrison JP, Coleman MC, Aunan ES, Walsh SA, Spitz DR, Kregel KC (2005). Aging reduces responsiveness to BSO- and heat stress-induced perturbations of glutathione and antioxidant enzymes. American journal of physiology. Regulatory, integrative and comparative physiology, 289: R1035-1041 [DOI] [PubMed] [Google Scholar]
- [48].Clarke KA, Still J (1999). Gait analysis in the mouse. Physiol Behav, 66: 723-729 [DOI] [PubMed] [Google Scholar]
- [49].Glynn D, Drew CJ, Reim K, Brose N, Morton AJ (2005). Profound ataxia in complexin I knockout mice masks a complex phenotype that includes exploratory and habituation deficits. Human molecular genetics, 14: 2369-2385 [DOI] [PubMed] [Google Scholar]
- [50].Serradj N, Jamon M (2009). The adaptation of limb kinematics to increasing walking speeds in freely moving mice 129/Sv and C57BL/6. Behavioural brain research, 201: 59-65 [DOI] [PubMed] [Google Scholar]
- [51].Veldink JH, Bar PR, Joosten EA, Otten M, Wokke JH, van den Berg LH (2003). Sexual differences in onset of disease and response to exercise in a transgenic model of ALS. Neuromuscul Disord, 13: 737-743 [DOI] [PubMed] [Google Scholar]
- [52].Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, et al. (2014). Rapamycin extends life and health in C57BL/6 mice. J Gerontol A Biol Sci Med Sci, 69: 119-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Parks RJ, Fares E, Macdonald JK, Ernst MC, Sinal CJ, Rockwood K, et al. (2012). A procedure for creating a frailty index based on deficit accumulation in aging mice. J Gerontol A Biol Sci Med Sci, 67: 217-227 [DOI] [PubMed] [Google Scholar]
- [54].Whitehead JC, Hildebrand BA, Sun M, Rockwood MR, Rose RA, Rockwood K, et al. (2014). A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci, 69: 621-632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Batka RJ, Brown TJ, McMillan KP, Meadows RM, Jones KJ, Haulcomb MM (2014). The need for speed in rodent locomotion analyses. Anat Rec (Hoboken), 297: 1839-1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Broom L, Ellison BA, Worley A, Wagenaar L, Sorberg E, Ashton C, et al. (2017). A translational approach to capture gait signatures of neurological disorders in mice and humans. Scientific reports, 7: 3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bohannon RW (1997). Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age and ageing, 26: 15-19 [DOI] [PubMed] [Google Scholar]