Abstract
The benefits of stable pair bonds (that persist between breeding attempts) have been well described, but are relatively less well known in cooperatively breeding species. If pair bonds are beneficial, then it is possible that the bond between the behaviorally and socially dominant pair may influence factors such as reproductive success and group stability in cooperative species. Here, we used long‐term data to investigate the relationships between pair bond tenure, reproductive success, and group stability in the cooperatively breeding pied babbler (Turdoides bicolor). Pair bond tenure positively influenced both the number of offspring recruited annually per pair and total reproductive success (over entire pair bond duration), indicating that pair bond tenure has an important influence on reproductive success. The likelihood of immigration into the group was lower for groups containing a bonded pair with long tenure, indicating that the duration of pair bonds may impact group stability. These findings suggest that pair tenure, a hitherto relatively unexplored factor in cooperative species, may have an important influence on group dynamics.
Keywords: cooperative breeding, pair bond, pair tenure, pied babblers, reproductive success
1. INTRODUCTION
Long‐term sequential monogamy, where a pair stay together for consecutive reproductive attempts, has been well described in biparental species (reviewed in Wittenberger & Tilson, 1980; Dillard & Westneat, 2016). Pairs that form and maintain pair bonds, rather than repeatedly divorcing and remating, may reap fitness benefits from such long‐term bonds (Forslund & Larsson, 1991). Increased mate familiarity over time through pair bonding may also facilitate better coordination in reproductive activities, territory acquisition and defence, and antipredator behaviors, for example, barnacle geese, Branta leucopsis (Black, 2001); blue‐footed boobies, Sula nebouxii (Sánchez‐Macouzet, Rodríguez, & Drummond, 2014).
Monogamous pair bonds can also be present in cooperative species, where the breeding pair in a cooperative group stay together over an extended time period (e.g., cichlids, Neolamprologus pulcher (Bergmüller, Heg, & Taborsky, 2005); red wolves, Canis rufus (Sparkman, Adams, Steury, Waits, & Murray, 2011)). For many cooperative breeders, reproductive success is an important predictor of annual group persistence, due to the benefits accrued through group augmentation (Keynan & Ridley, 2016; Kokko, Johnstone, & Clutton‐Brock, 2001; Wiley, 2017). Groups can also increase in size through the recruitment of unrelated individuals; however, nonkin group compositions can be more likely to lead to within‐group conflict due to reproductive competition (Goldstein, Woolfenden, & Hailman, 1998; Leimar & Hammerstein, 2010; Nelson‐Flower & Ridley, 2016; Ridley, 2016). In cooperative species, the bond between sequentially monogamous pairs may be important to the stability of the group, via direct influences on reproductive success, immigration, and within‐group conflict. Thus far, this possibility has rarely been investigated.
Pied babbler pairs form monogamous long‐term pair bonds within groups, with very low extrapair parentage: 92.3% of offspring are progeny of the dominant pair (Nelson‐Flower, Flower, & Ridley, 2018). Pied babblers are a long‐lived passerine, with some individuals reaching more than 10 years of age in the wild (Ridley, 2016); thus, pair bonds can persist for many years. Pied babblers live in stable groups consisting of a single breeding pair (Nelson‐Flower et al., 2011), and sexually mature (over 1 year old posthatching) subordinate helpers, with an average group size (±standard error) of 4.29 ± 0.22 adults (range: 2–13 adults, Wiley, 2017).
Here, we aim to test whether the prevailing trend seen in biparental species, where longer pair bond tenure results in enhanced reproductive success (Black, 1996; Griggio & Hoi, 2011; Sánchez‐Macouzet et al., 2014; Wittenberger & Tilson, 1980), persists in cooperative species. We also aim to test the idea that longer term pair bonds confer group stability in cooperative species, analogous to the territory stability observed in biparental or pair bonded species with long‐term partner fidelity (Hall & Magrath, 2007; Nowicki et al., 2018). In some social species, immigration events are known to negatively impact the stability of groups, with consequences such as the eviction or infanticide of group members (Packer, Scheel, & Pusey, 1990; Silk, 2007). We therefore use the relationship between pair bond tenure and group immigration events as a proxy for group stability. We investigate the effect of pair bond tenure on reproductive success and group stability in the pied babbler by quantifying reproductive success, pair persistence (likelihood of remaining as a pair to the next breeding season), and group immigration events over the duration of the pair bond.
2. METHODS
2.1. Study site
We investigated monogamous pair bonds in pied babbler groups at the Pied Babbler Research Project, based in the 33 km2 Kuruman River Reserve, southern Kalahari, South Africa (26°58′S, 21°49′E). The study site has a subtropical climate and is primarily semi‐arid grassland and acacia savanna (see Ridley & Thompson, 2011 for description of habitat types).
2.2. Study species
The pied babbler is a cooperatively breeding, territorial, medium‐sized (75–95 g) passerine, in which all adult group members contribute to the provisioning of nestlings and fledglings (Ridley & Raihani, 2007). Pied babblers can raise up to three broods per season. Breeding normally occurs in summer months but can occur year‐round if ecological conditions permit (Ridley, 2016). Since 2003, a study population of uniquely ringed individuals has been habituated, monitored and maintained at the study site, typically comprising 18 habituated groups of pied babblers each year. Groups are visited at least once per week leading up to and during the peak breeding season (September–March), to check group composition and record life history events such as breeding, immigration, and dispersal. For a small food reward, individuals will hop onto a small top‐pan scale to be weighed. In this way, body condition can be monitored throughout each individual's lifetime noninvasively, thus avoiding any need for recapture. Individuals typically must be part of the dominant breeding pair (one such pair per group) in order to breed (Nelson‐Flower et al., 2011). Although subordinate reproduction does occur, it is very rare (Nelson‐Flower, Flower, et al., 2018). In each group, the dominant pair enforce their dominance through agonistic displays and physical attacks on subordinates (Raihani, 2008). The dominant pair is also readily identifiable through regular duetting and affiliative behavior (Golabek, 2010; Wiley, 2017) and as the primary individuals engaging in nest‐building and breeding activity (Nelson‐Flower et al., 2013). Thus assignation of the breeding pair in each group is readily determined and unambiguous (Figure 1). Age of acquisition of dominance varies between males and females, with females typically acquiring dominance at 882 days posthatching (range 204–2,761), while males typically acquire dominance at 1,085 days posthatching (range 319–2,679). Inbreeding avoidance in this species results in the regular dispersal of individuals from their natal group to access breeding opportunities (Nelson‐Flower, Hockey, O'Ryan, & Ridley, 2012). Genetic research has revealed that individuals will gain the dominant breeding position on their natal territory only if the opposite‐sex dominant individual is not a close relative (Nelson‐Flower, Wiley, Flower, & Ridley, 2018).
Figure 1.

A pied babbler pair allopreening before going to roost. Tactile affiliative interactions between individuals may serve to strengthen social bonds. Observations of behavior can be recorded at a distance of 1–2 m from these habituated birds. (Photo by EMWiley)
Long‐term monitoring of cooperative behavior and lifetime reproductive success in the population precluded experimental manipulation of breeding pairs. However, by controlling for body mass and age we were able to account, at least in part, for the potential confounds of variation in individual quality acting on pair tenure and reproductive success.
2.3. Data collection
For every pair monitored from 2003 to 2015, individual data on the sex, age (in days since hatching) of each member of the pair, body mass (an average of all morning mass measurements per individual per year) and previous breeding experience as an individual (total breeding attempts. An attempt being defined as when eggs were laid and incubated, per individual regardless of current pairing) were extracted from the long‐term database.
For each pair, data on the pair length (total consecutive days since pair bonded, cumulative across years), previous reproductive success as a pair (total offspring recruited to one year of age, cumulative across years extant as a pair), group size (adults present at the start of each year), likelihood of pair persistence (whether a pair was still together the next year (yes/no)), number of immigrants (number of new adult individuals immigrating into the group per year: Individuals that spent more than 30 consecutive days in a group were considered to have successfully immigrated) and annual chick recruitment (number of offspring produced by the pair that survived to at least 1 year for each year the pair were together) were extracted. Each “year” started on 1 September (at the beginning of the breeding season) and ended on 31 August the next year.
There were a total of 64 pairs and 86 individuals available for analysis, of which 47 were female and 39 male. There was a difference in the number of males and females available for analysis because of differential mortality between the sexes. Where possible, we used known age (from hatching records). However, there were four (female) individuals that did not have an exact age. For these four, we assigned to them the average age at which a female attained a dominant position in a group (882 days, calculated from the other 43 females that acquired dominance rank) as their age on the date they became dominant, in the first year that they were part of a known breeding pair in the study population.
Pair lifetime reproductive success was estimated as the total number of offspring (that survived to 1 year of age) recruited over the duration of the pair tenure. We also calculated the total number of offspring (that survived to 1 year) produced from the total number of hatched broods per pair over the duration of the pair tenure. Calculating this way measured reproductive output per (viable) nesting attempt and thus avoided having time as a confounding factor. These analyses comprised a subset of pairs from the database (n = 57) because this variable could only be calculated for pair bonds that were no longer extant.
2.4. Statistical analysis
2.4.1. Chick recruitment
To determine which variables influenced the number of chicks each pair recruited per year, generalized linear mixed models (hereafter GLMMs) with Poisson distributions were employed for males and females separately. Previous reproductive success (as a pair; some individuals were present as members of different pairs at different times, thus offspring produced per pairing was analyzed separately), previous breeding experience, breeder age, group size, body mass and pair tenure were included as predictor terms. Year and individual identity nested within pair identity were included as random effects in all models.
2.4.2. Pair persistence
To determine which variables influenced pair persistence likelihood per year, GLMMs with binomial distributions (where 0 = pair bond ended, 1 = pair bond remained extant) were used with breeder age, group size, body mass, chick recruitment, previous reproductive success and pair tenure as predictor terms. Year and individual identity nested within pair identity were included as random effects in all models.
2.4.3. Reproductive success over entire duration of the pair bond
To determine what was influencing reproductive success (total offspring recruited to 1 year of age over entire pair tenure), GLMMs with Poisson distributions were employed, with average group size (average number of adults in the group for the duration of the pair tenure), and pair tenure (total days) as predictor terms. Group identity and year were included as random effects.
To investigate which parameters were affecting reproductive success (per hatched brood), we analyzed data in LMMs, with average group size (average number of adults in the group for the duration of the pair tenure), and pair tenure (total days) as predictor terms. Group identity and year were included as random effects.
2.4.4. Group stability
We investigated factors influencing immigration events into a group as a measure of group stability, using GLMMs with Poisson distributions, with pair tenure (total days), chick recruitment (total number of offspring recruited each year) and group size (adult group size in each year of pair tenure) as predictor terms. Pair identity nested within group identity and year was included as random effects.
Data from paired males and females were analyzed separately where individual‐level data were used as predictor terms in models, to avoid a lack of independence in the predictor variables of annual chick recruitment or pair persistence values. Correlated terms were not used together in the same models (see Supporting Information Table S7).
Model selection using the Akaike's information criterion corrected for small sample size (AICc) was employed to determine the model/s that best explained the patterns of variation in the data. Using AICc (with maximum likelihood estimation) a series of models were tested, with each model representing a biological hypothesis. Lower AICc values represented more parsimonious models, as per Johnson and Omland (2004). The best‐supported models were selected and where there were several models within 2AICc, the model with the fewest explanatory terms, that is, the simplest, was selected (Burnham & Anderson, 2003). All data were analyzed in the program “R” v 3.3.2 (2017), using the package “lme4” (Bates, Maechler, Bolker, & Walker, 2015). All continuous explanatory variables were scaled following Grueber, Nakagawa, Laws, & Jamieson, 2011 to allow model comparison, using the function “scale” in the program “R” (2017).
3. RESULTS
3.1. Pair bond tenure and reproductive success
In pied babblers, completed pair bonds (where the pair bond had ended and total pair tenure length was known) averaged 609 days, and ranged widely, from 19 to 1,940 days (Figure 2). On average, males formed pair bonds with 1.5 females over their lifetime, while females on average formed pair bonds with an average of 1.3 males.
Figure 2.
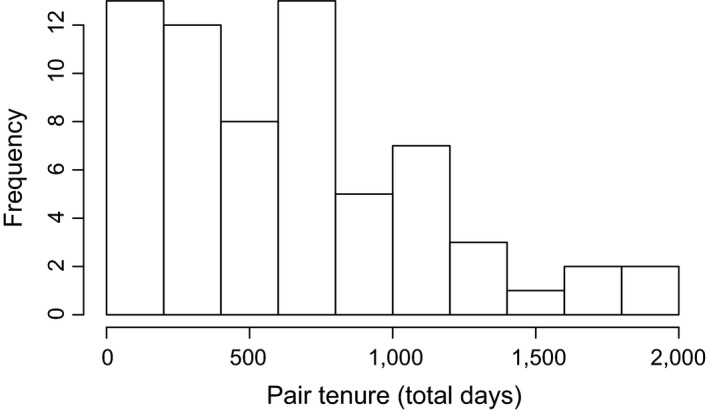
The frequency and distribution of known pair tenures in the pied babbler population, from 2003 to 2015
Pairs with longer tenure had significantly higher chick recruitment per year (Figure 3, Table 1 and for full model lists see Supporting Information Tables S1 and S2).
Figure 3.
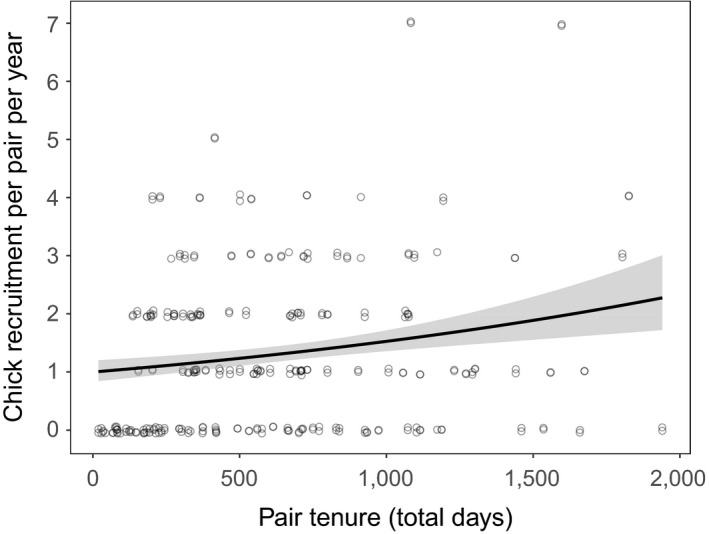
The relationship between chick recruitment (per year) and pair tenure. The fitted regression line is shown with shaded 95% confidence intervals. Data points are integers and have been jittered for better visibility
Table 1.
The top model set for the GLMM analysis of the terms influencing the number of chicks successfully recruited to 1 year of age per year, for females and males separately
| Model | AICc | ΔAICc | ωί |
|---|---|---|---|
| Females | |||
| Pair length | 453.96 | 0.57 | 0.42 |
| Pair length + body mass | 453.39 | 0 | 0.55 |
| Null | 462.48 | 9.09 | 0.01 |
| Parameter estimates | Estimate | SE | Z |
| Intercept | 0.18 | 0.18 | 0.97 |
| Pair length | 0.26 | 0.07 | 3.62 |
| Males | |||
| Pair length | 448.09 | 0.04 | 0.48 |
| Pair length + body mass | 448.06 | 0 | 0.49 |
| Null | |||
| Parameter estimates | Estimate | SE | Z |
| Intercept | 0.19 | 0.18 | 1.05 |
| Pair length | 0.27 | 0.07 | 3.74 |
3.2. Pair persistence
Pairs that successfully raised chicks that survived to 1 year of age were more likely to still be a pair in the following year (Figure 4, Table 2 and for full model lists see Supporting Information Tables S3 and S4).
Figure 4.
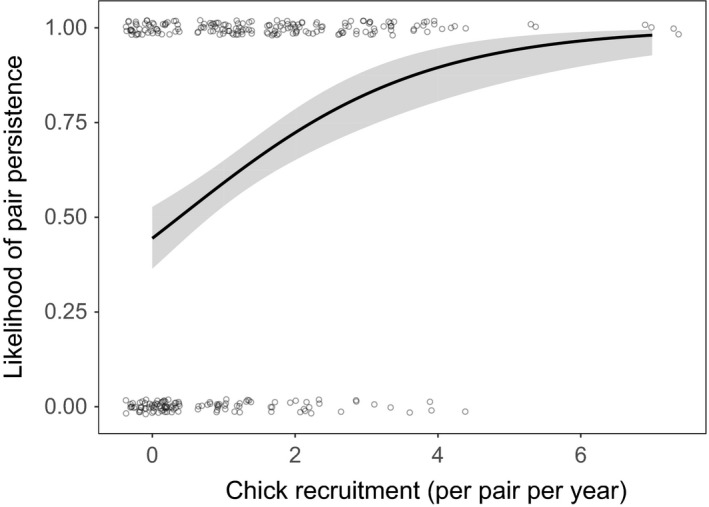
The relationship between the number of chicks (raised to 1 year of age posthatch) and the likelihood a pair persisted to the next year. A value of “1” on the y‐axis denotes persistence. The fitted regression line is shown with shaded 95% confidence intervals. Data points are integers and have been jittered for better visibility
Table 2.
The top model set for the GLMM analysis of the terms influencing the likelihood of pair persistence to the following year, for females and males separately
| Model | AICc | ΔAICc | ωί |
|---|---|---|---|
| Females | |||
| Chick recruitment | 190.28 | 0 | 0.99 |
| Null | 204.21 | 13.92 | 0 |
| Parameter estimates | Estimate | SE | Z |
| Intercept | 0.55 | 0.19 | 2.83 |
| Chick recruitment | 0.83 | 0.23 | 3.57 |
| Males | |||
| Chick recruitment | 188.07 | 0 | 1 |
| Null | 200.21 | 12.14 | 0 |
| Parameter estimates | Estimate | SE | Z |
| Intercept | 0.55 | 0.19 | 2.88 |
| Chick recruitment | 0.81 | 0.23 | 3.51 |
3.3. Reproductive success (total pair duration)
Patterns of pair lifetime reproductive success were best explained by pair tenure for both (a) total reproductive success and (b) reproductive success per hatched brood (Figures 5a and 5b, Table 3 and for a full list of models tested, see Supporting Information Tables S5 and S6).
Figure 5.
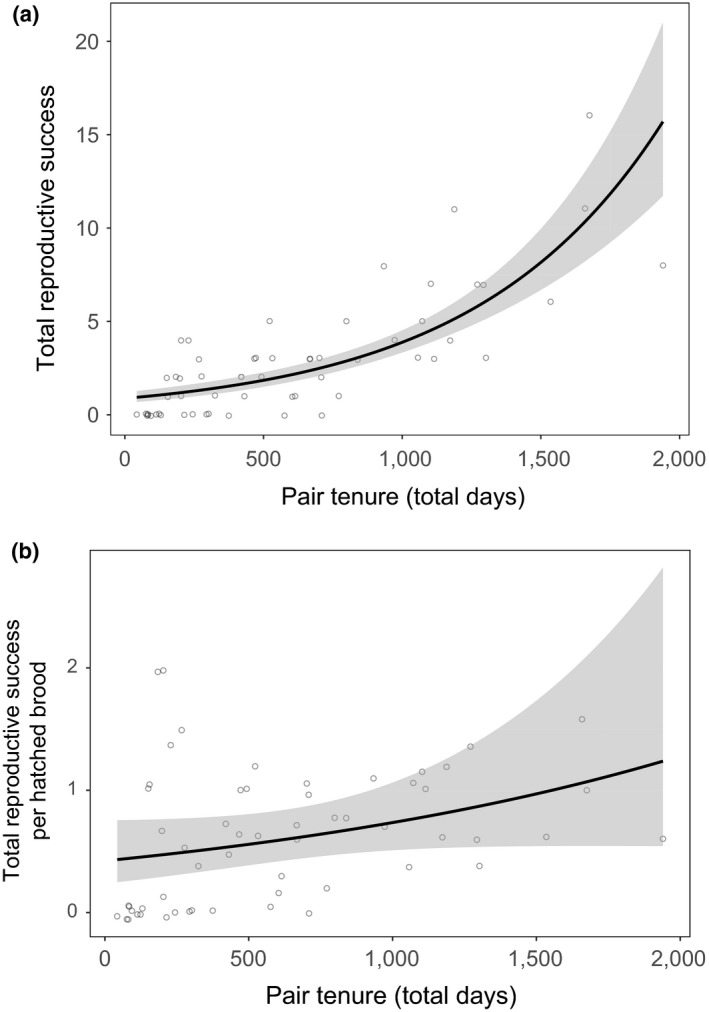
(a) The relationship between reproductive success (total number of chicks reaching adulthood that a pair raise together) and complete pair tenure (total length in days). The fitted regression line is shown with shaded 95% confidence intervals. Data points are integers and have been jittered for better visibility. (b) The relationship between total reproductive success per hatched brood (over entire pair duration) and complete pair tenure (total length in days). The fitted regression line is shown with shaded 95% confidence intervals. Data points are integers and have been jittered for better visibility
Table 3.
The top model set for the GLMM analysis of the terms influencing within‐pair lifetime reproductive success and within‐pair lifetime reproductive success as a proportion of hatched broods
| Model | AICc | ΔAICc | ωί |
|---|---|---|---|
| Lifetime reproductive success | |||
| Pair length | 215.49 | 0 | 1 |
| Null | 275.96 | 60.47 | 0 |
| Parameter estimates | Estimate | SE | Z |
| Intercept | 0.74 | 0.12 | 6.18 |
| Pair length | 0.71 | 0.07 | 9.001 |
| Lifetime reproductive success per hatched brood | |||
| Pair length | 35.77 | 0 | 1 |
| Null | 44.14 | 8.37 | 0 |
| Parameter estimates | Estimate | SE | X 2 |
| Intercept | 0.42 | 0.04 | |
| Pair length | 0.13 | 0.04 | 10.64 |
3.4. Group stability
Immigration events were less likely to occur at groups where the bonded pair had longer pair tenure (Figure 6 and Table 4). This effect was so strong, that groups with pairs that were bonded for more than 2 years (730 days) experienced less than 2% of all the immigration events observed in the population (n = 52 immigration events), despite the fact that pairs bonded for this long comprised more than 30% of our dataset.
Figure 6.
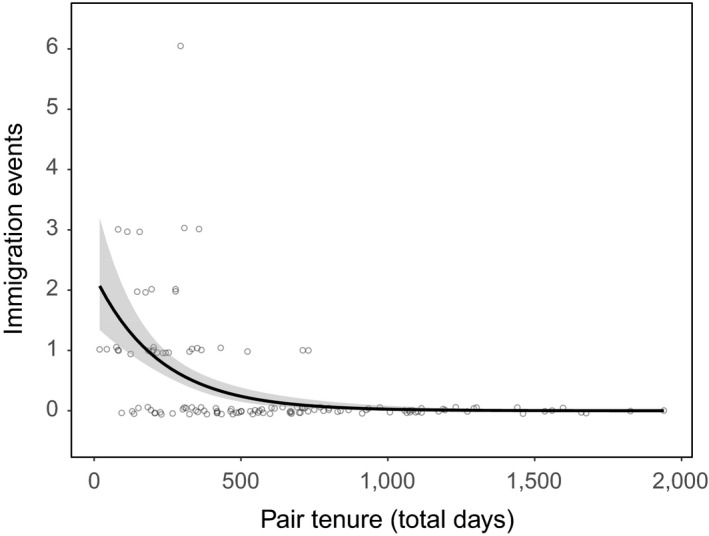
The relationship between immigration events and complete pair tenure (total length in days). The fitted regression line is shown with shaded 95% confidence intervals. Data points are integers and have been jittered for better visibility
Table 4.
The top model set for the GLMM analysis of the terms influencing immigration to the group for each year of each pair bond tenure. Analysis was conducted on the total number of immigration events in each year a pair were extant. The best‐supported model is bolded
| Model | df | AICc | ΔAICc | ωί | logLik |
|---|---|---|---|---|---|
| Pair length | 5 | 178.98 | 0 | 1 | −84.24 |
| Chick recruitment | 5 | 223.44 | 44.46 | 0 | −106.47 |
| Null | 4 | 224.38 | 45.40 | 0 | −108.02 |
| Group size | 5 | 224.53 | 45.55 | 0 | −107.01 |
| Parameter estimates | Estimate | SE | z |
|---|---|---|---|
| Intercept | −2.27 | 0.42 | −5.45 |
| Pair length | −2.11 | 0.41 | −5.09 |
N = 126. Random effects: Year: 13 (SD 0), Group ID: 18 (SD 0), Group ID/Pair ID: 57 (SD 0.69).
4. DISCUSSION
The benefits of pair bonding have been widely studied and the general consensus has been that the function of monogamous pair bonds is to increase reproductive output (Bradley, Wooller, & Skira, 1995; Evans & Poole, 1983; Fowler, 1995). Under the recently proposed dual benefits framework (Shen, Emlen, Koenig, & Rubenstein, 2017), cooperation is associated with “collective action benefits” (which include increased per capita productivity), but the potential benefits of pair bonding for productivity in cooperatively breeding species has received relatively little attention. In this study, we were able to confirm several substantial benefits of pair bonds in a cooperatively breeding species.
First, reproductive success was higher for those individuals with a longer pair bond. Duration of pair tenure also had a positive influence on within‐pair lifetime reproductive success. This supports findings from biparental species (Black, 1996; Wittenberger & Tilson, 1980), but provides one of the first instances of this benefit in a cooperative species. Additionally, an increase in reproductive success per year may imply that long‐term pair bonds in pied babblers accrue benefits through mate familiarity. Although further testing is required, this could corroborate similar results in species with biparental care such as Steller's Jays, Cyanocitta stelleri (Gabriel & Black, 2013) and Pinyon Jays, Gymnorhinus cyanocephalus (Marzluff & Balda, 1988) in which longer lasting pairs had higher reproductive success (independent of individual age).
Second, annual reproductive success increased the likelihood of a pair still being bonded in the next breeding season. This result suggests that reproductive success is not only a benefit of pair tenure, but (barring the death of one partner) could also be a key determinant in whether a pair continues to remain bonded. This result supports findings from a recent meta‐analysis of monogamous bird species, where divorce was suggested to be an adaptive strategy to improve poor breeding success (Culina, Radersma, & Sheldon, 2015).
Our results revealed a significant impact of pair bond tenure on immigration events, suggesting a significant impact of pair bond tenure on group stability—a finding that has hitherto not been reported for cooperative species. In pied babblers, there is a greater likelihood of within‐group conflict in groups comprised of nonkin, where reproductive competition decreases group productivity (Nelson‐Flower et al., 2013). Thus, where a long‐term pair bond precluded the immigration of unrelated individuals, within‐group stability was high, possibly due to lower intragroup reproductive competition. Our analysis therefore reveals that there are selective benefits associated with pair bond tenure in a cooperatively breeding species.
Longer pair bonds resulted in both higher lifetime, but also higher annual reproductive success, and this in turn positively influenced pair persistence to the following breeding season. Pairs with longer tenure also gained fewer adult immigrants into their group, thus possibly minimizing the potential for within‐group conflict due to reproductive success. Pair bonds may therefore benefit the whole group: Higher reproductive success can confer direct (production of individual's own offspring) or indirect (individual is related to offspring produced) fitness benefits to group members (Clutton‐Brock, 2002). Pair bonds are a relatively unexplored aspect of the complex social dynamics of cooperative breeders, and we have found, using an extensive long‐term dataset that encompasses lifetime pairing for many individuals, that pair tenure may have an important influence on both within‐pair and within‐group dynamics of a cooperatively breeding species.
CONFLICT OF INTEREST
The authors declare we have no competing interests.
AUTHOR CONTRIBUTIONS
EMW and ARR conceived the study idea and contributed to data collection. EMW carried out data analysis and wrote the manuscript. ARR habituated the study population, maintained long‐term data collection at the field site, and critically contributed to manuscript drafts.
ETHICAL NOTE
Our research was approved by the Animal Ethics Committee, University of Western Australia (RA/3/100/1263) and the Science Faculty Animal Ethics Committee, University of Cape Town (Ethics number R2012/2006/V15/AR).
DATA ACCESSIBILITY
The datasets supporting this article have been deposited in Dryad https://doi.org/10.5061/dryad.r3mk2ng
Supporting information
ACKNOWLEDGMENTS
We thank all past and present Pied Babbler Research Project staff, students, and assistants for their help in population monitoring and maintenance. We thank three anonymous reviewers for their constructive comments. We thank the University of Cape Town for long‐term logistical support at the field site. We are grateful to the Kuruman Reserve Trust for permission to work at the Kalahari Research Centre (KRC), Tim Clutton‐Brock & Marta Manser, and surrounding land owners for continued land access. The long‐term field site KRC was financed by the Universities of Cambridge and Zurich, ERC (grant No 294494 to Tim Clutton‐Brock) and received logistical support from the Mammal Research Institute of the University of Pretoria.
Wiley EM, Ridley AR. The benefits of pair bond tenure in the cooperatively breeding pied babbler (Turdoides bicolor). Ecol Evol. 2018;8:7178–7185. 10.1002/ece3.4243
Funding information
This work was supported by a University of Western Australia scholarship to EMW, the Percy FitzPatrick Institute, University of Cape Town and an Australian Research Council Future Fellowship (FT110100188) to ARR.
REFERENCES
- Bates, D. , Maechler, M. , Bolker, B. , & Walker, S. (2015). lme4: Linear mixed‐effects models using Eigen and S4. R package version 1.0–5. Retrieved from http://CRAN.R-project.org/package=lme4.
- Bergmüller, R. , Heg, D. , & Taborsky, M. (2005). Helpers in a cooperatively breeding cichlid stay and pay or disperse and breed, depending on ecological constraints. Proceedings of the Royal Society of London B. Biological Sciences, 272, 325–331. 10.1098/rspb.2004.2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. M. (Ed.) (1996). Partnerships in birds: The study of monogamy: The study of monogamy. Oxford, UK: Oxford University Press. [Google Scholar]
- Black, J. (2001). Fitness consequences of long‐term pair bonds in barnacle geese‐ monogamy in the extreme. Behavioral Ecology, 12, 640–645. 10.1093/beheco/12.5.640 [DOI] [Google Scholar]
- Bradley, A. J. S. , Wooller, R. D. , & Skira, I. J. (1995). The relationship of pair‐bond formation and duration to reproductive success in short‐ tailed shearwaters Puffinus tenuirostris . Journal of Animal Ecology, 64, 31–38. 10.2307/5825 [DOI] [Google Scholar]
- Burnham, K. P. , & Anderson, D. R. (2003). Model selection and multimodel inference: A practical information‐theoretic approach. New York, NY: Springer Science & Business Media. [Google Scholar]
- Clutton‐Brock, T. (2002). Breeding together: Kin selection and mutualism in cooperative vertebrates. Science, 296(5565), 69–72. 10.1126/science.296.5565.69 [DOI] [PubMed] [Google Scholar]
- Culina, A. , Radersma, R. , & Sheldon, B. C. (2015). Trading up: The fitness consequences of divorce in monogamous birds. Biological Reviews, 90, 1015–1034. 10.1111/brv.12143 [DOI] [PubMed] [Google Scholar]
- Dillard, J. R. , & Westneat, D. F. (2016). Disentangling the correlated evolution of monogamy and cooperation. Trends in Ecology and Evolution, 31(7), 503–513. 10.1016/j.tree.2016.03.009 [DOI] [PubMed] [Google Scholar]
- Evans, S. , & Poole, T. B. (1983). Pair‐bond formation and breeding success in the common marmoset Callithrix jacchus jacchus . International Journal of Primatology, 4, 83–97. 10.1159/000156142 [DOI] [Google Scholar]
- Forslund, P. , & Larsson, K. (1991). The effect of mate change and new partner's age on reproductive success in the barnacle goose, Branta leucopsis . Behavioral Ecology, 2, 116–122. 10.1093/beheco/2.2.116 [DOI] [Google Scholar]
- Fowler, G. S. (1995). Stages of age‐related reproductive success in bird: Simultaneous effects of age, pair bond duration and reproductive experience. The American Zoologist, 35, 318–328. 10.1093/icb/35.4.318 [DOI] [Google Scholar]
- Gabriel, P. O. , & Black, J. M. (2013). Correlates and consequences of the pair Bond in Steller's Jays. Ethology, 119, 178–187. 10.1111/eth.12051 [DOI] [Google Scholar]
- Golabek, K. A. (2010). Vocal communication and the facilitation of social behaviour in the southern pied babbler (Turdoides bicolor). PhD thesis, The University of Bristol.
- Goldstein, J. M. , Woolfenden, G. E. , & Hailman, J. P. (1998). A same‐sex stepparent shortens a prebreeder's duration on the natal territory: Tests of two hypotheses in Florida scrub‐jays. Behavioral Ecology and Sociobiology, 44(1), 15–22. 10.1007/s002650050510 [DOI] [Google Scholar]
- Griggio, M. , & Hoi, H. (2011). An experiment on the function of the long‐term pair bond period in the socially monogamous bearded reedling. Animal Behaviour, 82(6), 1329–1335. 10.1016/j.anbehav.2011.09.016 [DOI] [Google Scholar]
- Grueber, C. E. , Nakagawa, S. , Laws, R. J. , & Jamieson, I. G. (2011). Multimodel inference in ecology and evolution: Challenges and solutions. Journal of Evolutionary Biology, 24, 699–711. 10.1111/j.1420-9101.2010.02210 [DOI] [PubMed] [Google Scholar]
- Hall, M. L. , & Magrath, R. D. (2007). Temporal coordination signals coalition quality. Current Biology, 17, 406–407. 10.1016/j.cub.2007.04.022 [DOI] [PubMed] [Google Scholar]
- Johnson, J. B. , & Omland, K. S. (2004). Model selection in ecology and evolution. Trends in Ecology and Evolution, 19, 101–108. 10.1016/j.tree.2003.10.013 [DOI] [PubMed] [Google Scholar]
- Keynan, O. , & Ridley, A. R. (2016). Component, group and demographic Allee effects in a cooperatively breeding bird species, the Arabian babbler (Turdoides squamiceps). Oecologia, 182(1), 153–161. 10.1007/s00442-016-3656-8 [DOI] [PubMed] [Google Scholar]
- Kokko, H. , Johnstone, R. A. , & Clutton‐Brock, T. H. (2001). The evolution of cooperative breeding through group augmentation. Proceedings of the Royal Society of London B. Biological Sciences, 268(1463), 187–196. 10.1098/rspb.2000.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimar, O. , & Hammerstein, P. (2010). Cooperation for direct fitness benefits. Philosophical Transactions of the Royal Society B: Biological Sciences, 365(1553), 2619–2626. 10.1098/rstb.2010.0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzluff, J. M. , & Balda, R. P. (1988). Pairing patterns and fitness in a free‐ranging population of Pinyon Jays: What do they reveal about mate choice? Condor, 90, 201–213. 10.2307/1368449 [DOI] [Google Scholar]
- Nelson‐Flower, M. J. , Flower, T. P. , & Ridley, A. R. (2018). Sex differences in the drivers of reproductive skew in a cooperative breeder. Molecular Ecology, 27, 2435–2446. [DOI] [PubMed] [Google Scholar]
- Nelson‐Flower, M. J. , Hockey, P. A. R. , O'Ryan, C. , English, S. , Thompson, A. M. , Bradley, K. , … Ridley, A. R. (2013). Costly reproductive competition between females in a monogamous cooperatively breeding bird. Proceedings of the Royal Society of London B. Biological Sciences, 280, 20130728 10.1098/rspb.2013.0728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson‐Flower, M. J. , Hockey, P. A. R. , O'Ryan, C. , Raihani, N. J. , du Plessis, M. A. , & Ridley, A. R. (2011). Monogamous dominant pairs monopolize reproduction in the cooperatively breeding pied babbler. Behavioral Ecology, 22, 559–565. 10.1093/beheco/arr018 [DOI] [Google Scholar]
- Nelson‐Flower, M. J. , Hockey, P. A. , O'Ryan, C. , & Ridley, A. R. (2012). Inbreeding avoidance mechanisms: Dispersal dynamics in cooperatively breeding southern pied babblers. Journal of Animal Ecology, 81(4), 876–883. 10.1111/j.1365-2656.2012.01983.x [DOI] [PubMed] [Google Scholar]
- Nelson‐Flower, M. J. , & Ridley, A. R. (2016). Nepotism and subordinate tenure in a cooperative breeder. Biology Letters, 12(8), 20160365 10.1098/rsbl.2016.0365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson‐Flower, M. J. , Wiley, E. M. , Flower, T. P. , & Ridley, A. R. (2018). Individual dispersal decisions in a cooperative breeder: Ecological constraints, the benefits of philopatry, and the social queue for dominance. Journal of Animal Ecology, 10.1111/1365-2656.12814 [DOI] [PubMed] [Google Scholar]
- Nowicki, J. P. , Walker, S. P. , Coker, D. J. , Hoey, A. S. , Nicolet, K. J. , & Pratchett, M. S. (2018). Pair bond endurance promotes cooperative food defense and inhibits conflict in coral reef butterflyfish. Scientific Reports, 8(1), 6295 10.1038/s41598-018-24412-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer, C. , Scheel, D. , & Pusey, A. E. (1990). Why lions form groups: Food is not enough. The American Naturalist, 136(1), 1–9. 10.1086/285079 [DOI] [Google Scholar]
- R Core Team (2017). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from https://www.R-project.org/ [Google Scholar]
- Raihani, N. J. (2008). Cooperation and conflict in pied babblers. PhD thesis, University of Cambridge.
- Ridley, A. R. (2016). Southern pied babblers: The dynamics of conflict and cooperation in a group‐living society In Koenig W. D., & Dickinson J. L. (Eds.), Cooperative breeding in vertebrates: Studies of ecology, evolution and behavior (pp. 115–132). Cambridge, UK: Cambridge University Press; 10.1017/CBO9781107338357 [DOI] [Google Scholar]
- Ridley, A. R. , & Raihani, N. J. (2007). Variable postfledging care in a cooperative bird: Causes and consequences. Behavioral Ecology, 18, 994–1000. 10.1093/beheco/arm074 [DOI] [Google Scholar]
- Ridley, A. R. , & Thompson, A. M. (2011). Heterospecific egg destruction by Wattled Starlings and the impact on Pied Babbler reproductive success. Ostrich, 82, 201–205. 10.2989/00306525.2011.618247 [DOI] [Google Scholar]
- Sánchez‐Macouzet, O. , Rodríguez, C. , & Drummond, H. (2014). Better stay together: Pair bond duration increases individual fitness independent of age‐related variation. Proceedings of the Royal Society of London B. Biological Sciences, 281, 20132843 10.1098/rspb.2013.2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, S. F. , Emlen, S. T. , Koenig, W. D. , & Rubenstein, D. R. (2017). The ecology of cooperative breeding behaviour. Ecology Letters, 20(6), 708–720. 10.1111/ele.12774 [DOI] [PubMed] [Google Scholar]
- Silk, J. B. (2007). Social components of fitness in primate groups. Science, 317(5843), 1347–1351. 10.1126/science.1140734 [DOI] [PubMed] [Google Scholar]
- Sparkman, A. M. , Adams, J. R. , Steury, T. D. , Waits, L. P. , & Murray, D. L. (2011). Direct fitness benefits of delayed dispersal in the cooperatively breeding red wolf (Canis rufus). Proceedings of the Royal Society of London B. Biological Sciences, 278, 1381–1389. 10.1093/beheco/arq194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley, E. M. (2017). Examining how multilevel population dynamics and climate influence breeding behaviour, within‐group stability and demography in a cooperatively breeding bird. PhD thesis, The University of Western Australia.
- Wittenberger, J. F. , & Tilson, R. L. (1980). The evolution of monogamy: Hypotheses and evidence. Annual Review of Ecology, Evolution, and Systematics, 11(1), 197–232. 10.1146/annurev.es.11.110180.001213 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been deposited in Dryad https://doi.org/10.5061/dryad.r3mk2ng


