Abstract
Patients with chronic conditions frequently experience behavioral comorbidities to which primary care cannot easily respond. This study observed a Vermont family medicine practice with integrated medical and behavioral health services that use a structured approach to implement a chronic care management system with Lean. The practice chose to pilot a population-based approach to improve outcomes for patients with poorly controlled Type 2 diabetes using a stepped-care model with an interprofessional team including a community health nurse. This case study observed the team’s use of Lean, with which it designed and piloted a clinical algorithm composed of patient self-assessment, endorsement of behavioral goals, shared documentation of goals and plans, and follow-up. The team redesigned workflows and measured reach (patients who engaged to the end of the pilot), outcomes (HbA1c results), and process (days between HbA1c tests). The researchers evaluated practice member self-reports about the use of Lean and facilitators and barriers to move from pilot to larger scale applications. Of 20 eligible patients recruited over 3 months, 10 agreed to participate and 9 engaged fully (45%); 106 patients were controls. Relative to controls, outcomes and process measures improved but lacked significance. Practice members identified barriers that prevented implementation of all changes needed but were in agreement that the pilot produced useful outcomes. A systematized, population-based, chronic care management service is feasible in a busy primary care practice. To test at scale, practice leadership will need to allocate staffing, invest in shared documentation, and standardize workflows to streamline office practice responsibilities.
Keywords: Behavioral care integration, Chronic care coordination, Population management, Diabetes, Lean workflow
A primary care team-based approach to coordinating care for patients with hard-to-manage diabetes was feasible but will need leadership support to succeed in larger trials.
Implications
Practice: The collection and use of patient-reported behavioral risk data will fuel integrated medical behavioral pathways to assist management of chronic disease.
Policy: Integrating behavioral care into chronic care coordination may improve outcomes for expensive, complex patient populations if supported by an industry-wide culture of data collection and comparison.
Research: We need to identify and evaluate the factors that facilitate and limit scaling up this intervention for generalizability, as well as determine how best to evaluate and demonstrate outcomes.
BACKGROUND
Chronic disease management often requires attention to a behavioral component, including tobacco use, poor diet, inadequate physical activity, alcohol and substance use, nonadherence to treatment, insomnia, anxiety, depression, chronic pain, and stress [1–4]. Because these problems are so often comorbid with chronic medical problems such as diabetes, heart disease, arthritis, and lung disease, many have suggested integrating behavioral care into primary care by creating team-based care to coordinate the work of medical and behavioral providers [5, 6]. Examples of this work have included applications of the chronic care model [7] and the primary care behavioral health consultant model [8]. Both have demonstrated effectiveness in treating behavioral health problems, while applications to chronic disease continue to emerge [6].
With the exceptions of the Department of Defense, the Department of Veterans Affairs, and some health maintenance organizations, it is unusual for behavioral care and primary care to be systematically integrated [6]. Although the data are sparse, recent survey data of a sample of patient-centered medical homes found approximately 40% of respondent practices had some behavioral staff [9]. Inclusion of behavioral care in practice systems and operating processes calls for a deliberate redesign of workflows supporting patient care [10], including coordination of care for patients with chronic diseases. Chronic care coordination is complex:
a multidimensional concept that includes effective communication among healthcare providers, patients, families, and caregivers (regarding chronic conditions); safe care transitions; a longitudinal view of care that considers the past, while monitoring present delivery of care and anticipating future needs; and the facilitation of linkages between communities and the healthcare system to address medical, social, educational, and other support needs that align with patient goals. [11]
Chronic care coordination can further aid the integration of behavioral health by leveraging needed resources through well-designed population-based care [6].
Population-based care is a systematic method of providing measurement-based, stepped treatment and team-based care for specified populations [12]. Berwick et al. [13] suggest that improving health care must include a shift to focusing on care for populations of patients. “Population” denotes a defined group of people over time characterized by a unifying characteristic(s). Characterizing the population of interest allows knowledge about the experience of care, its health status, and its costs, as well as identification of a unified care delivery system. Applying this approach to chronic care coordination heightens the opportunity to improve patient outcomes.
The goal of this study was to observe a rural Vermont family medicine practice with integrated medical and behavioral health services that used a structured approach to implement a chronic care management system with Lean management [10], a systematic method of redesigning processes and systems of service and product delivery. The practice chose to design and pilot an algorithm as a demonstration of population-based integrated medical and behavioral chronic care coordination supported by an interprofessional team, including a community health nurse in a stepped model of care.
One element of this goal was to test a team-based model of care in which service delivery does not funnel through the primary care provider but leverages the care delivery capacity of all members of the team, up to the scope of their licenses [6]. In this model, the primary care providers agreed not be the initial point of contact in identifying the need for behavioral care, nor a necessary part of the referral process. In addition, the behavioral health providers agreed to support a community health nurse with tools and coaching to screen and provide initial interventions for patients in need, stepping up the intensity of care to higher level staff as needed [14].
Psychology and integrated care generally focus on the treatment of mental health and substance abuse disorders. Colocation of behavioral clinicians within primary care results in increased referral rates and treatment initiation [15, 16]. Recently, given the broad range of behavioral need and high expense of untreated behavioral comorbidities among patients with multiple chronic diseases, the most effective focus of behavioral clinicians in primary care may not be mental health and substance abuse disorders but comorbid health behavior issues such as poor sleep, limited activity, food consumption, and engagement in care.
The primary outcomes of this project looked for changes in patient outcome and delays in service. In addition to improving patient outcomes, the project had secondary goals of creating a plan that could be scaled up to population-based care for many patients with chronic conditions, engaging patients with chronic illnesses in self-management skills, improving accessibility to care providers, lowering the cost per patient, and improving the process of care.
The practice chose to pilot this approach with patients diagnosed with diabetes mellitus Type 2 who were judged to need assistance with managing their condition, a decision influenced by expectations of the emerging accountable care organizations in the state of Vermont. The prevalence of diabetes for individuals 65 years of age and older who live in Vermont (the location of the practice) is 8%; nationally for those over 65, it is 11% [17].
Over the last 20 years, accumulated evidence recognizes that effective control of diabetes requires management of behavioral elements [18, 19]. Furthermore, it suggests comorbid diabetes and depression predicts negative care outcomes [20–22]. Psychological and behavioral barriers to optimize diabetes self-management are large challenges to contemporary diabetes treatment. Given the strength of this observation, the American Diabetes Association (ADA) published Practical Psychology for Diabetes Clinicians [23], and in 1999, the National Institutes of Health (NIH) sponsored a conference on behavioral science and diabetes [24].
Complex medical and behavioral comorbidities are prevalent in the diabetic population. Nationally, 30% of patients with diabetes have a diagnosis of depression [17]. If these comorbidities are left untreated, patient care is compromised, whereas additional overall health care cost increases [25, 26]. Given these findings, research has spurred investigation of the utility of integrating behavioral interventions to improve diabetic outcomes, with a focus on diabetic patients with depression.
There is emerging support for diabetes treatment in integrated medical behavioral primary care [27]. Integrated models may generate greater frequency of patient participation and engagement in behavioral care with co-occurring physical and mental health concerns [28]. Integrating evidence-based psychotherapy with comprehensive health services improves mental health outcomes for those with diabetes [29–32]. Integrated care generates higher rates of referral and treatment initiation than referral to the specialty system [15, 16].
A key distinction is emerging between behavioral health and behavioral care or health behavior. Behavioral health refers to care delivered to patients whose primary presentation involves a mental health or a substance abuse diagnosis or condition. Behavioral care or health behavior primarily focuses on behaviors that contribute to decreased function and that limit the effectiveness of medical care. In caring for patients with chronic conditions, some of the identified issues may include mental health conditions or substance use problems, but the primary focus of integrated care is lifestyle issues that affect health status. One prominent example is the diabetes prevention program, with multiple trials indicating the salutary effects of modifying behavioral risks on progression from prediabetes to diabetes [33]. However, when follow-up is referred outside the primary care practice, patient reach and patient uptake drop. Referral within the practice can result in treatment engagement rates from 75% to 90% [15, 16].
The rural primary care practice examined in this case study had a strong foundation in integrated behavioral care based on 25 years of increasingly coordinated team-based care among medical and behavioral health providers [16, 34]. Based on this success, clinical leaders were strongly motivated to build a population-based, chronic care coordination algorithm for patients with poorly controlled diabetes. This project offered these leaders the opportunity to use Lean management to accomplish their goal.
Lean management is a mechanism to assist in the change of health care [35] by improving processes of care, for example, by reducing the time patients wait before receiving treatment [36–38]. Lean uses a structured, team-based problem-solving method to redesign work processes and center care delivery on the needs of patients [39]. Process improvement methods have a record of success in primary care [40]. The specific use of Lean management in primary care has emerged over the past 20 years, with a step-by-step process for conducting Lean in a clinic published in 2006 [41]. These clinic-based efforts usually focused on improving productivity and patient satisfaction. However, the value of Lean, as seen by leaders within and beyond health care [42, 43], highlights it as a method for improving the quality of services provided and patient outcomes. The use of Lean management to integrate primary care and behavioral health services is novel, with a recent case study being the first to report on the use of Lean to implement integrated care in a clinic setting [10]. The Lean principles involved in improving the quality and outcomes of care in this case study include matching clinical staff and provider services with the level and timing of patient need, without utilizing more resources (and costs) than necessary [42, 44].
PURPOSE
This case study describes the structured approach used by one rural primary care practice to develop and pilot a clinical algorithm for integrated medical/behavioral chronic care coordination. This algorithm used a stepped-care model with an interprofessional team that included a community health nurse, rather than a physician or psychologist, to engage patients in need of integrated care. The algorithm focused on patients with diabetes and elevated blood sugars and sought to improve their glycemic control, frequency of laboratory testing, and engagement in self-management skills. This case study captured the results of the pilot, as well as the perceptions of providers and staff on the processes of care and outcomes of the project.
METHODS
Design
This prospective, multi-methods case study observed a primary care practice as the unit of study over the course of development of a clinical algorithm. The primary outcome was the change in HbA1c values in pre/postimplementation comparisons. Secondary outcomes included the change in time between lab tests, engagement of patients in the intervention, staff time in conducting the intervention, and provider and staff perceptions about the project outcomes.
Setting
This case study took place in a family medicine practice in a northern Vermont academic health system. In 2014–2015, the practice provided 17,700 adult and child primary care visits/year. These visits provided care for approximately 7,400 unique individuals, 9.6% diagnosed with diabetes, served by six physicians, one advanced practice registered nurse, and two psychologists 5 days/week. The practice used an Epic electronic health record (EHR) to manage and document care. It included a registry identifying patients diagnosed with diabetes. This registry maintained updated data on patient visits and HbA1c laboratory results, used by clinical staff to identify patients with poor glycemic control for follow-up phone calls, additional lab tests, and new visits.
At the start of this project, the practice used an integrated behavioral health model employing two behavioral health providers (BHPs) (total 1.0 full time equivalent employee [FTE]). The BHPs, one a Ph.D. psychologist and the other a Diplomate of Clinical Psychology (UK), provided behavioral health services integrated into the practice’s primary care services and systems, consulted directly with medical providers on coexisting medical conditions, and provided a bridge to community mental health specialists. They provided interventions for substance use and mental health disorders and supported diet, exercise, medication adherence, and stress management. They also counseled on the behavioral aspects of medical conditions such as diabetes, lung disease, heart disease, asthma, and others. Separately, an on-site community health nurse (1.0 FTE at study start), funded by the state Department of Health, served high need patients by assisting in resource identification and connection, coordination of medical services, patient education, and other support services. Prior to this initiative, the behavioral clinicians and the community health nurse had limited interactions, did not seek out populations needing health behavior support, did not assess such patients for health behavior risks and assist them in developing plans outside clinic visits, and did not provide support for those plans outside the clinic visits. The clinical algorithm addressed all of these elements.
The medical providers in the practice were paid on a salary basis; one BHP was paid on salary and the other per patient contact. Most were assigned to 50% or more of clinic time. Although no financial incentives were provided for improving care quality or exploring innovations, providers demonstrated a long-term commitment to quality improvement (QI) projects, for example, by committing themselves to the integration of behavioral health as part of the practice since 1985. Support of integration has also been assisted by regulatory and reimbursement changes in Vermont since 2010, when state policy makers and leading local health insurers allowed primary care to bill directly for behavioral health services using traditional psychotherapy codes and health and behavior codes and reduced prior approval requirements [15, 16].
The practice leaders responded to an invitation to participate in a project funded by the National Institute of Mental Health, the purpose of which was to demonstrate implementation strategies of integrated behavioral health. The five-member team included a physician, a psychologist, a registered nurse, a community health nurse, and the practice manager and was facilitated by two of the authors (CvE, RK). One, RK, was also a psychologist member of the practice.
Subjects
The clinical algorithm targeted a population of patients with Type 2 diabetes and poor glycemic control whose care was overseen by two primary care providers who volunteered for the pilot. As described in the words of a QI team member, these were patients who were “…not following their diets or using chem strips, who might say ‘I can’t bring myself to do it’”. The team wanted to include patients who understood what they should do but were not sufficiently motivated or who were noncompliant for other behavioral reasons that are difficult to detect during medical visits. The team sought to include all patients with an HbA1c laboratory result greater than or equal to 8.0 within the past 6 months, a benchmark chosen empirically to avoid engaging patients who were less likely to need an intervention and to focus the practice’s scarce resources on patients with the highest need. The team excluded patients who were under the care of an endocrinology service or receiving end-of-life care, resided in a nursing home or skilled nursing facility, were cognitively impaired, less than 18 years of age, or without a practice visit in more than 2 years. The team selected subjects using a diabetes patient registry to identify patients who met the eligibility criteria as of February 1, 2015.
A manual chart review followed, conducted by a registered nurse from the team to exclude ineligible patients. This selection process resulted in a total of 20 eligible patients, recruited over 3 months from February 12, 2015 to May 5, 2015, to pilot a demonstration of chronic care coordination by a community health nurse supported by an interprofessional care team, including medical and behavioral providers, a registered nurse, and nonclinical staff. This represented a patient caseload that the team felt comfortable supporting as it tested its clinical algorithm. Eligible patients were invited to participate. Those who agreed were assigned to the intervention arm. Patients who met clinical criteria but preferred to continue usual care with their primary care providers or were cared for by other providers in the practice were included in the control arm.
Intervention
The team developed a chronic care coordination algorithm for patients with diabetes and poor glycemic control by using A3 problem-solving [39] to redesign workflow with Lean tactics such as reducing wait times and engaging underutilized human resources. The algorithm built on previous studies demonstrating the effectiveness of self-management training for diabetes patients [45, 46]. The team studied the current workflow starting from registry notification of an overdue visit, through visit scheduling, the primary care visit, referral for behavioral health (BH), and finishing with a scheduled appointment for a BH visit.
The team created a systems’ diagram of this process, identifying problems with their current process and changes needed to achieve coordinated, team-based care. Team members identified an ideal future workflow for between-visit-care, in which participating patients would automatically receive invitations to complete a health risk assessment survey, identify health risks, and endorse those that they would be willing to improve. The team developed a clinical algorithm guiding clinical staff to make follow-up phone calls to each patient with a completed survey, confirm survey results, assess patient needs, support goal setting, and engage the patient in treatment options tailored to the patients’ goals. Using a stepped-care model, patients needing advanced behavioral health services beyond the clinical algorithm were seen by the behavioral health provider, an approach consistent with the chronic care model. In this pilot, a successfully “engaged” patient was one who created a self-management plan and agreed to carry it out. All patients continued to receive usual care with their primary care providers.
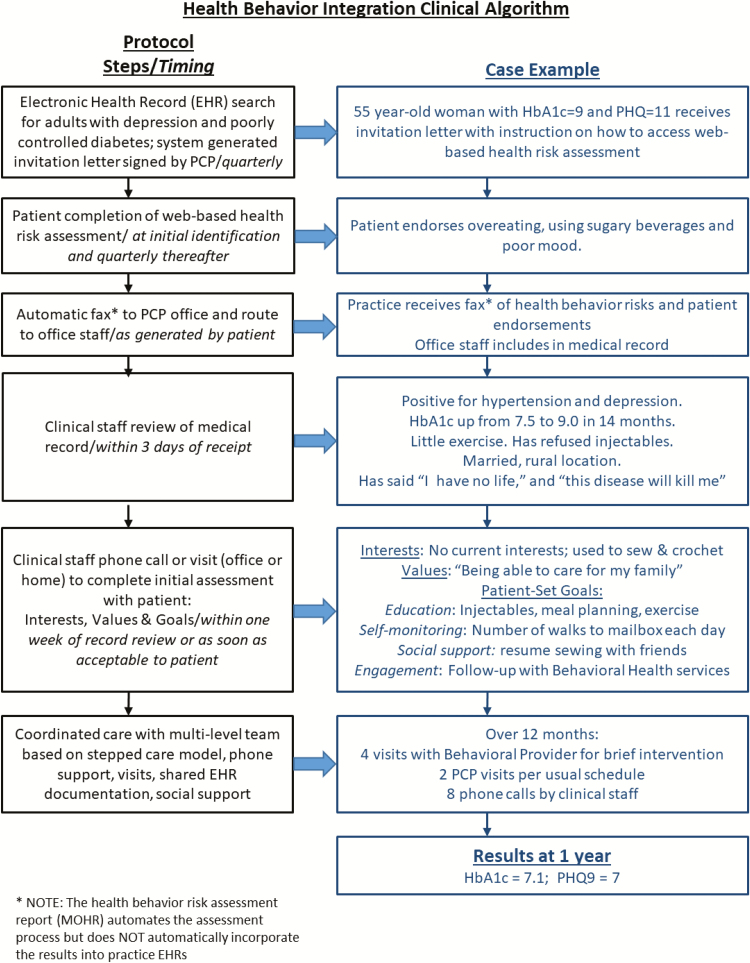
The clinical algorithm steps through a series of coordinated actions, which the team illustrated in a flow diagram and case example (Fig. 1). Prior to this project, the hypothetical patient in Fig. 1 would be identified for outreach on a quarterly basis if she missed an expected lab test or primary care provider (PCP) visit or had an HbA1c of 8 or higher. This project supplemented this workflow by using data from the EHR system to generate letters and create a patient log to document follow-up patient contact.This new workflow was supported by the community health nurse who provided a care management role for patients with chronic conditions. She initiated contact with patients and followed up with assessment and patient-generated care plans that could include education, self-education, self-monitoring, development of social support among friends and family, and connection with community resources. Through this process, patient identification and engagement in the project were driven by data generated from population-based registry and data sources, rather than relying on the inefficiencies of physician referrals to behavioral health providers. The medical and behavioral providers’ primary role was assisting in the design and development of the clinical algorithm. They did not provide the intervention, chronic care coordination, but became involved based on patient need using the stepped-care model, which occurred for one patient who was stepped up for treatment with a behavioral health provider. The community health nurse continued to provide support for patients who did not need more intensive care and maintained communications with the rest of the care team through shared EHR documentation and EHR-supported messaging.
Fig 1.
Health behavior integration clinical algorithm. *Note: The health behavior risk assessment report (MyOwnHealthReport) automates the assessment process but does not automatically incorporate the results into practice electronic health records.
After identifying eligible subjects for the pilot, the practice manager (a member of the team) set up a macro (a “dot phrase” in the EPIC medical record system) to generate form letters including a web-site link to “MyOwnHealthReport” (MOHR), a health behavior risk assessment. The MOHR was selected because it is a brief 10-dimension behavioral risk appraisal, validated for use in primary care settings [47]. The letter was personalized with the patient’s name, address, the signature of the patient’s PCP underneath and explained the pilot, invited participation, and noted future telephone follow-up. Two weeks post-mailing, the community health nurse initiated follow-up telephone calls, either to assist in the completion of the assessment or to discuss its results. To assist those conversations, the team developed a set of telephone call scripts to support completion of the assessment with the patient, review the assessment results with the patient, help set patient-determined goals, and provide tailored interventions. Each conversation ended with a plan for another follow-up telephone call or a scheduled visit with the community health nurse or with the patient’s PCP or a BHP. The community health nurse reinitiated unreturned phone calls for up to 1 month after making the first call, with a maximum of five attempts.
The pilot continued for 5 months from March 3, 2015 to August 3, 2015 with data collection from the EHR continuing to September 18, 2015. During this time, the community health nurse conducted outreach and phone calls, the practice manager programmed recruitment letters, and the providers and practice nurse coordinated their in-office care based on assessment and plan data in the EHR that was documented by the community health nurse. All met together regularly to fine-tune the intervention. The pilot concluded with team member interviews conducted by one of the authors (CvE) to identify facilitators and barriers to expand this pilot. The team reviewed the final evaluation of the work and provided confirmation of their agreement with its results.
Data collection
The practice’s EHR provided deidentified data for patients in this pilot, including HbA1c laboratory test values and test dates for the 5 years before the study through one and a half months afterwards. Clinical staff collected field notes on patient interactions by logging each contact into a standardized spreadsheet designed by the team for this purpose. Team members logged time spent in all efforts related to patient contact and engagement.
All practice providers and staff received invitations to participate in pre/postimplementation electronic surveys managed by REDCap [48], a secure web-based application designed to support data capture for research studies hosted at the University of Vermont. Study surveys collected perceptual data of the practice’s internal environment (climate and infrastructure) and changes in workflow processes (identifying and treating patients) using a Likert scale. Postproject surveys also collected ratings on perceived outcomes of the project (quality, efficiency, and access), the acceptability of the algorithm, and the acceptability of the Lean management method. Surveys were based on a previously used instrument [10] with minor modifications based on the intervention and then tested on and refined with a convenience sample of 12 people familiar with the study.
Three of the five team members, representing medical provider, nursing, and administrative perspectives, participated in semistructured interviews at the close of the project to identify facilitators, barriers, and outcomes of the study. These data were added to field notes collected during team meetings by facilitators. Results of the study were shared with all team members via email with follow-up feedback in-person and electronically.
All practice members received verbal information about this research study. The five team members who volunteered to work on the study based on practice role and leadership position were free to accept or decline this opportunity without penalty. The Committees on Human Research at the University of Vermont reviewed and approved the study protocol.
Analytic methods
The authors used Stata 13.1 (Stata Corporation, College Station, TX) for data management and descriptive statistics. These included comparisons across two time periods (preintervention and postintervention) using two-sample t tests for continuous data and Fisher’s exact tests for categorical data. All analyses were two-tailed with p < 0.05 required for statistical significance. Qualitative analysis used a grounded theory approach, subjecting the data to a constant comparative analysis as additional data were collected through field notes, comments made on surveys, and interviews of team members.
RESULTS
Patient characteristics
An electronic search of the diabetes registry identified 179 eligible patients. Manual chart review to identify exclusions reduced this number to 116. Patients under the care of the two participating providers were selected for recruitment: a total of 20. These patients received recruitment letters from their provider and follow-up phone calls. Four did not respond to telephone calls or messages, three refused, two were lost because of administrative or system failure to track the self-assessments, and one wished to be scheduled with her PCP rather than continue with the community health nurse. The characteristics of the 10 patients that agreed to participate were compared to the 106 that did not; these two groups did not differ significantly (Table 1).
Table 1.
Patient characteristics
| Patients in intervention arm | Patients in control arm | p value | |
|---|---|---|---|
| Patients | 10 | 106 | |
| Age, mean (SD) | 57.4 (10.6) | 57.9 (12.3) | 0.92 |
| Male (%, SD) | 60 (0.52) | 59 (0.49) | 1.00 |
| White (%) | 100 | 93 | 1.00 |
| Medicare (%) | 20 | 34 | |
| Medicaid, etc. (%) | 10 | 14 | 0.72 |
| Commercial (%) | 70 | 52 | |
| Baseline HgA1c (SD) (n) | 9.97 (1.98) (10) | 9.45 (1.78) (104)a | 0.39 |
aTwo patients in the control arm did not have HgA1c values in the electronic health record reporting system, although they had previously been identified as eligible via practice reports.
Patient outcomes
Of the 10 patients who agreed to participate in the pilot, nine (45% of the original 20) completed the self-assessment of health behavior risk and developed a self-management plan. One patient completed the self-assessment but did not respond to further contact or successfully engage, continuing with usual care with the primary care provider. There were no significant differences in clinical outcomes between the intervention and control groups. In particular, when compared to controls, the eight intervention patients with two HbA1c lab values during the study period experienced a nonsignificant improvement in an unadjusted analysis of these outcomes (a mean decrease in HbA1c of 0.45 compared with a decrease of 0.19) and a shorter lapse between lab tests (a mean of 145 days compared with 185; see Table 2). Examples drawn from the patient contact log demonstrate the processes and outcomes of the new algorithm for two patients (see Table 3).
Table 2.
Measures
| Intervention (n = 8) | Control (n = 71) | p value | |
|---|---|---|---|
| Preintervention period | |||
| HgA1c value, mean (SD) (range) | 9.21 (1.16) (8.2–11.1) | 9.43 (1.74) (6.1–14.4) | 0.74 |
| Postintervention period | |||
| HgA1c value, mean (SD) (range) | 8.76 (0.95) (7.2–10.3) | 9.24 (1.75) (5.6–13.7) | 0.45 |
| Comparison of pre- to postperiods | |||
| Change in HgA1c, mean (SD) (range) | −0.45 (0.86) (−1.8–1.0) | −0.19 (1.56) (−4.3–4.8) | 0.64 |
| Number of days between tests, mean (SD) (range) | 145 (39) (101–202) | 185 (91) (83–462) | 0.22 |
Table 3.
Example outcomes
| Patient S03 | Patient K10 | |
|---|---|---|
| My own health report completion | Completed | Completed |
| Patient endorsement | Weight loss | Stress management and exercise |
| Community health nurse support | Scripts and referral list for community health resources | Ongoing, follow-up phone calls |
| Plan | Weight watchers with husband and confirmation of next steps | Gym membership and behavioral health provider services; diet |
| Challenges | Orthopedic problem | Dislike of most vegetables |
| Facilitators | Exercise bike, orthotic boot | Reset meal timing |
| Follow-up status | Leg healing and blood sugars improved | Improvement in all goals except diet |
Operational impact on staff time
Staff made, on average, three calls to engage patients in self-management, with a maximum number of five calls before engagement. The pilot, in total, consumed 16.3 hr of staff effort over 153 days or 6.49 hr/month in reaching out to 20 patients. Staff spent an average of 0.47 hr total for each patient they did not engage and 0.49 hr/month for each patient they did engage (1.2 hr per patient over the 5 months of the pilot). The pilot did not affect the clinical time of the medical or behavioral providers with the exception of one new referral to a behavioral health provider.
Provider and staff survey respondents
A total of 33 providers and staff were present during the study, with 27 at the start (9 providers and 18 staff), of whom 59% responded to the preintervention survey. At the end of project, 17 months later, 29 providers and staff were present (9 providers and 20 staff), of whom 71% responded to the postintervention survey. Those that responded to either the last survey or both surveys accounted for 36% of the total eligible (12 of 33 respondents), a diminished response rate that was affected by turnover of 17% of staff during the study period. These respondents were, on average, 54 years of age (SD = 11.5) and in their profession for 13 years (10.2). Providers made up 33% of respondents; females made up 83%.
Survey results
The respondents’ perceptions of the practice context (Table 4, items 1–11) did not change significantly over the course of the project except for item 2, “We were able to implement changes in how behavioral health services are offered,” which decreased (worsened) by 0.86 points (p = 0.05) and item 9, “After the project was complete, all changes to our work were explained to us before being carried out,” which decreased by 1.0 (p = 0.02). These changes were explored in the team member interviews, described below.
Table 4.
Pre–post survey comparisons of practice context and process change
| n | Change in mean (SD) | Range of change | 95% CI | p value | |
|---|---|---|---|---|---|
| Practice context | |||||
| 1. We were committed to improving how behavioral health services are offered in our practice. | 9 | 0.22 (0.67) | −1 to 1 | −0.29, 0.73 | 0.35 |
| 2. We were able to implement changes in how behavioral health services are offered. | 7 | −0.86 (0.90) | −2 to 0 | −1.69, −0.03 | 0.05 |
| 3. The behavioral health services’ project was a good fit for the values of our practice. | 6 | 0.00 (0.63) | −1 to 1 | −0.66, 0.66 | 1.00 |
| 4. Our practice supported the time needed for a provider and at least two staff to meet together for 8 hr over 2 months to work on the behavioral health services’ project. | 4 | 0.50 (1.29) | −1 to 2 | −1.55, 2.55 | 0.50 |
| 5. We had a “champion” who supported the project by helping other practice providers and staff understand the project and help solve problems. | 6 | 0.50 (1.38) | −1 to 3 | −0.95, 1.95 | 0.42 |
| 6. Our practice provided the financial resources to carry the behavioral health services project out. | 5 | 0.00 (0.71) | −1 to 1 | −0.88, 0.88 | 1.00 |
| 7. We think it is important to do projects that improve the quality of care. | 8 | 0.00 (0.53) | −1 to 1 | −0.45, 0.45 | 1.00 |
| 8. During the project, everyone knew what the team was working on and what it was planning. | 7 | −0.71 (0.95) | −2 to 0 | −1.59, 0.17 | 0.09 |
| 9. After the project was completed, all changes to our work were explained to us before being carried out. | 7 | −1.00 (0.82) | −2 to 0 | −1.76, −0.24 | 0.02 |
| 10. Once the project team was finished, the project did not increase the amount of work we do in the practice but reduced the amount of work we do or kept it the same. | 6 | 0.50 (0.84) | −1 to 1 | −0.38, 1.38 | 0.20 |
| 11. After the project was complete, the project team took the time to think about how it worked. | 5 | −0.40 (0.55) | −1 to 0 | −1.08, 0.28 | 0.18 |
| Process change | |||||
| 12. Our practice usually identifies patients who need behavioral health services promptly and accurately. | 9 | 0.56 (1.74) | −1 to 4 | −0.78, 1.89 | 0.37 |
| 13. Our process for providers referring patients who need behavioral health services is usually completed smoothly and efficiently. | 8 | 0.38 (0.52) | 0 to 1 | −0.06, 0.81 | 0.08 |
| 14. Our front desk scheduling process for patients needing behavioral health appointments is usually timely, free of errors, and convenient for the patient. | 8 | 0.00 (0.53) | −1 to 1 | −0.45, 0.45 | 1.00 |
| 15. When one of our patients has been scheduled for behavioral health services, they are usually able to make their appointment and start treatment as scheduled. | 8 | 0.13 (0.83) | −1 to 1 | −0.82, 0.57 | 0.68 |
| 16. When one of our patients starts receiving behavioral health services, they usually continue with their care for as long as they need it. | 6 | 0.33 (1.21) | −2 to 1 | −1.60, 0.94 | 0.53 |
All response options were based on a five-point Likert scale: 1, strongly disagree; 2, disagree; 3, neutral; 4, agree; 5, strongly agree. Responses of “I Don’t Know” were not included in the analysis. A positive change in mean represents greater agreement than previously scored.
Perception of process measures regarding behavioral health services (Table 4, items 12–16) did not change significantly.
Postintervention perceptions of the impact of the pilot on quality, efficiency, and access to care were positive (mean scores higher than 3.0 on a Likert scale from 1 [strong disagreement] to 5 [strong agreement]) for two of six items (Table 5). Perceptions were positive for acceptance of the algorithm in two of four items and support of the pilot methods (Lean management) in five of six items. There was agreement that the method of conducting the project had useful outcomes (4.14), promoted a willingness to use a similar approach to make changes (3.86), elicited acknowledgment that the project resulted in improved quality of patient care (3.67), and resulted in more useful tasks (3.60).
Table 5.
Postsurvey evaluations
| Mean score | Range | SD | n | |
|---|---|---|---|---|
| Project outcomes | ||||
| 1. The behavioral health project has improved the quality of patient care. | 3.67 | 3–5 | 0.71 | 9 |
| 2. The behavioral health project has made us more efficient as a practice. | 2.89 | 2–5 | 1.17 | 9 |
| 3. Patients needing behavioral health services have access to a behavioral health provider inside the practice more quickly than before. | 2.91 | 2–4 | 0.94 | 11 |
| 4. Patients needing behavioral health services have access to a behavioral health provider outside the practice more quickly than before. | 2.82 | 1–5 | 1.25 | 11 |
| 5. Overall, patients needing behavioral health services do not wait as long for a behavioral health visit as they used to. | 2.80 | 1–5 | 1.40 | 10 |
| 6. We do a better job caring for patients needing behavioral health services than we did before. | 3.20 | 2–5 | 1.03 | 10 |
| Acceptability of algorithm | ||||
| 7. The behavioral health project made my job easier. | 2.82 | 2–4 | 0.75 | 11 |
| 8. The behavioral health project resulted in fewer steps or less work in helping patients needing behavioral health services. | 2.90 | 2–4 | 0.88 | 10 |
| 9. The behavioral health project improved how well I can do my job. | 3.18 | 2–5 | 0.98 | 11 |
| 10. The behavioral health project resulted in more useful tasks to help patients needing behavioral health services. | 3.60 | 2–5 | 0.84 | 10 |
| Acceptability of Lean management method | ||||
| 11. The method of conducting the behavioral health project was easy to do. | 3.25 | 1–4 | 1.16 | 8 |
| 12. The method of conducting the behavioral health project had useful outcomes. | 4.14 | 3–5 | 0.69 | 7 |
| 13. The Lean Toolkit helped us make changes that were part of the behavioral health project. | 3.71 | 1–5 | 1.38 | 7 |
| 14. I would be willing to use a similar Lean Toolkit to make changes in other parts of the office. | 3.86 | 2–5 | 1.07 | 7 |
| 15. I would recommend this Lean Toolkit to other practices interested in integrating behavioral health services. | 3.71 | 2–5 | 1.11 | 7 |
| 16. The time I took to do the behavioral health project using the Lean Toolkit was made up by time saved in the work I do in the practice. | 2.67 | 1–4 | 1.03 | 6 |
All response options were based on a five-point Likert scale: 1, strongly disagree; 2, disagree; 3, neutral; 4, agree; 5, strongly agree. Responses of “I Don’t Know” were not included in the analysis.
Qualitative results regarding barriers and facilitators
Grounded theory data analysis compared themes from field notes, survey comments, and team member interviews conducted at the close of the study. Facilitators that supported the pilot included:
The development of the algorithm depended heavily on a motivated physician leader and champion as well as a supportive practice manager.
Participants felt the Lean method provided structure as the project moved from a focused QI project to a broader process redesign activity. Lean was seen as successful in helping the team develop an algorithm for coordinating care for patients by the MOHR self-assessment and providing telephone contact to offer education, self-monitoring skills, and access to resources.
Barriers that challenged the pilot:
Deployment of the algorithm encountered multiple barriers, including the loss of key personnel (the community health nurse) at the point of implementation that delayed start-up by 6 months. The pilot restarted at the time a replacement was hired. However, the role was funded as a temporary and part-time position, limiting reach (number of patients) to 10.
Deploying the project in relative isolation from standard office procedures resulted in minimal disruption to providers and staff that facilitated its operation. However, this also created barriers to communicating what was needed to support the pilot, such as keeping track of faxed incoming patient self-assessments and preventing loss of patients to follow-up.
The needs for automated support to track incoming patient data and communicate changes in office procedures were not met because of competing priorities (implementation of new registries, arrival of a JCAHO surveyor team, and an NCQA survey). There was concern noted about the inability of higher administrative levels to support changes to the EHR. As a result, the population-based approach in coordinating the care for patients with chronic conditions such as diabetes was discontinued pending a future opportunity to align EHR improvements with these clinical goals.
Team members reviewed the results of the case study and endorsed them with minor changes.
DISCUSSION
Primary care is a complex system, within which the role of psychology is evolving. In our project, the primary role of the psychologist was to assist in design, with minimal involvement in delivery of the intervention. The proposed model of care was derived from the study by Kathol et al. [6] who posited a set of elements for successful integrated care such as team-based population care and engaging team members in the delivery of care up to the scope of license. In addition, it followed principles generated by the IMPACT model in Washington, suggesting stepped care involving only those staff members necessary to generate outcomes and engaging higher level staff if care was not progressing [14].
Therefore, the algorithm leveraged available time of nonprovider clinical staff, working at the highest level of their licenses, to provide chronic care coordination. In the course of this pilot, all initial and most follow-up interventions were delivered by the community health nurse with support from the practice nurse and the practice manager, guided by patient-reported and system data rather than by physician referral. This algorithm functioned successfully in part because of agreement by the participating medical and behavioral health providers to support the community health nurse’s activities. Given the limited resources for behavioral health clinicians in primary care, and the limited health care resources in rural areas in general, use of provider time in the development of a clinical algorithm can assist the spread (reach) of behavioral intervention to a broader primary care population needing chronic care coordination. On the other hand, bringing such an intervention to scale requires substantial care manager and nursing staff time to complete phone calls and follow-ups. From the results of this pilot, staffing support is likely to require a half hour of effort for every patent contacted and a half hour per month for every patient engaged. Bringing this pilot to scale requires significant practice planning and support.
The Lean management approach was successful for the duration of this pilot project. The practice provided behavioral services through chronic care coordination and supported patients who completed the intervention to manage their condition. The pilot did not reduce other BH service activities as both BH providers continued their regular schedules, uninterrupted except for one referral from the pilot group.
Perceptions of the pilot by providers and staff were mixed. Survey results indicated some frustrations in implementing the pilot and communicating those changes across all levels of providers and staff. Although the pilot was perceived as improving the quality of patient care and allowing practice members to do a better job in caring for patients, it did not improve efficiency or access to BH providers. Very likely, this was because the intervention improved access to the community health nurse, as an alternate provider to support BH needs. What was most striking about the response to the pilot was the strong support of the Lean management method, which was perceived as easy, useful, produced changes needed for the pilot and generated willingness to use this approach in the future.
The mixed message from these findings is rooted in the barriers that challenged the pilot, most notably the delay in replacing the loss of the community health nurse, exclusion of the pilot from standard office procedures to help prevent loss of patients to follow-up, and the need for changes in the EHR. These findings are consistent with the literature on Lean, in which challenges to successful Lean management are found in administrative barriers and the positive effects of Lean include increased staff satisfaction in improved healthcare performance [49–51]. Although the authors observed an improvement in outcomes related to delays in lab testing and physiologic control, these results were not significant. A larger sample size is needed for further study.
The use of an integrated chronic care coordination algorithm, developed using Lean, is novel to healthcare and rural primary care practices. The QI team needed the support of willing primary care providers and focused on their patients in recruitment and selection. This method of piloting an innovation in integrated care with a small group of providers in rapid tests of change can help demonstrate its value and recruit other providers who need time to evaluate its effect. This pilot suggests that a larger-scaled project will require other support as well: workflow design, consistent staffing in the absence of grant funding, and integrated documentation in EHR systems.
A real challenge is that organizations have extremely limited resources to respond to an innovation in care that requires significant EHR redesign/retooling. This is not a trivial issue. Without such retooling, the probability of a novel intervention being sustainable significantly decreases. Lack of EHR redesign resources limits patient access to, and therefore the number of patients reached by, behavioral care. Clinical algorithms of care rely on efficient, effective information communication across patients and multiple providers. When bringing such a pilot to scale, the ability to use the EHR is requisite. For instance, receiving MOHR patient survey results by fax (Fig. 1) created a workflow challenge around incorporating the data into the patient record. Without EHR engagement, such innovations are limited, as are reach and access. However, there is increasing interest in using data systems to influence care outcomes, with some early examples of applications in behavioral health [52]. Discussion is growing about the role of behavioral health in team-based population health with assistance from EHR technology, but uptake is slow.
The adaptation of EHR in practice-based improvement is a key facilitator of innovation uptake, including integration of care [53–55]. Key organizational features of EHR that systematically track patient data can improve diabetic outcomes [56–59]. To track patients effectively, practices may need to adapt or develop new work flows. Cykert and colleagues demonstrated that the facilitation of EHR implementation was associated with improvements in diabetes outcomes in practices that achieved meaningful use of their EHR [60]. Gill and coauthors found that practice-based quality-of-care improvement studies conducted with EHR resulted in greater control of diabetic risk factors. They suggested that this method has significant potential for effecting improvement across large numbers of outpatient practices [61].
The results of the pilot, while limited, support continued focus on an algorithmic approach to population-based integrated behavioral care, targeting a specified group of patients with shared criteria that require a structure for optimal treatment. This method may be scaled to a population-based approach, with the appropriate administrative support. There is an imperative to respond to the need for chronic care coordination with effective, efficient approaches. A corresponding investment of organizational resources is needed to bring this to fruition. Doing so will assist in chronic care coordination and improve patient outcomes for patients who have complex care needs and are at risk of high costs and poor health status.
Limitations
As a case study report, the results of this project are not expected to be generalizable. Participants were not blinded to their role in the intervention, and team members may have biased the results of some postproject surveys and any of their interviews, given their direct investment of time and effort. Patient outcomes drawn from the EHR are unlikely to have been affected by participation bias. Improvements noted in the results lacked statistical significance, because at least in part of the small size of intervention group, turnover in the community health nurse role, turnover among staff within the practice, and lack of ability to adapt the EHR to support documentation workflows. Nonetheless, patients appeared to respond to the clinical algorithm, and providers and staff voiced their support of the method as useful in moving towards the goals of the pilot.
The small size of the pilot and the low patient recruitment rate of 45% are consistent with the nature of QI projects and the need to provide a demonstration of proof of concept to the practice’s leadership. While the patients in the pilot reflected the community population, this community is mostly white, not reflecting the racial diversity of the U.S. population. The intervention was limited to a single, motivated site and collected a limited number of outcomes related to chronic care. The authors were not able to report clinical data on biomarkers and outcomes for individual patients. This practice benefited from three resources that are not universally available to primary care: a community health nurse funded by an outside agency (in this case, the state’s department of health), a committed medical leader, and knowledgeable, integrated psychologists. Consistent with the Lean management approach, however, practices can use the method to develop local solutions that take advantage of their own resources and iterate this development process over time to improve results using a structured and effective process.
CONCLUSIONS
Systematically designed clinical algorithms for chronic care coordination can contribute to an improved model of integrated medical behavioral care to help patients manage their conditions. A small sample, population-based, team-supported integrated care pilot demonstrated the feasibility of designing such an algorithm and implementing it in a busy primary care practice, although statistically significant changes in patient outcomes were not observed. The pilot was perceived to improve the quality of care, provide a better way to care for patients needing chronic care coordination, and produce useful outcomes. A clinical algorithm to coordinate care for patients with chronic conditions is a strategy worth continued study in a variety of settings to enhance patient self-management and improve clinical outcomes.
Compliance with Ethical Standards
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The Committees on Human Research at the University of Vermont reviewed and approved the study protocol.
Informed Consent: The requirement to obtain documentation of consent was waived. No animals were involved in this research.
Conflict of Interest: The authors have no conflict of interest to disclose.
Primary Data: The findings reported have not been previously published, and the article is not being simultaneously submitted elsewhere, nor has there been any previous reporting of data. The authors have full control of all primary data and they agree to allow the journal to review their data if requested.
Acknowledgments
The authors thank Kay Barrett DCP, Catherine Craig RN, Dale Stafford MD, Dorothy Martin RN, Valerie Smith BS (Berlin Family Practice, UVM Medical Center) and Stephanie Brennhofer MS RDN (Arizona State University). This project was supported by grant number 1R03MH099157-01A1 from the National Institute of Mental Health sponsored research under the Dissemination and Implementation Review Section.
References
- 1. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States. JAMA. 2000;291(10):1238–1245. [DOI] [PubMed] [Google Scholar]
- 2. Kathol RG, McAlpine D, Kishi Y, et al. General medical and pharmacy claims expenditures in users of behavioral health services. j Gen Intern Med. 2005;20(2):160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Melek S, Norris D, Chronic Conditions and Comorbid Psychological Disorders. Milliman Research Report2008. Available at https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=0ahUKEwjelaLn_e7YAhVlTd8KHblVAk0QFgguMAA&url=http%3A%2F%2Fus.milliman.com%2Finsight%2Fresearch%2Fhealth%2Fpdfs%2FChronic-conditions-and-comorbid-psychological-disorders%2F&usg=AOvVaw3eZ-zSckPu581BWbDlZZ7L. [Google Scholar]
- 4. Druss BG, Walker ER. The Synthesis Project. Research Synthesis Report. 2011. ;(21):1–26. [PubMed]
- 5. Baird ES, Blount A, Brungardt S, et al. Joint principles: integrating behavioral health care into the patient-centered medical home. Ann Fam Med. 2014;12(2):184–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kathol RG, Degruy F, Rollman BL. Value-based financially sustainable behavioral health components in patient-centered medical homes. Ann Fam Med. 2014;12(2):172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166(21):2314–2321. [DOI] [PubMed] [Google Scholar]
- 8. Strosahl K. Integrating behavioral health and primary care services: the primary mental health care mode. In: Blount A, ed. Integrated Primary Care: The Future of Medical and Mental Health Collaboration. New York, NY: W. W. Norton & Co; 1998:139–166. [Google Scholar]
- 9. Kessler R, Miller BF, Kelly M, et al. Mental health, substance abuse, and health behavior services in patient-centered medical homes. j Am Board Fam Med. 2014;27(5):637–644. [DOI] [PubMed] [Google Scholar]
- 10. van Eeghen C, Littenberg B, Holman MD, Kessler R. Integrating behavioral health in primary care using lean workflow analysis: a case study. j Am Board Fam Med. 2016;29(3):385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Care Coordination Endorsement Maintenance Project 2016–2017 Definition of Care Coordination; 2017. Available at http://www.qualityforum.org/ProjectDescription.aspx?projectID=83375. Accessibility verified January 29, 2017.
- 12. Hunter CL, Goodie JL. Operational and clinical components for integrated-collaborative behavioral healthcare in the patient-centered medical home. Fam Syst Health. 2010;28(4):308–321. [DOI] [PubMed] [Google Scholar]
- 13. Berwick DN, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood, Va). 2008;27(3):11. [DOI] [PubMed] [Google Scholar]
- 14. Hunkeler EM, Katon W, Tang L, et al. Long term outcomes from the IMPACT randomised trial for depressed elderly patients in primary care. BMJ. 2006;332(7536):259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Auxier A, Runyan C, Mullin D, Mendenhall T, Young J, Kessler R. Behavioral health referrals and treatment initiation rates in integrated primary care: a Collaborative Care Research Network study. Transl Behav Med. 2012;2(3):337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kessler R. Mental health care treatment initiation when mental health services are incorporated into primary care practice. j Am Board Fam Med. 2012;25(2):255–259. [DOI] [PubMed] [Google Scholar]
- 17. Vermont Department of Health. 1305 Surveillance: State public health actions to prevent and control diabetes, heart disease, obesity and associated risk factors and promote school health. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gonder-Frederick LA, Cox DJ, Ritterband LM. Diabetes and behavioral medicine: the second decade. j Consult Clin Psychol. 2002;70(3):611–625. [DOI] [PubMed] [Google Scholar]
- 19. Diabetes Control Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;1993(329):977–986. [DOI] [PubMed] [Google Scholar]
- 20. Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160(21):3278–3285. [DOI] [PubMed] [Google Scholar]
- 21. Katon W, Fan MY, Unützer J, Taylor J, Pincus H, Schoenbaum M. Depression and diabetes: a potentially lethal combination. j Gen Intern Med. 2008;23(10):1571–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ludman E, Katon W, Russo J, et al. Panic episodes among patients with diabetes. Gen Hosp Psychiatry. 2006;28(6):475–481. [DOI] [PubMed] [Google Scholar]
- 23. Anderson BJ, Rubin RR.. Practical Psychology for Diabetes Clinicians: How to Deal with the Key Behavioral Issues Faced by Patients and Health-care Teams. American Diabetes Association; Alexandria:1996. [Google Scholar]
- 24. Marrero DG, Peyrot M, Garfield S. Promoting behavioral science research in diabetes. Am Diabetes Assoc. 2001;24(1):1–2. [DOI] [PubMed] [Google Scholar]
- 25. Coleman KJ, Magnan S, Neely C, et al. The COMPASS initiative: description of a nationwide collaborative approach to the care of patients with depression and diabetes and/or cardiovascular disease. Gen Hosp Psychiatry. 2017;44:69–76. [DOI] [PubMed] [Google Scholar]
- 26. Chwastiak LA, Jackson SL, Russo J, et al. A collaborative care team to integrate behavioral health care and treatment of poorly-controlled type 2 diabetes in an urban safety net primary care clinic. Gen Hosp Psychiatry. 2017;44:10–15. [DOI] [PubMed] [Google Scholar]
- 27. Andrews AR, Gomez D, Larey A, et al. Comparison of integrated behavioral health treatment for internalizing psychiatric disorders in patients with and without type 2 diabetes. Fam Syst Health. 2016;34(4):367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blount A, Schoenbaum M, Kathol R, et al. The economics of behavioral health services in medical settings: a summary of the evidence. Prof Psychol Res Pr. 2007;38(3):290. [Google Scholar]
- 29. Hay JW, Katon WJ, Ell K, Lee PJ, Guterman JJ. Cost-effectiveness analysis of collaborative care management of major depression among low-income, predominantly Hispanics with diabetes. Value Health. 2012;15(2):249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Katon W, von Korff M, Ciechanowski P, et al. Behavioral and clinical factors associated with depression among individuals with diabetes. Diabetes Care. 2004;27(4):914–920. [DOI] [PubMed] [Google Scholar]
- 31. Katon WJ, Simon G, Russo J, et al. Quality of depression care in a population-based sample of patients with diabetes and major depression. Med Care. 2004;42(12):1222–1229. [DOI] [PubMed] [Google Scholar]
- 32. Ell K, Katon W, Xie B, et al. One-year postcollaborative depression care trial outcomes among predominantly Hispanic diabetes safety net patients. Gen Hosp Psychiatry. 2011;33(5):436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fisher EB, Fitzgibbon ML, Glasgow RE, et al. Behavior matters. Am j Prev Med. 2011;40(5):e15–e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Young J, Gilwee J, Holman M, Messier R, Kelly M, Kessler R. Mental health, substance abuse, and health behavior intervention as part of the patient-centered medical home: a case study. Transl Behav Med. 2012;2(3):345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gamm L, Kash B, Bolin J. Organizational technologies for transforming care: measures and strategies for pursuit of IOM quality aims. j Ambul Care Manage. 2007;30(4):291–301. [DOI] [PubMed] [Google Scholar]
- 36. Berwick DM. Eleven worthy aims for clinical leadership of health system reform. JAMA. 1994;272(10):797–802. [PubMed] [Google Scholar]
- 37. Miller D.Going Lean in Health Care. Cambridge, MA: Institute for Healthcare Improvement; 2005. [Google Scholar]
- 38. Smith M, Sauners R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. The National Academies Press; Washington, DC: 2012. Available at http://www.nap.edu/catalog.php?record_id=13444. Accessibility verified September 10, 2012. [PubMed] [Google Scholar]
- 39. Jimmerson C, Weber D, Sobek DK II. Reducing waste and errors: piloting lean principles at Intermountain Healthcare. Jt Comm j Qual Patient Saf. 2005;31(5):249–257. [DOI] [PubMed] [Google Scholar]
- 40. Fischer LR, Solberg LI, Kottke TE. Quality improvement in primary care clinics. Jt Comm j Qual Improv. 1998;24(7):361–370. [DOI] [PubMed] [Google Scholar]
- 41. Endsley S, Magill MK, Godfrey MM. Creating a lean practice. Fam Pract Manag. 2006;13(4):34–38. [PubMed] [Google Scholar]
- 42. Spear S, Bowen K. Decoding the DNA of the Toyota production system. Harv Bus Rev. 1999;77(6):97–106. [Google Scholar]
- 43. Berwick D, Kabcenell A, Nolan T. No Toyota yet, but a start. A cadre of providers seeks to transform an inefficient industry–before it’s too late. Mod Healthc. 2005;35(5):18–19. [PubMed] [Google Scholar]
- 44. Zidel TG. A Lean Guide to Transforming Healthcare. Milwaukee, WI: American Society for Quality, Quality Press; 2006. [Google Scholar]
- 45. Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001;24(3):561–587. [DOI] [PubMed] [Google Scholar]
- 46. Renders CM, Valk GD, Griffin SJ, Wagner EH, Eijk Van JT, Assendelft WJ. Interventions to improve the management of diabetes in primary care, outpatient, and community settings: a systematic review. Diabetes Care. 2001;24(10):1821–1833. [DOI] [PubMed] [Google Scholar]
- 47. Krist AH, Glenn BA, Glasgow RE, et al. ; MOHR Study Group. Designing a valid randomized pragmatic primary care implementation trial: the my own health report (MOHR) project. Implement sci. 2013;8(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. j Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Al-Hyari K, Abu Hammour S, Abu Zaid MK, Haffar M. The impact of Lean bundles on hospital performance: does size matter?Int j Health Care Qual Assur. 2016;29(8):877–894. [DOI] [PubMed] [Google Scholar]
- 50. Costa LBM, Filho MG, Rentes AF, Bertani TM, Mardegan R. Lean healthcare in developing countries: evidence from Brazilian hospitals. Int J Health Plann Manage. 2017;32(1):e99–e120. [DOI] [PubMed] [Google Scholar]
- 51. Lawal AK, Rotter T, Kinsman L, et al. Lean management in health care: definition, concepts, methodology and effects reported (systematic review protocol). Syst Rev. 2014;3:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Burdick TE, Kessler RS. Development and use of a clinical decision support tool for behavioral health screening in primary care clinics. Appl Clin Inform. 2017;8(2):412–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Persell SD, Kaiser D, Dolan NC, et al. Changes in performance after implementation of a multifaceted electronic-health-record-based quality improvement system. Med Care. 2011;49(2):117–125. [DOI] [PubMed] [Google Scholar]
- 54. Azad TD, Kalani M, Wolf T, et al. Building an electronic health record integrated quality of life outcomes registry for spine surgery. j Neurosurg Spine. 2016;24(1):176–185. [DOI] [PubMed] [Google Scholar]
- 55. Deutscher D, Hart DL, Dickstein R, Horn SD, Gutvirtz M. Implementing an integrated electronic outcomes and electronic health record process to create a foundation for clinical practice improvement. Phys Ther. 2008;88(2):270–285. [DOI] [PubMed] [Google Scholar]
- 56. Orzano AJ, Strickland PO, Tallia AF, et al. Improving outcomes for high-risk diabetics using information systems. j Am Board Fam Med. 2007;20(3):245–251. [DOI] [PubMed] [Google Scholar]
- 57. Herrin J, da Graca B, Nicewander D, et al. The effectiveness of implementing an electronic health record on diabetes care and outcomes. Health Serv Res. 2012;47(4):1522–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weber V, Bloom F, Pierdon S, Wood C. Employing the electronic health record to improve diabetes care: a multifaceted intervention in an integrated delivery system. j Gen Intern Med. 2008;23(4):379–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zahanova S, Tsouka A, Palmert MR, Mahmud FH. The iSCREEN electronic diabetes dashboard: a tool to improve knowledge and implementation of pediatric clinical practice guidelines. Can j Diabetes. 2017;41(6):603–612. [DOI] [PubMed] [Google Scholar]
- 60. Cykert S, Lefebvre A, Bacon T, Newton W. Meaningful use in chronic care: improved diabetes outcomes using a primary care extension center model. n c Med j. 2016;77(6):378–383. [DOI] [PubMed] [Google Scholar]
- 61. Gill JM, Foy AJ Jr, Ling Y. Quality of outpatient care for diabetes mellitus in a national electronic health record network. Am j Med Qual. 2006;21(1):13–17. [DOI] [PubMed] [Google Scholar]