Abstract
Apelin is a peptide hormone with anti-oxidative and anti-inflammatory activities and is proposed to be a potential therapeutic for many disease conditions, including sepsis. However, short in vivo half-life of the apelin peptide would limit its potential clinical applications. This study aims to investigate the effects of Fc-apelin, a novel long-acting apelin fusion protein, on lipopolysaccharide (LPS)-induced liver injury. Liver injury was induced by systemic injection of LPS in mice. Hepatoprotective activities of Fc-apelin against inflammation were evaluated in LPS mice and/or hepatoma Huh-7 cells with respect to serum ALT, apoptosis, oxidative stress, macrophage infiltration and gene expression. We found that LPS induced systemic inflammation and liver damage. Co-administration of Fc-apelin significantly attenuated serum ALT elevation, diminished LPS-induced apoptosis and ROS production in the liver and in Huh-7 cells, mitigated hepatic macrophage infiltration, and reduced TNFα and IL-6 gene expression. Collectively, Fc-apelin fusion protein exerts protective effects against LPS-induced liver damage and may serve as a potential therapeutic for endotoxin-induced liver injury.
Introduction
Endotoxemia is a common cause of multiple organ failure in the clinic. Hepatic injury by endotoxemia occurs early in multiorgan failure syndrome and is associated with poor prognosis. Despite advances in critical care, treatment for endotoxin-induced liver damage remains a challenge in clinical practice due to limited therapeutics. LPS-induced liver injury is a commonly-used model for mechanistic and therapeutic studies of endotoxemia-induced liver injury. Decades of research have revealed that activation of inflammatory pathways is the major mechanism for LPS-induced liver injury. One prevailing mechanism is that endotoxin activates macrophages in the liver1–3, which recruit and activate neutrophils, leading to over-production of reactive oxygen species (ROS) and oxidative stress in the liver4,5. Overproduction of ROS and small-scale inflammation induce transcription factor nuclear factor-кB (NF-кB) and subsequently results in a large scale inflammatory response and production of proinflammatory cytokines and chemokines6,7, leading to cell death by necrosis, apoptosis, and functional deterioration1.
Accordingly, suppression of oxidative stress and inflammation is found to alleviate LPS-induced liver injury. For example, ROS clearance and suppression of NF-κB alleviate liver injury8–10 in animals. However, clinical remedies for endotoxin-induced liver injury remain limited.
Apelin is a peptide hormone and ligand of the apelin receptor (APJ), a 7-transmembrane G protein-coupled receptor11,12. Numerous studies have demonstrated that apelin is a multifunctional hormone. Besides its inotropic activity, apelin has been reported to be anti-oxidative13–15. In addition, apelin can reduce inflammation and fibrosis and inhibit NF-κB activity in murine pancreatitis16, Moreover, apelin is known to activate PI3K/AKT/ERK signaling pathways and to promote cell survival17,18. We thus hypothesized that enhancement of apelin signaling would counteract LPS-mediated hepatic injury. Yet, apelin is a small peptide and has a very short in vivo half-life of about 8 mins19,20, which wound limit its clinical application21. To prolong its half-life, we engineered an apelin fusion protein by conjugating the Fc-fragment of human IgG with the apelin-13 peptide, resulting in Fc-apelin-13 (abbreviated as Fc-apelin hereafter)22. The fusion protein has an extended in vivo half-life of about 33 hours and has shown beneficial effects of improving insulin sensitivity and cardiac output in obese mice. In this report, we investigated whether Fc-apelin-13 fusion protein had protective effects against LPS-induced liver injury in mice.
Materials and Methods
Fc-apelin fusion protein
The production of Fc-apelin fusion protein is described previously22. In brief, the human IgG Fc-region is conjugated with apelin-13 at the N-terminus through the 3x linker of [Gly-Gly-Gly-Gly-Ser (GGGGS)]23. The protein is expressed in HEK293 cells and purified into homogeneity (>95% purity) through protein A affinity chromatography. Through buffer exchange, the fusion protein is dissolved in 1x PBS buffer, sterilized by filtering through a 0.02 μm filter, and stored at −20 °C until use.
Animal studies
Animal experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Maryland and all methods were performed in accordance with the relevant guidelines and regulations. C57/BL6 female mice, 6 weeks old, were obtained from Charles River Laboratories (Wilmington, MA) and used after one week of quarantine and acclimatization. Experimental animals were randomly divided into four groups according to the treatment they received: (1) control (Cont), receiving injection of vehicle PBS intraperitoneally (i.p.) and subcutaneously (s.c.); (2) Fc-apelin, receiving Fc-apelin (1 mg/kg/day), s.c. and PBS, i.p.; (3) LPS-challenged group (LPS), receiving LPS (1 mg/kg) i.p and PBS, s.c.; and (4); LPS/Fc-apelin group, receiving LPS (1 mg/kg), i.p. and Fc-apelin (1 mg/kg), s.c. Mice were administered PBS, LPS and/or Fc-apelin daily for five consecutive days. Six hours after final injection, the mice were sacrificed. Blood samples were collected, and sera separated and stored at −20 °C until analysis. Depending on the application, liver specimens were embedded in optimal cutting temperature compound Tissue-Teck (Sakura, CA), fixed in 10% neutral buffered formalin for histological analysis, or snap frozen in liquid nitrogen.
Serum ALT and IL-6 assay
Serum alanine transaminase (ALT) was measured by using the DiscretPak ALT substrate reagent (Catachem, Bridgeport, CT) and interleukin-6 (IL-6) levels were measured by ELISA kit (eBioscience, San Diego, CA), as described before24,25.
Cell culture
Human Huh-7 hepatoma cells obtained from the American Type Culture Collection (Manassas, VA) were grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin (complete medium) at 37 °C.
TUNEL assay
The extent of apoptosis was evaluated in cryostat liver tissue sections and Huh-7 cells using the terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) staining kit according to manufacturer’s instruction (Promega, Madison, WI). For TUNEL assay in cells, Huh-7 cells were cultured on glass coverslips in 6-well plates in complete medium up to 80% confluency. The cells were treated with or without, LPS (1 µg/ml) for 12 h in the presence or absence of Fc-apelin-13 (1 µM) and then fixed in 4% paraformaldehyde for 30 min. The fixed cells on cover slides were then processed for TUNEL assay. Nuclei were stained by 4′6-diamidino-2-phenylindole (DAPI) dye (1:800 dilution, Sigma). The number of TUNEL-positive nuclei was averaged over four randomly selected fields per section.
Dihydroethidium (DHE) staining
DHE is a lipophilic cell-permeable dye that can undergo oxidation to ethidium bromide or a structurally similar product in the presence of superoxide and, to a lesser extent, hydrogen peroxide and hydroxyl radicals. Ethidium then binds irreversibly to the double-stranded DNA, causing amplification of a red fluorescent signal, and appears as punctate nuclear staining indicating ROS production26. To measure in situ ROS levels, frozen liver sections were incubated with DHE (5 μM, Invitrogen) at 37 °C for 30 min. For Huh-7 cells, the cells growing in the complete medium were pre-treated with Fc-apelin (1 μM) for 30 minutes and then LPS (1 μg/ml) for 1 hour before addition of DHE (5 μM) at 37 °C for 20 min, in wrapped aluminum foil. Three non-overlapping fluorescent images were obtained per slide with a microscope (Olympus IX-51) and staining density was quantified and averaged by ImageJ software (Bethesda, MD).
Immunostaining and histology studies
Liver tissue samples were fixed in 10% formaldehyde for 24 hours and stored in PBS. The fixed tissues were processed routinely, embedded in paraffin, cut into 5 μm thickness and stained with hematoxylin and eosin (H&E). For immunohistochemistry (IHC), the tissue sections were subjected to deparaffination, 3% H2O2 treatment and antigen retrieval, and then primary antibody F4/80 (clone BM8, eBioscience, San Diego, CA) and HRP-conjugated goat anti-rabbit IgG or goat anti-mouse IgG (KPL, Gaithersburg, MD). Staining was developed with Vectastain ABC kit (Vector Laboratories, Burlingame, CA) and nuclei were stained by hematoxylin. Three non-overlapping areas were obtained and averaged per slide (x 200 magnification) with a microscope (Olympus IX-51).
Quantitative real-time PCR analysis
Total RNAs were prepared from the snap-frozen tissue specimens using TRIzol (Invitrogen), and reverse transcription was carried out in a reaction containing 1 μg of total RNA, poly(dT) primer, and Moloney murine leukemia virus reverse transcriptase using a first-strand cDNA synthesis kit (Promega). Quantitative real-time PCR (qRT-PCR) was conducted on a LightCycler480 using the LightCycler 480 SYBR Green I Master Mix (Roche Diagnostics). The amplification protocol was as follows: 95 °C/5 minutes and 45 × (95 °C/10 second, 60 °C/20 second, and 72 °C/30 second). Following amplification, a dissociation curve analysis was performed to ensure purity of the PCR product. Primers for TNFα and IL-6 were described previously27. Beta-actin mRNA was used for normalization of cDNA loading.
Data analysis
All data are presented as mean ± SEM. One-way analysis of variance (ANOVA) with Bonferroni post-hoc test was used for comparing group differences. Differences were considered statistically significant at the level of P < 0.05.
Results
Effects of Fc-apelin on serum ALT and IL-6
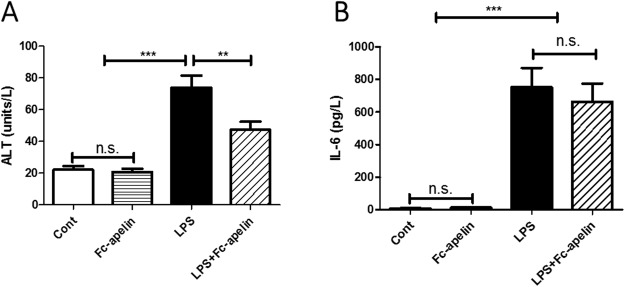
The effect of Fc-apelin on LPS-induced inflammatory liver injury was evaluated by measuring ALT levels in serum. As shown in Fig. 1A, compared with the control group, LPS administration significantly increased serum ALT levels by 3.3 fold from 22.2 to 73.8 unit/L (p < 0.001). Co-treatment with Fc-apelin significantly attenuated the LPS-induced increase in ALT activities, limiting the increase to 47.5 unit/L or 2.1 fold (p < 0.01), though the ALT levels were still higher than the control group. Fc-apelin alone had no effect on ALT in mice. Next, we assessed possible systemic effects of Fc-apelin on inflammation by measuring proinflammatory cytokine IL-6 levels in circulation (Fig. 1B). LPS greatly increased IL-6 with significant individual variations. Although Fc-apelin treatment appeared to decrease IL-6 levels, this difference was not significant. These data suggest that Fc-apelin may be protective against LPS-induced liver injury, without affecting systemic inflammation status.
Figure 1.
Effect of Fc-apelin on serum ALT and IL-6 in LPS-treated mice. (A) Effect on ALT (B) Effect on IL-6. Mice were administered with PBS (Cont) or Fc-apelin (1 mg/kg) with or without LPS (1 mg/kg) daily for five days. Data are expressed as mean + SE; ***P < 0.001 (n = 9 mice/group).
Fc-apelin is protective against LPS-induced apoptosis
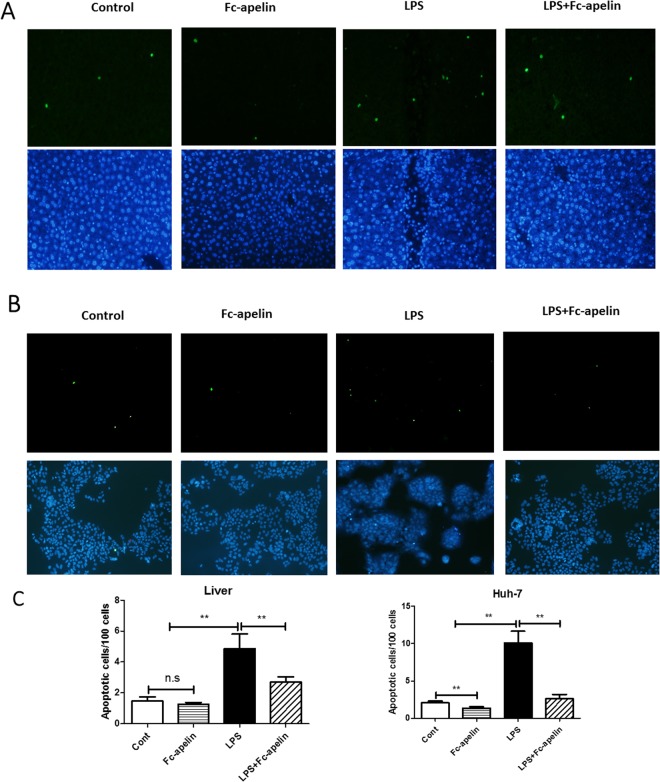
Hepatocyte apoptosis is an important early event underlying acute liver damage28. We therefore measured apoptosis in the liver. As depicted in Fig. 2, TUNEL staining indicated that hepatic apoptosis was increased by about 1.6 fold in mice receiving LPS, which was significantly attenuated by Fc-apelin treatment to levels near the control group. As controls, few apoptotic cells were observed in mice receiving PBS or Fc-apelin alone. To further determine whether Fc-apelin had anti-apoptotic effects at the cellular level, we treated human hepatoma Huh-7 cells with LPS and/or Fc-apelin and found that Fc-apelin could ameliorate LPS-induced apoptosis. Thus, Fc-apelin was protective against LPS-induced hepatic apoptosis and exerted direct anti-apoptotic effects on cultured hepatocytes in vitro.
Figure 2.
Fc-apelin prevents LPS-induced apoptosis. TUNEL assay was used to assess apoptosis of liver tissue sections (A) of mice receiving PBS, Fc-apelin (1 mg/kg) and/or LPS were assessed by TUNEL assay for apoptosis or Huh-7 cells (B) which were treated with PBS/albumin (Cont) or Fc-apelin (0.5 μM) with or without LPS (1 μg/ml) for 12 hours. Green staining indicates apoptotic cells and DAPI staining was used to visualize nuclei (low panel). The representative images are presented and data (C) are expressed as mean + SE; n = 5; ***p < 0.001and n.s.: no significance.
Reduction of LPS-induced ROS production by Fc-apelin
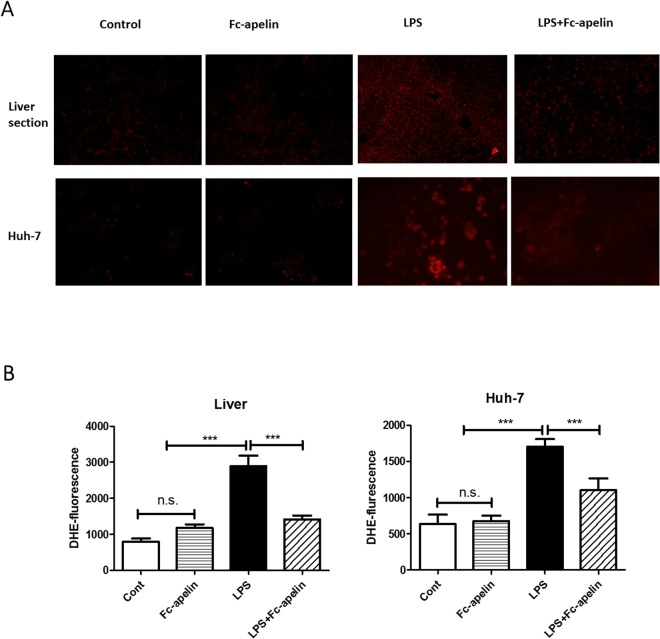
ROS production is an initiator of hepatic inflammation by LPS and apelin has been found to be anti-oxidative. To determine whether the protective effect of Fc-apelin might be attributable to its anti-oxidative activities, we determined ROS levels in liver tissue sections and cultured hepatocytes by DHE fluorescence. As shown in Fig. 3, compared with the control group, mice injected with LPS showed a significant increase (P < 0.01) in the liver DHE staining. Co-treatment with Fc-apelin markedly lowered the DHE intensity. In contrast, PBS or Fc-apelin alone had no effect on ROS production (Fig. 3). In cultured Huh-7 cells, LPS also induced ROS production, which was ameliorated by Fc-apelin (Fig. 3). Thus, Fc-apelin suppressed LPS-induced ROS production both in vivo and in vitro.
Figure 3.
Detection of ROS in mouse livers by DHE staining. (A, upper panel) Liver tissue sections of mice receiving PBS, Fc-apelin (1 mg/kg) and/or LPS were stained with DHE and the fluorescence intensity was quantified by fluorescence microscope. (A, lower panel) Huh-7 cells were pre-treated with PBS/albumin (Cont) or Fc-apelin (0.5 μM) for 30 min and then with or without LPS (1 μg/ml) for one hour. The cells were incubated with DHE for 20 min and quantified by microscopy. (B) Quantification of ROS production was done by measuring fluorescence intensity by Image J. Data are expressed as mean + SE; n = 5; ***p < 0.001and n.s.: no significance.
Effects of Fc-apelin on LPS-induced liver injury and macrophage infiltration
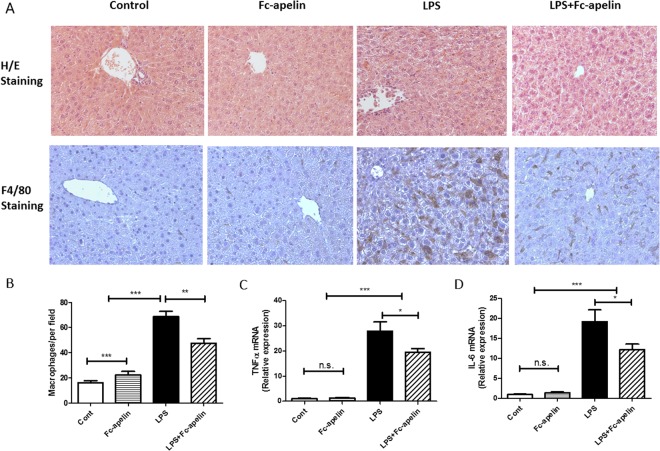
Macrophages play a pivotal role in pathogenesis of LPS-induced liver damage29,30. We next conducted histological studies to examine the effect of Fc-apelin on liver histology. In H/E staining (Fig. 4A), liver morphology appeared normal in mice receiving PBS and Fc-apelin. However, in the LPS-treated mice, focal necrosis and tissue structure distortion were apparent, which was significantly attenuated by co-treatment of Fc-apelin. The effect of Fc-apelin on macrophage infiltration was measured by IHC by using F4/80, a macrophage-specific marker31,32. As shown in Fig. 4B, LPS remarkably increased the macrophage labeling, which was substantially blunted by Fc-apelin treatment. Nevertheless, the extent of macrophage staining in the LPS + Fc-apelin group was still higher than the PBS or Fc-apelin-treated group. Thus, the apelin fusion protein can reduce liver injury and diminish macrophage infiltration induced by LPS.
Figure 4.
Fc-apelin ameliorates LPS-induced liver damage and inflammation responses. (A) Representative H/E staining and immunohistochemistry of macrophage marker F4/80 of liver sections. (B) Quantification of F4/80 + cells per view (200 x magnification). Quantitative PCR (qPCR) of gene expression for TNFα (C) and IL-6 (D). Gene expression is normalized with β-actin. Data are expressed as mean + SE; n = 5 for microphage infiltration analyses and n = 8 for qPCR; *p < 0.05; **p < 0.01; ***p < 0.001 and n.s.: no significance.
Effects of Fc-apelin on mRNA expression of TNFα and IL-6
Given the pronounced changes of the macrophages in the liver of mice receiving LPS and Fc-apelin treatment, we next measured mRNA expression of tumor necrosis factor-alpha (TNFα) and IL-6 in the liver. Figure 4B shows that the administration of LPS induced increases in TNF-α and IL-6 by about 28 and 19-fold, respectively, compared to PBS or Fc-apelin controls. The increase was attenuated by about 30 and 36%, respectively, by co-administration of Fc-apelin.
Discussion
Besides its prominent inotropic activity and cell protective activities, apelin has been demonstrated to possess a broad range of anti-inflammatory properties from suppression of the NF-κB pathway and pro-inflammatory cytokines to anti-oxidation. Thus, apelin appears to be a potential therapeutic for endotoxemia-induced tissue injuries at multiple levels. However, the short in vivo half-life of apelin would limit its potential clinic application.
In the present study, we show a protective role of the long-acting Fc-apelin fusion protein22 against LPS-induced liver injury. The administration of LPS induced serum ALT elevation, indicating a liver damage. LPS-induced liver injury develops in a step-wise, but complex fashion involving activation of NF-κB, subsequent production of a variety of inflammatory mediators33,34, activation of liver macrophages (Kupffer cells), infiltration of blood-derived macrophages and neutrophils, and over-generation of ROS. This excessive inflammation leads to cell death and tissue destruction. In this study, we treated mice with relatively a low dose of LPS for 5 days and thus established a sub-acute model of endotoxemia in which multiple inflammatory events occur concurrently.
Apelin is a peptide hormone acting on APJ which is expressed in multiple tissues including the liver35 and in hepatoma cells36. Many studies have shown apelin’s protective activities against infection through anti-inflammation and promoting cell survival. For example, apelin reduces inflammatory cytokines in cerulein-induced chronic pancreatitis, partially through suppression of NF-κB signaling37. Luo et al.38. report that apelin suppresses the inflammatory response and promotes cell survival partially via activating the PI3K and AKT pathways in a mouse model of postburn sepsis. Moreover, apelin can directly reduce gene expression and secretion of inflammatory cytokines in Raw276.4 monocytes39, and lower serum inflammatory cytokines TNFα and IL-6. In this study, we basically reproduced those anti-inflammation effects observed in animals by apelin peptide. We demonstrated that administration of Fc-apelin fusion protein attenuated the apoptosis and ROS production both in cultured hepatoma cells and in mice challenged by LPS, suggesting that the fusion protein may exert both direct and indirect protective effects against LPS-inflicted cell damage. Notably, we found that Fc-apelin greatly reduced macrophage infiltration and liver tissue injury by histology, which is in agreement with the observed reduction of serum ALT. However, although we observed a decreased expression of inflammation markers of IL-6 and TNFα in the liver, we did not see a statistically significant decrease of systemic IL-6 levels by Fc-apelin treatment in LPS mice, which is not in line with the report where systemic levels of IL-6, TNFα and IL-1β are reduced by apelin peptide infusion in a sepsis model of rats38. The difference might be attributable to the model of inflammation and/or apelin dose.
It is worth noting that apelin’s protective effects against inflammation are not restricted to the liver. Activation of apelinergic system by apelin and ELA, a new ligand of APJ40–42 have been shown to improve survival and exert strong protective effects against cardiac and renal dysfunction in rats with sepsis43–45. Combining cardiac and renal functional benefits with anti-inflammatory activities in the liver makes the activation of the apelinergic system an appealing therapeutic approach for systemic inflammation. Our long-acting Fc-apelin is potentially a novel biologic for this disease condition. More studies are warranted to investigate the Fc-apelin’s protective activities in other inflammation animal models.
Acknowledgements
Partially supported by Maryland Stem Cell Research Fund.
Author Contributions
H.Z., R.Y., W.W., F.X., Y.X., R.B., H.Z. and L.S. performed the experiments. H.Z., R.Y., W.W., F.X., Y.X., and D.G. prepared the figures. H.Z., R.Y., W.W., D.Z. and D.G. participated in design of the experiments. H.Z., R.Y., Y.X., R.B., D.Z. and D.G. contributed to the writing of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/29/2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has not been fixed in the paper.
References
- 1.Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol. 2011;26(Suppl 1):173–179. doi: 10.1111/j.1440-1746.2010.06592.x. [DOI] [PubMed] [Google Scholar]
- 2.Hamesch K, Borkham-Kamphorst E, Strnad P, Weiskirchen R. Lipopolysaccharide-induced inflammatory liver injury in mice. Lab Anim. 2015;49:37–46. doi: 10.1177/0023677215570087. [DOI] [PubMed] [Google Scholar]
- 3.Ju C, Tacke F. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell Mol Immunol. 2016;13:316–327. doi: 10.1038/cmi.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bautista AP, Meszaros K, Bojta J, Spitzer JJ. Superoxide anion generation in the liver during the early stage of endotoxemia in rats. Journal of leukocyte biology. 1990;48:123–128. doi: 10.1002/jlb.48.2.123. [DOI] [PubMed] [Google Scholar]
- 5.Leng W, et al. Aspartate alleviates liver injury and regulates mRNA expressions of TLR4 and NOD signaling-related genes in weaned pigs after lipopolysaccharide challenge. J Nutr Biochem. 2014;25:592–599. doi: 10.1016/j.jnutbio.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Macdonald J, Galley HF, Webster NR. Oxidative stress and gene expression in sepsis. Br J Anaesth. 2003;90:221–232. doi: 10.1093/bja/aeg034. [DOI] [PubMed] [Google Scholar]
- 7.Cadenas S, Cadenas AM. Fighting the stranger-antioxidant protection against endotoxin toxicity. Toxicology. 2002;180:45–63. doi: 10.1016/S0300-483X(02)00381-5. [DOI] [PubMed] [Google Scholar]
- 8.Sun B, Karin M. NF-kappaB signaling, liver disease and hepatoprotective agents. Oncogene. 2008;27:6228–6244. doi: 10.1038/onc.2008.300. [DOI] [PubMed] [Google Scholar]
- 9.Farghali H, Kgalalelo Kemelo M, Wojnarova L, Kutinova Canova N. In vitro and in vivo experimental hepatotoxic models in liver research: applications to the assessment of potential hepatoprotective drugs. Physiol Res. 2016;65:S417–S425. doi: 10.33549/physiolres.933506. [DOI] [PubMed] [Google Scholar]
- 10.Coldewey SM, Rogazzo M, Collino M, Patel NS, Thiemermann C. Inhibition of IkappaB kinase reduces the multiple organ dysfunction caused by sepsis in the mouse. Disease models & mechanisms. 2013;6:1031–1042. doi: 10.1242/dmm.012435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Dowd BF, et al. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136:355–360. doi: 10.1016/0378-1119(93)90495-O. [DOI] [PubMed] [Google Scholar]
- 12.Tatemoto K, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochemical and biophysical research communications. 1998;251:471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Q, Cao J, Chen L. Apelin/APJ system: A novel therapeutic target for oxidative stress-related inflammatory diseases (Review) International journal of molecular medicine. 2016;37:1159–1169. doi: 10.3892/ijmm.2016.2544. [DOI] [PubMed] [Google Scholar]
- 14.Than A, et al. Apelin attenuates oxidative stress in human adipocytes. The Journal of biological chemistry. 2014;289:3763–3774. doi: 10.1074/jbc.M113.526210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foussal C, et al. Activation of catalase by apelin prevents oxidative stress-linked cardiac hypertrophy. FEBS letters. 2010;584:2363–2370. doi: 10.1016/j.febslet.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 16.Han S, et al. Pancreatitis activates pancreatic apelin-APJ axis in mice. Am J Physiol Gastrointest Liver Physiol. 2013;305:G139–150. doi: 10.1152/ajpgi.00370.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou Y, et al. Apelin-13 Protects PC12 Cells from Corticosterone-Induced Apoptosis Through PI3K and ERKs Activation. Neurochem Res. 2016;41:1635–1644. doi: 10.1007/s11064-016-1878-0. [DOI] [PubMed] [Google Scholar]
- 18.Zeng X, Yu SP, Taylor T, Ogle M, Wei L. Protective effect of apelin on cultured rat bone marrow mesenchymal stem cells against apoptosis. Stem cell research. 2012;8:357–367. doi: 10.1016/j.scr.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Japp AG, et al. Vascular effects of apelin in vivo in man. Journal of the American College of Cardiology. 2008;52:908–913. doi: 10.1016/j.jacc.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Yamaleyeva LM, Shaltout HA, Varagic J. Apelin-13 in blood pressure regulation and cardiovascular disease. Curr Opin Nephrol Hypertens. 2016;25:396–403. doi: 10.1097/MNH.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 21.van Witteloostuijn SB, Pedersen SL, Jensen KJ. Half-Life Extension of Biopharmaceuticals using Chemical Methods: Alternatives to PEGylation. ChemMedChem. 2016;11:2474–2495. doi: 10.1002/cmdc.201600374. [DOI] [PubMed] [Google Scholar]
- 22.Wang Weimin, Zhang Dongming, Yang Rongze, Xia Wei, Qian Kun, Shi Zhengrong, Brown Robert, Zhou Huifen, Xi Yue, Shi Lin, Chen Ling, Xu Feng, Sun Xiaojian, Zhu Dalong, Gong Da-Wei. Hepatic and cardiac beneficial effects of a long-acting Fc-apelin fusion protein in diet-induced obese mice. Diabetes/Metabolism Research and Reviews. 2018;34(5):e2997. doi: 10.1002/dmrr.2997. [DOI] [PubMed] [Google Scholar]
- 23.Klein JS, Jiang S, Galimidi RP, Keeffe JR, Bjorkman PJ. Design and characterization of structured protein linkers with differing flexibilities. Protein Eng Des Sel. 2014;27:325–330. doi: 10.1093/protein/gzu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang RZ, et al. Alanine aminotransferase isoenzymes: molecular cloning and quantitative analysis of tissue expression in rats and serum elevation in liver toxicity. Hepatology. 2009;49:598–607. doi: 10.1002/hep.22657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang RZ, et al. Acute-phase serum amyloid A: an inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS medicine. 2006;3:e287. doi: 10.1371/journal.pmed.0030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benov L, Sztejnberg L, Fridovich I. Critical evaluation of the use of hydroethidine as a measure of superoxide anion radical. Free radical biology & medicine. 1998;25:826–831. doi: 10.1016/S0891-5849(98)00163-4. [DOI] [PubMed] [Google Scholar]
- 27.Chen M, et al. From the Cover: Lung-Specific Overexpression of Constitutively Active IKK2 Induces Pulmonary and Systemic Inflammations but Not Hypothalamic Inflammation and Glucose Intolerance. Toxicological sciences: an official journal of the Society of Toxicology. 2017;160:4–14. doi: 10.1093/toxsci/kfx154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craig DG, et al. Circulating apoptotic and necrotic cell death markers in patients with acute liver injury. Liver Int. 2011;31:1127–1136. doi: 10.1111/j.1478-3231.2011.02528.x. [DOI] [PubMed] [Google Scholar]
- 29.Hirano K, et al. Overexpression of granulocyte-macrophage colony-stimulating factor in mouse liver enhances the susceptibility of lipopolysaccharide leading to massive apoptosis of hepatocytes. Liver Int. 2005;25:1027–1035. doi: 10.1111/j.1478-3231.2005.01136.x. [DOI] [PubMed] [Google Scholar]
- 30.Tacke F. Targeting hepatic macrophages to treat liver diseases. Journal of hepatology. 2017;66:1300–1312. doi: 10.1016/j.jhep.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 31.Dos Anjos Cassado A. F4/80 as a Major Macrophage Marker: The Case of the Peritoneum and Spleen. Results Probl Cell Differ. 2017;62:161–179. doi: 10.1007/978-3-319-54090-0_7. [DOI] [PubMed] [Google Scholar]
- 32.Leenen PJ, de Bruijn MF, Voerman JS, Campbell PA, van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods. 1994;174:5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 33.Akira S, Takeda K. Toll-like receptor signalling. Nature reviews. Immunology. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 34.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nature reviews. Immunology. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 35.O’Carroll AM, Lolait SJ, Harris LE, Pope GR. The apelin receptor APJ: journey from an orphan to a multifaceted regulator of homeostasis. The Journal of endocrinology. 2013;219:R13–35. doi: 10.1530/JOE-13-0227. [DOI] [PubMed] [Google Scholar]
- 36.Melgar-Lesmes P, et al. Hypoxia and proinflammatory factors upregulate apelin receptor expression in human stellate cells and hepatocytes. Gut. 2011;60:1404–1411. doi: 10.1136/gut.2010.234690. [DOI] [PubMed] [Google Scholar]
- 37.Han S, Englander EW, Gomez GA, Greeley GH., Jr. Apelin Regulates Nuclear Factor-kappaB’s Involvement in the Inflammatory Response of Pancreatitis. Pancreas. 2017;46:64–70. doi: 10.1097/MPA.0000000000000740. [DOI] [PubMed] [Google Scholar]
- 38.Luo K, Long H, Xu B, Luo Y. Apelin attenuates postburn sepsis via a phosphatidylinositol 3-kinase/protein kinase B dependent mechanism: A randomized animal study. Int J Surg. 2015;21:22–27. doi: 10.1016/j.ijsu.2015.06.072. [DOI] [PubMed] [Google Scholar]
- 39.Yang F, Bai Y, Jiang Y. Effects of Apelin on RAW264.7 cells under both normal and hypoxic conditions. Peptides. 2015;69:133–143. doi: 10.1016/j.peptides.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 40.Chng SC, Ho L, Tian J, Reversade B. ELABELA: a hormone essential for heart development signals via the apelin receptor. Developmental cell. 2013;27:672–680. doi: 10.1016/j.devcel.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Pauli A, et al. Toddler: an embryonic signal that promotes cell movement via Apelin receptors. Science. 2014;343:1248636. doi: 10.1126/science.1248636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z, et al. Elabela-apelin receptor signaling pathway is functional in mammalian systems. Scientific reports. 2015;5:8170. doi: 10.1038/srep08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan CS, et al. Apelin antagonizes myocardial impairment in sepsis. Journal of cardiac failure. 2010;16:609–617. doi: 10.1016/j.cardfail.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Chagnon F, et al. Apelin Compared With Dobutamine Exerts Cardioprotection and Extends Survival in a Rat Model of Endotoxin-Induced Myocardial Dysfunction. Critical care medicine. 2017;45:e391–e398. doi: 10.1097/CCM.0000000000002097. [DOI] [PubMed] [Google Scholar]
- 45.Coquerel David, Chagnon Frédéric, Sainsily Xavier, Dumont Lauralyne, Murza Alexandre, Côté Jérôme, Dumaine Robert, Sarret Philippe, Marsault Éric, Salvail Dany, Auger-Messier Mannix, Lesur Olivier. ELABELA Improves Cardio-Renal Outcome in Fatal Experimental Septic Shock. Critical Care Medicine. 2017;45(11):e1139–e1148. doi: 10.1097/CCM.0000000000002639. [DOI] [PubMed] [Google Scholar]