Abstract
Efficient and timely diagnosis of head and neck squamous cell carcinoma (HNSCC) is a critical challenge, particularly in low and middle income countries. These regions, which are expected to witness a drastic increase in HNSCC rates, are ill-prepared to handle the diagnostic burden due to limited resources, especially the low ratio of pathologists per population, resulting in delayed diagnosis and treatment. Here, we demonstrate the potential of an alternative diagnostic method as a low-cost, resource-efficient alternative to histopathological analysis. Our novel technology employs unique surface-enhanced Raman scattering (SERS) “nanorattles” targeting cytokeratin nucleic acid biomarkers specific for HNSCC. In this first study using SERS diagnostics for head and neck cancers, we tested the diagnostic accuracy of our assay using patient tissue samples. In a blinded trial, our technique demonstrated a sensitivity of 100% and specificity of 89%, supporting its use as a useful alternative to histopathological diagnosis. The implications of our method are vast and significant in the setting of global health. Our method can provide a rapid diagnosis, allowing for earlier treatment before the onset of distant metastases. In comparison to histopathology, which can take several months in remote limited-resources regions, our method provides a diagnosis within a few hours.
Introduction
Affecting over 600,000 people worldwide each year1, head and neck cancers present a major issue in the field of global health. These tumors can significantly impact basic life functions such as swallowing, breathing, and speaking. Head and neck squamous cell carcinomas (HNSCCs), which comprise over 90% of these tumors2, can be curable if diagnosed at an early stage. Despite advances in diagnostic and therapeutic technology, HNSCCs continue to comprise a large portion of the global cancer burden. This is largely due to an epidemiological shift towards low and middle income countries (LMICs). While rates of head and neck squamous cell carcinomas are declining in the United States, their incidence is on the rise in LMICs countries3 due to risk factors including smoking, drinking, betel nut chewing, and HPV infection4,5. Two thirds of HNSCCs now occur in developing countries6. By 2030, the projected incidence of HNSCC will exceed 1 million, 73% of which will arise from less developed countries6,7.
The rise of HNSCC in LMICs is alarming and poses a major challenge to global health. Diagnostic capacity in these regions is limited and inadequate to handle the increased burden. In developed countries, HNSCC is traditionally diagnosed using the gold standard of histopathological analysis and immunohistochemical staining for cytokeratin. However, in much of the world, a single pathologist often serves a population of several million people8,9. Diagnosis of HNSCC can take weeks or months, thereby delaying treatment. As the incidence of HNSCC rises, particularly in LMICs, there is a growing need for alternative diagnostic approaches.
A common presentation of HNSCC is an enlarged cervical lymph node. However, lymphadenopathy is not specific for HNSCC and may be attributed to a multitude of diagnoses including thyroid carcinoma, viral infection, tuberculosis, and lymphoma, among others. In regions with limited access to pathologists, this poses a barrier to treatment for patients suffering from HNSCC. Our group sought to develop a resource-efficient method to distinguish the presence of HNSCC from other pathologies in human lymph nodes, permitting earlier diagnosis and treatment before widespread metastasis, thereby improving survival.
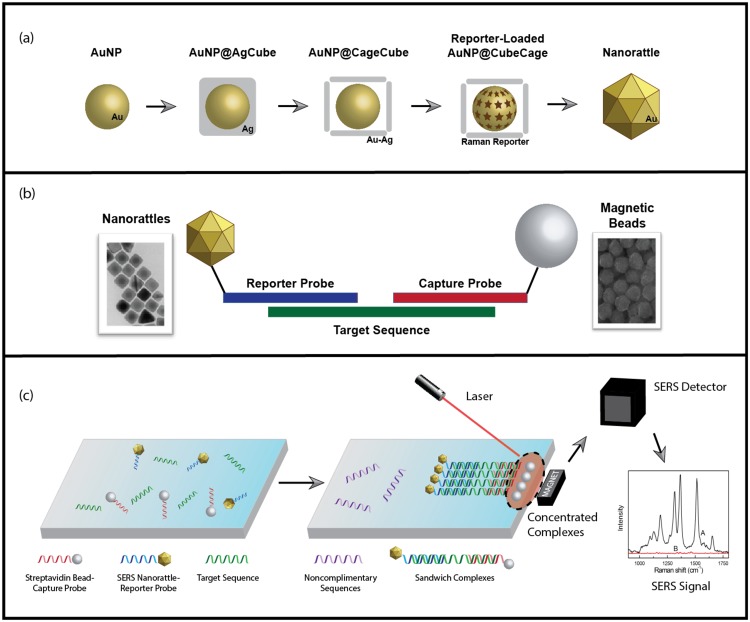
Raman spectroscopy offers an approach to molecular diagnosis by providing unique chemical fingerprints produced from the inelastic scattering of light upon interacting with distinct molecules. Surface-enhanced Raman scattering (SERS) further enhances the signals by providing magnification on the order of millions, allowing for single molecule detection10–12. SERS has been developed in our laboratory for many applications including chemical monitoring, biosensing and molecular diagnostics13–17. Recently we have developed a sensitive SERS sandwich hybridization assay using unique SERS nanorattles for nucleic acid detection18. Unlike previous applications of sandwich hybridization, which utilized gold and silver nanoparticles, the unique core-gap-shell structure of our nanorattles results in ultrabright SERS signals, and thus higher sensitivity for nucleic acid detection19,20. This method is summarized in Fig. 1. Firstly, SERS nanorattles and magnetic beads are hybridized to unique nucleic acid sequences. Following magnetic isolation, a laser is applied to the hybridized complexes. When successful hybridization occurs in the presence of target nucleic acid sequences, an increase in SERS signal can be detected.
Figure 1.
Overview of SERS Diagnostic Method. (a) Synthesis of cubic nanorattles, beginning with single-crystal, spherical gold nanoparticle (AuNP) cores. The AuNP cores are coated with cubic Ag shells to obtain AuNP@AgCube. Galvanic replacement transforms cubic Ag shells into cubic Au-Ag cages containing AuNP in the interior. Raman reporters are loaded, and the porous cubic cages are turned into complete shells by a final Au coating to obtain cube nanorattles. (b) Structure of individual hybridization complex. Gold nanorattles (Au-NR) are functionalized with DNA reporter probes, and streptavidin beads are functionalized with DNA capture probes. Both probes are complimentary to the specific cytokeratin sequence. (c) Extracted nucleic acids are incubated with functionalized nanorattles and beads, then washed away. Remaining complexes undergoing successful hybridization are isolated using a strong magnet, and concentrate to a spot for laser excitation in order to yield a SERS signal.
We have previously described a surface-enhanced SERA sandwich assay utilizing unique nanoparticles to detect cytokeratin nucleic acid biomarkers in HNSCC21. Cytokeratin 14 (CK14) mRNA has been demonstrated to be both sensitive and specific for detection of HNSCC micrometastases in lymph nodes22. In contrast to other members of the cytokeratin family of proteins, CK14 is specific for head and neck SCC, and can distinguish SCC from thyroid tumors23–25. In the present study, we employ our diagnostic method in human tissue samples. This study is the first of its kind to use SERS nanoparticles to diagnose HNSCC ex vivo using clinical specimens. Using our novel sandwich hybridization protocol, we demonstrate the diagnostic capacity of our method in a blinded trial of 25 patient samples.
Results
A total of 25 samples were obtained from human cervical lymph nodes, tonsils, oropharyngeal mucosa, sinus mucosa, and thyroid gland tissue. Histopathological diagnoses ranged from HNSCC, normal lymphoid tissue, follicular lymphoid hyperplasia, normal oral mucosa, normal thyroid tissue, papillary thyroid carcinoma, and melanoma. A summary of tissue sample characteristics is shown in Table 1.
Table 1.
Tissues and diagnoses of clinical samples.
| Sample | Tissue Site | Histopathological Diagnosis |
|---|---|---|
| 1 | thyroid | papillary carcinoma |
| 2 | thyroid | normal thyroid tissue |
| 3 | lymph node | squamous cell carcinoma |
| 4 | oral mucosa | squamous cell carcinoma |
| 5 | oral mucosa | squamous cell carcinoma |
| 6 | tongue | normal squamous mucosa |
| 7 | lymph node | papillary carcinoma |
| 8 | thyroid | papillary carcinoma |
| 9 | thyroid | normal thyroid tissue |
| 10 | lymph node | squamous cell carcinoma |
| 11 | tongue | normal squamous mucosa |
| 12 | lymph node | papillary carcinoma |
| 13 | sinus | squamous cell carcinoma |
| 14 | tongue | squamous cell carcinoma |
| 15 | lymph node | papillary carcinoma |
| 16 | tongue | squamous cell carcinoma |
| 17 | lymph node | follicular lymphoid hyperplasia |
| 18 | tonsil | normal lymphoid tissue |
| 19 | tonsil | normal lymphoid tissue |
| 20 | lymph node | melanoma |
| 21 | tonsil | normal lymphoid tissue |
| 22 | tonsil | normal lymphoid tissue |
| 23 | lymph node | melanoma |
| 24 | tonsil | normal lymphoid tissue |
| 25 | tonsil | normal lymphoid tissue |
Using our cytokeratin detection probes, our method was applied to each tissue sample in triplicate. The SERS intensities were quantified by the peak intensity at 930 cm−1. In our previous study using human cell lines, we derived a threshold of 3000 counts to distinguish HNSCC from other cell types. We applied the same threshold to our tissue samples, as depicted in Fig. 2. Using our method, we diagnosed samples above this threshold as HNSCC and those below as negative for HNSCC.
Figure 2.
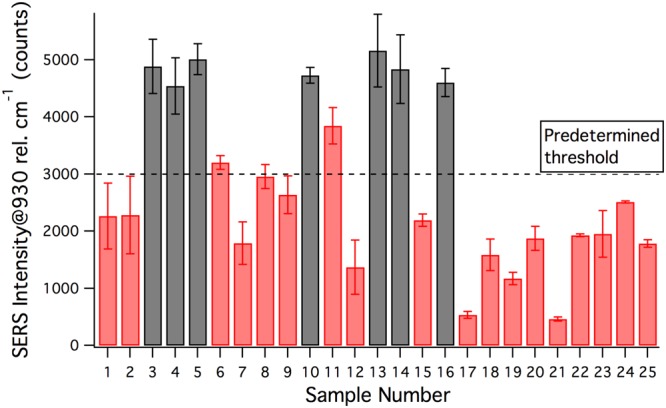
Blinded Test: Intensities of 930 cm−1 peaks against threshold.
Our diagnostic impressions were then compared to the histopathological diagnosis of each tissue sample. The sensitivity and specificity of our method was calculated, as shown in Table 2.
Table 2.
Sensitivity and specificity of SCC detection in blinded samples.
| SCC | Non-SCC | Total | |
|---|---|---|---|
| Total | 7 | 18 | 25 |
| Test Positive | 7 | 2 | 9 |
| Test Negative | 0 | 16 | 16 |
| Sensitivity 7/7 = 100% | Specificity 16/18 = 89% |
Following the calculation of sensitivity and specificity, the distribution of samples, now un-blinded, was analyzed. Figure 3 depicts the distribution of non-HNSCC compared to HNSCC. A threshold of 4000 counts was observed to demarcate the two groups with 100% sensitivity and 100% specificity.
Figure 3.
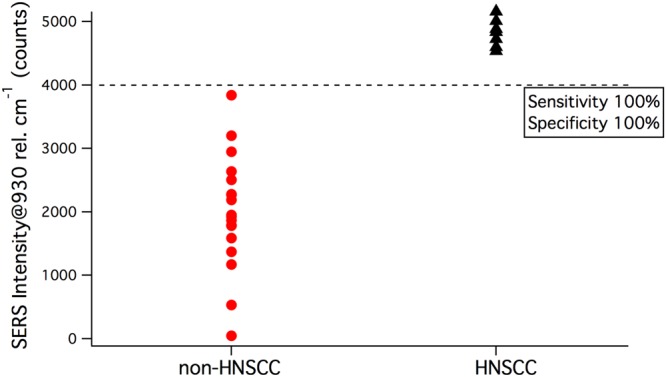
Distribution of SERS peaks by diagnosis after unblinding.
Discussion
SERS-based methods are becoming increasingly popular in nucleic acid detection due to their high sensitivity. We previously developed a SERS method to detect HNSCC as a diagnostic alternative to histopathology using human cell lines21. In the present study, we validated our unique SERS method in human tissue samples. By targeting cytokeratin 14 RNA with ultrabright SERS nanorattles, we demonstrated the capability of our method in distinguishing HNSCC from other tissue types such as thyroid cancer and benign lymphoid tissue. In a blinded trial of 25 samples, our method demonstrated 100% sensitivity and 89% specificity for HNSCC detection, supporting its use as an alternative to histopathological diagnosis.
In our blinded test, we used a previously derived threshold of 3000 counts to diagnose HNSCC. Using this threshold value, our method was able to correctly diagnose all 7 HNSCC samples. Out of the remaining 18 negative samples, 16 were correctly diagnosed as non-HNSCC. The 2 negative samples that tested positive were both squamous tissue from the oral mucosa. While the signals from these tissues exceed the threshold, the data demonstrate that these samples exhibit lower SERS signals than HNSCC. Furthermore, upon unblinding, we identified a new threshold of 4000 counts to delineate HNSCC from non-HNSCC. Compared to the previous threshold, which was derived using data from human cell lines, the new threshold resulted in 100% sensitivity and 100% specificity. As this was derived from human clinical samples, it represents a significant improvement to our previous method, and will be further evaluated in future studies using larger sample sets.
This is the first study using SERS diagnostics for head and neck cancers in clinical specimens. The use of solid tissue samples demonstrates the capacity of our method to detect nucleic acid sequences within the complex, structural framework of the tissues. However, for practical application of our method in the clinical setting, fine needle aspirate (FNA) samples will be required as they are less invasive. In future studies, we will optimize our method for detection in fine needle aspirate (FNA) samples. We anticipate that due to the relative purity of cells in FNA samples compared to solid tissue, and previous work supporting molecular testing in FNA samples26, our method will be effective.
The results of our study serve to establish the use of SERS detection of HNSCC in human tissue samples. We anticipate that ultimately this method can be incorporated into an integrated device that can be used in the field for point-of-care testing as a diagnostic alternative to histopathology. Our team recently developed a prototype of an integrated diagnostic device capable of detecting malaria in human blood lysate27. For patients presenting with cervical lymphadenopathy, our method can provide a diagnosis in a more timely manner than the current standard, thereby allowing for earlier treatment.
The implications of our method are vast and significant in the setting of global health. For patients presenting cervical lymphadenopathy, our method can provide a rapid diagnosis, allowing for earlier treatment before the onset of metastases. Compared to diagnosis by histopathology, which can take several months in remote regions, our method can be completed in a matter of a few hours. While digital pathology services have emerged to meet the growing diagnostic demand, these telemedicine technologies face many challenges, including limited infrastructure, incompatibility among different platforms, security, and resistance to cultural change28,29. Our method can be completed by a local technician and provide patients with a point-of-care diagnosis without transferring personal health data.
Additionally, our method has the advantage of incorporating molecular testing. For head and neck cancers, this is of particular importance as HPV status is an important prognosticator30. Furthermore, our method does not require the expensive equipment necessary for qRT-PCR; a simple thermal cycler powered by solar energy is adequate and affordable31. Finally, as our method yields an output of a binary diagnosis based on SERS signal intensity, rather than a qPCR curve, it can be easily interpreted in the field. Our method thus provides a cheaper, user-friendly, resource-efficient alternative for use in LMICs.
Methods
Patient Selection
For this prospective study, eligible patients included adults with HNSCC, thyroid papillary carcinoma, lymphoma, or benign lymphoid or tonsillar disease. Patients with cutaneous squamous cell carcinoma were excluded from the study. Patients were identified on the basis of undergoing head and neck surgery at Duke University in 2017. The study was approved by the Duke institutional review board. All experiments were performed in compliance with the relevant guidelines and regulations. Informed consent was obtained from all patients by the Duke Biospecimen Repository and Processing Core (BRPC). Participation in the study did not alter the management of patients.
Collection of Clinical samples
Fresh tissue samples were collected intraoperatively by the primary surgeon. After adequate tissue was dissected by Duke Pathology for standard histopathological diagnosis, remaining tissue was collected by the Duke BRPC. Samples were flash frozen in liquid nitrogen and maintained at −80 °C. Our team was blinded to all diagnostic information and received de-identified tissues containing only a unique numerical identifier.
RNA Extraction and Amplification
All steps involving fresh tissues and RNA were conducted on ice. 30 mg of tissue was required for RNA extraction. Tissues were first homogenized using a mortar and pestle and subsequently with a 21 gauge needle and syringe. RNA extraction was performed on all tissue samples using the Qiagen RNAeasy Mini Kit. RNA was eluted in RNAse free deionized water (dH2O) to final concentrations between 100–300 ng/uL. RNA was normalized to a concentration of 100 ng/uL. then immediately used for amplification as per the Qiagen OneStep PCR Kit with our previously designed primers and probes21,22. Briefly, 5uL of RNA was added to a mixture containing 1000 nM forward primer, 50 nM reverse primer, 200 nM fluorescent probe, 2uL RT-PCR Buffer 5X, 2uL OneStep RT-PCR Enzyme Mix, and 30uL RNase-free DI to a final volume of 50 mL. Samples were heated for 5 minutes at 95 C, then amplified by heating to 60 C for 1 minute. This was followed by 40 cycles of 15 s at 95 C. The Qiagen PCR Purifcation Kit was used to remove any excess products, and the final single stranded cDNA was eluted in RNase-free DI to final volumes of 7–18 ng/mL.
Nanorattles and Magnetic Beads Preparation
Materials
Sodium borohydride (NaBH4), Au chloride solution (HAuCl4) 200 mg/dL in deionized water, ascorbic acid (AA), hexadecyltrimethylammonium chloride (CTAC) solution 25 wt% in water, polyvinylpyrrolidone Mw ~55,000 (PVP), ethanol (EtOH), 1,3,3,1′,3′,3′,-hexamethyl-2,2′-indotricarbocyanine iodide (HITC), phosphate buffer saline (PBS), tris-EDTA buffer solution (TE), tween 20, sodium chloride (NaCl), hydrochloric acid (HCl) were purchased from Sigma-Aldrich. Sodium citrate dihydrate was purchased from BDH. Methoxy polyethylene glycol thiol Mw 5000 (mPEG-SH) was purchased from Nanocs. Magnetic beads were purchased from Life Technologies. All DNA sequences were synthesized by Integrated DNA Technologies (IDT, Coralville, IA). Millipore Synergy ultrapure water (DI) of resistivity = 18.2 MΩ cm was used in all nanoparticle synthesis solutions. Nuclease-free water was used in all experiments relating to RNA and DNA.
Nanorattles Synthesis
Nanorattles were synthesized with a previously developed procedure18. In brief, the gold nanoparticles (GNPs) were prepared using a seed-mediated method, resulting in 20 nm GNPs32. This method consisted in the synthesis of gold seeds by the reduction of gold chloride by NaBH4 in ice bath and the further growth of the seeds obtained by reduction of gold chloride by ascorbic acid the presence of CTAC. After washing the GNPs suspension, a silver shell was coated on the GNPs by reducing AgNO3 with ascorbic acid in the presence of CTAC at 60 °C for 4 h. This reaction yields gold-silver core-shell structures with a cubic shape (GNP@AgCubes). The silver shells were then converted into cages using galvanic replacement. A solution of Au chloride was slowly added to the GNP@AgCubes in the presence of PVP and CTAC at 90 °C, obtaining the porous mixed gold-silver shells. For Raman reporter loading, the stock GNP@AgCages were mixed with 1 mM HITC in ethanol. After 2 h under shaking, the suspension was washed for the final gold coating, which was performed by reducing gold chloride with ascorbic acid in the presence of CTAC.
Nanorattles Functionalization
Nanorattles were functionalized with DNA reporter probes using a pH-assisted method with slight modification18. First, 5 µL of 100 mM TCEP in TE 1X were added to 50 μL of 100 μM thiolated DNA reporter probes. The mixture was added to 1 mL of nanorattles after 1 h incubation at rom temperature. This new mixture was incubated for 1 h under shaking. Then 10 μL of citrate-HCl buffer (300 mM trisodium citrate, pH adjusted to 3.1 using 1 M HCl) were added to promote loading of DNA onto nanorattles and incubated for 1 more hour. The mixture was centrifuged at 6,500 rpm for 5 minutes. Fifty µL of 1 mM SH-PEG 5 K (freshly prepared, sonicated 5 minutes) were added to the pellet followed by addition of 1 mL PBS 1X with 0.01% tween20 and sonication. The resulting nanorattles were washed once with TE 1X followed by resuspension in TE 1X and storage at 4 °C before use. The functionalized nanorattles are good for use within 3–4 months.
Magnetic Beads Functionalization
Magnetic beads (Dynabeads MyOne Streptavidin C1, 1 μm diameter) were functionalized with DNA capture probes using the manufacturer’s protocol. Briefly, 800 µL of10 mg/mL stock magnetic beads were washed three times using washing buffer 1X (5 mM Tris-HCl pH 7.5, 0.5 mM EDTA, 1 M NaCl) and suspended in 400 µL of washing buffer 2X. To load DNA capture probes on magnetic beads, 400 μL of 5 μM biotinylated DNA capture probe were added. The mixture was incubated for 0.5 h under shaking. DNA capture probe-loaded magnetic beads were washed three times using washing buffer 1X and resuspended in 1600 μL TE 1X (magnetic bead final conc. 1.25 mg/ml). The capture probe-loaded magnetic beads solution was stored at 4 °C and used within 3–4 months.
DNA Detection
Target sequence detection capability of the method was demonstrated by testing sample solutions containing purified PCR products of extracted RNA in hybridization buffer (0.5 M NaCl, 10 mM phosphate-buffered solution at pH 7.4) with Tween-20 0.01%. For each sample, 3 µL DNA, 2 μL of magnetic beads loaded with capture probes, and 3 μL of nanorattles functionalized with reporter probes were added to 27 µL of buffer. The samples were incubated at 40 C for 3 hours, then washed three times before being pipetted into glass capillary tubes (5–25 μL volume, Sigma-Aldrich). The magnetic bead complexes were concentrated at small spots near the middle of the capillary tubes using a permanent magnet positioned under the tubes.
SERS Measurement
Using a lab-built system, a laser beam was focused onto the concentrated magnetic bead complexes. The lab-built SERS measurement system was composed of a 785 nm laser source (Rigaku Xantus-1), a fiber optic probe (InPhotonics RamanProbe), a spectrometer (Princeton Instruments Acton LS 785), and a CCD camera (Princeton Instruments PIXIS: 100BR_eXcelon). Laser power of the Xantus-1 was set at 300 mW and the CCD camera exposure time was set at 1 second.
Acknowledgements
This work was sponsored by the National Institutes of Health (R21 AI120981-01) and the Duke Faculty Exploratory Research Fund.
Author Contributions
This project was conceived and designed by P.V., H.T.N., W.T.L., and T.V. The S.E.R.S. method used in this study was developed by H.T.N., with guidance from T.V. W.T.L. facilitated the collection of tissue samples. R.N.A. extraction and amplification was performed by P.V. S.E.R.S. nanorattles were synthesized by P.S. and H.T.N. Functionalization of probes, testing of method was conducted by P.V. Statistical analysis and manuscript preparation was done by P.V. and P.S. All authors have provided input into the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/23/2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA. Cancer J. Clin. 2002;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Pezzuto, F., Caponigro, F. & Lina, M. Update on Head and Neck Cancer: Current Knowledge on Epidemiology, Risk Factors, Molecular Features and Novel Therapies. 125–136. 10.1159/000381717 (2015). [DOI] [PubMed]
- 3.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Vecchia, C. L et al. Epidemiology and Prevention of Oral Cancer. 33, 302–312 (1997). [DOI] [PubMed]
- 5.Herrero, R. et al. Human Papillomavirus and Oral Cancer: The International Agency for Research on Cancer Multicenter Study. 95, (2003). [DOI] [PubMed]
- 6.Byakodi R, et al. Oral cancer in India: An epidemiologic and clinical review. J. Community Health. 2012;37:316–319. doi: 10.1007/s10900-011-9447-6. [DOI] [PubMed] [Google Scholar]
- 7.Gupta, B., Johnson, N. W. & Kumar, N. Global Epidemiology of Head and Neck Cancers: A Continuing Challenge. 4222, 13–23 (2016). [DOI] [PubMed]
- 8.Adeyi, O. A. & Histopathology, C. Pathology Services in Developing Countries — The West African Experience. 183–186 [DOI] [PubMed]
- 9.Harder HI. Pathology services in developing countries. Arch. Pathol. Lab. Med. 2009;133:1911. doi: 10.5858/133.12.1911. [DOI] [PubMed] [Google Scholar]
- 10.Nie S. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science (80-.). 1997;275:1102–1106. doi: 10.1126/science.275.5303.1102. [DOI] [PubMed] [Google Scholar]
- 11.Kneipp K, Wang Y, Kneipp H, Perelman LT, Itzkan I. Single molecule detection using surface-enhanced Raman scattering (SERS) Phys. Rev. …. 1997;78:1667–1670. [Google Scholar]
- 12.Lane LA, Qian X, Nie S. SERS Nanoparticles in Medicine: From Label-Free Detection to Spectroscopic Tagging. Chemical Reviews. 2015;115:10489–10529. doi: 10.1021/acs.chemrev.5b00265. [DOI] [PubMed] [Google Scholar]
- 13.VoDinh T, Hiromoto MYK, Begun GM, Moody RL. Surface-Enhanced Raman Spectrometry for Trace Organic Analysis. Anal. Chem. 1984;56:1667–1670. doi: 10.1021/ac00273a029. [DOI] [Google Scholar]
- 14.Bello JM, Anantha Narayanan V, Stokes DL, Vo-Dinh T. Fiber-Optic Remote Sensor for in Situ Surface-Enhanced Raman Scattering Analysis. Anal. Chem. 1990;62:2437–2441. doi: 10.1021/ac00221a005. [DOI] [Google Scholar]
- 15.Yuan H, Fales AM, Khoury CG, Liu J, Vo-Dinh T. Spectral characterization and intracellular detection of Surface-Enhanced Raman Scattering (SERS)-encoded plasmonic gold nanostars. J. Raman Spectrosc. 2013;44:234–239. doi: 10.1002/jrs.4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vo-Dinh T, et al. SERS Nanosensors and Nanoreporters: Golden Opportunities in Biomedical Applications. Wiley Interdiscip. Rev. Nanomedicine. Nanobiotechnology. 2015;7:17–33. doi: 10.1002/wnan.1283. [DOI] [PubMed] [Google Scholar]
- 17.Wang HN, et al. Multiplexed Detection of MicroRNA Biomarkers Using SERS-Based Inverse Molecular Sentinel (iMS) Nanoprobes. J. Phys. Chem. C. 2016;120:21047–21055. doi: 10.1021/acs.jpcc.6b03299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ngo HT, Gandra N, Fales AM, Taylor SM, Vo-Dinh T. Sensitive DNA detection and SNP discrimination using ultrabright SERS nanorattles and magnetic beads for malaria diagnostics. Biosens. Bioelectron. 2016;81:8–14. doi: 10.1016/j.bios.2016.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang, H., Harpster, M. H., Park, H. J., Johnson, P. A. & Wilson, W. C. Surface-Enhanced Raman Scattering Detection of DNA Derived from the West Nile Virus Genome Using Magnetic Capture of Raman-Active Gold Nanoparticles. 83, 254–260 (2011). [DOI] [PubMed]
- 20.Donnelly T, Smith WE, Faulds K, Graham D. Silver and magnetic nanoparticles for sensitive DNA detection by SERS. Chem. Commun. 2014;50:12907–12910. doi: 10.1039/C4CC06335J. [DOI] [PubMed] [Google Scholar]
- 21.Vohra, P., Ngo, H. T., Lee, W. T. & Vo-Dinh, T. Squamous cell carcinoma DNA detection using ultrabright SERS nanorattles and magnetic beads for head and neck cancer molecular diagnostics. Anal. Methods. 10.1039/C7AY01913K (2017). [DOI] [PMC free article] [PubMed]
- 22.Shores C, Yin X, Funkhouser W, Yarbrough W. Clinical evaluation of a new molecular method for detection of micrometastases in head and neck squamous cell carcinoma. Arch. Otolaryngol. Head. Neck Surg. 2004;130:937–42. doi: 10.1001/archotol.130.8.937. [DOI] [PubMed] [Google Scholar]
- 23.Chu, P. G., Weiss, L. M., Chu, P. G. & Weiss, L. M. Keratin expression in human tissues and neoplasms. 403–439 (2002). [DOI] [PubMed]
- 24.Lal A, et al. A gene expression profile test to resolve head & neck squamous versus lung squamous cancers. Diagn. Pathol. 2013;8:1. doi: 10.1186/1746-1596-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim, H. et al. Overexpression of cytokeratin 17 is associated with the development of papillary thyroid carcinoma and the presence of lymph node metastasis. 8, 5695–5701 (2015). [PMC free article] [PubMed]
- 26.Ladd AC, et al. Preservation of fine-needle aspiration specimens for future use in RNA-based molecular testing. Cancer Cytopathol. 2011;119:102–110. doi: 10.1002/cncy.20130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoan Thanh Ngo, Elizabeth Freedman, Ren Odion, Pietro Strobbia, A. Swarnapali De Silva Indrasekara, Priya Vohra, Steve M Taylor, T. V.-D. Direct Detection of Unamplified Pathogen RNA in Blood Lysate Using an Integrated Lab-in-a-Stick Device and Ultrabright SERS Nanorattles. Sci. Rep. (2018). [DOI] [PMC free article] [PubMed]
- 28.Digital Pathology Systems Market (By Technology - Virtual Microscopy, Telepathology (Static Telepathology and Dynamic Telepathology); By Applications - Drug Discovery and Development, Disease Diagnosis and Academic Research) - Global Industry Analysis, Si. (2014).
- 29.Benediktsson H, Whitelaw J, Roy I. Pathology services in developing countries: A challenge. Archives of Pathology and Laboratory Medicine. 2007;131:1636–1639. doi: 10.5858/2007-131-1636-PSIDCA. [DOI] [PubMed] [Google Scholar]
- 30.Kimple RJ, Harari PM. The prognostic value of HPV in head and neck cancer patients undergoing postoperative chemoradiotherapy. Ann. Transl. Med. 2015;3:S14. doi: 10.3978/j.issn.2305-5839.2015.01.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang, L. et al. Solar thermal polymerase chain reaction for smartphone-assisted molecular diagnostics. Sci. Rep. 4 (2014). [DOI] [PMC free article] [PubMed]
- 32.Ma Y, et al. Au@Ag core-shell nanocubes with finely tuned and well-controlled sizes, shell thicknesses, and optical properties. ACS Nano. 2010;4:6725–6734. doi: 10.1021/nn102237c. [DOI] [PMC free article] [PubMed] [Google Scholar]