Abstract
Melanoma is one of the most fatal and therapy-resistant types of cancer; therefore, identifying novel therapeutic candidates to improve patient survival is an ongoing effort. Previous studies have revealed that pimozide is not sufficient to treat melanoma; therefore, enhancing the treatment is necessary. Indoleamine 2, 3-dioxygenase (IDO) is an immunosuppressive, intracellular rate-limiting enzyme, which contributes to immune tolerance in various tumours, including melanoma, and inhibition of IDO may be considered a novel therapeutic strategy when combined with pimozide. The present study aimed to assess the antitumour activities of pimozide in vitro, and to investigate the effects of pimozide combined with L-methyl-tryptophan (L-MT) in vivo. For in vitro analyses, the B16 melanoma cell line was used. Cell cytotoxicity assay, cell viability assay, wound-healing assay and western blotting were conducted to analyse the effects of pimozide on B16 cells. Furthermore, B16 cell-bearing mice were established as the animal model. Haematoxylin and eosin staining, immunohistochemistry, terminal deoxynucleotidyl transferase dUTP nick end-labelling staining, western blotting and flow cytometry were performed to determine the effects of monotherapy and pimozide and L-MT cotreatment on melanoma. The results demonstrated that pimozide exhibited potent antitumour activity via the regulation of proliferation, apoptosis and migration. Furthermore, the antitumour effects of pimozide were enhanced when combined with L-MT, not only via regulation of proliferation, apoptosis and migration, but also via immune modulation. Notably, pimozide may regulate tumour immunity through inhibiting the activities of signal transducer and activator of transcription (Stat)3 and Stat5. In conclusion, the present study proposed the use of pimozide in combination with the IDO inhibitor, L-MT, as a potential novel therapeutic strategy for the treatment of melanoma.
Keywords: melanoma, pimozide, IDO inhibitor, L-MT, combination therapy
Introduction
Melanoma is the most severe form of skin cancer, with ~232,000 new cases diagnosed and 55,000 cases of melanoma-associated mortality estimated annually worldwide (1). The current treatment of melanoma is based on surgery plus chemotherapy or radiation therapy; however, even dacarba-zine, the standard first-line treatment for melanoma, does not improve the overall survival benefit (2). Previous studies have reported that numerous Food and Drug Administration (FDA)-approved drugs may be useful as novel pharmacotherapies in the treatment of malignant tumours (3–6). Consequently, additional novel drugs need to be identified to suppress cancer cell growth, diversify options and enhance the effectiveness of antitumour therapy.
As an FDA-approved neuroleptic drug, pimozide belongs to the diphenylpiperidine class of drugs, and is commonly used in the treatment of Tourette syndrome and schizophrenia (7). Previous studies have revealed that pimozide is efficacious in the treatment of various types of leukaemia and carcinoma, including acute or chronic myelogenous leukaemia (8,9), breast cancer (10), liver carcinoma (11) and prostate cancer (12). However, the effects of pimozide on melanoma are not sufficient, and the molecular mechanism has yet to be fully elucidated (13,14). Therefore, it is necessary to determine the mechanism underlying the progression of melanoma, in order to identify a novel strategy to enhance pimozide treatment.
It has previously been reported that a major impediment to cancer immunotherapy is the induction of indoleamine 2, 3-dioxygenase (IDO), which is an enzyme that may contribute to tumour immune tolerance (15). IDO is an immunosuppressive, intracellular rate-limiting enzyme, which initiates the catabolism of essential amino acids along the kynurenine pathway (16). IDO expression has been detected in various stromal and immune cells, and is best characterized in dendritic cells (17). In cancer, IDO is expressed in various tumour cells, and tumours expressing IDO can resist immune reaction by tumour-associated antigen-specific host cytotoxic T lymphocyte cells in mouse models (18). Numerous studies and clinical trials have evaluated the role of IDO and its inhibitors in animal models and in patients with cancer, and have revealed the benefit of IDO inhibitors in cancer treatment (19–22). Therefore, the suppression of IDO expression may be considered a novel, effective therapeutic strategy (18,23).
The present study aimed to investigate the mechanism underlying the antitumour effects of pimozide on melanoma cells in vitro. Since the promising effects of combination therapies on melanoma have been verified in previous basic and clinical studies (24–28), the present study combined an IDO inhibitor, L-methyl-tryptophan (L-MT), with pimozide and investigated the antitumour effects of the combination in vivo.
Materials and methods
Cell lines, mice and reagents
The B16 mouse melanoma cell line was provided by Professor Liying Wang (Department of Molecular Biology, Jilin University, Changchun, China). The cells were cultured in RPMI-1640 medium (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% foetal bovine serum (MP Biomedicals, LLC, Santa Ana, CA, USA) at 37°C in an atmosphere containing 5% CO2. A total of 120 male C57BL/6 mice (weight, 18–22 g; age, 6 weeks) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) and were maintained at 22±2°C with a 12-h light/dark cycle under pathogen-free conditions. All mice had free access to food and water, and the animal studies were approved by the Ethics Committee of Xinxiang Medical University (Xinxiang, China). Pimozide was obtained from Shanghai ZZBIO Co., Ltd. (Shanghai, China) and L-MT was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Cell cytotoxicity and cell viability assays
Cell cytotoxicity following treatment with various concentrations of pimozide was monitored according to morphological observation, whereas cell viability was detected using the Cell Counting kit (CCK)-8 assay (Beyotime Institute of Biotechnology, Shanghai, China), according to the manufacturer's protocol. Briefly, 1×104 B16 cells were seeded onto 96-well plates (Corning Incorporation, Corning, NY, USA) and incubated at 37°C in a 5% CO2 humidified incubator for 24 h. Subsequently, the cells were treated with dimethyl sulfoxide or 0–40 µg/ml pimozide for 24 and 48 h. Cells in culture medium alone served as the untreated control group. For the cell cytotoxicity assay, images of the cells were captured under an inverted microscope at 24 or 48 h. For cell viability assays, CCK-8 regent (10 µl) was added to each well, and cells were incubated for 2 h at 37°C, after which the cells were analysed by measuring absorbance at 450 nm using a plate reader. Cell viability was exhibited as the optical density value.
Wound-healing assay
A total of 3×105 B16 cells were seeded onto 6-well plates (Corning Incorporated) and were incubated at 37°C in a 5% CO2 humidified incubator for 24 h. Subsequently, each well was scratched using a thin, disposable pipette tip, in order to generate a wound in the cell monolayer. B16 cells were then treated with 0–5 µg/ml pimozide and incubated for a further 24 or 48 h. Images of cell migration were captured and analysed under a light microscope (TI-S, Nikon Corporation, Tokyo, Japan).
Tumour challenge and animal treatment
Melanoma-bearing mice were established via the subcutaneous (s.c.) inoculation of B16 cells. Briefly, 5×105 B16 cells (s.c.) were injected into the right side of the back of C57BL/6 mice. A total of 7 days after tumour inoculation, mice were randomly assigned into four groups and received daily intraperitoneal injections with PBS, pimozide (200 µg/mouse), L-MT (2 mg/mouse) or pimozide (200 µg/mouse) plus L-MT (2 mg/mouse) for 1 week (n=30/group). Tumour incidence and tumour weight were measured daily, and the experiment was terminated on day 30.
Haematoxylin and eosin (HE) staining, immunohistochemistry (IHC) and terminal deoxynucleotidyl transferase dUTP nick end-labelling (TUNEL) staining
A total of 21 days after inoculation, nine of the mice in each group were sacrificed, and the tumours were excised and fixed in 4% formalin at room temperature for >24 h. Formalin-fixed and paraffin-embedded specimens were cut into 5-µm sections, which were mounted on glass slides. The sections underwent standard HE staining (29). Images were captured under a light microscope (TI-S; Nikon Corporation). For IHC, sections were deparaffinized and dehydrated in a series of xylene and alcohol washes. Following quenching of endogenous peroxidase activity with 3% (vol/vol) H2O2 in methanol for 15 min, sections were microwaved (10 min) in citrate buffer for antigen retrieval. The tissues were then blocked with 1% (wt/vol) bovine serum albumin (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at room temperature for 15 min and incubated with monoclonal antibodies directed against CD4 (1:100) and CD8 (1:100) (cat. nos. 25229 and 98941; both Cell Signaling Technology, Inc., Danvers, MA, USA) overnight at 4°C. After washing with PBS, the sections were incubated with a horseradish peroxidase-conjugated immunoglobulin G secondary antibody (1:1,000; cat. no. BS13278; Bioworld Technology, Inc., St. Louis Park, MN, USA) for 30 min at room temperature. Immunostaining for CD4 and CD8 was evaluated by light microscopy (TI-S; Nikon Corporation) in a blinded fashion. The intensity of CD4 or CD8 cell staining was scored on a 0 to 3+ scale: 0, no staining identified; 1+, <25% of positive cells; 2+, 25–75% positive cells; and 3+, >75% positive cells. A TUNEL assay was used to detect tumour cell apoptosis and was performed according to the manufacturer's protocol (Beyotime Institute of Biotechnology). TUNEL-positive cells were detected under light microscopy.
Western blotting
Cells were harvested at the 24 or 48 h following pimozide treatment. For animal experiments, nine mice were sacrificed from each group, and tumours were harvested and immediately frozen in liquid nitrogen at day 14 after treatment. Proteins were extracted from the samples using radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology) and the protein concentrations were determined using a bicinchoninic acid protein assay (Beyotime Institute of Biotechnology). Protein samples (50 µg/lane) were subsequently separated by SDS-PAGE on 10–15% resolving gels, and were transferred onto poly-vinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA). After blocking with 5% non-fat milk for 1 h at room temperature, the membranes were incubated with the following primary antibodies: IDO (1:1,000; cat. no. 68572), cyclin D1 (1:1,000; cat. no. 2978), proliferating cell nuclear antigen (PCNA) (1:1,000; cat. no. 13110), signal transducer and activator of transcription (Stat)3 (1:1,000; cat. no. 9139), phosphorylated (p)-Stat3 (1:500; cat. no. 9145), Stat5 (1:1,000; cat. no. 9363), p-Stat5 (1:500; cat. no. 4322), cleaved caspase 3 (1:500; cat. no. 9664s), cleaved caspase 7 (1:500; cat. no. 9491), matrix metalloproteinase 2 (MMP2) (1:1,000; cat. no. 87809) and α/β-tubulin (1:1,000; cat. no. 2148) (all from Cell Signaling Technology, Inc.) overnight at 4°C. Appropriate, horseradish peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin G secondary antibodies (1:2,000; cat. nos. 7074 and 7076; Cell Signaling Technology, Inc.) were subsequently used for 1 h at room temperature. Specific immune complexes were visualized using enhanced chemiluminescence (Beyotime Institute of Biotechnology). Blots were semi-quantifed using Quantity One software (version 4.62; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Flow cytometry
A total of 14 days post-treatment, spleens were excised from nine mice in each group, lysed with Red Blood Cell Lysis Buffer (Beyotime Institute of Biotechnology), centrifuged at 204 × g for 5 min at 4°C and washed with PBS. Cell suspension was prepared at a concentration of 1×107/ml. Subsequently, 100 µl cell suspension was incubated with appropriate fluorochrome-labelled CD3 (2 µl; cat. no. 100204), CD4 (1.5 µl; cat. no. 100412), CD8 (1.5 µl; cat. no. 100708) and natural killer (NK)1.1 (0.7 µl; cat. no. 108706) antibodies (all from BioLegend, Inc., San Diego, CA, USA) in the dark for 30 min at 4°C. Regulatory T cell (Treg) detection was performed using the Mouse Regulatory T Cell Staining kit (eBioscience; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The fluorescence intensity of cells was measured using a flow cytometer (Guava easyCyte; EMD Millipore) with a minimum of 10,000 cells collected.
Statistical analysis
All values are expressed as the means ± standard deviation of three independent experiments. Statistical analyses were performed using SPSS software 21.0 (SPSS Inc., Chicago, IL, USA). Differences of measurement data were compared using one-way analysis of variance (ANOVA) followed by the least significant difference post hoc test. For survival analysis, the Kaplan-Meier method with log-rank test was used. P<0.05 was considered to indicate a statistically significant difference.
Results
Pimozide inhibits B16 cell growth in vitro without toxicity when the concentration is <10 µg/ml
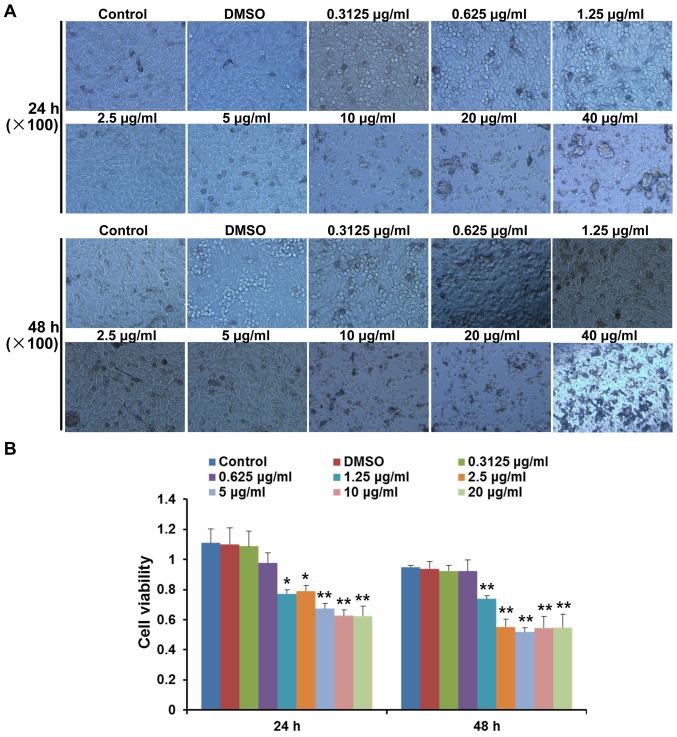
The present study analysed the cytotoxic effects of various concentrations of pimozide on B16 cell growth at 24 and 48 h. Morphological alterations and cell detachment were observed at concentrations >10 µg/ml (Fig. 1A). To further determine whether pimozide exerted direct effects on B16 cell growth, the viability of B16 cells was detected following pimozide treatment for 24 and 48 h using the CCK-8 assay. Pimozide, at a concentration between 1.25 and 20 µg/ml, decreased the viability of B16 cells following treatment for 24 and 48 h (P<0.05), whereas pimozide at concentrations between 0.3125 and 0.625 µg/ml did not exert antitumour effects (P>0.05; Fig. 1B). These results suggested that the 1.25–5 µg/ml pimozide did not exhibit toxicity but inhibited B16 cell viability.
Figure 1.
Effects of pimozide on B16 cell cytotoxicity and viability. (A) B16 cells were treated with various concentrations (0–40 µg/ml) of pimozide for 24 and 48 h. Images of the cytotoxic effects of pimozide were captured. (B) Growth inhibitory effects of pimozide on B16 cells were assessed by Cell Counting kit-8 assay. Values are presented as the means ± standard deviation (n=3, in triplicate). *P<0.05 vs. the control group; **P<0.01 vs. the control group. DMSO, dimethyl sulfoxide.
Apoptosis- and proliferation-associated proteins are regulated following treatment with pimozide
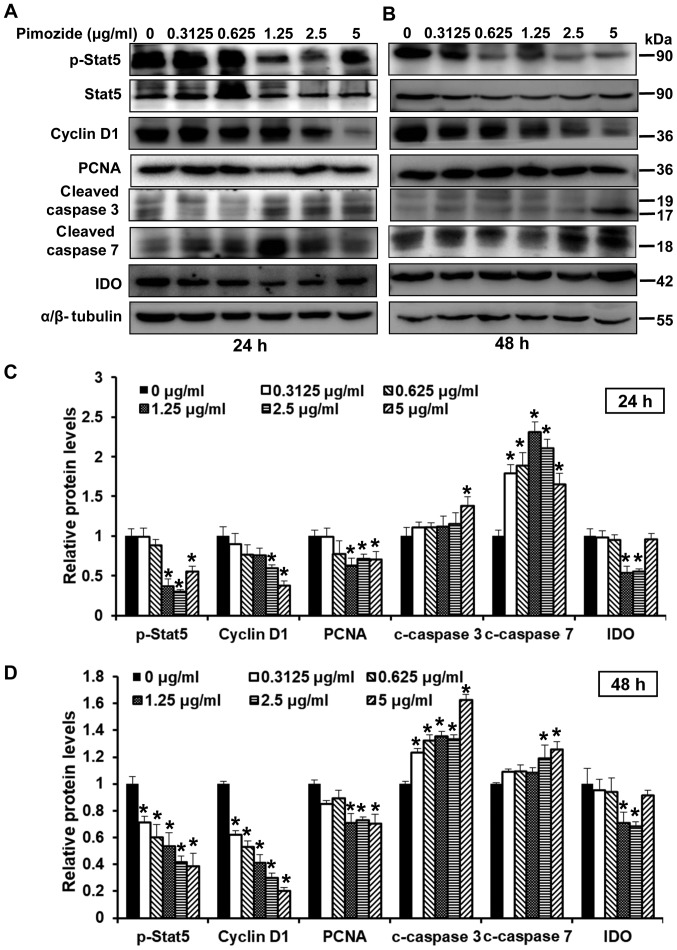
To determine the effects of pimozide on proliferation and apoptosis following 24 and 48-h treatment, the expression levels of related proteins, including Stat5, p-Stat5, cyclin D1, PCNA, cleaved caspase 3 and cleaved caspase 7 were detected. As shown in Fig. 2A–D, treatment with pimozide at various concentrations altered protein expression; proliferation-associated proteins (cyclin D1, PCNA, Stat5 and p-Stat5) were suppressed and the expression of apoptosis-associated proteins (cleaved caspase 3 and cleaved caspase 7) were increased. In addition, the present study detected whether pimozide could affect the expression levels of IDO. The results demonstrated that 1.25 and 2.5 µg/ml pimozide inhibited the expression of IDO (P<0.01; Fig. 2C and D). Conversely, when the concentration of pimozide reached 5 µg/ml, IDO expression was increased (Fig. 2C and D). These results suggested that apoptotic and proliferative proteins were regulated when treated with the appropriate concentration of pimozide.
Figure 2.
Effects of pimozide on the expression of proliferation- and apoptosis-associated proteins in B16 cells. B16 cells were treated with pimozide at the indicated doses. Western blotting was conducted to detect the expression of proliferation- and apoptosis-associated proteins, including p-Stat5, Stat5, cyclin D1, PCNA, cleaved caspase 3, cleaved caspase 7 and IDO, following (A) 24 and (B) 48-h treatment. α/β-tubulin was used as a standard. Semi-quantitative results of the relative protein levels at (C) 24 and (D) 48 h. Values are presented as the means ± standard deviation (n=3, in triplicate). *P<0.01 vs. the control group. IDO, indoleamine 2, 3-dioxygenase; p-, phosphorylated; PCNA, proliferating cell nuclear antigen; Stat, signal transducer and activator of transcription.
Pimozide inhibits the migration of B16 cells
Wound-healing assays were used to analyse the effects of pimozide on cell migration. As shown in Fig. 3A and B, the wound-healing assay indicated that treatment with >0.3125 µg/ml pimozide significantly inhibited the migration of B16 cells at 24 and 48 h (P<0.01), whereas in the control group, the cells migrated into the wound area in 48 h. Furthermore, the present study investigated whether MMP2, which has been reported to be associated with cell migration (30), was involved in the inhibitory effects of pimozide on migration. The results of the western blot analysis indicated that treatment with pimozide (≥1.25 µg/ml) significantly inhibited the expression levels of MMP2 in B16 cells (P<0.01; Fig. 3C–E). Taken together, these results indicated that pimozide may inhibit the migration of B16 cells.
Figure 3.
Effects of pimozide on the migration of B16 cells. (A) Wound-healing assay. Cells were seeded onto 6-well plates and the cell monolayer was wounded manually. Images were captured at 0, 24 and 48 h following treatment with pimozide. (B) Quantification of the wound-healing assay. Western blotting was conducted to detect MMP2 expression in B16 cells following various concentrations of pimozide for (C) 24 and (D) 48 h. (E) Semi-quantitative results of relative MMP2 protein levels. Values are presented as the means ± standard deviation (n=3, in triplicate). **P<0.01 vs. the control group. MMP2, matrix metalloproteinase 2.
Antitumour efficacy of pimozide and L-MT combined treatment in a mouse B16 xenograft model
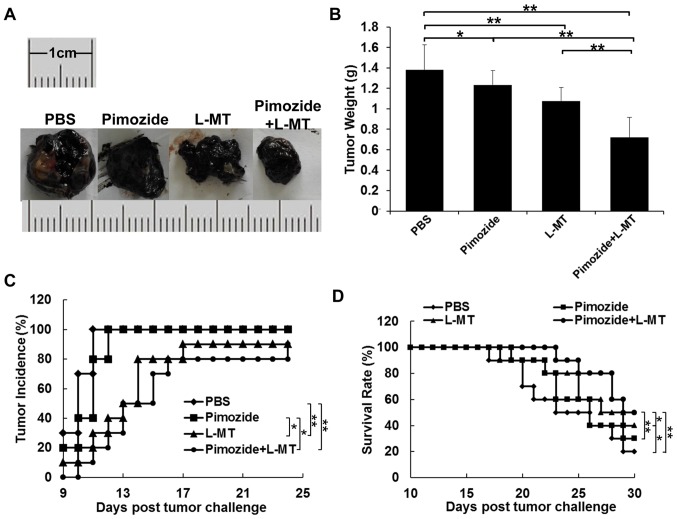
To explore whether inhibiting IDO expression could enhance the antitumour effects of pimozide in mice, the present study administered pimozide or L-MT single therapy, and pimozide and L-MT combined treatment. According to the results of previous studies (9,12,31,32) and a preliminary experiment, the optimal dosage of pimozide and L-MT was 200 µg/mouse and 2 mg/mouse, respectively. As shown in Fig. 4A and B, the average tumour weight of the L-MT and pimozide groups was less than that of the PBS group after 2 weeks of treatment; tumour weight was decreased by 22 (P<0.01) and 11% (P<0.05), respectively. In addition, the average tumour weight following combination therapy was decreased by 33, 41 and 48% compared with that of the L-MT, pimozide and PBS groups (P<0.01), respectively, thus indicating that combination therapy exhibited the most substantial antitumour effect. As shown in Fig. 4C, tumour incidence in the PBS and pimozide groups was highest (100%) on days 11 and 12, respectively, compared with in the L-MT and combination groups, where tumour incidence rate was 90 and 80% on day 24, respectively; this finding was consistent with tumour weight alterations. Finally, treatment with L-MT significantly prolonged the survival rate of B16 tumour-bearing mice compared with in the PBS group (P<0.05). Notably, combined treatment with pimozide and L-MT significantly prolonged the survival rate when compared with the three other treatment groups (Fig. 4D).
Figure 4.
Antitumour effects of pimozide + L-MT on a mouse B16 xenograft model in vivo. B16 cell-bearing mice were treated with pimozide, L-MT or a combination of the two drugs. (A) Images of representative tumours from each group. (B) Tumour weight. (C) Tumour incidence curves. (D) Survival rate curves for each group. n=10/group. *P<0.05; **P<0.01. L-MT, L-methyl-tryptophan.
Pimozide + L-MT treatment significantly inhibits melanoma cell proliferation and induces apoptosis in vivo
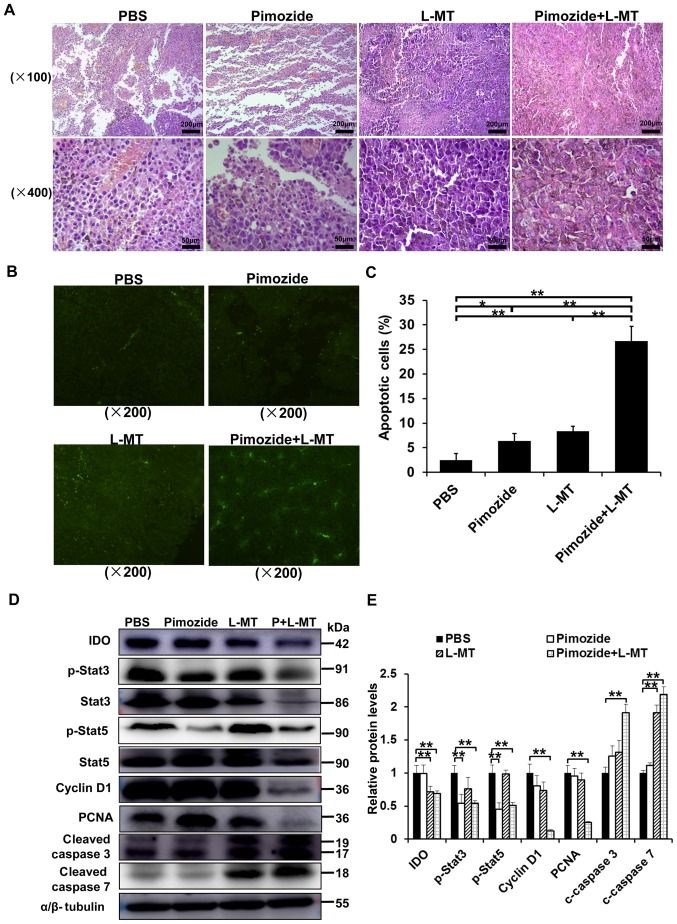
The marked inhibitory effects of pimozide on cell growth in vitro suggested that pimozide may suppress tumour cell proliferation or induce B16 cell apoptosis in vivo. To verify this hypothesis, and to detect the effects of combination therapy, the underlying mechanisms were assessed. HE staining detected obvious morphological differences in the tumour cells in both monotherapy groups and particularly in the combination group (Fig. 5A). TUNEL staining detected an increase in the amount of tumour cells undergoing apoptosis in the combination group (Fig. 5B and C). To further characterize pimozide-induced tumour growth inhibition and apoptosis, the expression levels of some associated proteins were detected by western blotting. IDO, p-Stat3, Stat3, p-Stat5, Stat5, cyclin D1, PCNA, cleaved caspase 3 and cleaved caspase 7 expression levels were detected in tumour tissues following various treatments for 2 weeks. As shown in Fig. 5D and E, IDO expression was significantly inhibited in the L-MT and combination therapy groups, thus indicating that the IDO inhibitor L-MT could efficiently suppress IDO expression. Furthermore, the proliferation-associated proteins, including p-Stat3, p-Stat5, cyclin D1 and PCNA, were markedly decreased, whereas the apoptosis-associated proteins, cleaved caspase 3 and cleaved caspase 7, were significantly increased in the combination therapy group (P<0.01; Fig. 5D and E), suggesting that combination therapy-induced apoptosis might occur via the mitochondrial apoptotic pathway. Collectively, these results indicated that combination therapy with pimozide and L-MT could delay tumour growth in vivo, potentially via inhibiting tumour cell proliferation and inducing apoptosis.
Figure 5.
Effects of pimozide and L-MT treatment on proliferation and apoptosis in vivo. B16 cell-bearing mice were treated with pimozide, L-MT or a combination of the two drugs. After 1 week, the mice were sacrificed and tumours were excised. (A) Haematoxylin and eosin staining was conducted to observe tumour tissue morphology. (B) Apoptosis of tumour cells was detected by TUNEL staining. (C) Quantitative analysis of apoptotic cells using the TUNEL assay. (D) Western blotting was performed to detect the expression levels of related proteins. (E) Semi-quantitative results of relative protein levels. n=9/group. *P<0.05; **P<0.01. IDO, indoleamine 2, 3-dioxygenase; L-MT, L-methyl-tryptophan; p-, phosphorylated; PCNA, proliferating cell nuclear antigen; Stat, signal transducer and activator of transcription; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end-labelling.
Effects of combination therapy on T-lymphocyte infiltration into tumour tissue
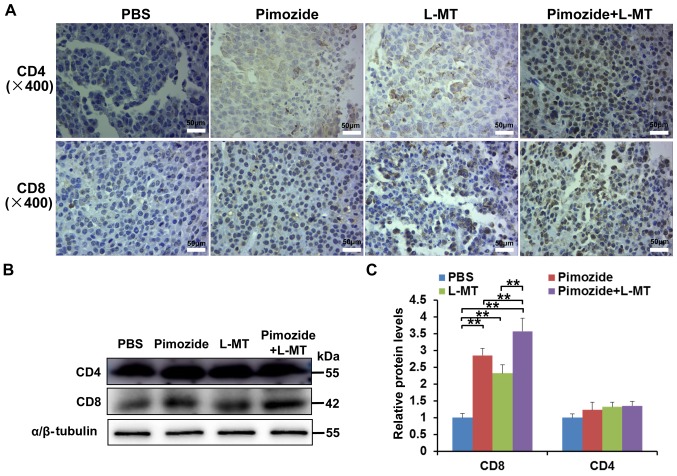
To determine the effects of combination therapy on T lymphocytes, an IHC assay was performed on tumour tissue samples. The results demonstrated that following treatment with pimozide and L-MT, the number of CD4+ and CD8+ T cells in tumour tissues increased, particularly CD8+ T cells (Fig. 6A). In addition, the results of western blotting confirmed that the expression levels of CD8 were markedly increased in the three treatment groups, particularly in the pimozide + L-MT group (P<0.01, Fig. 6B and C). These results suggested that the therapeutic effects of combination therapy on melanoma may be enhanced by promoting the T-lymphocyte response.
Figure 6.
Effects of combination therapy on T-lymphocyte infiltration into tumour tissues. Tumour tissue samples were harvested on day 21 after tumour inoculation. (A) Infiltration of CD4+ and CD8+ T cells in tumour tissues was determined by immunohistochemical analyses (each group contained three independent samples). (B) Protein expression levels of CD4 and CD8 in tumour tissues were analysed using western blotting. (C) Semi-quantitative results of CD4 and CD8 protein levels. n=9/group. **P<0.01. CD, cluster of differentiation; L-MT, L-methyl-tryptophan.
Effects of combination therapy on the proportion of immune cells in the spleen
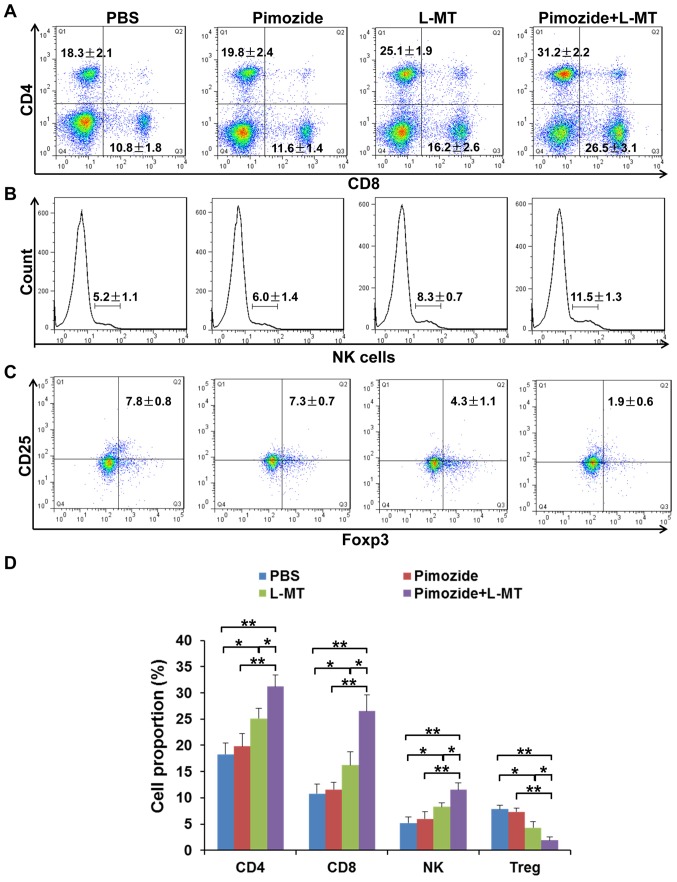
The spleen is the centre of cellular immunity; in order to analyse whether tumour-infiltrating T cells multiplied following treatment, the proportion of CD4+ and CD8+ T cells, NK cells and CD4+CD25+Foxp3+ Treg cells was detected in the spleens of mice. As shown in Fig. 7, after combination therapy, the proportion of CD4+ and CD8+ T cells, and NK cells, was increased; among these cells, the proportion of CD8+ T cells was most significantly increased. In addition, Treg cells, as representatives of CD25+Foxp3+ T cells, were significantly inhibited following combination therapy. These results suggested that L-MT enhanced the pimozide-induced therapeutic effect partly through regulation of the immune response.
Figure 7.
Effects of combination therapy on the proportion of immune cells in the spleen. A total of 2 weeks post-treatment, the spleens were obtained and immune cells were evaluated by flow cytometry. (A) CD4+ and CD8+ T cells, (B) NK1.1+ cells and (C) CD25+Foxp3+ T cells were detected by flow cytometry. (D) Statistical analysis of the proportion of CD4, CD8, NK and Treg cells. n= 9/group. *P<0.05; **P<0.01. CD, cluster of differentiation; Foxp3, forkhead box P3; L-MT, L-methyl-tryptophan; NK, natural killer; Treg, regulatory T cells.
Discussion
Melanoma is a drug-resistant malignancy that is found worldwide, the incidence of which is rapidly increasing (33). This neoplasm can be surgically removed at the early stages of disease (34); however, when the tumour is detected at the advanced stage, there are few therapeutically effective options that arrest or prevent tumour progression (35). Therefore, there is an urgent requirement for a low-cost novel drug that is more effective against melanoma (36,37). The present study provided evidence to suggest that treatment with a combination of pimozide and an IDO inhibitor exerted a significant therapeutic effect against malignant melanoma in vivo.
In the present study, the antipsychotic agent pimozide was investigated for its potential action against melanoma. The results of a CCK-8 assay demonstrated that pimozide (1.25–5 µg/ml) inhibited the growth of B16 cells with little cytotoxicity. To evaluate the mechanism underlying the anti-growth effects of pimozide, the expression levels of some key proteins associated with proliferation and apoptosis were detected by western blotting. Generally, as the target protein for pimozide, Stat5 phosphorylation serves an important role in melanoma cell survival (38). In the present study, Stat5 phosphorylation was significantly suppressed in B16 cells following treatment with pimozide at low micromolar concentrations. Cell cycle dysregulation has a vital role in modulating cell proliferation regulated by cyclins and cyclin-dependent kinases (39,40). Additionally, the nuclear protein, PCNA, is expressed during the G1-M phases of the cell cycle and promotes cell cycle progression (41). The present data indicated that treatment of B16 cells with pimozide decreased the protein expression levels of cyclin D1 and PCNA. As a fundamental component of cancer pathogenesis, apoptosis has a major role in melanoma progression (42). Therefore, inducing apoptosis is an important therapeutic approach to treat cancer (43). In the present study, pimozide-induced apoptotic death of B16 cells was confirmed by upregulation of cleaved caspase 3 and cleaved caspase 7. Melanoma is a malignancy that can spread to the lungs, lymph nodes and other organs (44), and the poor effectiveness of melanoma treatment might be ascribed to its high metastatic potential (45). The occurrence of cancer cell metastasis is associated the activation of MMP (46). In the present study, pimozide inhibited cell migration and significantly reduced MMP2 expression. Therefore, the present study indicated that pimozide treatment may inhibit B16 cell growth and migration.
Drugs with potent anticancer activity in vitro do not necessarily exhibit antitumour activity in vivo. To date, the elimination of large tumours in the advanced stages of cancer has been reliably achieved using a combination of different therapies in clinical trials and animal models (25,47–50). In addition, the IDO pathway has been reported to serve a central role in regulating the immunological tolerance of tumours (51–54). Numerous preclinical studies examined the application of IDO-targeted therapy (55–57). Among the inhibitors of IDO, L-MT is the most widely studied compound with a small molecular weight (18,23,58). In the present study, the results of western blotting demonstrated that pimozide, at a concentration of 5 µg/ml, increased IDO expression, which may be the cause of the insufficient effects of pimozide against melanoma in the clinic. Therefore, an IDO inhibitor combined with pimozide was used to generate an improved antitumour effect. The results suggested that tumour growth was markedly inhibited, and survival rate was prolonged in the combination therapy group compared with in the PBS or other monotherapy groups. Additionally, significantly reduced proliferation and induced apoptosis were observed following treatment with pimozide and L-MT, as detected by the alternative levels of associated proteins. Unexpectedly, regardless of Stat5 suppression, activation of Stat3 was also reduced following pimozide single treatment or pimozide + L-MT combination therapy, thus suggesting that pimozide was not only an inhibitor of Stat5 but also a potential inhibitor of Stat3, which is consistent with the results of a similar study in prostate cancer (12).
Tumours can escape immune attack through various mechanisms of immunosuppression (59,60). CD8+ T cells have an important role in antitumour immunity (61,62), and the importance of innate immune effector cells, such as NK cell, has also been described. A large body of evidence has indicated that the tumour microenvironment may prompt tumour development, progression and immune evasion (63,64). In the present study, CD4+ and CD8+ T cells were increased, not only in the spleen, but also in the tumour microenvironment following combination therapy; in particular, the proportion of CD8+ T cells was increased. Consistent with the importance of the innate immune response, this enhanced tumour immunity in response to combination therapy was attributed not only to T lymphocytes, but also to NK cells. Unexpectedly, immune stimulation was detected, to some extent, in the pimozide monotherapy group; the underlying mechanism may be attributed to the suppression of Stat3 activation, which is relevant to immune escape (58). In addition, Tregs have been identified as key components to induce the immune tolerance of cancer cells (65). As expected, the number of Tregs was significantly decreased following treatment with pimozide and L-MT.
In conclusion, the present study provided evidence to suggest that pimozide and L-MT have a combined effectiveness against melanoma. Notably, L-MT enhanced the antitumour immunity of pimozide against melanoma in vivo through regulating tumour proliferation, apoptosis, migration and immunity. Therefore, these results suggested that combination therapy with pimozide and L-MT may be considered a novel treatment strategy for melanoma.
Acknowledgments
This study has been presented as an abstract at The 12th National Academic Congress of Immunology (303; 2017.10.26-29; Tianjin, China).
Funding
The present study was financially supported through grants from the National Natural Science Foundation of China (grant nos. 81301947, 81300442 and U1404816), the Scientific Research Fund of Xinxiang Medical University (grant nos. 2013QN112 and 2014QN115), the Doctor Launch Fund of Xinxiang Medical University (grant nos. 505016 and 505017), the Key Projects of Scientific Research for Higher Education of Henan Province (grant no. 17A310026), the Medical Science and Technology Research Project in Henan Province of China (grant no. 201502016), the Natural Science Foundation of Henan Province (grant no. 162300410225), the Xinxiang Science and Technology Research Program (grant no. CXGG16020) and the Graduate Student Innovation Support Plan (grant no. YJSCX201648Y).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
ZWT and ZWF designed the experiments. HW and MYW participated in designing the experiment, analysing the data and revising the manuscript. HJJ, WJR, YCF, TW, MMG, JG, JJZ, XFS and TSZ carried out the experiments. HJJ wrote the manuscript.
Ethics approval and consent to participate
All animal studies were approved by the Ethics Committee of Xinxiang Medical University (Xinxiang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Eggermont AM, Kirkwood JM. Re-evaluating the role of dacarbazine in metastatic melanoma: What have we learned in 30 years. Eur J Cancer. 2004;40:1825–1836. doi: 10.1016/j.ejca.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 3.Zhao T, Jia H, Cheng Q, Xiao Y, Li M, Ren W, Li C, Feng Y, Feng Z, Wang H, et al. Nifuroxazide prompts antitumor immune response of TCL-loaded DC in mice with orthotopically-implanted hepatocarcinoma. Oncol Rep. 2017;37:3405–3414. doi: 10.3892/or.2017.5629. [DOI] [PubMed] [Google Scholar]
- 4.Del Barco S, Vazquez-Martin A, Cufí S, Oliveras-Ferraros C, Bosch-Barrera J, Joven J, Martin-Castillo B, Menendez JA. Metformin: Multi-faceted protection against cancer. Oncotarget. 2011;2:896–917. doi: 10.18632/oncotarget.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hossain MA, Kim DH, Jang JY, Kang YJ, Yoon JH, Moon JO, Chung HY, Kim GY, Choi YH, Copple BL, et al. Aspirin induces apoptosis in vitro and inhibits tumor growth of human hepatocellular carcinoma cells in a nude mouse xenograft model. Int J Oncol. 2012;40:1298–1304. doi: 10.3892/ijo.2011.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Triscott J, Lee C, Hu K, Fotovati A, Berns R, Pambid M, Luk M, Kast RE, Kong E, Toyota E, et al. Disulfiram, a drug widely used to control alcoholism, suppresses the self-renewal of glioblastoma and over-rides resistance to temozolomide. Oncotarget. 2012;3:1112–1123. doi: 10.18632/oncotarget.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egolf A, Coffey BJ. Current pharmacotherapeutic approaches for the treatment of Tourette syndrome. Drugs Today (Barc) 2014;50:159–179. doi: 10.1358/dot.2014.50.2.2097801. [DOI] [PubMed] [Google Scholar]
- 8.Nelson EA, Walker SR, Xiang M, Weisberg E, Bar-Natan M, Barrett R, Liu S, Kharbanda S, Christie AL, Nicolais M, et al. The STAT5 inhibitor pimozide displays efficacy in models of acute myelogenous leukemia driven by FLT3 mutations. Genes Cancer. 2012;3:503–511. doi: 10.1177/1947601912466555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson EA, Walker SR, Weisberg E, Bar-Natan M, Barrett R, Gashin LB, Terrell S, Klitgaard JL, Santo L, Addorio MR, et al. The STAT5 inhibitor pimozide decreases survival of chronic myelogenous leukemia cells resistant to kinase inhibitors. Blood. 2011;117:3421–3429. doi: 10.1182/blood-2009-11-255232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strobl JS, Kirkwood KL, Lantz TK, Lewine MA, Peterson VA, Worley JF., III Inhibition of human breast cancer cell proliferation in tissue culture by the neuroleptic agents pimozide and thioridazine. Cancer Res. 1990;50:5399–5405. [PubMed] [Google Scholar]
- 11.Sun J, Jiang J, Lu K, Chen Q, Tao D, Chen Z. Therapeutic potential of ADAM17 modulation in gastric cancer through regulation of the EGFR and TNF-α signalling pathways. Mol Cell Biochem. 2017;426:17–26. doi: 10.1007/s11010-016-2877-9. [DOI] [PubMed] [Google Scholar]
- 12.Zhou W, Chen MK, Yu HT, Zhong ZH, Cai N, Chen GZ, Zhang P, Chen JJ. The antipsychotic drug pimozide inhibits cell growth in prostate cancer through suppression of STAT3 activation. Int J Oncol. 2016;48:322–328. doi: 10.3892/ijo.2015.3229. [DOI] [PubMed] [Google Scholar]
- 13.Taub RN, Baker MA. Treatment of metastatic malignant melanoma with pimozide. Lancet. 1979;1:605. doi: 10.1016/S0140-6736(79)91025-0. [DOI] [PubMed] [Google Scholar]
- 14.Neifeld JP, Tormey DC, Baker MA, Meyskens FL, Jr, Taub RN. Phase II trial of the dopaminergic inhibitor pimozide in previously treated melanoma patients. Cancer Treat Rep. 1983;67:155–157. [PubMed] [Google Scholar]
- 15.Mellor AL, Munn DH. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 16.Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2.3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516–2522. doi: 10.1096/fasebj.5.11.1907934. [DOI] [PubMed] [Google Scholar]
- 17.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 18.Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 19.Löb S, Königsrainer A, Rammensee HG, Opelz G, Terness P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: Can we see the wood for the trees. Nat Rev Cancer. 2009;9:445–452. doi: 10.1038/nrc2639. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Shin N, Koblish HK, Yang G, Wang Q, Wang K, Leffet L, Hansbury MJ, Thomas B, Rupar M, et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010;115:3520–3530. doi: 10.1182/blood-2009-09-246124. [DOI] [PubMed] [Google Scholar]
- 21.Su C, Zhang P, Liu J, Cao Y. Erianin inhibits indoleamine 2,3-dioxygenase-induced tumor angiogenesis. Biomed Pharmacother. 2017;88:521–528. doi: 10.1016/j.biopha.2017.01.090. [DOI] [PubMed] [Google Scholar]
- 22.Jiang GM, Wang HS, Du J, Ma WF, Wang H, Qiu Y, Zhang QG, Xu W, Liu HF, Liang JP. Bortezomib relieves immune tolerance in nasopharyngeal carcinoma via STAT1 suppression and indoleamine 2,3-dioxygenase downregulation. Cancer Immunol Res. 2017;5:42–51. doi: 10.1158/2326-6066.CIR-16-0102. [DOI] [PubMed] [Google Scholar]
- 23.Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, Mellor AL, Prendergast GC, Munn DH. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67:792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 24.Rogiers A, Wolter P, Bechter O. Dabrafenib plus trametinib rechallenge in four melanoma patients who previously progressed on this combination. Melanoma Res. 2017;27:164–167. doi: 10.1097/CMR.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 25.Dummer R, Hoeller C, Gruter IP, Michielin O. Combining talimogene laherparepvec with immunotherapies in melanoma and other solid tumors. Cancer Immunol Immunother. 2017;66:683–695. doi: 10.1007/s00262-017-1967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmer L, Apuri S, Eroglu Z, Kottschade LA, Forschner A, Gutzmer R, Schlaak M, Heinzerling L, Krackhardt AM, Loquai C, et al. Ipilimumab alone or in combination with nivolumab after progression on anti-PD-1 therapy in advanced melanoma. Eur J Cancer. 2017;75:47–55. doi: 10.1016/j.ejca.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Hermel DJ, Ott PA. Combining forces: The promise and peril of synergistic immune checkpoint blockade and targeted therapy in metastatic melanoma. Cancer Metastasis Rev. 2017;36:43–50. doi: 10.1007/s10555-017-9656-2. [DOI] [PubMed] [Google Scholar]
- 28.Deniger DC, Kwong ML, Pasetto A, Dudley ME, Wunderlich JR, Langhan MM, Lee CR, Rosenberg SA. A pilot trial of the combination of vemurafenib with adoptive cell therapy in patients with metastatic melanoma. Clin Cancer Res. 2017;23:351–362. doi: 10.1158/1078-0432.CCR-16-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia H, Cui J, Jia X, Zhao J, Feng Y, Zhao P, Zang D, Yu J, Zhao T, Wang H, et al. Therapeutic effects of STAT3 inhibition by nifuroxazide on murine acute graft graft-vs.-host disease: Old drug, new use. Mol Med Rep. 2017;16:9480–9486. doi: 10.3892/mmr.2017.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauvois B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: Outside-in signaling and relationship to tumor progression. Biochim Biophys Acta. 2012;1825:29–36. doi: 10.1016/j.bbcan.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Lim JY, Lee SE, Park G, Choi EY, Min CK. Inhibition of indoleamine 2,3-dioxygenase by stereoisomers of 1-methyltryptophan in an experimental graft-versus-tumor model. Exp Hematol. 2014;42:862–866 e863. doi: 10.1016/j.exphem.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Qian F, Liao J, Villella J, et al. Effects of 1-methyltryptophan stereoisomers on IDO2 enzyme activity and IDO2-mediated arrest of human T cell proliferation. Cancer Immunol Immunother. 2012;61:2013–2020. doi: 10.1007/s00262-012-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 34.Cho YR, Chiang MP. Epidemiology, staging (new system), and prognosis of cutaneous melanoma. Clin Plast Surg. 2010;37:47–53. doi: 10.1016/j.cps.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Damsky WE, Theodosakis N, Bosenberg M. Melanoma metastasis: New concepts and evolving paradigms. Oncogene. 2014;33:2413–2422. doi: 10.1038/onc.2013.194. [DOI] [PubMed] [Google Scholar]
- 36.Holmes D. The cancer that rises with the sun. Nature. 2014;515:S110–S111. doi: 10.1038/515S110a. [DOI] [PubMed] [Google Scholar]
- 37.Schadendorf D, Fisher DE, Garbe C, Gershenwald JE, Grob JJ, Halpern A, Herlyn M, Marchetti MA, McArthur G, Ribas A, et al. Melanoma. Nat Rev Dis Primers. 2015;1:15003. doi: 10.1038/nrdp.2015.3. [DOI] [PubMed] [Google Scholar]
- 38.Mirmohammadsadegh A, Hassan M, Bardenheuer W, Marini A, Gustrau A, Nambiar S, Tannapfel A, Bojar H, Ruzicka T, Hengge UR. STAT5 phosphorylation in malignant melanoma is important for survival and is mediated through SRC and JAK1 kinases. J Invest Dermatol. 2006;126:2272–2280. doi: 10.1038/sj.jid.5700385. [DOI] [PubMed] [Google Scholar]
- 39.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: A changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz GK, Shah MA. Targeting the cell cycle: A new approach to cancer therapy. J Clin Oncol. 2005;23:9408–9421. doi: 10.1200/JCO.2005.01.5594. [DOI] [PubMed] [Google Scholar]
- 41.Kelman Z, Hurwitz J. Protein-PCNA interactions: A DNA-scanning mechanism. Trends Biochem Sci. 1998;23:236–238. doi: 10.1016/S0968-0004(98)01223-7. [DOI] [PubMed] [Google Scholar]
- 42.Hersey P, Zhang XD. How melanoma cells evade trail-induced apoptosis. Nat Rev Cancer. 2001;1:142–150. doi: 10.1038/35101078. [DOI] [PubMed] [Google Scholar]
- 43.Xia Y, Song X, Li D, Ye T, Xu Y, Lin H, Meng N, Li G, Deng S, Zhang S, et al. YLT192, a novel, orally active bioavailable inhibitor of VEGFR2 signaling with potent antiangiogenic activity and antitumor efficacy in preclinical models. Sci Rep. 2014;4:6031. doi: 10.1038/srep06031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fidler IJ. The pathogenesis of cancer metastasis: The 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 45.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, Nickerson E, Auclair D, Li L, Place C, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27:5287–5297. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bauer D, Werth F, Nguyen HA, Kiecker F, Eberle J. Critical role of reactive oxygen species (ROS) for synergistic enhancement of apoptosis by vemurafenib and the potassium channel inhibitor TRAM-34 in melanoma cells. Cell Death Dis. 2017;8:e2594. doi: 10.1038/cddis.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Booth L, Roberts JL, Sander C, Lee J, Kirkwood JM, Poklepovic A, Dent P. The HDAC inhibitor AR42 interacts with pazopanib to kill trametinib/dabrafenib-resistant melanoma cells in vitro and in vivo. Oncotarget. 2017;8:16367–16386. doi: 10.18632/oncotarget.14829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thakur V, Lu J, Roscilli G, Aurisicchio L, Cappelletti M, Pavoni E, White WL, Bedogni B. The natural compound fucoidan from New Zealand Undaria pinnatifida synergizes with the ERBB inhibitor lapatinib enhancing melanoma growth inhibition. Oncotarget. 2017;8:17887–17896. doi: 10.18632/oncotarget.14437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lugini L, Sciamanna I, Federici C, Iessi E, Spugnini EP, Fais S. Antitumor effect of combination of the inhibitors of two new oncotargets: Proton pumps and reverse transcriptase. Oncotarget. 2017;8:4147–4155. doi: 10.18632/oncotarget.13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117:1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frumento G, Rotondo R, Tonetti M, Ferrara GB. T cell proliferation is blocked by indoleamine 2,3-dioxygenase. Transplant Proc. 2001;33:428–430. doi: 10.1016/S0041-1345(00)02078-9. [DOI] [PubMed] [Google Scholar]
- 54.Munn DH, Mellor AL. IDO in the tumor microenvironment: Inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37:193–207. doi: 10.1016/j.it.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Solms M. A previously-untranslated report by Freud of a lecture on the mechanism of obsessional ideas and phobias. Int J Psychoanal. 1989;70:91–94. [PubMed] [Google Scholar]
- 56.Holtzhausen A, Zhao F, Evans KS, Tsutsui M, Orabona C, Tyler DS, Hanks BA. Melanoma-derived Wnt5a promotes local dendritic-cell expression of IDO and immunotolerance: Opportunities for pharmacologic enhancement of immunotherapy. Cancer Immunol Res. 2015;3:1082–1095. doi: 10.1158/2326-6066.CIR-14-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen D, Koropatnick J, Jiang N, Zheng X, Zhang X, Wang H, Yuan K, Siu KS, Shunnar A, Way C, et al. Targeted siRNA silencing of indoleamine 2, 3-dioxygenase in antigen-presenting cells using mannose-conjugated liposomes: A novel strategy for treatment of melanoma. J Immunother. 2014;37:123–134. doi: 10.1097/CJI.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 58.Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 59.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 61.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: Impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 62.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kortylewski M, Swiderski P, Herrmann A, Wang L, Kowolik C, Kujawski M, Lee H, Scuto A, Liu Y, Yang C, et al. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat Biotechnol. 2009;27:925–932. doi: 10.1038/nbt.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15:409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jia H, Li Y, Zhao T, Li X, Hu J, Yin D, Guo B, Kopecko DJ, Zhao X, Zhang L, et al. Antitumor effects of Stat3-siRNA and endostatin combined therapies, delivered by attenuated Salmonella, on orthotopically implanted hepatocarcinoma. Cancer Immunol Immunother. 2012;61:1977–1987. doi: 10.1007/s00262-012-1256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.