Abstract
Polycystic ovary syndrome (PCOS), the most common endocrine disorder in women of reproductive age, is characterized by androgen excess and ovarian dysfunction and presents with increased cardiometabolic risk factors such as obesity, insulin resistance, and elevated blood pressure (BP). We previously reported that administration of dihydrotestosterone (DHT) to female rats elicits cardiometabolic derangements similar to those found in women with PCOS. In this study, we tested the hypothesis that the DHT-mediated cardiometabolic derangements observed in PCOS are long lasting despite DHT withdrawal. Four-week-old female Sprague Dawley rats were treated with DHT (7.5 mg/90 days) or placebo for 6 months. DHT was discontinued (ex-DHT), and rats were followed for 6 additional months. After 6 months of DHT withdrawal, food intake, body weight, fat and lean mass, fasting plasma insulin, leptin, and adiponectin were elevated in ex-DHT rats. BP remained significantly elevated, and enalapril, an angiotensin-converting enzyme (ACE) inhibitor, normalized BP in ex-DHT rats. Expression of components of the intrarenal renin-angiotensin system was increased in ex-DHT rats. The cardiometabolic features found in ex-DHT rats were associated with lower plasma androgen levels but increased expression of renal and adipose tissue androgen receptors. In summary, androgen-induced cardiometabolic effects persisted after DHT withdrawal in a PCOS experimental model. Activation of intrarenal renin-angiotensin system plays a major role in the androgen-mediated increase in BP in ex-DHT. Upregulation of the renal and adipose tissue androgen receptor may explain the long-lasting effects of androgens. In clinical scenarios characterized by hyperandrogenemia in women, prompt normalization of androgen levels may be necessary to prevent their long-lasting cardiometabolic effects.
Keywords: androgen receptor, androgens, blood pressure, cardiometabolic risk factors, polycystic ovary syndrome, renin angiotensin system
Cardiometabolic derangements persist after 6 months of dihydrotestosterone withdrawal in a rat model of androgen-induced polycystic ovary syndrome.
Polycystic ovary syndrome (PCOS), the most common endocrine disorder in women of reproductive age, is characterized by androgen excess and ovarian dysfunction [1–5]. PCOS is also frequently associated with an increased prevalence of cardiometabolic risk factors, such as obesity, insulin resistance, and elevated blood pressure (BP) [6–10]. Diagnosis of PCOS remains controversial because three different criteria exist. Depending on the diagnostic criteria applied [National Institutes of Health (NIH), Rotterdam, or the Androgen Excess and PCOS Society] [11–13], the prevalence of PCOS is between 6% and 18% among women of reproductive age [1, 14]. More recently, the NIH Evidence-based Methodology Workshop on PCOS recommended the use of the broad, inclusionary diagnostic criteria of Rotterdam (which includes the NIH and Androgen Excess and PCOS Society criteria) while specifically identifying the phenotype [15]. Several lines of evidence show a positive correlation between hyperandrogenemia in PCOS and obesity, insulin resistance, and elevated BP [16–21], suggesting that hyperandrogenemia is a key factor underling the cardiometabolic derangements present in PCOS.
Activation of the renin-angiotensin system (RAS) plays a major role in the pathophysiology of several models of hypertension [22, 23]. Women with PCOS have elevated levels of renin that positively correlate with circulating androgen levels [24]. Furthermore, telmisartan, an angiotensin II type 1 receptor antagonist, reduces BP in patients with PCOS [25]. Whether hyperandrogenemia leads to activation of RAS and subsequently to elevation of BP in women with PCOS has not been entirely elucidated.
Hyperandrogenemia is considered a key factor in the clinical manifestations of PCOS [11, 21, 26]. A recent worldwide internet survey of 1385 women with a diagnosis of PCOS showed that more than a third of women spent 2 years and saw at least three separate medical providers seeking a diagnosis to explain their symptoms [27]. These data suggest that hyperandrogenemia is present for a long period before treatment is initiated in patients with PCOS. Moreover, there are limited pharmacological tools available to treat the cardiovascular abnormalities [28] or to normalize androgen levels in women with PCOS.
We have previously reported that administration of dihydrotestosterone (DHT), a nonaromatizable androgen, to 4-week-old female Sprague Dawley rats for 3 months results in several negative cardiometabolic features, such as increase in food intake, body weight, adiposity, BP, insulin resistance, and renal injury [29]. Those changes were associated with a significant upregulation of intrarenal angiotensinogen [29]; however, whether changes in renal angiotensinogen and the subsequent activation of the RAS mediate the increase in BP in women with PCOS remains unknown.
In this study, we tested the hypothesis that the DHT-mediated cardiometabolic derangements observed in PCOS are long lasting despite DHT withdrawal.
1. Materials and Methods
A. Animals
Female Sprague Dawley rats were obtained from Envigo (Indianapolis, IN) at 3 weeks of age. Rats were maintained throughout the study on standard rat chow diet (Teklad 22/5 Rodent Diet #8640; Envigo), housed in temperature-controlled rooms with a constant light/dark cycle (12 h/12 h) and free access to food and water. At 4 weeks of age, rats were randomly assigned to be implanted subcutaneously on the back of the neck with a continuous-release DHT (7.5 mg/90 days; Innovative Research of America, Sarasota, FL) or placebo pellets (n = 10 per group), as we previously reported [29]. Pellets were replaced every 90 days. After 6 months of DHT or placebo exposure, pellets were discontinued (ex-DHT), and animals were followed for an additional 6 months and compared with control groups. At the end of the study, blood samples and tissues were collected.
All experimental protocols were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals, 8th Edition, and were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
B. Body Weight, Food Intake, and Body Composition by Echo-MRI
Monthly body weight and food intake were measured for 6 months after DHT withdrawal. At 13 months of age, body composition of ex-DHT and control rats (n = 10/group), including total body fat mass, total body lean mass, and total body water, were measured (4 in1 900 model Body Composition Analyzer; Echo-MRI, Houston, TX) according to the manufacturer’s instructions, as we previously reported [30].
C. Metabolic Parameters
Fasting plasma insulin (RRID: AB_2732074), leptin (RRID: AB_2732075), and adiponectin (RRID: AB_2732076) were measured by ELISA according to the manufacturer’s instructions using commercially available kits (Linco Research, St. Charles, MO). An oral glucose tolerance test was performed after 6 hours of fasting, as we previously published [29]. After glucose administration, blood glucose was measured at 0, 15, 30, 60, and 120 minutes in blood collected from the tail using a Contour Next Bayer glucometer. Data are expressed as area under the curve. Metabolic determinations were performed at 13 months of age (6 months after DHT withdrawal).
D. Measurement of BP
At 12 months of age, under gas anesthesia with isoflurane using aseptic technique, a subset of ex-DHT rats and controls (n = 6 per group) were implanted with radiotelemetry transmitters (HD-SD10; Data Sciences International, St. Paul, MN) into the abdominal aorta below the renal arteries, as we previously described [31]. The transmitter was secured to the abdominal muscle. Each animal was housed with one roommate rat above a receiver (RLA-3000, Data Sciences International, St. Paul, MN) and allowed 10 days of recovery. Thereafter, mean systolic and diastolic arterial pressure and heart rate were monitored continuously for a total of 3 weeks in freely moving conscious animals. Telemetry BP measurements were obtained during a 10-second sampling period (500 Hz), recorded, and averaged every 5 minutes for 24 h/d [31]. After a week of baseline BP, the ACE inhibitor enalapril (250 mg/L) was administered via drinking water for 1 week, and BP was measured throughout. Thereafter, enalapril administration was discontinued, and BP was recorded for an additional 5 days (washout period).
E. Urinary Protein and Albumin Excretion
At 13 months of age, rats were placed in metabolic cages with free access to food and water for 24-hour urine collection. Urinary protein excretion was measured using the Bradford method with a commercially available reagent (Bio-Rad, Richmond, CA), and urinary albumin excretion was measured using the Nephrat ELISA (Exocell, Philadelphia, PA, RRID: AB_2732077).
F. Assessment of Glomerular Sclerosis
Formalin-fixed, paraffin-embedded, 5-µm kidney sections were stained with hematoxylin and eosin and Masson’s Trichrome stains. Kidney sections were examined by a pathologist who was unaware of the identity of the groups. At least 300 glomeruli from each kidney were examined, and each one was graded for segmental glomerular sclerosis as follows: <25% of glomerulus affected; 25% to 50% of glomerulus affected; 50% to 75% of the glomerulus affected; >75% of the glomerulus affected; and global sclerosis, as we previously reported [29]. Data are expressed as the average percentage of total glomeruli in each kidney exhibiting each injury level.
G. Gene Expression
Total RNA was extracted, DNAse treated, quantified, and reverse transcribed as we previously reported [32, 33]. Gene expression was quantified by quantitative RT-PCR using Sybr-Green I technology as we previously reported [32]. PCR product quantification was performed by the relative quantification method and expressed as arbitrary units standardized against GAPDH or β-actin [34, 35]. Primers, annealing temperature, and PCR amplicon size are reported in Table 1.
Table 1.
Quantitative PCR Primers
| Gene (Standard Abbreviation, Gene Symbol ) | Accession # |
Sequence
|
Annealing Temperature (°C) | Amplicon Size (bp) | |
|---|---|---|---|---|---|
| Sense | Antisense | ||||
| Angiotensiongen (AGTN, Agt) | NM_134432 | AGCACGGACAGCACCCTATT | AGAACTCATGGAGCCCAGTCA | 67.5 | 91 |
| Renin (Ren) | NM_012642 | GCTACATGGAGAATGGGACTGAA | ACCACATCTTGGCTGAGGAAAC | 67.5 | 79 |
| Angiotensin I converting enzyme (ACE, Ace) | NM_012544 | CTGCCTCCCAACGAGTTAGAA | CGGGACGTGGCCATTATATT | 60 | 140 |
| Angiotensin II type 1 receptor (AT1R, Agtr1) | NM_030985 | TATCACAGTGTGCGCGTTTCA | TGGTAAGGCCCAGCCCTAT | 67.5 | 68 |
| Mineralocorticoid receptor (MR, Nr3c2) | NM_013131 | ATCTGTTTGGTGTGTGGAGATG | CACGGCTCTTTTGAAGAAGACT | 60.0 | 90 |
| Androgen receptor (AR, Ar) | NM_012502 | GTGTCGTCTCCGGAAATGTT | GGAATCAGGCTGGTTGTTGT | 60.0 | 250 |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Gapdh) | NM_017008 | AAGATGGTGAAGGTCGGTGT | GTTGATGGCAACAATGTCCACT | 60.0 | 99 |
| Actin beta (ACTB, Actb) | V01217 | AAGTCCCTCACCCTCCCAAAAG | AAGCAATGCTGTCACCTTCCC | 60.0 | 97 |
H. Plasmatic Steroids and Estrous Cycle
Plasma levels of DHT were measured using a commercially available RIA kit after oxidation and extraction (DHT: DSL4900 Active DHT kit; Diagnostic Systems Laboratories, Inc., Beckman Coulter, RRID: AB_2732078) as we previously reported [29]. Plasma levels of estradiol, estrone, and testosterone were measured by liquid chromatography-mass spectroscopy at the Mayo Clinic Clinical Laboratory (Rochester, MN) as we previously reported [30]. DHT, estrone, and estradiol concentrations are expressed as picograms per milliliter, and testosterone concentration is expressed as nanograms per milliliter. Vaginal smears to determine estrus cycling were done as we previously reported [36].
I. Statistical Analyses
All data are expressed as mean ± SEM. Data were analyzed by Student t test (for two groups) or two-way ANOVA with Dunnett post hoc test. Differences were considered statistically significant at P < 0.05. Statistical analyses were performed with GraphPad Prism 6 software package version 6.07 (GraphPad Software Inc., La Jolla, CA).
2. Results
A. Body Weight
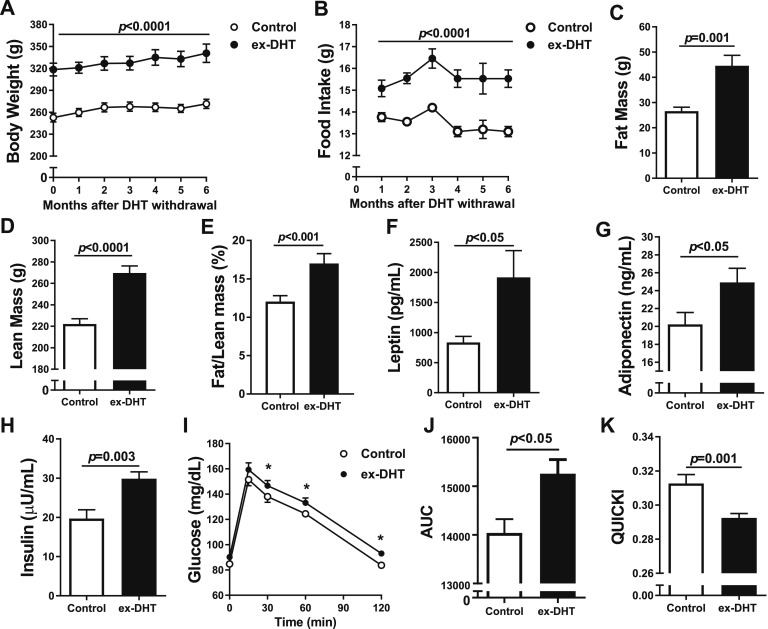
After 6 months of DHT withdrawal, ex-DHT rats showed a 25% higher body weight (340.7 ± 41 g vs 271.4 ± 18.9 g; P < 0.001) and a persistent increased food consumption (15.6 ± 0.1 g vs 13.2 ± 0.2 g; P < 0.0001) than controls (Fig. 1A and 1B).
Figure 1.
Body composition and metabolic parameters in ex-DHT and control rats. (A) Body weight and (B) food intake were elevated in ex-DHT rats and sustained throughout the 6 months after DHT withdrawal. (C) Fat mass, (D) lean mass, and (E) fat/lean mass ratio were increased in ex-DHT rats. (F) Leptin, (G) adiponectin, and (H) fasting insulin levels were higher in ex-DHT rats. (I–K) Insulin resistance measured by AUC (I and J) during oral glucose tolerance test was increased, whereas results obtained with the quantitative insulin sensitivity check index (QUICKI) (K) were decreased in ex-DHT rats. Determinations were performed at 13 months of age (6 months after DHT withdrawal). AUC, area under the curve. *P < 0.05.
B. Metabolic Parameters
Fat mass, lean mass, and fat/lean mass ratio, determined by Echo-MRI, were significantly higher in ex-DHT rats (Fig. 1C–1E). Plasma levels of leptin were approximately twofold higher, adiponectin was increased by 23%, and fasting plasma insulin was increased by 45% in ex-DHT rats (Fig. 1F–1H). Oral glucose tolerance test was significantly higher in ex-DHT rats (Fig. 1I and 1J). Insulin sensitivity, calculated by the quantitative insulin sensitivity check index (QUICKI), was significantly lower in ex-DHT rats (Fig. 1K). Taken together with the elevated insulin levels, these data suggest that ex-DHT rats remained insulin resistant.
C. BP and Response to Enalapril
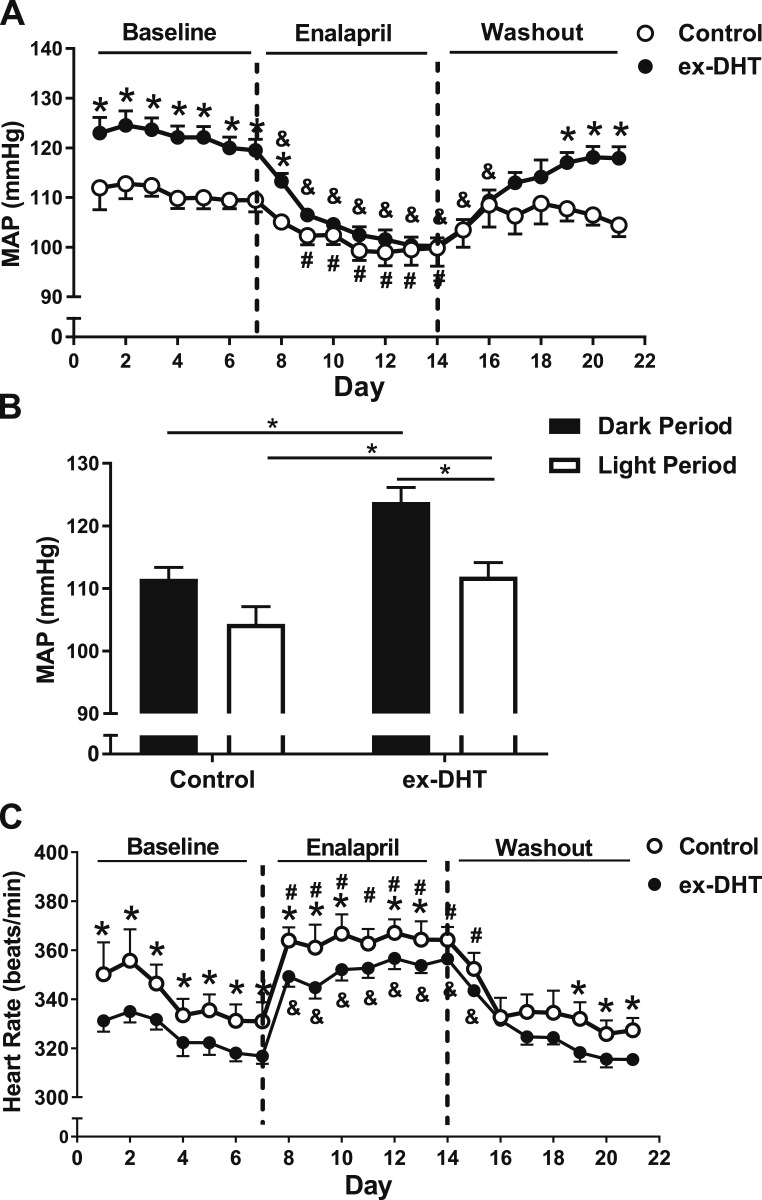
After 6 months of DHT withdrawal, mean arterial pressure (MAP) was significantly higher at baseline in ex-DHT rats (122.1 ± 0.2 vs 110.2 ± 0.2 mm Hg; P < 0.001) (Fig. 2A) and was higher during dark- and light-cycle periods compared with controls (Fig. 2B). Baseline differences were observed in systolic (143.4 ± 0.3 vs 131.6 ± 0.3 mm Hg; P < 0.001) and diastolic BPs (101.3 ± 0.9 vs 91.68 ± 0.6 mm Hg; P < 0.001) between ex-DHT and control rats. However, no significant differences in pulse pressure were observed between groups (42.13 ± 1.88 vs 39.52 ± 2.56 mm Hg). The difference observed in MAP at baseline was abolished after 5 days of enalapril treatment (103.9 ± 1.4 vs 100.9 ± 2.1 mm Hg; P = 0.28). The reduction in MAP values after enalapril treatment was significantly greater in ex-DHT rats than in controls (21.4 ± 0.6 vs 13.3 ± 0.8 mm Hg; P < 0.001). Heart rate was significantly lower in ex-DHT rats at baseline and during enalapril treatment (Fig. 2C). Hearts were also heavier (1.12 ± 0.03 vs 1.00 ± 0.02 g; P < 0.05) in ex-DHT rats than in controls.
Figure 2.
Blood pressure, heart rate, and effect of enalapril in ex-DHT and control rats. (A and B) ex-DHT rats had elevated MAP during light and dark cycles compared with controls. (C) ex-DHT rats had decreased heart rate compared with controls under both baseline and enalapril treatment. *P < 0.05, ex-DHT vs control. &P < 0.05 vs baseline ex-DHT rats, same treatment. #P < 0.05 vs baseline control rats, same treatment.
D. Renal Function and Morphology
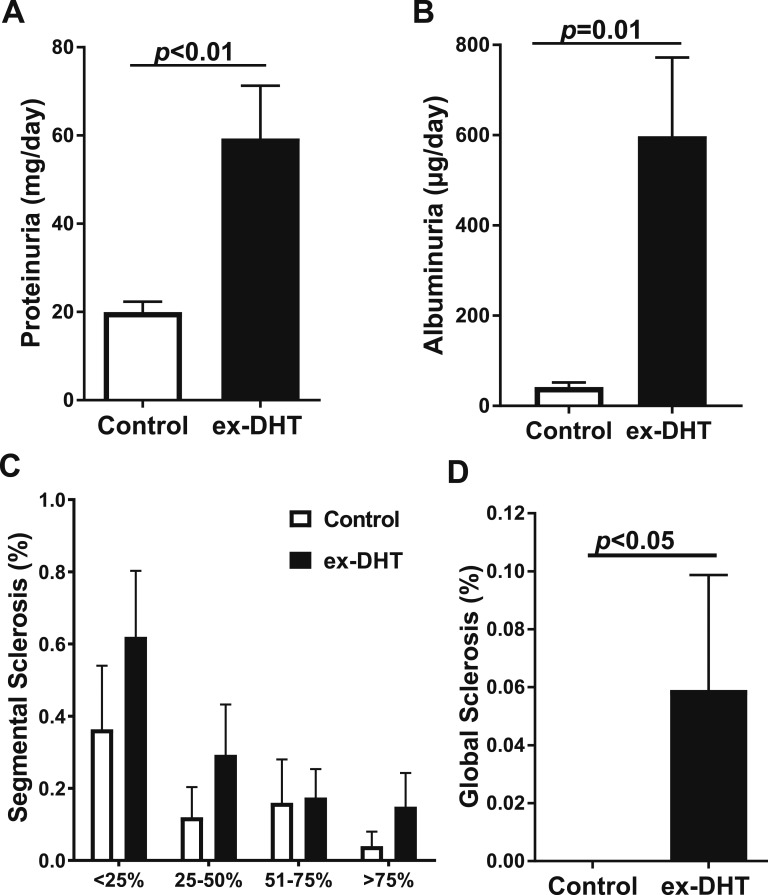
Urinary protein excretion (59.3 ± 12 vs 19.9 ± 2.3 mg/d; P < 0.05) and urinary albumin excretion (597.8 ± 174.3 vs 42.0 ± 9.7 µg/d; P < 0.05) were increased in ex-DHT rats (Fig. 3A and 3B). Kidneys from ex-DHT rats were heavier than controls (2.46 ± 0.11 vs 1.92 ± 0.13 g; P < 0.05). Renal injury was greater in ex-DHT rats, with histological analysis of glomeruli showing increased percentage of glomeruli with segmental and global sclerosis (Fig. 3C and 3D).
Figure 3.
Renal function and histology in ex-DHT and control rats. (A) ex-DHT rats had increased urinary protein excretion compared with control rats. (B) ex-DHT rats had elevated urinary albumin excretion compared with control rats. (C and D) Histological analysis of glomerular injury showed that segmental sclerosis at all grades and global sclerosis was increased in ex-DHT rats.
E. Expression of Androgen Receptor and RAS components
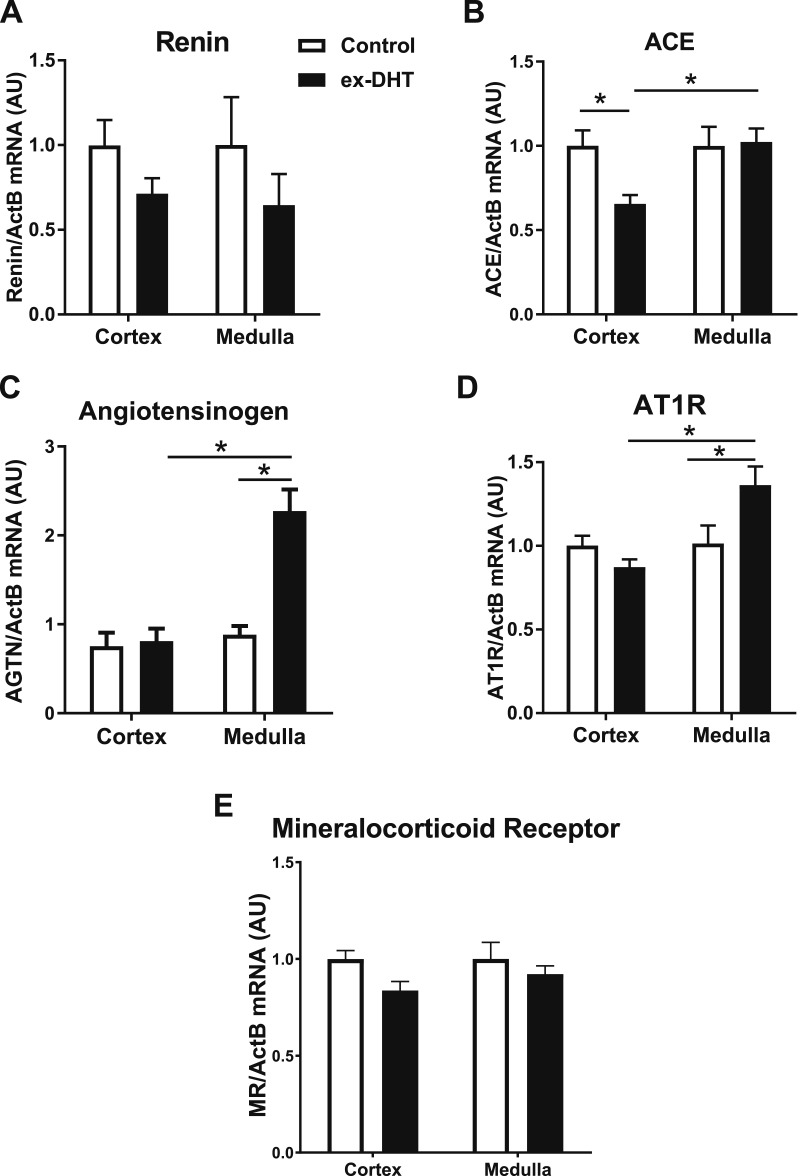
Renal cortical and medullar mRNA expression of renin had a tendency to be lower in ex-DHT rats, but this difference did not reach significance (Fig. 4A). Renal cortical expression of ACE was significantly decreased in ex-DHT rats (Fig. 4B). However, medullary mRNA expression of angiotensinogen and AT-1 receptors was significantly increased (by 2.3- and 1.4-fold, respectively) in ex-DHT rats (Fig. 4C and 4D). No difference in mRNA expression of the mineralocorticoid receptor was observed among the groups (Fig. 4E).
Figure 4.
Expression of intrarenal renin-angiotensin system components in ex-DHT and control rats. (A) Renal cortical and medullary renin mRNA expression had a tendency to be lower in ex-DHT rats but did not reach statistical significance. (B) Renal cortical expression of ACE was significantly decreased in ex-DHT rats; however, no differences were observed in the renal medulla. (C and D) Medullary mRNA expression of angiotensinogen (AGTN) and angiotensin II type 1 receptor (AT1R) was significantly increased in ex-DHT rats. (E) No differences were observed cortically nor medullary in mineralocorticoid receptor (MR) mRNA expression among the groups. *P < 0.05. ActB, β-actin; AU, arbitrary units.
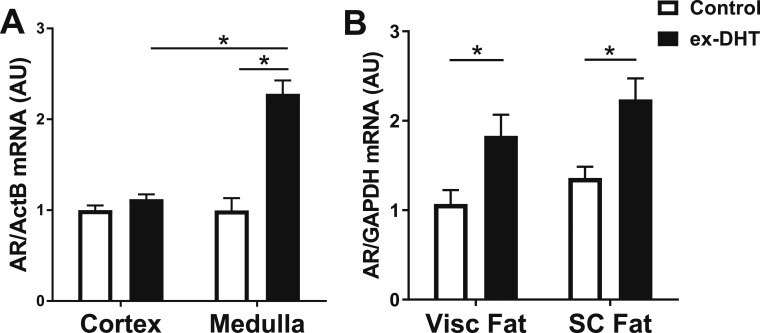
Renal and adipose androgen receptor mRNA expressions were also determined at the end of the experimental protocol. Androgen receptor expression was increased in the renal medulla in ex-DHT rats. No differences were observed in androgen receptor mRNA expression in the renal cortex (Fig. 5A). Finally, androgen receptor expression was increased in both visceral and subcutaneous adipose tissue in ex-DHT rats (Fig. 5B).
Figure 5.
Expression of androgen receptor in ex-DHT and control rats. (A) Renal medullary androgen receptor (AR) mRNA expression was increased in ex-DHT rats; however, no differences were observed in the renal cortex. (B) Androgen receptor mRNA expression was upregulated in both visceral (Visc fat) and subcutaneous (SC fat) adipose tissue in ex-DHT rats. ActB, β-actin; AU, arbitrary units; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. *P < 0.05.
F. Circulating Steroids and Estrous Cycle
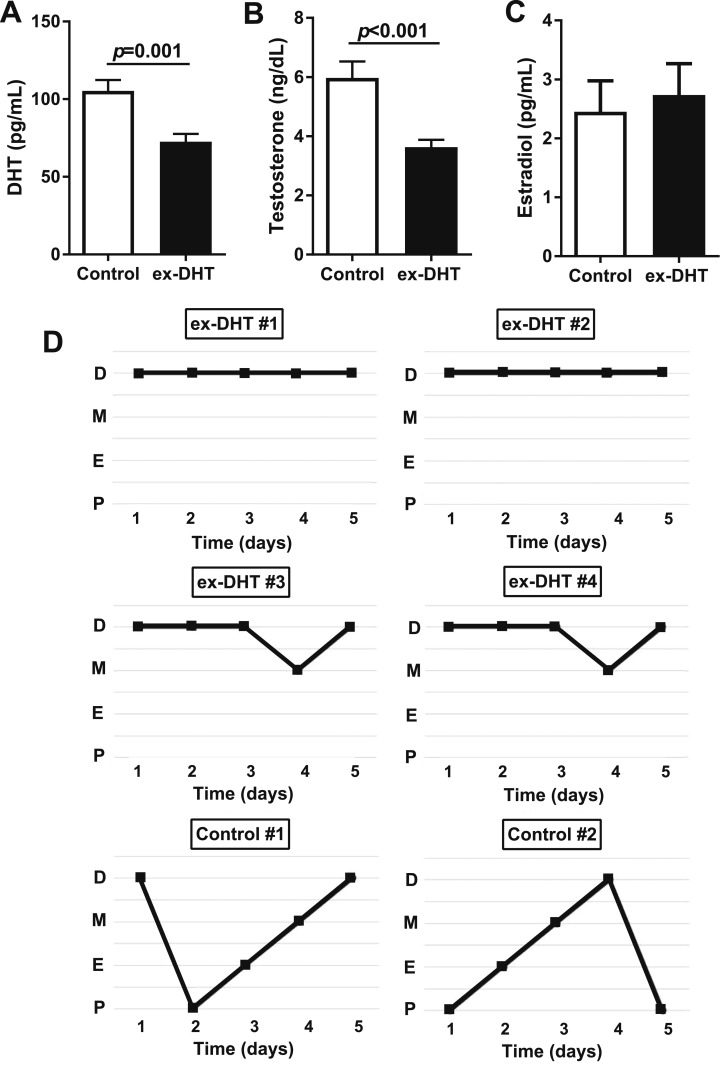
Six months after DHT withdrawal, plasma DHT (72.6 ± 5.0 vs 105.4 ± 6.9 pg/mL; P < 0.001) and testosterone levels (3.6 ± 0.2 vs 5.9 ± 0.5 ng/dL; P < 0.001) were significantly lower in ex-DHT rats (Fig. 6A and 6B). Plasma estradiol (2.3 ± 0.5 vs 3.9 ± 1.4 pg/mL) and estrone (2.73 ± 0.53 vs 2.45 ± 0.52 pg/mL) levels were not significantly different between the groups (Fig. 6C). The estrous cycle in ex-DHT rats was abnormal (constant diestrus with some incursions to metestrus) compared with the regular 4-day cycle in control rats (Fig. 6D).
Figure 6.
(A and B) Sex hormones and (C) plasma DHT and testosterone levels were decreased in ex-DHT rats. Plasma estradiol levels were similar among groups. (D) Vaginal cytology was performed daily for 5 consecutive days at the end of the study. The figure shows the pattern of four representative ex-DHT rats (#1 to #4) and two controls (#5 and #6). D, diestrus; E, estrus; M, metestrus; P, proestrus.
3. Discussion
PCOS is characterized by ovarian dysfunction, hyperandrogenism, and increased cardiometabolic risk factors, such as insulin resistance, obesity, and hypertension [6–10, 21]. Hypertension is a major risk factor for cardiovascular disease and mortality [37, 38]. Several clinical studies have shown that the prevalence of hypertension, across multiple ethnic groups, is significantly increased in women with PCOS [8, 9, 39–44]. However, the mechanisms that are responsible for the increase in BP in PCOS are unclear. We and other investigators have demonstrated that female DHT-treated rats have higher BP than controls [29, 45, 46]. In the current study we demonstrated that the increase in BP also persists after DHT withdrawal in our PCOS model. Similar findings on androgen’s long-lasting effects were described by Lenders et al. [47], who reported that, 5 months after discontinuing anabolic androgenic steroids, systolic BP remained higher in women ex-users compared with anabolic-free women in the control group.
The RAS plays a major role in hypertension, and for BP to be chronically elevated, a rightward shift in the pressure-natriuresis relationship must occur [48]. Our results show that the increase in BP in ex-DHT rats was abolished by administration of the ACE inhibitor enalapril, suggesting that the RAS plays an important role in mediating increases in BP in the PCOS model. It has been shown that androgen replacement in castrated male rats increases renin and angiotensinogen synthesis [31]. If renin is not working at maximum velocity, then an increase in the substrate (i.e., angiotensinogen) will increase angiotensin II production because renin is the rate-limiting enzyme for angiotensin II production [49]. Furthermore, androgen-induced hypertension in ovariectomized spontaneously hypertensive rats is mediated by activation of the RAS [50]. In clinical practice, RAS blockers are one of the first-line therapeutic options for hypertension; however, RAS blockers are contraindicated in pregnancy-seeking patients with PCOS due to their teratogenic and developmental effects on the fetal kidney [51, 52]. Our data suggest that the increase in BP in PCOS is mediated by long-lasting effects of androgens upon activation of intrarenal RAS, further demonstrating the importance of early remediation of excess androgens in women with PCOS.
In a large cohort of women with PCOS in the United States, ∼60% of the patients studied were obese [body mass index (BMI) >30 kg/m2], and 20% were severely obese (BMI >40 kg/m2) [53]. Obesity is a well-known risk factor for hypertension. Whether the increase in BP in PCOS is due to obesity remains unclear. In a large, community-based population study, women with PCOS were 40% more likely to have elevated BP than women without PCOS independent of age, BMI, diabetes, or dyslipidemia [8]. Similar findings were reported in a Czech population [41]. Women with hypertension and PCOS seem to have a worse metabolic profile compared with normotensive women [54]. We have previously reported that implantation of DHT pellets in female SD rats causes an increase in food intake, body weight, and adiposity, similar to the characteristics of women with elevated androgens in PCOS [29]. Using the same animal experimental model of PCOS, a recent study showed that hyperandrogenemia and insulin resistance, but not changes in body weight, mediated endothelial dysfunction [55]. Our present study shows that the increase in body weight, food intake, and adiposity persisted after DHT withdrawal. A recent study indicated that difficulty in losing weight, even after the syndrome was treated, was the primary health concern reported among women with PCOS [27].
Testosterone has different effects on adipose tissue in women compared with men. For example, in men, testosterone deficiency is associated with increases in obesity and visceral adiposity [56, 57], whereas in women with PCOS, there is a positive correlation between circulating levels of testosterone and obesity [57–59]. Moreover, androgen levels are positively correlated with BMI not only in PCOS but also in simple obesity in women [60]. Enhancement in the steroidogenic activity and androgen levels in the subcutaneous adipose tissue in PCOS has been demonstrated in recent studies [61, 62]. Our present study supports these findings because adipose androgen receptor expression was significantly increased in ex-DHT rats despite the low levels of plasma androgens, suggesting that activation of the adipose androgen receptor may be responsible for, or at least contribute to, the increase in fat mass observed in ex-DHT rats; however, this must be tested in future studies.
The mechanisms by which androgens increase BP in PCOS and the persistence of this effect despite discontinuation of hyperandrogenemia in the PCOS model remain unclear. Our study shows that the expression of the androgen receptor is upregulated in renal medulla in ex-DHT rats. Moreover, immunohistochemical studies have shown expression of the androgen receptor in the collecting ducts and proximal tubule in rats [63] and in proximal and distal tubules in humans [64]. Additionally, several lines of evidence point to the major role of the androgen receptor in the reproductive manifestations in patients with PCOS [65]. It has been reported that women with PCOS have higher expression of endometrial androgen receptor compared with subjects without PCOS [65]. Furthermore, endometrial cells from patients with PCOS treated in vitro with DHT expressed higher levels of androgen receptor compared with the ones from control subjects [65]. There are many remaining questions about the regulation of the androgen receptor in nonreproductive tissues in patients with PCOS. Taken together, our findings on the upregulation of renal and adipose tissue androgen receptor expression in ex-DHT women suggest that the androgen-induced upregulation of the androgen receptor may be a key factor in the pathogenesis of the cardiometabolic abnormalities observed in patients with PCOS.
Our observations have several important clinical implications. A recent cross-sectional study using an online questionnaire showed that there is a substantial delay in the diagnosis of PCOS in affected women despite their consulting with health care professionals; therefore, most women with PCOS are exposed to high levels of androgens for an extended period before the diagnosis of PCOS is made [27]. Moreover, there are limited pharmacological tools available to normalize the level of androgens in patients with PCOS. The standard pharmacological therapeutic approach in women with PCOS is oral contraceptives and insulin-sensitizing agents. Neither treatment is effective in lowering BP or other cardiometabolic risk factors. Furthermore, compliance with the standard pharmacological agents is very poor, likely due to side effects [66]. Androgen blockers are used to treat hirsutism in women with PCOS but are seldom used to treat the cardiometabolic manifestations of the syndrome [67]. Therefore, women with PCOS are exposed to hyperandrogenemia for a significant amount of time even after receiving treatment, and thus their cardiometabolic features and risk factors are allowed to remain elevated, setting them up for future adverse health outcomes.
In addition to PCOS, there are several clinical scenarios in which plasma androgen levels are increased, such as congenital adrenal hyperplasia, adrenal and ovarian tumors, treatment of sexual dysfunction, androgen anabolic use in athletes, and female-to-male transsexuals. Increased cardiovascular risk factors have also been described in these populations [68–70]. Our study suggests that hyperandrogenemia will play a major role in mediating the cardiometabolic abnormalities in these populations just as in women with PCOS and may contribute to increased cardiometabolic risk factors in them.
In our experimental model of PCOS, animals present multiple cardiometabolic abnormalities even after 6 months of DHT treatment discontinuation. These findings suggest that the deleterious effects of high androgen levels in female animals are long-lasting and may be irreversible after a period of time. Moreover, these data highlight the critical importance of early detection and treatment of hyperandrogenemia to prevent the cardiometabolic derangements found in patients with PCOS. Time is of the essence to normalize androgen levels in PCOS and other androgen excess pathological conditions because, once cardiometabolic dysregulations have been established, normalization of the androgenic profile may have little beneficial effect.
Acknowledgments
Financial Support: This work was supported by American Heart Association Grants 0830239N (L.L.Y.C.) and 12SDG8980032 (D.G.R.), Endocrine Fellows Foundation Endocrine Research Grant (L.L.Y.C.), National Institute of General Medical Sciences of the National Institutes of Health Grant P20GM-121334 (L.L.Y.C. and R.O.M.), and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health Grant R21DK-113500 (D.G.R.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ACE
angiotensin-converting enzyme
- BMI
body mass index
- BP
blood pressure
- DHT
dihydrotestosterone
- MAP
mean arterial pressure
- NIH
National Institutes of Health
- PCOS
polycystic ovary syndrome
- RAS
renin-angiotensin system
References and Notes
- 1. Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841–2855. [DOI] [PubMed] [Google Scholar]
- 2. Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83(9):3078–3082. [DOI] [PubMed] [Google Scholar]
- 3. Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ, Yildiz BO. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057. [DOI] [PubMed] [Google Scholar]
- 4. McCartney CR, Marshall JC. Polycystic ovary syndrome. N Engl J Med. 2016;375(1):54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270–284. [DOI] [PubMed] [Google Scholar]
- 6. Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, Lobo R, Norman RJ, Talbott E, Dumesic DA. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010;95(5):2038–2049. [DOI] [PubMed] [Google Scholar]
- 7. Aziz M, Sidelmann JJ, Faber J, Wissing ML, Naver KV, Mikkelsen AL, Nilas L, Skouby SO. Polycystic ovary syndrome: cardiovascular risk factors according to specific phenotypes. Acta Obstet Gynecol Scand. 2015;94(10):1082–1089. [DOI] [PubMed] [Google Scholar]
- 8. Lo JC, Feigenbaum SL, Yang J, Pressman AR, Selby JV, Go AS. Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(4):1357–1363. [DOI] [PubMed] [Google Scholar]
- 9. Elting MW, Korsen TJ, Bezemer PD, Schoemaker J. Prevalence of diabetes mellitus, hypertension and cardiac complaints in a follow-up study of a Dutch PCOS population. Hum Reprod. 2001;16(3):556–560. [DOI] [PubMed] [Google Scholar]
- 10. Dokras A. Cardiovascular disease risk factors in polycystic ovary syndrome. Semin Reprod Med. 2008;26(1):39–44. [DOI] [PubMed] [Google Scholar]
- 11. Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF; Androgen Excess Society . Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91(11):4237–4245. [DOI] [PubMed] [Google Scholar]
- 12. Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach In: Dunaif A, Givens JR, Haseltine FP, and Merrian GR, eds. Polycystic Ovary Syndrome. Boston, MA: Blackwell Scientific Publications; 1992:377–384. [Google Scholar]
- 13. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. [DOI] [PubMed]
- 14. March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–551. [DOI] [PubMed] [Google Scholar]
- 15. National Institutes of Health. Evidence-Based Methodology Workshop on Polycystic Ovary Syndrome. 3–5 December 2012; Bethesda, MD.
- 16. Daan NM, Louwers YV, Koster MP, Eijkemans MJ, de Rijke YB, Lentjes EW, Fauser BC, Laven JS. Cardiovascular and metabolic profiles amongst different polycystic ovary syndrome phenotypes: who is really at risk? Fertil Steril. 2014;102(5):1444–1451.e3. [DOI] [PubMed] [Google Scholar]
- 17. Valkenburg O, Steegers-Theunissen RP, Smedts HP, Dallinga-Thie GM, Fauser BC, Westerveld EH, Laven JS. A more atherogenic serum lipoprotein profile is present in women with polycystic ovary syndrome: a case-control study. J Clin Endocrinol Metab. 2008;93(2):470–476. [DOI] [PubMed] [Google Scholar]
- 18. Espinós-Gómez JJ, Corcoy R, Calaf J. Prevalence and predictors of abnormal glucose metabolism in Mediterranean women with polycystic ovary syndrome. Gynecol Endocrinol. 2009;25(3):199–204. [DOI] [PubMed] [Google Scholar]
- 19. Chen MJ, Yang WS, Yang JH, Chen CL, Ho HN, Yang YS. Relationship between androgen levels and blood pressure in young women with polycystic ovary syndrome. Hypertension. 2007;49(6):1442–1447. [DOI] [PubMed] [Google Scholar]
- 20. Golden SH, Ding J, Szklo M, Schmidt MI, Duncan BB, Dobs A. Glucose and insulin components of the metabolic syndrome are associated with hyperandrogenism in postmenopausal women: the atherosclerosis risk in communities study. Am J Epidemiol. 2004;160(6):540–548. [DOI] [PubMed] [Google Scholar]
- 21. Yanes Cardozo LL, Romero DG, Reckelhoff JF. Cardiometabolic features of polycystic ovary syndrome: role of androgens. Physiology (Bethesda). 2017;32(5):357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prieto-Carrasquero MC, Botros FT, Kobori H, Navar LG. Collecting duct renin: a major player in angiotensin II-dependent hypertension. J Am Soc Hypertens. 2009;3(2):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39(2):316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jaatinen TA, Matinlauri I, Anttila L, Koskinen P, Erkkola R, Irjala K. Serum total renin is elevated in women with polycystic ovarian syndrome. Fertil Steril. 1995;63(5):1000–1004. [PubMed] [Google Scholar]
- 25. Jensterle M, Janez A, Vrtovec B, Meden-Vrtovec H, Pfeifer M, Prezelj J, Kocjan T. Decreased androgen levels and improved menstrual pattern after angiotensin II receptor antagonist telmisartan treatment in four hypertensive patients with polycystic ovary syndrome: case series. Croat Med J. 2007;48(6):864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barber TM, Wass JA, McCarthy MI, Franks S. Metabolic characteristics of women with polycystic ovaries and oligo-amenorrhoea but normal androgen levels: implications for the management of polycystic ovary syndrome. Clin Endocrinol (Oxf). 2007;66(4):513–517. [DOI] [PubMed] [Google Scholar]
- 27. Gibson-Helm M, Teede H, Dunaif A, Dokras A. Delayed diagnosis and a lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(2):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mahalingaiah S, Diamanti-Kandarakis E. Targets to treat metabolic syndrome in polycystic ovary syndrome. Expert Opin Ther Targets. 2015;19(11):1561–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yanes LL, Romero DG, Moulana M, Lima R, Davis DD, Zhang H, Lockhart R, Racusen LC, Reckelhoff JF. Cardiovascular-renal and metabolic characterization of a rat model of polycystic ovary syndrome. Gend Med. 2011;8(2):103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dalmasso C, Maranon R, Patil C, Bui E, Moulana M, Zhang H, Smith A, Yanes Cardozo LL, Reckelhoff JF. Cardiometabolic effects of chronic hyperandrogenemia in a new model of postmenopausal polycystic ovary syndrome. Endocrinology. 2016;157(7):2920–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am J Physiol Renal Physiol. 2009;296(4):F771–F779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ball JP, Syed M, Marañon RO, Hall ME, Kc R, Reckelhoff JF, Yanes Cardozo LL, Romero DG. Role and regulation of microRNAs in aldosterone-mediated cardiac injury and dysfunction in male rats. Endocrinology. 2017;158(6):1859–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yanes LL, Romero DG, Iles JW, Iliescu R, Gomez-Sanchez C, Reckelhoff JF. Sexual dimorphism in the renin-angiotensin system in aging spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2006;291(2):R383–R390. [DOI] [PubMed] [Google Scholar]
- 34. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 35. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. [DOI] [PubMed] [Google Scholar]
- 36. Fortepiani LA, Zhang H, Racusen L, Roberts LJ II, Reckelhoff JF. Characterization of an animal model of postmenopausal hypertension in spontaneously hypertensive rats. Hypertension. 2003;41(3 Pt 2):640–645. [DOI] [PubMed] [Google Scholar]
- 37. Lawes CM, Vander Hoorn S, Rodgers A; International Society of Hypertension . Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371(9623):1513–1518. [DOI] [PubMed] [Google Scholar]
- 38. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies C; Prospective Studies Collaboration . Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. [DOI] [PubMed] [Google Scholar]
- 39. Holte J, Gennarelli G, Berne C, Bergh T, Lithell H. Elevated ambulatory day-time blood pressure in women with polycystic ovary syndrome: a sign of a pre-hypertensive state? Hum Reprod. 1996;11(1):23–28. [DOI] [PubMed] [Google Scholar]
- 40. Wild S, Pierpoint T, McKeigue P, Jacobs H. Cardiovascular disease in women with polycystic ovary syndrome at long-term follow-up: a retrospective cohort study. Clin Endocrinol (Oxf). 2000;52(5):595–600. [DOI] [PubMed] [Google Scholar]
- 41. Vrbíková J, Cífková R, Jirkovská A, Lánská V, Platilová H, Zamrazil V, Stárka L. Cardiovascular risk factors in young Czech females with polycystic ovary syndrome. Hum Reprod. 2003;18(5):980–984. [DOI] [PubMed] [Google Scholar]
- 42. Chang AY, Oshiro J, Ayers C, Auchus RJ. Influence of race/ethnicity on cardiovascular risk factors in polycystic ovary syndrome, the Dallas Heart Study. Clin Endocrinol (Oxf). 2016;85(1):92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Morgan CL, Jenkins-Jones S, Currie CJ, Rees DA. Evaluation of adverse outcome in young women with polycystic ovary syndrome versus matched, reference controls: a retrospective, observational study. J Clin Endocrinol Metab. 2012;97(9):3251–3260. [DOI] [PubMed] [Google Scholar]
- 44. Pinola P, Puukka K, Piltonen TT, Puurunen J, Vanky E, Sundström-Poromaa I, Stener-Victorin E, Lindén Hirschberg A, Ravn P, Skovsager Andersen M, Glintborg D, Mellembakken JR, Ruokonen A, Tapanainen JS, Morin-Papunen LC. Normo- and hyperandrogenic women with polycystic ovary syndrome exhibit an adverse metabolic profile through life. Fertil Steril. 2017;107(3):788–795.e2. [DOI] [PubMed] [Google Scholar]
- 45. Mishra JS, More AS, Hankins GDV, Kumar S. Hyperandrogenemia reduces endothelium-derived hyperpolarizing factor-mediated relaxation in mesenteric artery of female rats. Biol Reprod. 2017;96(6):1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hoang V, Bi J, Mohankumar SM, Vyas AK. Liraglutide improves hypertension and metabolic perturbation in a rat model of polycystic ovarian syndrome. PLoS One. 2015;10(5):e0126119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lenders JW, Demacker PN, Vos JA, Jansen PL, Hoitsma AJ, van ’t Laar A, Thien T. Deleterious effects of anabolic steroids on serum lipoproteins, blood pressure, and liver function in amateur body builders. Int J Sports Med. 1988;9(1):19–23. [DOI] [PubMed] [Google Scholar]
- 48. Hall JE, Guyton AC, Brands MW. Pressure-volume regulation in hypertension. Kidney Int Suppl. 1996;55:S35–S41. [PubMed] [Google Scholar]
- 49. Luther RR, Stein HH, Glassman HN, Kleinert HD. Renin inhibitors: specific modulators of the renin-angiotensin system. Arzneimittelforschung. 1989;39(1):1–5. [PubMed] [Google Scholar]
- 50. Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension. 2000;35(1 Pt 2):480–483. [DOI] [PubMed] [Google Scholar]
- 51. Barr M., Jr Teratogen update: angiotensin-converting enzyme inhibitors. Teratology. 1994;50(6):399–409. [DOI] [PubMed] [Google Scholar]
- 52. Quan A. Fetopathy associated with exposure to angiotensin converting enzyme inhibitors and angiotensin receptor antagonists. Early Hum Dev. 2006;82(1):23–28. [DOI] [PubMed] [Google Scholar]
- 53. Glueck CJ, Dharashivkar S, Wang P, Zhu B, Gartside PS, Tracy T, Sieve L. Obesity and extreme obesity, manifest by ages 20-24 years, continuing through 32-41 years in women, should alert physicians to the diagnostic likelihood of polycystic ovary syndrome as a reversible underlying endocrinopathy. Eur J Obstet Gynecol Reprod Biol. 2005;122(2):206–212. [DOI] [PubMed] [Google Scholar]
- 54. Shi Y, Cui Y, Sun X, Ma G, Ma Z, Gao Q, Chen ZJ. Hypertension in women with polycystic ovary syndrome: prevalence and associated cardiovascular risk factors. Eur J Obstet Gynecol Reprod Biol. 2014;173:66–70. [DOI] [PubMed] [Google Scholar]
- 55. Hurliman A, Keller Brown J, Maille N, Mandala M, Casson P, Osol G. Hyperandrogenism and insulin resistance, not changes in body weight, mediate the development of endothelial dysfunction in a female rat model of polycystic ovary syndrome (PCOS). Endocrinology. 2015;156(11):4071–4080. [DOI] [PubMed] [Google Scholar]
- 56. Cai X, Tian Y, Wu T, Cao CX, Li H, Wang KJ. Metabolic effects of testosterone replacement therapy on hypogonadal men with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Asian J Androl. 2014;16(1):146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Grossmann M, Gianatti EJ, Zajac JD. Testosterone and type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17(3):247–256. [DOI] [PubMed] [Google Scholar]
- 58. LaZovic G, Radivojevic U, Milicevic S, Spremovic S. Influence of adiposity on leptin, LH and androgen levels in lean, overweight and obese PCOS patients. Int J Fertil Womens Med. 2007;52(2-3):82–88. [PubMed] [Google Scholar]
- 59. Varlamov O, White AE, Carroll JM, Bethea CL, Reddy A, Slayden O, O’Rourke RW, Roberts CT Jr. Androgen effects on adipose tissue architecture and function in nonhuman primates. Endocrinology. 2012;153(7):3100–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Valderhaug TG, Hertel JK, Nordstrand N, Dale PO, Hofsø D, Hjelmesæth J. The association between hyperandrogenemia and the metabolic syndrome in morbidly obese women. Diabetol Metab Syndr. 2015;7(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Quinkler M, Sinha B, Tomlinson JW, Bujalska IJ, Stewart PM, Arlt W. Androgen generation in adipose tissue in women with simple obesity: a site-specific role for 17beta-hydroxysteroid dehydrogenase type 5. J Endocrinol. 2004;183(2):331–342. [DOI] [PubMed] [Google Scholar]
- 62. O’Reilly M, Gathercole L, Capper F, Arlt W, Tomlinson J. Effect of insulin on AKR1C3 expression in female adipose tissue: in-vivo and in-vitro study of adipose androgen generation in polycystic ovary syndrome. Lancet. 2015;385(Suppl 1):S16. [DOI] [PubMed] [Google Scholar]
- 63. Boulkroun S, Le Moellic C, Blot-Chabaud M, Farman N, Courtois-Coutry N. Expression of androgen receptor and androgen regulation of NDRG2 in the rat renal collecting duct. Pflugers Arch. 2005;451(2):388–394. [DOI] [PubMed] [Google Scholar]
- 64. Quinkler M, Bujalska IJ, Kaur K, Onyimba CU, Buhner S, Allolio B, Hughes SV, Hewison M, Stewart PM. Androgen receptor-mediated regulation of the alpha-subunit of the epithelial sodium channel in human kidney. Hypertension. 2005;46(4):787–798. [DOI] [PubMed] [Google Scholar]
- 65. Li X, Pishdari B, Cui P, Hu M, Yang HP, Guo YR, Jiang HY, Feng Y, Billig H, Shao R. Regulation of androgen receptor expression alters AMPK phosphorylation in the endometrium: in vivo and in vitro studies in women with polycystic ovary syndrome. Int J Biol Sci. 2015;11(12):1376–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC, Taylor K, Boots LR. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89(2):453–462. [DOI] [PubMed] [Google Scholar]
- 67. Radosh L. Drug treatments for polycystic ovary syndrome. Am Fam Physician. 2009;79(8):671–676. [PubMed] [Google Scholar]
- 68. Streed CG Jr, Harfouch O, Marvel F, Blumenthal RS, Martin SS, Mukherjee M. Cardiovascular disease among transgender adults receiving hormone therapy: a narrative review. Ann Intern Med. 2017;167(4):256–267. [DOI] [PubMed] [Google Scholar]
- 69. Velho I, Fighera TM, Ziegelmann PK, Spritzer PM. Effects of testosterone therapy on BMI, blood pressure, and laboratory profile of transgender men: a systematic review. Andrology. 2017;5(5):881–888. [DOI] [PubMed] [Google Scholar]
- 70. Grace F, Sculthorpe N, Baker J, Davies B. Blood pressure and rate pressure product response in males using high-dose anabolic androgenic steroids (AAS). J Sci Med Sport. 2003;6(3):307–312. [DOI] [PubMed] [Google Scholar]