Abstract
Background
We analyzed the 96-week results in the overall population and in prespecified subgroups from the ongoing STARTMRK study of treatment-naive HIV-infected patients.
Methods
Eligible patients with HIV-1 RNA (vRNA) levels >5000 copies per milliliter and without baseline resistance to efavirenz, tenofovir, or emtricitabine were randomized in a double-blind noninferiority study to receive raltegravir or efavirenz, each combined with tenofovir/emtricitabine.
Results
At week 96 counting noncompleters as failures, 81% versus 79% achieved vRNA levels <50 copies per milliliter in the raltegravir and efavirenz groups, respectively [Δ (95% confidence interval) = 2% (−4 to 9), noninferiority P < 0.001]. Mean change in baseline CD4 count was 240 and 225 cells per cubic millimeter in the raltegravir and efavirenz groups, respectively [Δ (95% confidence interval) = 15 (−13 to 42)]. Treatment effects were consistent across prespecified baseline demographic and prognostic subgroups. Fewer drug-related clinical adverse events (47% versus 78%; P < 0.001) occurred in raltegravir than efavirenz recipients. Both regimens had modest effects on serum lipids and glucose levels and on body fat composition.
Conclusions
When combined with tenofovir/emtricitabine in treatment-naive patients, raltegravir exhibited durable antiretroviral activity that was noninferior to the efficacy of efavirenz through 96 weeks of therapy. Subgroup analyses were generally consistent with the overall findings. Both regimens were well tolerated.
Keywords: efavirenz, integrase inhibitor, MK-0518, raltegravir, STARTMRK, treatment-naive patients
INTRODUCTION
Combination antiretroviral therapy has resulted in longer survival and a better quality of life for many HIV-infected patients.1 Unfortunately, some patients cannot tolerate reverse transcriptase or protease inhibitors, and transmission of drug-resistant HIV jeopardizes the response to standard first-line regimens in certain treatment-naive patients.2–8 Additional agents from novel drug classes are needed to expand options for the initial treatment of HIV-1 infection and enhance the probability of achieving and maintaining virologic suppression.8–12 Raltegravir as part of combination therapy has been efficacious and generally well tolerated in patients infected with HIV-1 susceptible or resistant to other classes of antiretroviral drugs.10–14 In the Phase III STARTMRK study of treatment-naive patients, the efficacy of raltegravir was noninferior to the results with efavirenz when used in combination with tenofovir/emtricitabine through 48 weeks of therapy.11 Raltegravir recipients experienced significantly fewer clinical adverse events and central nervous system side effects than efavirenz recipients. In a Phase II trial, virologic suppression with a raltegravir-based regimen was maintained in most treatment-naive patients for at least 96 weeks.12 We now present the 96-week results from the ongoing Phase III STARTMRK study, including the first presentation of data regarding changes in body fat composition.
METHODS
Study Design
STARTMRK (MK-0518 Protocol 021) is an ongoing blinded, randomized, active-controlled Phase III clinical trial enrolling patients from 67 sites: 21 in North America (including 18 in the United States and 3 in Canada); 19 in Australia and Europe (including 1 in Australia, 5 in France, 5 in Germany, 5 in Italy, and 3 in Spain); 19 in Latin America (including 3 in Brazil, 3 in Chile, 4 in Colombia, 5 in Mexico, and 4 in Peru); and 8 in South Asia (including 4 in India and 4 in Thailand).11 The protocol was approved by the Institutional Review Boards or Ethical Review Committees at each site. All participants provided written informed consent. The primary analysis was performed at week 48 as specified in the protocol. Participants were to continue on blinded therapy at least through week 96.
Briefly, treatment-naive HIV-infected patients ≥18 years of age were eligible if their vRNA levels were >5000 copies per milliliter without genotypic resistance to tenofovir, emtricitabine, and/or efavirenz. Patients were stratified by baseline vRNA levels (>50,000 vs. ≤50,000 copies/mL) and viral hepatitis co-infection status, defined by hepatitis B surface antigen positivity and/or detection of hepatitis C RNA by polymerase chain reaction. Patients with renal insufficiency or with acute or decompensated chronic hepatitis were excluded, but patients with chronic hepatitis remained eligible if their serum aminotransferase levels were ≤5-fold the upper limit of the normal range.
After stratification, patients were randomly assigned in a 1:1 ratio through a central interactive voice response system according to a computer-generated randomized allocation schedule to receive raltegravir or efavirenz, each in combination with coformulated tenofovir and emtricitabine. Investigators, study site personnel, patients, monitors, and central laboratory personnel remained blinded to treatment allocation; masking was accomplished through use of matching-image placebo tablets of raltegravir and efavirenz. Participants were instructed to take tenofovir 300 mg and emtricitabine 150 mg coformulated as a single tablet (Truvada) in the morning with food, a 400-mg tablet of raltegravir or identical placebo twice daily without regard to food intake, and efavirenz 600 mg or identical placebo on an empty stomach at the hour of sleep.
Patients were asked to complete diary cards for all study drugs, and the information was reviewed with the patients. Site personnel collected the used study drug bottles at each visit and counted the returned tablets to ensure information provided on the diary card was accurate. A patient was considered adherent for a particular day if he/she took at least 1 study medication on that day. Clinical status was assessed at regularly scheduled visits and as needed. A series of prespecified laboratory tests were performed periodically. To measure changes in body fat composition over time on study drugs, dual energy x-ray absorptiometry (DEXA) scans were to be obtained on a subset of patients from sites in the United States which had access to the necessary equipment at the baseline, week-48, and week-96 visits. All DEXA scans were submitted to a central reader for interpretation. An independent Data and Safety Monitoring Board monitored the trial for evidence of beneficial or adverse effects.
Virologic Studies and Definitions
HIV RNA levels were measured at a central laboratory using the standard COBAS Amplicor HIV-1 Monitor assay (version 1.5; Roche Diagnostics, Branchburg, NJ) with a lower limit of quantification of 400 vRNA copies per milliliter and the Ultrasensitive Amplicor HIV-1 Monitor assay (version 1.5; Roche Diagnostics) with a lower quantification limit of 50 vRNA copies per milliliter. Possible efavirenz, emtricitabine, and tenofovir resistance was assessed using commercially available assays (Monogram Biosciences, South San Francisco, CA). Genotyping of the integrase coding sequence was performed at Merck Research Laboratories before week 48 and by Monogram after week 48 on viruses from patients after virologic failure.
Statistical Analyses
All treated patients were included in the efficacy and safety analyses. This report presents efficacy results through week 96 and all available safety data through May 6, 2009 (the date when the last patient remaining in the study completed the week-96 visit). As prespecified in the protocol, analyses of the nervous system adverse events, metabolic parameters, and DEXA results were based on 96-week data.
The main efficacy hypothesis was that a raltegravir-based regimen would have noninferior antiretroviral activity compared with an efavirenz-based regimen determined by the proportion of patients achieving vRNA levels <50 copies per milliliter; the primary and secondary time points were at week 48 and week 96, respectively. After adjustment for stratification of baseline vRNA concentration, raltegravir would be judged noninferior to efavirenz if the lower bound of the 2-sided 95% confidence interval (CI) for the proportion of patients who responded in the raltegravir group minus the efavirenz group was higher than −12% (noninferiority margin), using the method of Miettinen and Nurminen.15 For calculation of virologic response rates, the primary approach to handling missing data was to include non-completers (NC) as failures (F). In the NC = F analysis, missing vRNA measurements regardless of the reason were imputed as failures unless the values immediately before and after the missing value were both successes, in which case the absent value was left as missing. An observed-failure approach, which allowed evaluation of efficacy without confounding by discontinuations due to intolerability or nontreatment-related reasons, was used for assessing changes from baseline CD4 cell counts and for the prespecified subgroup analyses based on demographic and prognostic factors at baseline. With the observed-failure approach, baseline values were carried forward for patients who discontinued due to lack of efficacy; patients who discontinued for other reasons were not included in the analyses at subsequent time points.
Time to virologic response was calculated as the time on study to the first of 2 consecutive vRNA measurements <50 copies per milliliter at least a week apart. Time to loss of virologic response was defined for patients who had confirmed vRNA levels <50 copies per milliliter on 2 consecutive visits (at least 1 week apart) as the time between randomization and the first of 2 consecutive vRNA values >50 copies per milliliter or loss to follow-up and for patients who never achieved vRNA levels <50 copies per milliliter on 2 consecutive visits as time 0. Kaplan–Meier estimates of the time to attainment or loss of virologic response were calculated by treatment group and compared by the log-rank test, using all available data as of May 6, 2009.
Adverse events occurring during the double-blind phase of the study or within 14 days after discontinuation were included in this analysis. Adverse event terms were adopted from the Medical Dictionary for Regulatory Activities (MedDRA version 12.0 http://www.meddramsso.com/MSSOWeb/index.htm). Investigators were to assess the relationship of each adverse event to study therapy; adverse events were counted as drug related if judged by the investigator as definitely, probably, or possibly related to any of the study drugs. The intensity of clinical adverse events was graded by the investigator as mild, moderate, or severe. Severity of laboratory abnormalities was graded according to the 1992 DAIDS toxicity guidelines for adults (http://rcc.tech-res-intl.com/tox_tables.htm).
For analysis of lipid levels at week 96, missing data were handled by carrying the last observation forward. If patients initiated or increased the dosage of lipid-lowering therapy, the last available lipid values before the medication change were used in the analysis. No missing data were imputed for the analyses of glucose levels and body composition measurements by DEXA; results had to be available at both baseline and the later time point (week 48 or week 96) to be included in these analyses.
RESULTS
Patient Accounting and Baseline Characteristics
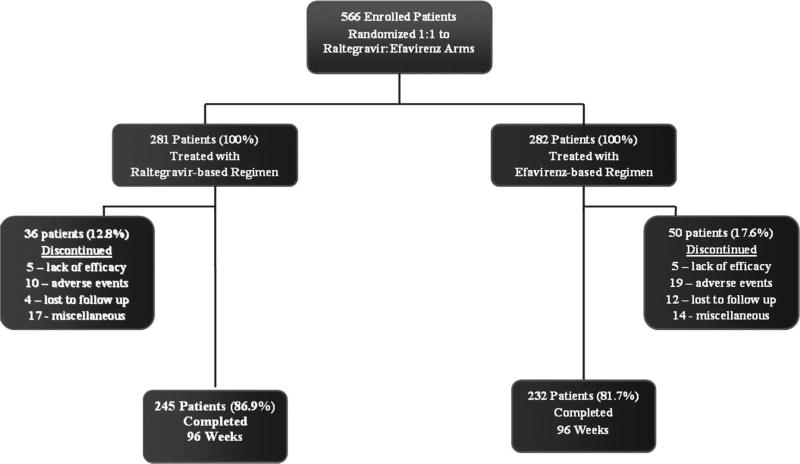
Figure 1 summarizes subject accounting through week 96. A total of 281 of 282 and 282 of 284 patients randomized to the raltegravir and efavirenz groups, respectively, received study drug. Overall, 36 patients (13%) in the raltegravir group and 50 patients (18%) in the efavirenz group discontinued the study before week 96. Discontinuations between week 48 and week 96 occurred in 12 raltegravir recipients and 15 efavirenz recipients. No clinically important difference in adherence between the 2 treatment groups was identified: 276 (98%) patients in the raltegravir group and 273 (97%) patients in the efavirenz group took study medication on ≥90% of the days on study, with 86% and 82%, respectively, taking their study drug(s) every day.
FIGURE 1.
Subject accounting. A CONSORT diagram shows patient disposition through study week 96. A total of 3 randomized patients, including 1 patient in the raltegravir arm and 2 patients in the efavirenz arm, never received study drugs. For the calculation of percentages subsequent to entry, the number of treated patients in each group was assigned a value of 100%.
Baseline characteristics were generally balanced across treatment groups in the study as a whole (Table 1). However, Asian and Latin American patients tend to be slightly younger than patients from Europe/Australia or North America on average. Mean and median baseline CD4 counts (cells/mm3) were the lowest in patients from Asia (173; 162) and highest in patients from Europe/Australia (244; 238). Median log vRNA levels ranged from 5.0 to 5.2 across the 4 regions.
TABLE 1.
Selected Baseline Characteristics by Treatment Assignment for Participants in the Parent Study and DEXA Substudy
| All Treated Patients | Patients in the DEXA Substudy | |||
|---|---|---|---|---|
|
|
|
|||
| Raltegravir Group | Efavirenz Group | Raltegravir Group | Efavirenz Group | |
|
|
|
|
|
|
| (n = 281) | (n = 282) | (n = 55)* | (n = 57)* | |
| Gender, n (%) | ||||
| Male | 227 (81) | 231 (82) | 51 (93) | 48 (84) |
| Female | 54 (19) | 51 (18) | 4 (7) | 9 (16) |
| Race/ethnicity, n (%) | ||||
| White | 116 (41) | 123 (44) | 34 (62) | 33 (58) |
| Black | 33 (12) | 23 (8) | 14 (25) | 9 (16) |
| Asian | 36 (13) | 32 (11) | 0 (0) | 1 (2) |
| Hispanic | 60 (21) | 67 (24) | 5 (9) | 11 (19) |
| Native American | 1 (0.4) | 1 (0.4) | 0 (0) | 1 (2) |
| Multiracial | 35 (12) | 36 (13) | 2 (4) | 2 (4) |
| Region, n (%) | ||||
| Latin America | 99 (35) | 97 (34) | — | — |
| South Asia | 34 (12) | 29 (10) | — | — |
| North America | 82 (29) | 90 (32) | 55 (100) | 57 (100) |
| Europe/Australia | 66 (23) | 66 (23) | — | — |
| Age, in yrs | ||||
| Mean (SD) | 38 (9) | 37 (10) | 37 (9) | 40 (10) |
| Median (min to max) | 37 (19–67) | 36 (19–71) | 38 (20–61) | 39 (21–67) |
| CD4 cell count, cell/mm3 | ||||
| Mean (SD) | 219 (124) | 217 (134) | 236 (157) | 226 (149) |
| Median (min to max) | 212 (1–620) | 204 (4–807) | 231 (1–609) | 202 (6–567) |
| Plasma HIV RNA, log 10 copies/mL | ||||
| Mean (SD) | 5.0 (0.6) | 5.0 (0.6) | 5.0 (0.6) | 5.0 (0.6) |
| Median (min to max) | 5.1 (3–6) | 5.0 (4–6) | 4.9 (4–6) | 5.0 (4–6) |
| Investigator-reported history of AIDS | ||||
| Yes | 52 (19) | 59 (21) | 10 (18) | 8 (14) |
| Stratum, n (%) | ||||
| Screening HIV RNA level ≤50,000 | 75 (27) | 80 (28) | 16 (29) | 15 (26) |
| Hepatitis B or C positive | 18 (6) | 16 (6) | 2 (4) | 4 (7) |
| Viral Subtype, n (%) | ||||
| Clade B | 219 (78) | 230 (82) | 53 (96) | 52 (91) |
| Non-Clade B | 59 (21) | 47 (17) | 2 (4) | 3 (5) |
| Missing | 3 (1) | 5 (2) | 0 (0) | 2 (4) |
| Baseline plasma HIV RNA, n (%) | ||||
| ≤50,000 copies/mL | 79 (28) | 84 (30) | 19 (35) | 19 (33) |
| >50,000 copies/mL | 202 (72) | 198 (70) | 36 (65) | 38 (67) |
| ≤100,000 copies/mL | 127 (45) | 139 (49) | 31 (56) | 27 (47) |
| >100,000 copies/mL | 154 (55) | 143 (51) | 24 (44) | 30 (53) |
| Baseline CD4 cell counts, n (%) | ||||
| ≤50 cells/mm3 | 27 (10) | 31 (11) | 8 (15) | 9 (16) |
| >50 cells/mm3 and ≤200 cells/mm3 | 104 (37) | 105 (37) | 15 (27) | 19 (33) |
| >200 cells/mm3 | 150 (53) | 145 (51) | 32 (58) | 29 (51) |
| Missing | 0 (0) | 1 (0.4) | 0 (0) | 0 (0) |
| Body mass at baseline | ||||
| Mean weight in kg (SD) | 72 (15) | 70 (16) | 83 (15) | 77 (23) |
| Mean BMI in kg/M2(SD) | 24 (5) | 24 (5) | 27 (6) | 25 (6) |
There were 111 patients with Dual Energy X-ray Absorptiometry (DEXA) scans at baseline: 86 patients were evaluable at Week 48 and 75 patients were evaluable Week 96, including 68 patients evaluable at both time points. One patient enrolled in the substudy from the efavirenz arm did not have a baseline scan and therefore was excluded from the DEXA analyses.
Dash indicates that only participants at US sites were eligible for the DEXA substudy.
n, number of patients; BMI, body mass index; SD, standard deviation.
Virologic and Immunologic Responses
In the NC = F analysis at week 96, viral suppression to <50 vRNA copies per milliliter was achieved in 81% of raltegravir recipients compared with 79% of efavirenz recipients. The treatment difference [Δ (95% CI)] was 2% (−4 to 9), demonstrating noninferiority of raltegravir to efavirenz (P < 0.001) (Table 2). The week-96 response rates slightly declined from the 86% and 82% week-48 response rates in both the raltegravir and efavirenz arms, respectively.
TABLE 2.
Comparisons of Virologic and Immunologic Responses at Week 48 and Week 96
| %* Patients (95% CI)† With HIV RNA <50 Copies/mL |
Change‡ From Baseline CD4 cells/mm3 (95% CI) |
|||
|---|---|---|---|---|
|
|
|
|||
| 48-Week | 96-Week | 48-Week | 96-Week | |
| Raltegravir group | n = 280 | n = 281 | n = 280 | n = 281 |
| 86 (82 to 90) | 81 (76 to 86) | 189 (174 to 204) | 240 (220 to 259) | |
| Efavirenz group | n = 281 | n = 282 | n = 281 | n = 282 |
| 82 (77 to 86) | 79 (74 to 83) | 163 (148 to 178) | 225 (206 to 244) | |
| Difference between treatment groups§ | 4‖ (−2 to 10) | 2‖ (−4 to 9) | 26 (4 to 47) | 15 (−13 to 42) |
The numbers differ at the 2 time points because data in 1 patient in each treatment arm was available at Week 96, but not at Week 48.
Missing data were handled by counting non-completers as failures.
The 95% CI were calculated using the method of Miettinen and Nurminen.15
Missing data were handled by the observed-failure approach with baseline values carried forward for virologic failures.
Difference was calculated as the value in the raltegravir group minus the value in the efavirenz.
The P value for noninferiority was <0.001.
n, number of patients.
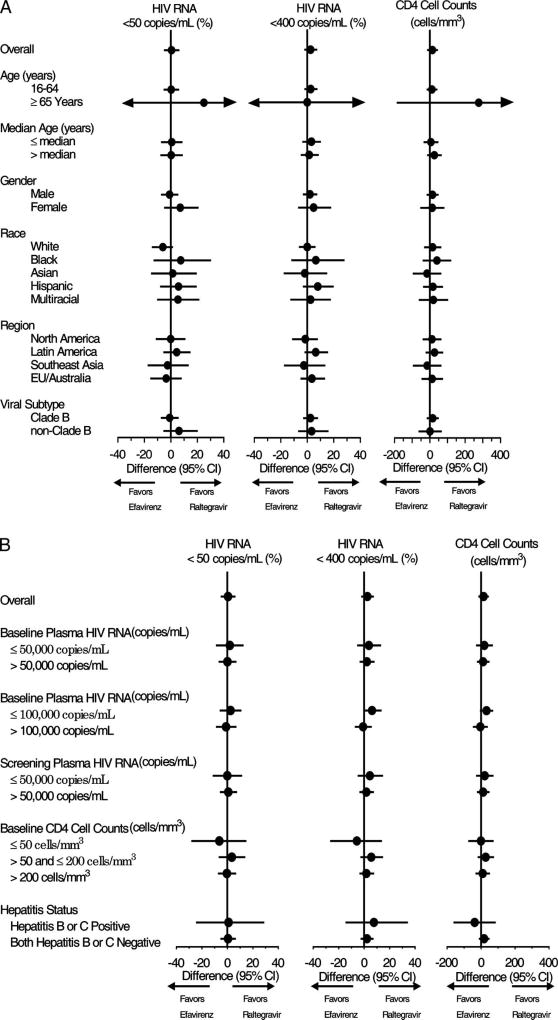
Using an observed-failure approach for missing data, suppression to <50 copies per milliliter was achieved at week 96 in 90.1% of the raltegravir group versus 89.5% of the efavirenz group [Δ (95% CI) = 1% (−5, 6), noninferiority P < 0.001]. Mean (95% CI) change in CD4 counts from baseline to week 96 was 240 (220 to 259) cells per cubic millimeter for raltegravir recipients and 225 (206 to 244) cells per cubic millimeter for efavirenz recipients [Δ (95% CI) = 15 (−13 to 42) cells/mm3], increased on average by 51 and 62 CD4 cells per cubic millimeter from week 48 in the raltegravir and efavirenz groups, respectively. Both regimens showed generally consistent virologic and immunologic effects across demographic and baseline prognostic factors, including the subgroup with baseline vRNA levels >100,000 copies per milliliter (Fig. 2). Time to confirmed virologic response was significantly shorter for raltegravir recipients than efavirenz recipients (P < 0.001). Time to loss of confirmed virologic response did not significantly differ by treatment arm (P = 0.276).
FIGURE 2.
Forest plots showing the between-treatment group differences in the proportions (%) of patients with vRNA <50 copies per milliliter and vRNA <400 copies per milliliter alongside the mean change from baseline CD4 cell counts (cells/mm3) at week 96 by (A) subpopulations and (B) prognostic subgroups at baseline. Point estimates of treatment effects with 95% CIs are presented in Forest plots to provide an estimate of treatment effects at week 96 in subpopulations and prognostic subgroups identified at baseline using the observed failure approach to missing data. The vertical line indicates no treatment difference. For reference, the overall treatment effect is given for each parameter at the top of the graph. The 95% CIs were calculated using the method of Miettinen and Nurminen.15 The arrowheads on the horizontzal bars indicate that the 95% CIs exceed the scale used for the x axis.
Safety and Tolerability
At least 1 clinical adverse event had occurred in 95% of raltegravir recipients and 98% of efavirenz recipients by the time the last patient remaining in the study completed the 96-week visit (P = 0.086) (Table 3). Drug-related clinical adverse events occurred less often in the raltegravir group (47%) than in the efavirenz group (78%) (P < 0.001); the rates were 44% among raltegravir recipients and 77% among efavirenz recipients at the corresponding 48-week time point. Significant differences were not demonstrated between raltegravir and efavirenz recipients for serious clinical adverse events (14% versus 12%; P = 0.457) or discontinuations due to clinical adverse events (4% versus 6%; P = 0.333). Only 1 serious musculoskeletal adverse event (myopathy in a raltegravir recipient) occurred in the study; the serum creatine kinase levels measured off-study peaked at 1131 IU/L in this patient, which returned to normal although continuing raltegravir. Table 4 shows drug-related clinical adverse events of moderate or severe intensity occurring in ≥2% of either treatment group.
TABLE 3.
Types and Frequencies of AE*
| Clinical Adverse Events | Laboratory Adverse Events | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Raltegravir Group N = 281 |
Efavirenz Group N = 282 |
Raltegravir Group N = 281 |
Efavirenz Group N = 282 |
|||||
| Number of Participants | n (%) | n (%) | Δ† (95% CI) | P‡ | n (%) | n (%) | Δ† (95% CI) | P‡ |
| With one or more AE | 266 (95) | 275 (98) | −3 (−6 to 0.4) | 0.086 | 36 (13) | 59 (21) | −8 (−14 to −1.9) | 0.013 |
| With drug-related AE§ | 132 (47) | 220 (78) | −31 (−38 to −23) | <0.001 | 19 (7) | 35 (12) | −6 (−11 to −1) | 0.031 |
| With serious AE‖ | 40 (14) | 34 (12) | 2 (−4 to 8) | 0.457 | 0 (0) | 2 (1) | −1 (−3 to 1) | 0.499 |
| With serious drug-related AE§ | 6 (2) | 5 (2) | 0.4 (−2 to 3) | 0.772 | 0 (0) | 12 (0.4) | −0.4 (−2 to 1) | 1.000 |
| Who discontinued due to AE¶ | 11 (4) | 17 (6) | −2 (−6 to 2) | 0.333 | 0 (0) | 3 (1) | −1 (−3 to 0.3) | 0.249 |
| Who discontinued due to drug-related AE§ | 3 (1) | 12 (4) | −3 (−6 to −1) | ND | 0 (0) | 2 (7) | −0.7 (−3 to 0.7) | ND |
| Who discontinued due to serious AE | 9 (3) | 5 (2) | 1 (−1 to 4) | ND | 0 (0) | 1 (0.4) | −0.4 (−2 to 1) | ND |
| Who discontinued due to serious drug-related AE§ | 1 (0.4) | 2 (0.7) | −0.4 (−2.2 to 1.3) | ND | 0 (0) | 1 (0.4) | −0.4 (−2 to 1) | ND |
All treated patients were included in the safety analysis. All adverse events occurring during the study or within 14 days of study discontinuation through May 6, 2009 (the day when the last patient remaining in the study had their 96-week assessment) were counted. The frequencies of adverse events were not adjusted for the duration of follow-up.
Difference (Δ) and 95% CI was calculated as the response rate in the raltegravir group minus the response rate in the efavirenz group. The 95% CIs were calculated using the method of Miettinen and Nurminen.15
Tests of significance were performed on the percentage of patients with at least one adverse experience in a prespecified category per protocol. P-values were generated using the Fisher exact test.
Determined by investigator to be possibly, probably, or definitely drug-related to any drug in the study regimen.
Three patients in the raltegravir group died; none of which was judged to be drug-related.
The discontinuations in the table refer to discontinuation of study medications (even if the patient remained in the study), whereas Figure 1 describes study discontinuations. The discordance between table and figure arises from patients who stopped study medication due to an adverse event but remained on study at the time of the Week 96 analysis.
N, Number of patients in each group; n (%) = number (percent) of patients in each category; ND, not done (because the test was not prespecified in the data analysis plan).
AE, adverse event.
TABLE 4.
| Raltegravir Group N = 281 |
Efavirenz Group N = 282 |
|
|---|---|---|
| n (%) | n (%) | |
| Rash§ | 0 (0.0) | 19 (6.7) |
| Headache | 11 (3.9) | 13 (4.6) |
| Dizziness | 4 (1.4) | 18 (6.4) |
| Insomnia | 10 (3.6) | 9 (3.2) |
| Nausea | 8 (2.8) | 10 (3.5) |
| Fatigue | 5 (1.8) | 8 (2.8) |
| Diarrhea | 3 (1.1) | 8 (2.8) |
All treated patients were included in the safety analysis. All adverse events occurring during the study or within 14 days of study discontinuation through 6-May-2009 (the day when the last patient remaining in the study had their 96-week assessment) were counted. The frequencies of adverse events were not adjusted for the duration of follow-up.
Determined by investigator to be possibly, probably, or definitely related to any drug in the study regimen.
Present in ≥2% of either treatment group.
Rash includes the MedDRA terms for unspecified, generalized, macular, and/or papular rashes (but not for allergic dermatitis, drug eruption, eczema, and skin lesion) under the category of “Skin and Subcutaneous Tissue Disorders”. The tabulation excluded 1 efavirenz recipient with a “viral rash” under the “Infections and Infestations” category.
N, total number of treated patients in each group.
n, number of patients with the specified clinical adverse event.
Through week 96, raltegravir recipients experienced cumulatively fewer nervous system side effects compared with efavirenz recipients [29% vs. 61%, Δ (95% CI) = −32% (−39 to −24); P < 0.001]. Between weeks 48 and 96, neuropsychiatric adverse experiences were reported in an additional 3% of patients in the raltegravir arm and 2% of patients in the efavirenz arm. The large majority of patients with neuropsychiatric complaints had symptoms during the first 8 weeks of treatment. Depression occurred in 21 (8%) patients in the raltegravir group and 25 (9%) patients in the efavirenz group and was classified as a serious adverse event in 2 patients in each group. Suicidal behavior or ideation was reported in 1 raltegravir recipient and 2 efavirenz recipients.
Immune reconstitution syndromes were reported as adverse events in 19 (7%) raltegravir recipients and 13 (5%) efavirenz recipients, with 2 cases in each treatment arm developing after 48 weeks. New or recurrent cancers were diagnosed in 3 (1%) raltegravir and 11 (4%) efavirenz recipients. The malignancies in the raltegravir group were Kaposi sarcoma, basal cell carcinoma, and metastatic lung cancer. The malignancies in the efavirenz group were Kaposi sarcoma in 6 cases; basal cell carcinoma in 2 cases; and B-cell lymphoma, squamous cell carcinoma of the anus, and bone cancer in 1 case each. Three patients died, all of whom were in the raltegravir arm; 1 death occurred between week 48 and week 96. Causes of death were Kaposi sarcoma, cerebral hemorrhage, and metastatic lung cancer. None of the deaths were judged to be drug related.
At least 1 laboratory adverse event was reported in 36 (13%) patients in the raltegravir group and 59 (21%) patients in the efavirenz group. Grades 3/4 laboratory abnormalities are reported in Table 5. Elevations of serum aminotransferase levels were more common in patients coinfected with hepatitis B and/or C than in HIV-monoinfected patients. Increased alanine aminotransferase levels of any grade occurred in 68 of 263 (26%) monoinfected versus 12 of 18 (67%) coinfected raltegravir recipients, and in 90 of 263 (34%) monoinfected versus 8 of 16 (50%) coinfected efavirenz recipients. Grade 3/4 elevations of alanine aminotransferase levels occurred in 4 of 263 (2%) monoinfected versus 1 of 18 (6%) coinfected raltegravir recipients and in 5 of 263 (2%) monoinfected versus 2 of 16 (13%) coinfected efavirenz recipients. Other adverse events occurred at similar rates in patients with or without hepatitis regardless of treatment group.
TABLE 5.
| Laboratory Test§ | Toxicity Criteria† |
Raltegravir Group N = 281, m/n (%) |
Efavirenz Group N = 282, m/n (%) |
|---|---|---|---|
| Absolute neutrophil count | <750 cells/µL | 7/281 (2.5) | 3/278 (1.1) |
| Hemoglobin | <7.5 gm/dL | 2/281 (0.7) | 2/278 (0.7) |
| Platelet count | <50,000/µL | 0/276 (0.0) | 1/276 (0.4) |
| Fasting total cholesterol | >300 mg/dL | 0/276 (0.0) | 11/267 (4.1) |
| Fasting LDL-cholesterol | ≥190 mg/dL | 3/271 (1.1) | 17/262 (6.5) |
| Fasting triglycerides | >750 mg/dL | 1/276 (0.4) | 4/267 (1.5) |
| Fasting glucose | >250 mg/dL | 3/274 (1.1) | 0/266 (0.0) |
| Total bilirubin | >2.5 × ULN | 2/281 (0.7) | 0/279 (0.0) |
| Alkaline phosphatase | >5 × ULN | 0/281 (0.0) | 2/279 (0.7) |
| Aspartate aminotransferase | >5 × ULN | 9/281 (3.2) | 8/279 (2.9) |
| Alanine aminotransferase | >5 × ULN | 5/281 (1.8) | 7/279 (2.5) |
Grades 3/4 by DAIDS criteria [http://rcc.tech-res-intl.com/tox_tables.htm].
All treated patients were included in the safety analysis. All laboratory abnormalities exceeding the predefined limit of change through May 6, 2009 (the day when the last patient remaining in the study had their 96-week assessment) were tallied. Only patients with a worsened grade from baseline were included.
Although there were no grade 3 or 4 abnormalities of serum creatinine levels (≥1.9 × ULN), the frequency of grade 1 elevations in serum creatinine concentration from baseline was 5.7% (16 of 281) in the raltegravir group compared with 1.1% (3 of 279) in the efavirenz group, and the frequency of grade 2 elevations was 0.4% (1 of 281) in the raltegravir group compared with 0.4% (1 of 279) in the efavirenz group. The mean change from baseline was 0.01 for raltegravir recipients and −0.03 for efavirenz recipients.
N, total number of treated patients in each group.
m, number of patients with Grade 3 or 4 abnormalities of the specified laboratory test.
n, number of patients with results for the specified laboratory test.
ULN, upper limit of normal range.
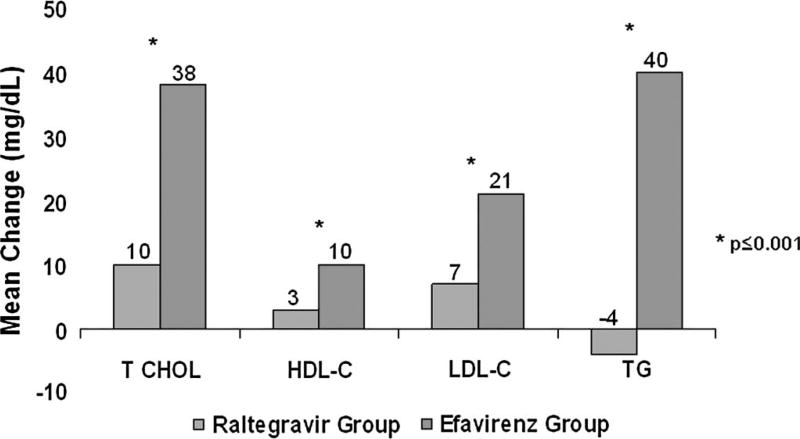
Consistent with the week-48 findings, mean changes from baseline in total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglyceride levels were smaller in raltegravir than efavirenz recipients at week 96 (P ≤ 0.001 for each comparison) (Fig. 3). There was no statistically significant difference between treatment groups in the change from baseline in the total cholesterol to HDL-cholesterol ratio (P = 0.192). Lipid-lowering medication was a concomitant treatment in 5% of subjects in the raltegravir group and 3% of subjects in the efavirenz group at entry and in 7% of subjects in the raltegravir group and 9% of subjects in the efavirenz group at some point through week 96. The mean change from baseline glucose levels at week 96 was slightly less in the raltegravir group (2 mg/dL) than in the efavirenz group (6 mg/dL) (P = 0.025).
FIGURE 3.
Changes in lipid values over the first 96 weeks of therapy by treatment group. The graph shows the mean change from baseline to week 96 in total cholesterol, HDL-cholesterol, LDL-cholesterol, and TG in mg/dL between the raltegravir group (left column) and the efavirenz (right column). All 4 between-group comparisons were significantly different (P ≤ 0.001). The mean change in the total cholesterol:HDL-cholesterol ratio did not significantly differ between the raltegravir group (−0.18) and efavirenz group (0.04) (P = 0.192). HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; TG, triglyceride.
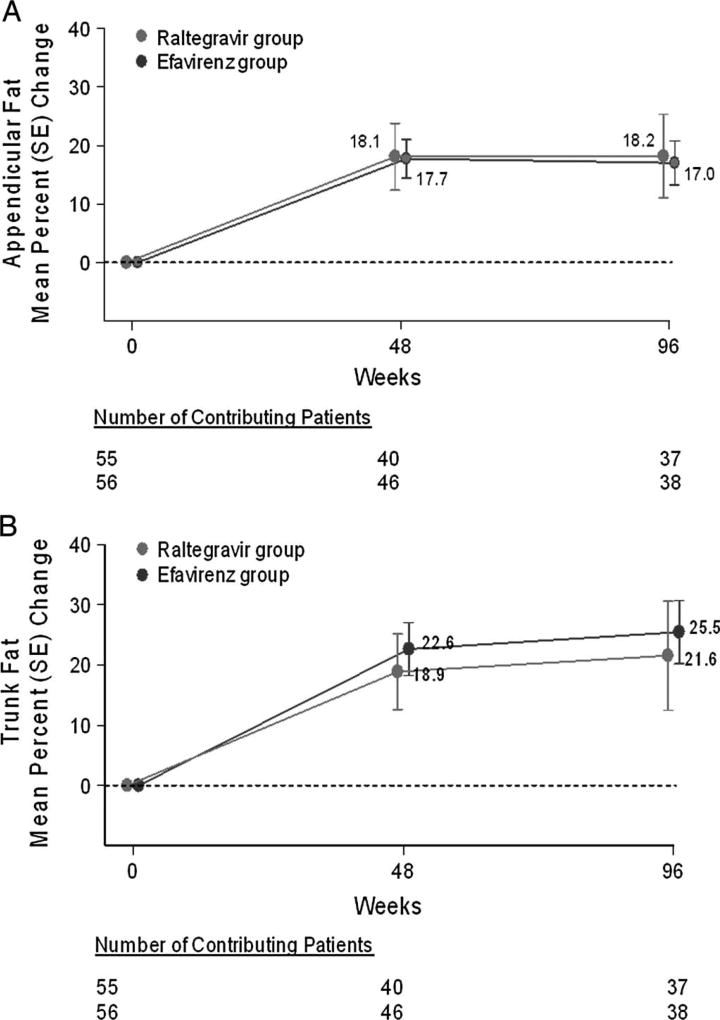
DEXA scans were obtained on a convenience sample of 86 patients at both baseline and week 48, and on a distinct but overlapping sample of 75 patients at both baseline and Week 96 (Fig. 4). Patients who missed their follow-up visit were not included in the dataset at that time point. Changes in fat content at each time point were comparable in both treatment groups. At week 96, the majority of patients in both treatment groups had experienced fat gain, which was modestly more prominent in the trunk and arms than in the legs. Lipoatrophy (defined as loss of ≥20% appendicular fat) occurred in 3 of 37 raltegravir recipients (8%) and 2 of 38 efavirenz recipients (5%).
FIGURE 4.
Mean percent changes in body fat over time by treatment group. The graph displays the mean percent change from baseline in appendicular (A) and trunk (B) fat at week 48 and week 96. Measurements were made by DEXA. The mean % change in fat content from baseline was calculated as the difference between the measurements at baseline and at the specified time point (week 48 or week 96). The number of evaluable patients is shown for the treatment groups below each time point. Bars represent standard errors. There were 111 patients with DEXA scans at baseline; 86 patients had repeat scans at week 48; and 75 patients had repeat scans at week 96, including 68 patients evaluable at both time points. Repeat DEXA scans at the baseline visit were used as the baseline measurements in 7 patients for whom the original baseline scans were not available. Baseline total fat content was 20.4 kg and 17.5 kg for the raltegravir and efavirenz participants, respectively (Δ = 2.9 kg).
Virologic Failure and Antiretroviral Drug Resistance
Between weeks 48 and 96, 18 additional patients (12 in the raltegravir group and 6 in the efavirenz group) met the protocol definition of virologic failure, 7 of whom (4 raltegravir recipients and 3 efavirenz recipients) had vRNA >400 copies per milliliter (allowing genotypic susceptibility testing to be performed). None of the viruses from the 4 evaluable raltegravir recipients (2 with data for both the integrase and reverse transcriptase genes, 1 with data for only the integrase gene, and 1 with data for only the reverse transcriptase gene) had detectable resistance to any of the drugs in their regimen. Two of the 3 evaluable efavirenz recipients had detectable resistance to drugs in their regimen: 1 had virus with resistance only to efavirenz, and 1 had virus with resistance to efavirenz and emtricitabine.
Overall, 84 patients experienced virologic failure by week 96. Among these patients, 16 of 39 (41%) raltegravir recipients and 11 of 45 (24%) efavirenz recipients had both vRNA levels >400 copies per milliliter and available genotyping results. Raltegravir-resistant virus was demonstrated in 4 of 12 patients in the raltegravir group in which the integrase gene was amplified (1 case each showing Q148H + G140S, Q148R + G140S, Y143H + L74L/M+E92Q + T97A, Y143R); in the 3 cases with data on the reverse transcriptase gene, the viruses were sensitive to tenofovir and resistant to emtricitabine. In the 4 remaining cases where the integrase gene could not be amplified, there were 2 patients who developed resistance to emtricitabine. The reverse transcriptase gene could not be amplified in 2 of 11 patients in the efavirenz arm. Five of the 9 evaluable patients in the efavirenz group had efavirenz-resistant virus (1 case each showing K103N, K103N + V108I, K103K/N + V106V/M, K103N, K103N + V108I + P225H); the efavirenz-resistant virus was emtricitabine resistant but sensitive to tenofovir in 2 cases and susceptible to both emtricitabine and tenofovir in the other 3 cases.
DISCUSSION
The extended STARTMRK results demonstrate that raltegravir combined with tenofovir/emtricitabine exerted a durable antiretroviral effect in treatment-naive patients, which was statistically noninferior to the results obtained with efavirenz combined with tenofovir/emtricitabine through 96 weeks of therapy. At week 48, there was a significantly greater increase in the mean CD4 cell counts in the raltegravir compared with the efavirenz group. By week 96, the difference between the 2 groups was no longer apparent. Both regimens showed consistent efficacy across baseline stratification and prognostic factors, including high viral loads at entry. In the second 48 weeks of follow-up, there were no new cases with raltegravir resistance and 2 new cases of efavirenz resistance. The ultimate frequency of resistance to the nucleos(t)ide backbone was also similar in both treatment groups, although longitudinal analyses are ongoing to elucidate whether these mutations appeared concurrently or sequentially relative to the raltegravir or efavirenz mutations.
Raltegravir exhibited a favorable safety profile relative to efavirenz. Raltegravir recipients experienced significantly fewer drug-related clinical adverse events. Efavirenz was more likely to be associated with rash than raltegravir; although not seen in our study, rash has been reported in raltegravir recipients.13 The cumulative proportion of patients who developed predefined central nervous system side effects remained greater in the efavirenz group than in the raltegravir group. Exacerbation of pre-existing depression subsequent to initiation of raltegravir therapy has recently been described in 4 patients outside of STARTMRK.16 In the present study, the incidence of depression with raltegravir was similar to that with efavirenz.
Relatively few patients who had not experienced drug-related clinical adverse events during the first 48 weeks of the study later developed such side effects. Serious adverse events and discontinuations due to adverse events were comparably infrequent in both treatment groups. Late immune reconstitution syndromes were diagnosed in only 2 cases in each treatment group after week 48. In contrast to the BENCHMRK studies of treatment-experienced patients with virologic failure and multiclass-resistant virus,13 malignancies were less common in raltegravir-treated patients than in the control group during the present study of treatment-naive patients.
Both raltegravir and efavirenz regimens exerted modest effects on serum lipid and glucose levels. Analysis of body fat content by DEXA scanning in a subset of patients showed similar degrees of fat gain through 96 weeks for each treatment group, with a greater increase in trunk than limb adiposity. Lipoatrophy was uncommon in both arms of the study. Laboratory abnormalities occurred with comparable frequency in hepatitis co-infected patients compared with those patients without hepatitis B or C, except that more patients with hepatitis co-infection developed elevated serum aminotransferase levels on both treatment regimens.
Limitations of our study include the use of placebos, which would tend to minimize any potential advantage of a 1-pill once-daily regimen on patient adherence. Postmarking reports have associated myositis and rhabdomyolysis with raltegravir use.17 In our trial, serious musculoskeletal complications were seen in only 1 patient who developed myopathy while receiving raltegravir. Creatine phosphokinase levels were not routinely monitored during this study. We did not compare regimens using raltegravir with ritonavir-boosted protease inhibitors in treatment-naive patients, although a large Phase III study is ongoing (NCT00811954). Nucleos(t)ide backbones other than tenofovir/emtricitabine were not evaluated.
STARTMRK establishes raltegravir combined with tenofovir/emtricitabine as a durably efficacious and generally well-tolerated regimen for treatment-naive patients.12 Raltegravir had noninferior antiretroviral efficacy relative to efavirenz through 96 weeks of therapy. Although raltegravir was associated with significantly fewer drug-related clinical adverse events of any intensity than efavirenz, the rates of serious clinical adverse events and discontinuations due to clinical adverse events were similar in each treatment arm. Metabolic perturbations were modest in both treatment groups.18 Raltegravir provides another potent and durable therapeutic option for the initial treatment of HIV-1–infected patients.19,20
Acknowledgments
Supported and funded by Merck and Co, Inc, which manufactures raltegravir under the brand name Isentress. The study was designed, managed, and analyzed by the sponsor in conjunction with external investigators.
Authors had access to all study data upon request. This report was principally drafted by Drs. J.L.L., P.S., J.Z., and M.J.D. and was critically reviewed and subsequently approved by each co-author in its essentially final form. The article underwent formal review by the sponsor.
Potential conflicts of interest: J.L.L. has been an investigator for Merck, Gilead, Pfizer, Schering, Tibotec, and Abbott, and has served as a paid consultant for Merck, Roche, and Abbott. E.D. has been an investigator for Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Merck, Roche Labs, Pfizer, Schering Plough, Tibotec, and Vertex, and has served as a paid consultant for Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Merck, and Tibotec. A.L. has been an investigator, consultant or speaker for Merck, Bristol-Myers Squibb, Gilead, Pfizer, Tibotec, Roche, GlaxoSmithKline, Boehringer-Ingelhelm, and Abbott. R.B.P. has been an investigator for Merck, Schering Plough, Idenix, Gilead, and Koronis, and has served as a paid consultant for Pfizer, Bristol-Myers Squibb, and Genetic Immunity. J.V.R.M. has been an investigator for Merck, Bristol-Myers Squibb, Gilead, Pfizer, Tibotec, Janssen, Boehringer-Ingelhelm, Schering Plough, and Abbott. D.S.B. has been an investigator for Merck, Gilead, Bristol-Myers Squibb, Tibotec, GlaxoSmithKline, Pfizer, Progenics, and Theratechnologies, has served as a paid consultant and speaker for Merck, Gilead, Tibotec, GlaxoSmithKline, and Theratechnologies, and owns stock in Gilead. Co-authors who are employees of Merck Research Laboratories (as indicated on the title page) may own stock and/or stock options in the company.
We thank all the patients and their caregivers who participated in this study. We gratefully recognize the essential contributions of the investigators who enrolled their patients in the study. The expert statistical support of Chengxing Lu and Bo Jin and the indispensable technical assistance of Joann DiLullo and Karyn Davis are greatly appreciated. We are also indebted to Calvin Cohen for his instructive advice regarding the analyses of the DEXA substudy results.
APPENDIX I
PRINCIPAL INVESTIGATORS
The MK-0518 Protocol 021 principal investigators by country are: Australia: Cooper D; Brazil: Madruga J, Netto EM, Zajdenverg R; Canada: Baril JG, Kovacs C, Smaill F; Chile: Afani A, Beltran C, Perez Godoy J; Colombia: Tobon AM, Arango A, Tamara J, Velez J; France: Cotte L, Girard P-M, Pialoux G, Salmon-Ceron D, Yazdanpanah, Y; Germany: Esser S, Fatkenheuer G, Rockstroh J, Schmidt R, Stellbrink H-J; India: Dinaker M, Pazare A, Rajendran J, Srivastava O; Italy: Carosi G, Chirianni A, Esposito R, Lazzarin A, Viscoli C; Mexico: Andrade J, Quintero Perez N, Reyes G, Sierra J, Torres I; Peru: Gotuzzo E, Lama J, Cabello-Chavez R, Salazar R; Spain: Portilla Sogorb J, Rivero-Roman A, Santamaria Jauregui J; Thailand: Manosuthi W, Sungkanuparph S, Supparatpinyo K, Vibhagool A; United States: Berger D*, DeJesus E*, Friel T, Hicks C*, Kozal M*, Kumar P*, Lennox J*, Liporace R*, Little S, Morales-Ramirez J, Novak R*, Pollard R*, Saag M*, Santiago S*, Schneider S*, Steigbigel R*, Towner W*, Wright D*. (*denotes investigators in the DEXA substudy).
Footnotes
ClinicalTrials.gov Identifier: NCT00369941.
References
- 1.Freedberg KA, Losina E, Weinstein MC, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001;344:824–831. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 2.Richman DD, Morton SC, Wrin T, et al. The prevalence of antiretroviral drug resistance in the United States. AIDS. 2004;18:1393–1401. doi: 10.1097/01.aids.0000131310.52526.c7. [DOI] [PubMed] [Google Scholar]
- 3.Truong HH, Grant RM, McFarland W, et al. Routine surveillance for the detection of acute and recent HIV infections and transmission of antiretroviral resistance. AIDS. 2006;20:2193–2197. doi: 10.1097/01.aids.0000252059.85236.af. [DOI] [PubMed] [Google Scholar]
- 4.Vercauteren J, Wensing AMJ, van de Vijver DMAC, et al. Transmission of drug-resistant HIV-1 is stabilizing in Europe. J Infect Dis. 2009;200:1503–1508. doi: 10.1086/644505. [DOI] [PubMed] [Google Scholar]
- 5.Welles SL, Bauer GR, Larussa PS, et al. Time trends for HIV-1 antiretroviral resistance among antiretroviral-experienced and -naïve pregnant women in New York City during 1991 to early 2001. J Acquir Immune Defic Syndr. 2007;44:329–335. doi: 10.1097/QAI.0b013e31802f1296. [DOI] [PubMed] [Google Scholar]
- 6.Hare CB, Mellors J, Krambrink A, et al. Detection of nonnucleoside reverse-transcriptase inhibitor-resistant HIV-1 after discontinuation of virologically suppressive antiretroviral therapy. Clin Infect Dis. 2008;47:421–424. doi: 10.1086/589867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuritzkes DR, Lalama CM, Ribaudo HJ, et al. Preexisting resistance to nonnucleoside reverse-transcriptase inhibitors predicts virologic failure of an efavirenz-based regimen in treatment-naive HIV-1-infected subjects. J Infect Dis. 2008;197:867–870. doi: 10.1086/528802. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch MS, Gunthard HF, Schapiro JM, et al. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society-USA panel. Clin Infect Dis. 2008;47:266–285. doi: 10.1086/589297. [DOI] [PubMed] [Google Scholar]
- 9.Landovitz RJ, Angel JB, Hoffmann C, et al. Phase II study of vicriviroc versus efavirenz (both with zidovudine/lamivudine) in treatment-naive subjects with HIV-1 infection. J Infect Dis. 2008;198:1113–1122. doi: 10.1086/592052. [DOI] [PubMed] [Google Scholar]
- 10.Markowitz M, Nguyen BY, Gotuzzo E, et al. Rapid and durable antiretroviral effect of the HIV-1 Integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J Acquir Immune Defic Syndr. 2007;46:125–133. doi: 10.1097/QAI.0b013e318157131c. [DOI] [PubMed] [Google Scholar]
- 11.Lennox JL, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naïve patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374:796–806. doi: 10.1016/S0140-6736(09)60918-1. [DOI] [PubMed] [Google Scholar]
- 12.Markowitz M, Nguyen BY, Gotuzzo E, et al. Sustained antiretroviral effect of raltegravir after 96 weeks of combination therapy in treatment-naive patients with HIV-1 infection. J Acquir Immune Defic Syndr. 2009;52:350–356. doi: 10.1097/QAI.0b013e3181b064b0. [DOI] [PubMed] [Google Scholar]
- 13.Steigbigel RT, Cooper DA, Kumar PN, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359:339–354. doi: 10.1056/NEJMoa0708975. [DOI] [PubMed] [Google Scholar]
- 14.Cooper DA, Steigbigel RT, Gatell JM, et al. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N Engl J Med. 2008;359:355–365. doi: 10.1056/NEJMoa0708978. [DOI] [PubMed] [Google Scholar]
- 15.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–226. doi: 10.1002/sim.4780040211. [DOI] [PubMed] [Google Scholar]
- 16.Harris M, Larsen G, Montaner JS. Exacerbation of depression associated with starting raltegravir: a report of four cases. AIDS. 2008;22:1890–1892. doi: 10.1097/QAD.0b013e32830e0169. [DOI] [PubMed] [Google Scholar]
- 17.Zembower TR, Gerzenshtein L, Coleman K, et al. Severe rhabdomyolysis associated with raltegravir use. AIDS. 2008;22:1382–1384. doi: 10.1097/QAD.0b013e328303be40. [DOI] [PubMed] [Google Scholar]
- 18.Riddler SA, Li X, Otvos J, et al. Antiretroviral therapy is associated with an atherogenic lipoprotein phenotype among HIV-1-infected men in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2008;48:281–288. doi: 10.1097/QAI.0b013e31817bbbf0. [DOI] [PubMed] [Google Scholar]
- 19.DHHS Panel-Office of AIDS Research Advisory Council. US Department of Health and Human Services 2008. [Accessed October 2, 2009];Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 20.Hammer SM, Eron JJ, Reiss PK, et al. Antiretroviral treatment for adult HIV infection: 2008 recommendations of the International AIDS Society—USA panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]