Abstract
Human T-lymphotropic virus type 1 (HTLV-1) is a pathogenic retrovirus that infects human CD4+ T lymphocytes. Despite its presence in T cells, HTLV-1 causes little overt immunosuppression. This host-virus relationship has therefore been exploited as an excellent model system for studying the dynamic interaction between a persistent retrovirus and the normal human immune system. We use a combination of mathematical and experimental techniques to identify key factors on both sides of the in vivo host-virus interaction that significantly determine HTLV-I proviral load and disease risk. We develop a model to describe how these factors interact to enable viral persistence.
Introduction
Human T-lymphotropic virus type 1 (HTLV-1) is a pathogenic, CD4+ T-lymphotropic human retrovirus that persists indefinitely in the infected host. Over 90% of HTLV-1-infected individuals remain lifelong asymptomatic carriers (ACs) of the virus. In the remaining 5%–10% of individuals, HTLV-1 causes one of two types of disease: an aggressive T-cell malignancy that typically kills the host within 12 months, and a range of chronic inflammatory diseases, of which the best recognized is a disease of the nervous system, HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP).
HTLV-I does not readily produce detectable extracellular virions; thus, viral burden is quantified as the proviral load – the proportion of peripheral blood mononuclear cells that carry an integrated HTLV-I provirus. HTLV-1 proviral load reaches a steady-state ‘set point.’ This set-point proviral load typically varies over time by less than 5-fold within each host, but it can differ by more than 1000-fold between hosts. The risk of HAM/TSP is strongly correlated with the proviral load. In a recent paper [1], we reviewed the evidence that the HTLV-1 proviral load and the risk of HAM/TSP are influenced by the efficiency of the cellular immune response to the virus. In the present article, we focus on a dynamic analysis of the fundamental mechanisms by which HTLV-1 persists, that is the means by which the virus avoids clearance by the immune system. This analysis includes an identification and quantification of key factors in the host-virus interaction and the development of a model describing how these factors interact to enable viral persistence.
HTLV-1 persistence
Evidence for latency of HTLV-1: the standard model
Superficially, it seems clear that HTLV-1 depends primarily on latency to persist in the host. There are four main lines of evidence for HTLV-1 latency. First, HTLV-1 mRNA and proteins such as Tax are usually undetectable in freshly isolated PBMCs, by using RT-PCR or flow cytometry techniques, respectively, although low levels of each can be detected in PBMCs in some infected individuals [2,3]. Second, HTLV-1 virions cannot be detected in plasma, either by electron microscopy or RT-PCR, and transfusion of cell-free blood products does not transmit HTLV-1 infection [4]. Third, HTLV-1 varies little in sequence, which suggests that HTLV-1 does not replicate by the infectious route [5], because this would require the action of the error-prone viral RNA polymerase [6]. Fourth, large clones of provirus-positive lymphocytes persist over months or years in the infected host [7], suggesting that HTLV-1 is maintained chiefly by T-cell proliferation [8], that is the ‘mitotic’ route.
These observations give rise to what we term the standard model of HTLV-1 persistence (Table 1). In this model, HTLV-1 is maintained by passive proliferation of lymphocytes that harbour a transcriptionally silent provirus [9]. A fraction of these provirus-containing cells might express HTLV-1, but the vast majority remain silent and thus invisible to the immune response. Consequently, the immune response does not make a significant impact on the proviral load. In this model, latency is essential for viral persistence, and an increase in viral expression would lead to a decrease in proviral load because of cell-mediated immune destruction of HTLV-I-expressing cells. Viral expression is essential, in this scheme, only at the stage of transmission between individuals.
Table 1. Summary of the key similarities and differences between two models of HTLV-1 persistence during stable chronic infection.
| Standard model: Passive proliferation of latently infected cells |
Alternative model: Active proliferation of Tax-expressing cells |
|
|---|---|---|
| Similarities | ||
| Level of Tax expression | The majority of infected cells do not express Tax in a day. | The majority of infected cells do not express Tax in a day. |
| Infected cell propagation | Most infected cells are produced by division of a provirus-positive cell; de novo infectious events make little, if any, contribution. | Most infected cells are produced by division of a provirus-positive cell; de novo infectious events make little, if any, contribution. |
| Differences | ||
| HTLV-1 persistence | Latency is essential for persistence. | Tax-driven proliferation is essential for persistence. |
| Production of new infected cells | Latent integrated proviruses are ‘passively’ replicated when the host-cell divides. There is no selective proliferation of infected cells. | Tax protein drives infected cell proliferation. There is selective proliferation of infected cells. |
| HTLV-1-specific CTL | CTL have few targets and thus have minimal impact on proviral load. | CTL inhibit the key pathway of viral persistence and thus have a significant impact on proviral load. |
| HAM/TSP occurrence | HAM/TSP is associated with reactivation of HTLV-1 after a long period of viral and clinical latency. | HAM/TSP is associated with a high continuous rate of Tax expression. |
| Impact of increased Tax expression (other factors unchanged) | Proviral load would decrease (because previously latent cells would be exposed to immune surveillance). | Proviral load would increase (because Tax-driven proliferation would exceed CTL lysis). |
Evidence against the standard model of HTLV-1 persistence
We identify two main objections to the standard model. First, there is a large, chronically activated cytotoxic T lymphocyte (CTL) response in most infected individuals [10,11], indicating persistent expression of HTLV-1 proteins. There is also a chronic, HTLV-1-specific IgM antibody response in 10%–40% of infected people [12]: this similarly implies persistent production of viral antigens. Indeed, if all proviruses remained transcriptionally silent indefinitely, the virus could not be transmitted to another host. Second, the proviral load must be maintained by selective proliferation of provirus-containing lymphocytes rather than normal homeostatic lymphocyte proliferation. The reason for this is that if even a small proportion of proviruses was spontaneously transcribed each day, the proviral load would steadily decline unless provirus-expressing cells were replaced, because the expressing cells would be killed by the immune response or by direct toxic effects of the virus. Because a steady decline in proviral load is not observed [13], the provirus-expressing cells must be continuously replaced in vivo. Passive proliferation cannot selectively replace provirus-carrying cells to balance their selective loss. We conclude that the standard model of HTLV-1 persistence is not compatible with current observations, and that an alternative understanding of HTLV-1 persistence and immune control in vivo is needed.
An alternative model of HTLV-1 persistence
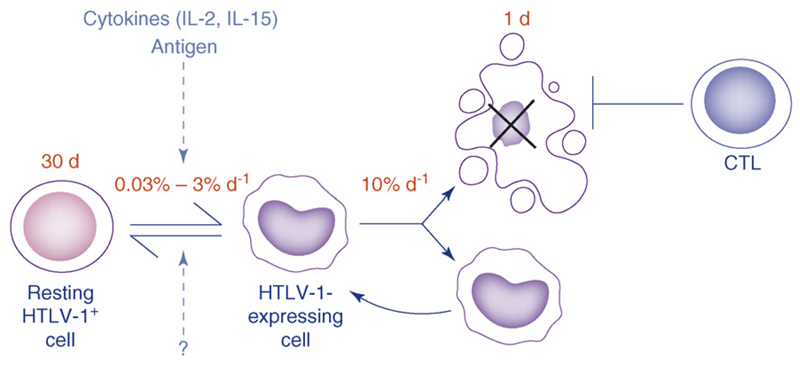
An alternative model (Table 1) involves HTLV-1-infected cells spontaneously expressing viral proteins, including Tax, which, in turn, induces infected cell proliferation by mechanisms including upregulation of promitotic cellular genes [14] and deregulation of cell cycle checkpoints [15]. In this model, as in the standard model, mitosis is the main route of viral replication: the crucial difference is that, in the alternative model, expression of HTLV-I proteins – particularly Tax – is required to promote the selective expansion of cells that harbour a provirus. Infected cells do not evade immune surveillance, and the immune response makes a significant impact on proviral load. Tax expression is then silenced in the majority of surviving cells, by mechanisms that are still poorly understood (Figure 1). As in the standard model, infectious cycle replication of HTLV-1 is necessary for transmission of the virus to a new host. We suggest that most cells that initiate viral protein expression during the chronic phase of infection do not complete a round of infectious viral replication because cells that continue to express viral protein are rapidly killed by the CTL response. Upon infection of a new host, before the establishment of an adaptive immune response, it is probable that a greater proportion of cells that express Tax will successfully produce virions.
Figure 1.
An alternative model of HTLV-I persistence: active proliferation of Tax-expressing cells. We propose that, at any one time, the majority of infected cells making up an individual’s proviral load are not expressing viral protein. Every day, a small proportion (0.03%–3%) of infected cells express Tax (and possibly other viral proteins). The trigger for Tax expression is unknown but might include cell division or the cytokine microenvironment (e.g. IL-2 or IL-15). These Tax-expressing cells proliferate more rapidly than silently infected and uninfected cells, leading to the selective expansion of infected cells and an increase in proviral load. This increase in proviral load is counterbalanced by an efficient HTLV-I-specific CTL response that rapidly kills Tax-positive cells. Tax expression is then silenced in the majority of surviving cells. The mechanism of such transcriptional shutdown is not fully understood: the viral proteins HBZ, Rex, and p30II, as well as epigenetic modifications have been shown to regulate HTLV-I gene expression and might play a role. In this model, an increase in Tax expression would lead to an increase in proviral load. In this model, we consider only nonmalignant HTLV-1 infection. The dynamics of adult T-cell leukaemia cells, which are malignantly transformed and usually proliferate independently of IL-2, will be different. Source of parameter estimates: average life span of a silently infected cell (30 d): assumed to be the same as the life span of an uninfected cell of the same phenotype (CD4+CD45RO+) [61]. Rate of Tax expression (0.03%–3% per d): [16]. Average life span of a Tax-expressing cell (1 d): the life span of Tax-expressing cells depicted is the upper limit in the infected cell life span imposed by CTLs (i.e. it is the life span of an infected cell if CTLs were the only factor contributing to infected cell death; life span = 1/rate of CTL lysis) [25]. In this figure, we depict average values of the parameters; in Figure 4, we show how the values of these parameters differ in examples of actual infected individuals.
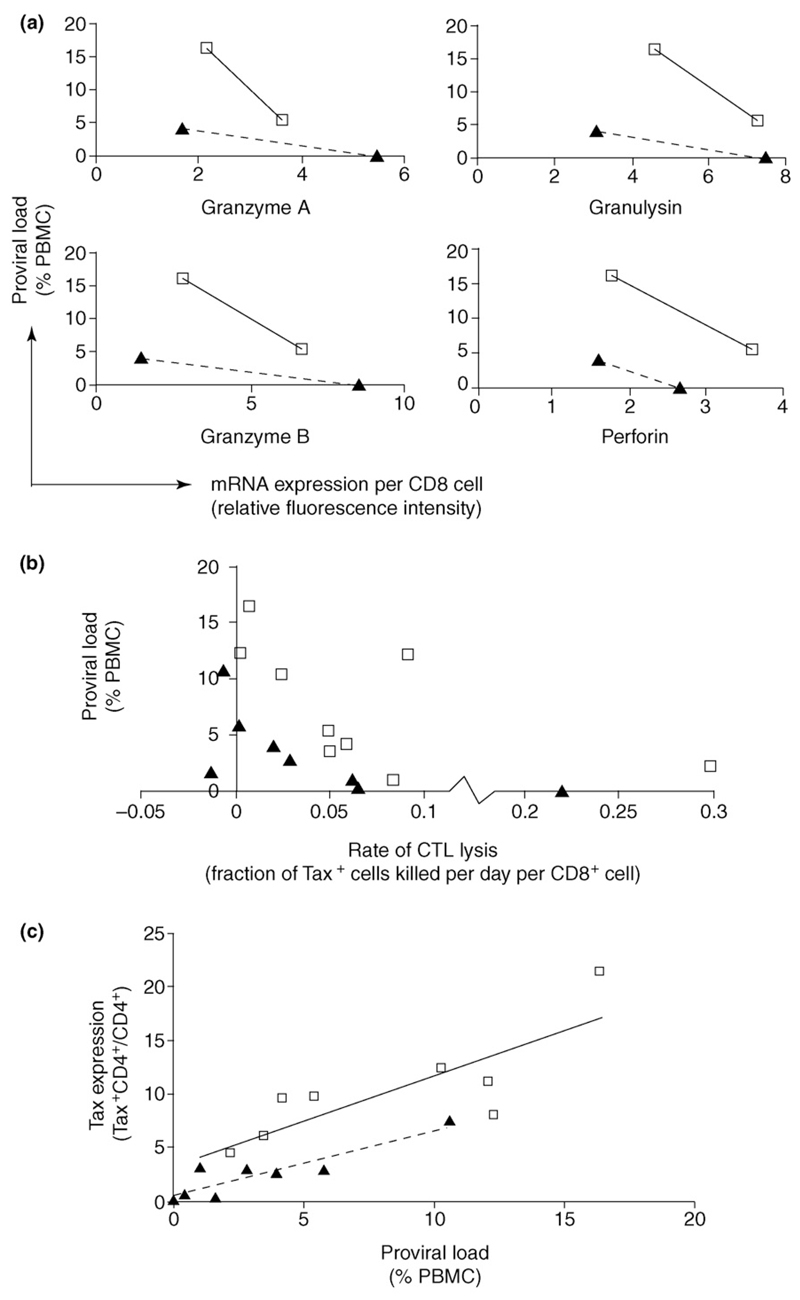
The presence of a large, chronically activated, lytic CTL response in HTLV-1-infected individuals indicates that such a model might be accurate. However, 95%–99% of infected cells do not express viral proteins at any one time [2,16], which raises the question of whether the CTL response can make a significant contribution to the control of proviral load. But, there are several independent lines of evidence that, despite low numbers of virus-expressing cells, CTL-mediated killing does indeed play a significant role in determining the set-point proviral load. This evidence, which has been extensively reviewed elsewhere [17], comes from studies of host genetics [18,19], viral genetics [20–22], gene expression microarrays [23], and in vitro T-cell function [24,25] (Figure 2a,b). Further, vaccination studies in several animal models [26–28] have shown that vaccine-induced CTLs can be associated with suppression of viral replication.
Figure 2.
CD8+ cell lytic activity, proviral load, HTLV-1 Tax expression, and disease. A negative correlation between proviral load and CD8+ cell lytic activity can be observed in both asymptomatic carriers (ACs) (solid triangles) and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients (open squares). (a and b) This was true whether lytic activity was measured (a) by expression of lytic genes or (b) by an ex vivo killing assay. When ACs and HAM/TSP patients with similar lytic activity were compared, the HAM/TSP patients consistently had higher proviral loads than the ACs. (c) We believe that this is because infected cells from HAM/TSP patients have a significantly higher probability of expressing Tax protein, which drives cell proliferation and increases proviral load [33].
However, it remains counterintuitive that CTLs can have a large impact on proviral load when most infected cells are invisible to the immune response at any one time. We hypothesize that expression of viral proteins is necessary to drive cell division and maintain proviral load in the chronic phase of infection. Any factor that kills virus-expressing cells will therefore block this pathway and will probably have a large effect on proviral load. Work to test this hypothesis is currently in progress.
The alternative model is consistent with the observations of the genetic stability of HTLV-1 [6,29] and the large population sizes of infected cell clones [7,30], which both suggest that infected cell division rather than error-prone reverse transcriptase-dependent replication is the dominant mode of infected cell production during the chronic phase of infection. Importantly, the existence of large infected cell clones also implies selective (we hypothesize Tax-driven) proliferation rather than passive proliferation of infected cells. Infected cell clones are frequently very large (1/1500–1/300 PBMC) [7,30], and such large selective clonal expansion could not occur by normal homeostatic or antigen-driven proliferation: antigen-specific CD4+ cell clones are typically very small [31] and do not reach frequencies of 1 cell in 10,000 even during chronic infection such as acute infectious mononucleosis [32].
Viral escape from CTLs
Unlike HIV, CTL escape mutants of HTLV-1 do not become established in the proviral population [21,22]. Two important differences could reduce the likelihood of escape in HTLV-1. First, because of the infrequency of infectious transmission, HTLV-I generates fewer mutants than HIV-1 [6]. Second, the immunodominant Tax protein is intolerant of mutations: naturally occurring escape mutations in Tax severely impair the transactivation function of Tax [21]. Furthermore, the tax gene overlaps with rex, which places further functional constraints on any mutations. Therefore, it seems that HTLV-1 depends on approaches other than CTL escape to persist in the host.
Determinants of proviral load
Expression of Tax both increases infected cell division (and thus the proviral load) and exposes infected cells to effective immune surveillance, thus decreasing proviral load. The balance between these two opposing factors will determine the net effect of Tax expression. Whereas the standard model predicts that Tax expression will decrease the proviral load, the alternative model predicts that Tax expression will increase the load.
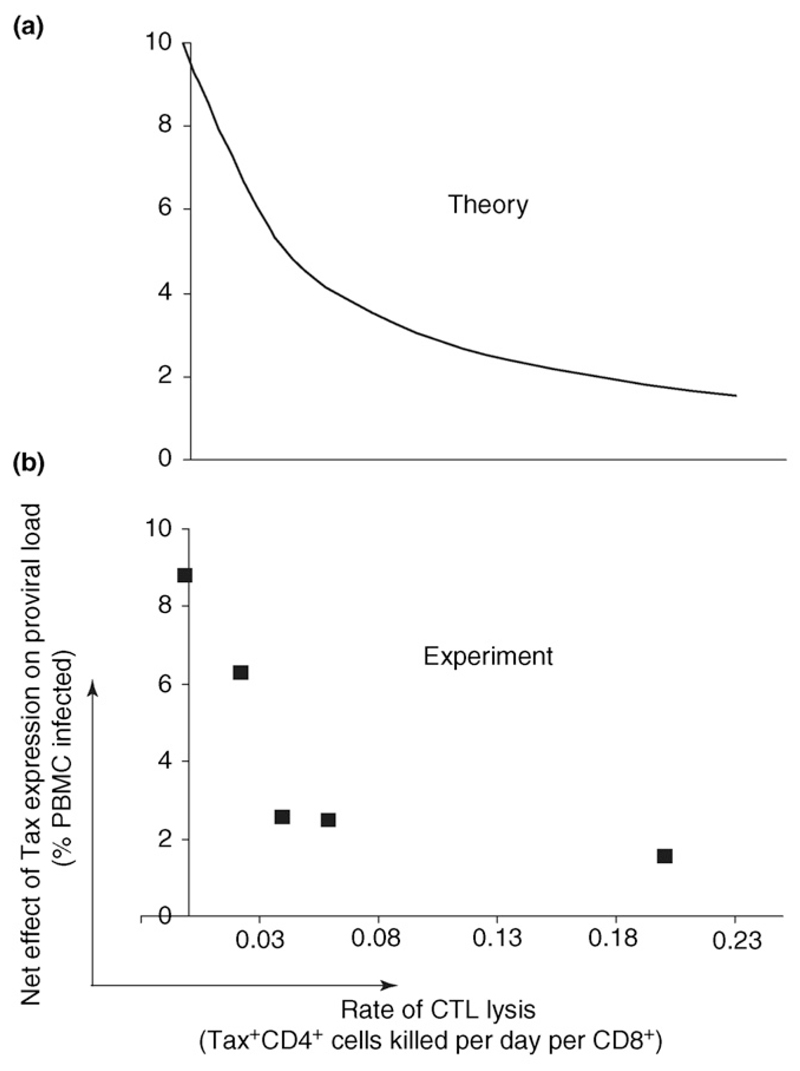
Both the rate of CD8+ cell lysis and the probability that an infected cell expresses Tax are independent, significant predictors of an individual’s proviral load [33]. A high proviral load was associated with a low rate of CTL lysis and with a high probability of Tax expression by an infected cell (Figure 2c). Together, the rate of lysis and the probability of Tax expression predicted almost half of the between-individual proviral load variation: 30% of the proviral load was predicted by the CTL lysis rate, and 13% was predicted by the probability of Tax expression in an infected cell. These data are consistent with the alternative model and suggest that the net effect of Tax expression is to increase proviral load. That is, the positive effect of Tax expression on cell division outweighs the negative effect of exposure to the immune response. Furthermore, as expected, the net benefit to the virus (increase in proviral load) conferred by Tax expression was lower in individuals with a strong CTL response than in individuals with a weaker CTL response [33] (Figure 3).
Figure 3.
The net effect of HTLV-1 Tax expression on proviral load. Tax has both positive and negative effects on proviral load. The former occurs because Tax expression drives host T-cell proliferation, and the latter occurs because Tax expression facilitates detection and elimination by the host cytotoxic T lymphocyte (CTL) response. The balance between these two opposing factors determines the net effect of Tax expression on proviral load. (a) The predicted net effect of Tax expression on proviral load within hosts with CTL responses of differing efficiency. As the CTL response becomes stronger, the net positive effect of Tax expression on proviral load is predicted to decrease [33]. (b) The net effect of Tax expression on HTLV-I proviral load was estimated by grouping individuals with similar rates of CTL lysis and then measuring the difference in proviral load between individuals whose infected cells had a high rate of Tax expression and individuals whose infected cells had a low rate of Tax expression; the difference in proviral load was plotted as a function of the group’s rate of CTL lysis. We found that Tax expression was always associated with an increase in HTLV-I proviral load, and that the net effect of Tax expression on proviral load decreased as the rate of CTL lysis increased [33]. There is thus a good correlation between the theoretically predicted pattern of provirus expression and actual patient data.
Determinants of HAM/TSP risk
A high proviral load is a significant risk factor for the development of HAM/TSP [34]. If CTLs do indeed play a major role in controlling proviral load, then a strong CTL response that reduces the proviral load should be associated with protection from HAM/TSP. This was borne out by an immunogenetics study in Kagoshima [18,35] in which two HLA class I alleles that were associated with a reduced proviral load in healthy carriers were also associated with a lower incidence of HAM/TSP.
How can a high proviral load cause disease when most of that proviral load is not expressed? The answer again appears to be that the small proportion of infected cells that do express viral proteins play a crucial role. The CD4+ cell population from HAM/TSP patients expresses higher levels of tax mRNA [3,36] than CD4+ cells from ACs. Moreover, infected cells from HAM/TSP patients had a significantly higher probability of expressing Tax protein than infected cells from ACs (Figure 2c) [33]. The probability of Tax expression was a significant predictor of HAM/TSP status [33] and correctly classified 89% of subjects independently of proviral load. We therefore suggest that proviral expression itself plays an active part in the pathogenesis of the inflammatory disease, and that the well-documented association between a high proviral load and HAM/TSP [34] arises because both result from proviral expression [37,38]. Consistent with both the observation that Tax expression is significantly higher in HAM/TSP patients and that Tax expression drives cell division, it has recently been shown that CD4+CD45RO+ (‘memory’) T cells proliferate significantly faster in vivo in HAM/TSP patients than the same cell population in healthy carriers [16], and that CD4+CD45RO+ T-cell proliferation in vivo is directly correlated with Tax expression.
The host-virus interaction
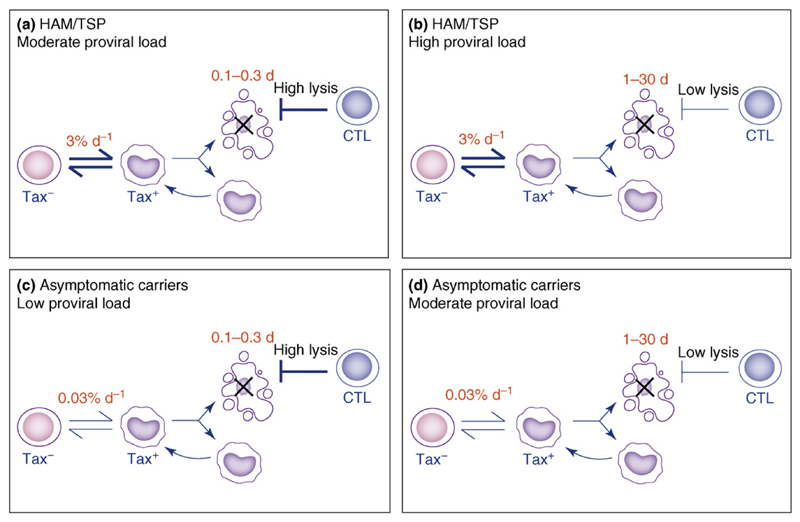
The emerging picture of HTLV-1 suggests that two chief aspects of the host-virus interaction – the efficiency of the cellular immune response and the rate of spontaneous HTLV-1 expression – determine the outcome of HTLV-I infection, that is, the HTLV-I proviral load and the risk of the associated inflammatory diseases HAM/TSP. We hypothesize that the reason why HAM/TSP patients have a higher proviral load at a given rate of lysis (Figure 2b) – a picture that is qualitatively repeated if CD8+-cell lytic activity is measured by mRNA expression (Figure 2a) – is that infected cells from HAM/TSP patients have a higher probability of Tax expression (Figure 2c), which drives both infected cell proliferation and disease. We find that subjects with the highest proviral loads (upper right-hand quadrant in Figure 4) have infected cells that express Tax readily combined with a weak CTL lytic response. Subjects with the lowest proviral loads (lower left-hand quadrant in Figure 4) have both an effective CTL response and infected cells that express Tax less frequently. Subjects with intermediate proviral loads (upper left and lower right-hand quadrants in Figure 4) either have a good CTL response but infected cells that rapidly express Tax or a poor CTL response but infected cells that are largely latent. The observed difference in probability of Tax expression can explain the previously puzzling observation that subjects with a low proviral load can develop HAM/TSP, whereas others with a higher proviral load can remain asymptomatic.
Figure 4.
Both sides of the host-virus interaction affect proviral load and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) risk. A total of 30% of between-individual variation in proviral load is explained by the rate of CTL lysis [25]; 13% is explained by the probability of Tax expression of infected cells [33]. The probability of Tax expression of infected cells predicts 89% of HAM/TSP cases [33]. We have observed HTLV-I-infected subjects in all four categories [25,33], e.g. (a) patient TAY has a high rate of CTL lysis of 600% per d (Tax+ cell life span = 0.2 d) and a high probability of Tax expression: the patient has HAM/TSP and a proviral load of 2%; (b) patient TBG has a low rate of CTL lysis of 20% per d (life span = 5 d) and a high probability of Tax expression: the patient has HAM/TSP and a proviral load of 16.4%; (c) patient HBD has a high rate of CTL lysis of 540% per d (life span = 0.2 d) and a low probability of Tax expression: the patient is asymptomatic and has a proviral load of 0.001%; (d) patient HS has a low rate of CTL lysis of 3% per d (life span = 30 d) and a low probability of Tax expression: the patient is asymptomatic and has a proviral load of 5.8%.
Lessons drawn from the HTLV-1 system
The increasingly detailed picture of HTLV-1 persistence makes possible certain inferences that are of general significance in persistent viral infections.
Importance of CTLs in determining viral load
The HTLV-1 system provides the most direct evidence to date that CTLs can determine load in a retroviral infection [17]. This conclusion has been widely suspected, but clear evidence in natural human infections has been hard to obtain. There is growing evidence, albeit less direct [39–41], that CTLs similarly make a significant impact in HIV-1 infection. CTL lysis of HTLV-1-infected cells appears to be considerably more rapid than CTL lysis of HIV-1-infected cells [42], although different methods of quantification make direct comparison problematic.
CTL efficacy in viral infections cannot be inferred from CTL frequency
Studies of the correlation between CTL frequency and viral load, notably in HIV-1 infection, have given contradictory results [43–45]. Recently, a hypothesis has been suggested to explain this apparent contradiction [46,47]: certain CTL responses are ‘drivers’ that effectively control viral infection and thus are negatively correlated with viral load, whereas other CTL responses are merely ‘passengers’ that have no impact on viral infection and passively follow viral load, leading to a positive correlation between the viral load and the frequency of virus-specific passenger CTLs. While such an explanation is intuitively appealing, we have previously shown that the feedback loop between CTL frequency and viral load (high CTL frequency lowers viral load, leading to reduced antigen stimulus and thus reduced CTL frequency) makes it impossible to predict the direction of correlation between the frequency of a driver CTL response and viral load without several implicit assumptions that cannot be justified [48]. This argument is supported by data from the HTLV-1 system. In HTLV-1 infection in Japan, HLA-A*02 was associated with a significantly lower proviral load and significantly reduced HAM/TSP prevalence [18]: HLA-A*02-restricted responses would thus be classified as ‘driver’ responses, and a negative correlation between frequency and proviral load would be predicted. It is not known which HLA-A2-restricted CTL response confers protection, but the HLA-A2:Tax11–19 response probably plays a significant role because the affinity of Tax11–19 for HLA-A2 is unusually high [49], and the Tax11–19-specific response is frequently immunodominant [50]. However, within HLA-A*02+ individuals, both the frequency of CTLs specific for the immunodominant epitope Tax11–19 and the frequency of all HTLV-I-specific CTLs was observed to correlate positively with proviral load rather than negatively [43,51,52]. We suggest that inferring CTL control from the correlation between CTL frequency and viral load might be misleading. Measurement of CTL function, for example by killing assays ex vivo [25,53] or in vivo [42,54] in both HIV and HTLV-1 infections, appears to provide the most direct and coherent understanding of the importance of the CTL response.
Comparison of strategies of viral persistence
Many persistent viruses have evolved mechanisms to evade or disrupt the host immune response; indeed, it has been hypothesized that such mechanisms are essential for viral persistence [55]. Some viruses such as HIV-1 or cytomegalovirus (CMV) subvert the immune response by disrupting major histocompatibility complex (MHC) class I presentation of antigen [56,57], while others such as Epstein Barr virus (EBV) have evolved complex, two-phase life cycles to restrict viral gene expression [58]. HTLV-I does not appear to rely on any such mechanisms. Putative CTL escape mutants of HTLV-1 fail to become established [21,22]. Partial MHC class I downregulation by p12I has been described in vitro [59], but, conversely, Tax upregulates class I expression [60]. Moreover, in primary cells, there is no evidence for loss of class I from the cell surface ([38] and S. Sowinski, pers. commun.). More directly, ex vivo CTLs from infected subjects are able to lyse HTLV-1-infected cells very efficiently [24,25]. It is possible that HTLV-1, a small (9 kb) genetically stable virus, has limited opportunity to use more sophisticated methods of persistence. Instead, it appears simply to out-pace the immune response, replicating faster than it is killed.
Future questions
This work raises several important questions. In particular:
What factors other than HLA genotype determine the efficiency of the antiviral CTL response? Variation between individuals in antiviral CTL efficiency might explain the different outcomes of viral infections and the effectiveness of antiviral vaccines.
What determines the rate of expression of the HTLV-1 provirus? There is significant variation between individuals in the probability that an HTLV-1 provirus is expressed in a given time, and a higher probability is associated with a higher risk of the inflammatory disease HAM/TSP. Possible factors include epigenetic changes and the genomic integration site of the HTLV-1 provirus.
How can we reconcile low-level viral expression with a large contribution of CTL in controlling proviral load? We suggest here that CTLs control the proviral load of HTLV-1 despite the low proviral expression rate in vivo because CTLs attack the key point in the viruses life cycle – virus-driven host-cell proliferation – that maintains the proviral load. While existing experimental data are consistent with this conclusion, further corroborative evidence needs to be obtained, for example, from assays of HTLV-1 expression and load in plausible animal models of HTLV-1 infection.
Answering these questions will enable us to understand what constitutes an effective CTL response, what determines an individual’s risk of developing HAM/TSP, and how the immune response can be effective despite frequent viral latency.
References
- 1.Asquith B, Bangham CR. Quantifying HTLV-I dynamics. Immunol Cell Biol. 2007;85:280–286. doi: 10.1038/sj.icb.7100050. [DOI] [PubMed] [Google Scholar]
- 2.Richardson JH, et al. Variable immortalizing potential and frequent virus latency in blood-derived T-cell clones infected with human T-cell leukemia virus type I. Blood. 1997;89:3303–3314. [PubMed] [Google Scholar]
- 3.Yamano Y, et al. Correlation of human T-cell lymphotropic virus type 1 (HTLV-1) mRNA with proviral DNA load, virus-specific CD8(+) T cells, and disease severity in HTLV-1-associated myelopathy (HAM/TSP) Blood. 2002;99:88–94. doi: 10.1182/blood.v99.1.88. [DOI] [PubMed] [Google Scholar]
- 4.Okochi K, Sato H. Transmission of ATLV (HTLV-I) through blood transfusion. Princess Takamatsu Symp. 1984;15:129–135. [PubMed] [Google Scholar]
- 5.Overbaugh J, Bangham CR. Selection forces and constraints on retroviral sequence variation. Science. 2001;292:1106–1109. doi: 10.1126/science.1059128. [DOI] [PubMed] [Google Scholar]
- 6.Ina Y, Gojobori T. Molecular evolution of human T-cell leukemia virus. J Mol Evol. 1990;31:493–499. doi: 10.1007/BF02102076. [DOI] [PubMed] [Google Scholar]
- 7.Cavrois M, et al. Persistent oligoclonal expansion of human T-cell leukemia virus type 1-infected circulating cells in patients with Tropical spastic paraparesis/HTLV-1 associated myelopathy. Oncogene. 1998;17:77–82. doi: 10.1038/sj.onc.1201906. [DOI] [PubMed] [Google Scholar]
- 8.Etoh K, et al. Persistent clonal proliferation of human T-lymphotropic virus type I-infected cells in vivo. Cancer Res. 1997;57:4862–4867. [PubMed] [Google Scholar]
- 9.Mortreux F, et al. Molecular and cellular aspects of HTLV-1 associated leukemogenesis in vivo. Leukemia. 2003;17:26–38. doi: 10.1038/sj.leu.2402777. [DOI] [PubMed] [Google Scholar]
- 10.Daenke S, et al. High activated and memory cytotoxic T-cell responses to HTLV-1 in healthy carriers and patients with tropical spastic paraparesis. Virology. 1996;217:139–146. doi: 10.1006/viro.1996.0101. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson S, et al. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature. 1990;348:245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- 12.Kira J, et al. Antibody titers to HTLV-I-p40tax protein and gagenv hybrid protein in HTLV-I-associated myelopathy/tropical spastic paraparesis: correlation with increased HTLV-I proviral DNA load. J Neurol Sci. 1992;107:98–104. doi: 10.1016/0022-510x(92)90215-7. [DOI] [PubMed] [Google Scholar]
- 13.Matsuzaki T, et al. HTLV-I proviral load correlates with progression of motor disability in HAM/TSP: analysis of 239 HAM/TSP patients including 64 patients followed up for 10 years. J Neurovirol. 2001;7:228–234. doi: 10.1080/13550280152403272. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida M. Multiple viral strategies of HTLV-1 for dysregulation of cell growth control. Annu Rev Immunol. 2001;19:475–496. doi: 10.1146/annurev.immunol.19.1.475. [DOI] [PubMed] [Google Scholar]
- 15.Gatza ML, et al. Cellular transformation by the HTLV-I Tax protein, a jack-of-all-trades. Oncogene. 2003;22:5141–5149. doi: 10.1038/sj.onc.1206549. [DOI] [PubMed] [Google Scholar]
- 16.Asquith B, et al. In vivo T lymphocyte dynamics in humans and the impact of human T-lymphotropic virus 1 infection. Proc Natl Acad Sci U S A. 2007;104:8035–8040. doi: 10.1073/pnas.0608832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bangham CR, Osame M. Cellular immune response to HTLV-1. Oncogene. 2005;24:6035–6046. doi: 10.1038/sj.onc.1208970. [DOI] [PubMed] [Google Scholar]
- 18.Jeffery KJ, et al. HLA alleles determine human T-lymphotropic virus-I (HTLV-I) proviral load and the risk of HTLV-I-associated myelopathy. Proc Natl Acad Sci U S A. 1999;96:3848–3853. doi: 10.1073/pnas.96.7.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vine AM, et al. Polygenic control of human T lymphotropic virus type I (HTLV-I) provirus load and the risk of HTLV-I-associated myelopathy/tropical spastic paraparesis. J Infect Dis. 2002;186:932–939. doi: 10.1086/342953. [DOI] [PubMed] [Google Scholar]
- 20.Niewiesk S, et al. The transactivator gene of human T-cell leukemia virus type I is more variable within and between healthy carriers than patients with tropical spastic paraparesis. J Virol. 1994;68:6778–6781. doi: 10.1128/jvi.68.10.6778-6781.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niewiesk S, et al. Naturally occurring variants of human T-cell leukemia virus type I Tax protein impair its recognition by cytotoxic T lymphocytes and the transactivation function of Tax. J Virol. 1995;69:2649–2653. doi: 10.1128/jvi.69.4.2649-2653.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubota R, et al. Genetic stability of human T lymphotropic virus type I despite antiviral pressures by CTLs. J Immunol. 2007;178:5966–5972. doi: 10.4049/jimmunol.178.9.5966. [DOI] [PubMed] [Google Scholar]
- 23.Vine AM, et al. The role of CTLs in persistent viral infection: cytolytic gene expression in CD8+ lymphocytes distinguishes between individuals with a high or low proviral load of human T cell lymphotropic virus type 1. J Immunol. 2004;173:5121–5129. doi: 10.4049/jimmunol.173.8.5121. [DOI] [PubMed] [Google Scholar]
- 24.Hanon E, et al. Abundant tax protein expression in CD4+ T cells infected with human T-cell lymphotropic virus type I (HTLV-I) is prevented by cytotoxic T lymphocytes. Blood. 2000;95:1386–1392. [PubMed] [Google Scholar]
- 25.Asquith B, et al. A functional CD8+ cell assay reveals individual variation in CD8+ cell antiviral efficacy and explains differences in human T-lymphotropic virus type 1 proviral load. J Gen Virol. 2005;86:1515–1523. doi: 10.1099/vir.0.80766-0. [DOI] [PubMed] [Google Scholar]
- 26.Hanabuchi S, et al. Regression of human T-cell leukemia virus type I (HTLV-I)-associated lymphomas in a rat model: peptide-induced T-cell immunity. J Natl Cancer Inst. 2001;93:1775–1783. doi: 10.1093/jnci/93.23.1775. [DOI] [PubMed] [Google Scholar]
- 27.Hislop AD, et al. Vaccine-induced cytotoxic T lymphocytes protect against retroviral challenge. Nat Med. 1998;4:1193–1196. doi: 10.1038/2690. [DOI] [PubMed] [Google Scholar]
- 28.Sundaram R, et al. Protective efficacy of multiepitope human leukocyte antigen-A*0201 restricted cytotoxic T-lymphocyte peptide construct against challenge with human T-cell lymphotropic virus type 1 Tax recombinant vaccinia virus. J Acquir Immune Defic Syndr. 2004;37:1329–1339. doi: 10.1097/00126334-200411010-00001. [DOI] [PubMed] [Google Scholar]
- 29.Kubota R, et al. Fluctuation of HTLV-I proviral DNA in peripheral blood mononuclear cells of HTLV-I-associated myelopathy. J Neuroimmunol. 1993;42:147–154. doi: 10.1016/0165-5728(93)90004-i. [DOI] [PubMed] [Google Scholar]
- 30.Cavrois M, et al. Proliferation of HTLV-1 infected circulating cells in vivo in all asymptomatic carriers and patients with TSP/HAM. Oncogene. 1996;12:2419–2423. [PubMed] [Google Scholar]
- 31.Maini MK, et al. A comparison of two techniques for the molecular tracking of specific T-cell responses; CD4+ human T-cell clones persist in a stable hierarchy but at a lower frequency than clones in the CD8+ population. Immunology. 1998;94:529–535. doi: 10.1046/j.1365-2567.1998.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maini MK, et al. Clonal expansions in acute EBV infection are detectable in the CD8 and not the CD4 subset and persist with a variable CD45 phenotype. J Immunol. 2000;165:5729–5737. doi: 10.4049/jimmunol.165.10.5729. [DOI] [PubMed] [Google Scholar]
- 33.Asquith B, et al. Quantification of the virus-host interaction in human T lymphotropic virus I infection. Retrovirology. 2005;2:75. doi: 10.1186/1742-4690-2-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagai M, et al. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J Neurovirol. 1998;4:586–593. doi: 10.3109/13550289809114225. [DOI] [PubMed] [Google Scholar]
- 35.Jeffery KJ, et al. The influence of HLA class I alleles and heterozygosity on the outcome of human T cell lymphotropic virus type I infection. J Immunol. 2000;165:7278–7284. doi: 10.4049/jimmunol.165.12.7278. [DOI] [PubMed] [Google Scholar]
- 36.Furukawa Y, et al. Human T-cell leukemia virus type-1 (HTLV-Tax is expressed at the same level in infected cells of HTLV-1-associated myelopathy or tropical spastic paraparesis patients as in asymptomatic carriers but at a lower level in adult T-cell leukemia cells. Blood. 1995;85:1865–1870. [PubMed] [Google Scholar]
- 37.Jacobson S. Immunopathogenesis of human T cell lymphotropic virus type I-associated neurologic disease. J Infect Dis. 2002;186(Suppl 2):S187–S192. doi: 10.1086/344269. [DOI] [PubMed] [Google Scholar]
- 38.Yamano Y, et al. Increased expression of human T lymphocyte virus type I (HTLV-I) Tax11-19 peptide-human histocompatibility leukocyte antigen A*201 complexes on CD4+ CD25+ T Cells detected by peptide-specific, major histocompatibility complex-restricted antibodies in patients with HTLV-I-associated neurologic disease. J Exp Med. 2004;199:1367–1377. doi: 10.1084/jem.20032042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carrington M, et al. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science. 1999;283:1748–1752. doi: 10.1126/science.283.5408.1748. [DOI] [PubMed] [Google Scholar]
- 40.Jin X, et al. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones NA, et al. Determinants of human immunodeficiency virus type 1 escape from the primary CD8+ cytotoxic T lymphocyte response. J Exp Med. 2004;200:1243–1256. doi: 10.1084/jem.20040511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asquith B, et al. Inefficient cytotoxic T lymphocyte-mediated killing of HIV-1-infected cells in vivo. PLoS Biol. 2006;4:e90. doi: 10.1371/journal.pbio.0040090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wodarz D, et al. Cytotoxic T-cell abundance and virus load in human immunodeficiency virus type 1 and human T-cell leukaemia virus type 1. Proc R Soc Lond B Biol Sci. 2001;268:1215–1221. doi: 10.1098/rspb.2001.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Betts MR, et al. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. J Virol. 2001;75:11983–11991. doi: 10.1128/JVI.75.24.11983-11991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogg GS, et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 46.Zafiropoulos A, et al. Analysis of ‘driver’ and ‘passenger’ CD8+ T-cell responses against variable viruses. Proc R Soc Lond B Biol Sci. 2004;271(Suppl 3):S53–S56. doi: 10.1098/rsbl.2003.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zuniga R, et al. Relative dominance of Gag p24-specific cytotoxic T lymphocytes is associated with human immunodeficiency virus control. J Virol. 2006;80:3122–3125. doi: 10.1128/JVI.80.6.3122-3125.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asquith B, Bangham CR. An introduction to lymphocyte and viral dynamics: the power and limitations of mathematical analysis. Proc R Soc Lond B Biol Sci. 2003;270:1651–1657. doi: 10.1098/rspb.2003.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parker KC, et al. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol. 1994;152:163–175. [PubMed] [Google Scholar]
- 50.Elovaara I, et al. High human T cell lymphotropic virus type 1 (HTLV-1)-specific precursor cytotoxic T lymphocyte frequencies in patients with HTLV-1-associated neurological disease. J Exp Med. 1993;177:1567–1573. doi: 10.1084/jem.177.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kubota R, et al. HTLV-I specific IFN-γ+ CD8+ lymphocytes correlate with the proviral load in peripheral blood of infected individuals. J Neuroimmunol. 2000;102:208–215. doi: 10.1016/s0165-5728(99)00175-7. [DOI] [PubMed] [Google Scholar]
- 52.Kubota R, et al. Increased HTLV type 1 tax specific CD8+ cells in HTLV type 1-asociated myelopathy/tropical spastic paraparesis: correlation with HTLV type 1 proviral load. AIDS Res Hum Retroviruses. 2000;16:1705–1709. doi: 10.1089/08892220050193182. [DOI] [PubMed] [Google Scholar]
- 53.Yang OO, et al. Efficient lysis of human immunodeficiency virus type 1-infected cells by cytotoxic T lymphocytes. J Virol. 1996;70:5799–5806. doi: 10.1128/jvi.70.9.5799-5806.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernandez CS, et al. Rapid viral escape at an immunodominant simian-human immunodeficiency virus cytotoxic T-lymphocyte epitope exacts a dramatic fitness cost. J Virol. 2005;79:5721–5731. doi: 10.1128/JVI.79.9.5721-5731.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oldstone MB. Viral persistence: parameters, mechanisms and future predictions. Virology. 2006;344:111–118. doi: 10.1016/j.virol.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 56.Mocarski ES., Jr Immune escape and exploitation strategies of cytomegaloviruses: impact on and imitation of the major histocompatibility system. Cell Microbiol. 2004;6:707–717. doi: 10.1111/j.1462-5822.2004.00425.x. [DOI] [PubMed] [Google Scholar]
- 57.Collins KL, et al. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 58.Tsurumi T, et al. Latent and lytic Epstein-Barr virus replication strategies. Rev Med Virol. 2005;15:3–15. doi: 10.1002/rmv.441. [DOI] [PubMed] [Google Scholar]
- 59.Johnson JM, et al. Free major histocompatibility complex class I heavy chain is preferentially targeted for degradation by human T-cell leukemia/lymphotropic virus type 1 p12(I) protein. J Virol. 2001;75:6086–6094. doi: 10.1128/JVI.75.13.6086-6094.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sawada M, et al. Human T-cell leukemia virus type I trans activator induces class I major histocompatibility complex antigen expression in glial cells. J Virol. 1990;64:4002–4006. doi: 10.1128/jvi.64.8.4002-4006.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Macallan DC, et al. Measurement and modeling of human T cell kinetics. Eur J Immunol. 2003;33:2316–2326. doi: 10.1002/eji.200323763. [DOI] [PubMed] [Google Scholar]