Highlights
-
•
One-dose HPV vaccination was cost-saving compared to no vaccination.
-
•
One-dose optimistically averted ~19% fewer cases than two-dose vaccination.
-
•
Two-dose vaccination was generally preferred to one-dose vaccination.
-
•
One-dose was cost-effective only when accompanied by higher coverage and lifelong protection.
Keywords: Human papillomavirus, Vaccination, Cervical cancer
Abstract
Background
Although guidelines for prophylactic human papillomavirus (HPV) vaccination recommend two doses for girls ages 9–14 years, several studies have demonstrated similar protection with one dose. Our objective was to evaluate the long-term health and economic impacts of routine one-dose HPV vaccination compared to (1) no vaccination and (2) two-dose HPV vaccination in a low-income country.
Methods
We used a three-tiered hybrid modeling approach that captured HPV transmission, cervical carcinogenesis, and population demographics to project long-term health and economic outcomes associated with one-dose HPV vaccination (assuming 80% efficacy against HPV-16/18 infections under three waning scenarios) and two-dose HPV vaccination (assuming 100% efficacy over the lifetime) in Uganda. Costs included the vaccine program (dosage and delivery) costs over a 10-year period and cervical cancer costs over the lifetimes of the current population of Ugandan women. Health outcomes included number of cervical cancer cases and disability-adjusted life years (DALYs). Incremental cost-effectiveness ratios (i.e., cost per DALY averted) were calculated and compared against the Ugandan per-capita gross domestic product.
Results
Routine one-dose HPV vaccination of 9-year-old girls required substantial upfront investment but was cost-saving compared to no vaccination when accounting for the cost-offsets from future cancers averted. Forty years after initiating routine vaccination and depending on assumptions of vaccine waning, one-dose HPV vaccination with equivalent coverage (70%) averted 15–16% of cervical cancer cases versus 21% with two-dose vaccination but required only half the upfront economic investment. Vaccination with two doses had an attractive cost-effectiveness profile except if one-dose vaccination enabled higher coverage (90% vs. 70%) and did not wane.
Conclusions
One-dose HPV vaccination resulted in cost-savings compared to no vaccination and could be cost-effective compared to two-dose vaccination if protection is longstanding and higher coverage can be achieved.
1. Introduction
Cervical cancer is caused by persistent infection with one of 13 sexually transmitted high-risk human papillomavirus (HPV) genotypes [1]. Globally, approximately 70% of cervical cancers are attributable to HPV genotypes 16 and 18 [2]. The World Health Organization recommends two-dose prophylactic HPV vaccination for young girls aged 9–14 years, with a 6-month interval between doses and completion prior to initiation of sexual activity [3].
Several studies—including the Costa Rica Vaccine Trial [4], a multicenter cohort study in India [5], and the industry-sponsored PATRICIA trial [6]—have indicated similar vaccine protection among those receiving one or two doses of HPV vaccine. Compared with a two-dose HPV vaccination schedule, one-dose HPV vaccination could potentially reduce programs costs, ease administration, enable the delivery of multi-cohort vaccination, and increase HPV vaccine program adoption in populations with limited access to healthcare and a high burden of cervical cancer. Yet many uncertainties remain, including the efficacy, duration of vaccine protection and value of a single HPV vaccine dose. Several vaccine trials are underway to evaluate the properties of a one-dose vaccine schedule, but it will be several years before those data become available.
As we await additional empirical data, mathematical models that integrate available evidence on sexual behavior and cervical cancer natural history can project epidemiological, health, and economic outcomes over the considerable time horizon between intervention and prevention of cancer. Such analyses can help inform stakeholder decision-making in light of data gaps and uncertainties. In particular, countries eligible for funding from Gavi, the Vaccine Alliance face severe resource constraints, prompting decision makers to consider the value – or cost-effectiveness – of reduced HPV vaccine dosing schedules prior to policy adoption or augmentation. Our objective was to evaluate the long-term health and economic impacts of routine one-dose HPV vaccination, compared to (1) no vaccination and (2) two-dose HPV vaccination in the context of a low-income Gavi-eligible country.
2. Methods
2.1. Analytic overview
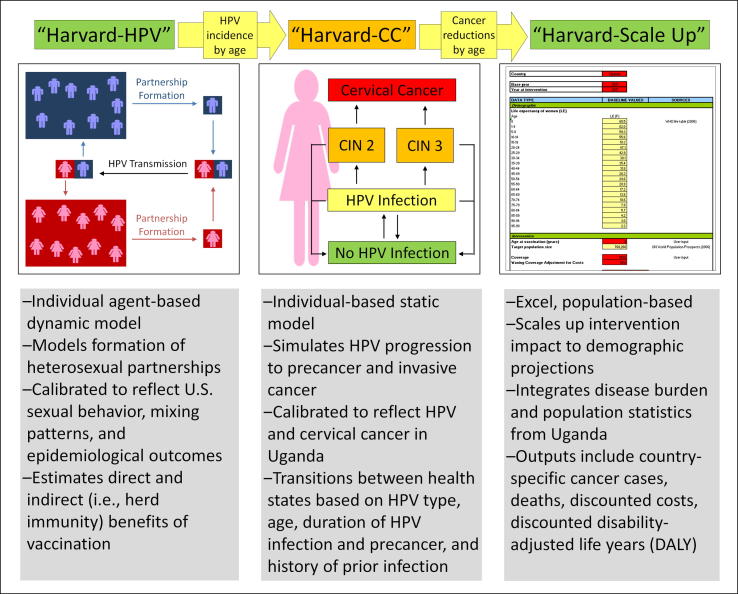
We used a three-tiered hybrid modeling approach (Fig. 1) to capture important behavioral, epidemiological and demographic information in order to estimate the health and economic outcomes associated with alternative one-dose HPV vaccination scenarios in Uganda. We linked a dynamic agent-based model of HPV transmission (“Harvard-HPV”) to a static individual-based model of cervical carcinogenesis (“Harvard-CC”) in order to capture both the direct and indirect “herd immunity” benefits of HPV vaccination, as well as the complex natural history of HPV-induced cervical cancer. Finally, we used a companion population-based model (“Harvard-Scale Up”) to project the health and economic consequences for the population of Ugandan women over time.
Fig. 1.
Overview of three-tiered model-based approach. Abbreviations. CC: cervical cancer, CIN: Cervical intraepithelial neoplasia, HPV: Human papillomavirus.
2.2. Simulation models
Harvard-HPV is an agent-based dynamic model of partnership acquisition and HPV transmission that was based on a previous compartmental model of HPV-16/18 transmission [7]. The model allows the interaction of heterosexual men and women guided by individual-level attributes such as HPV infection duration, natural immunity, partner concurrency, number of lifetime partners, and duration of partnership(s) in order to capture both the direct and indirect benefits under alternative HPV vaccination scenarios. In contrast to the previous HPV-16/18 transmission model [7], Harvard-HPV is an individual (i.e., agent-based) model and includes additional stratified HPV genotypes (HPV-16, -18, -31, -33, -45, -52, and -58). As Harvard-HPV requires highly-detailed data on sexual behavior that are limited in the setting of Uganda, we used a version of the model that reflects sexual mixing patterns in the U.S. population (see Technical Appendix). We used Harvard-HPV to generate HPV incidence reductions (including herd immunity) by genotype and age over time associated with alternative HPV vaccination scenarios that served as inputs into Harvard-CC.
Harvard-CC is a static, individual-based (i.e., microsimulation) model that tracks women from age 9 years as they transition through HPV-related health states (i.e., no HPV infection, HPV infection, cervical intraepithelial neoplasia grades 2 and 3, and cervical cancer) until death, either from all causes or cervical cancer after its onset. Monthly transitions depend on age, duration (i.e., time since acquisition of HPV infection or precancer), HPV genotype (HPV-16, -18, -31, -33, -45, -52, and -58, other grouped high-risk types, and grouped low-risk types), and history of HPV infection. Baseline model input parameters were derived from large, empirical studies [8], [9], [10], [11] and adapted to reflect epidemiological outcomes in Uganda such as high-risk HPV prevalence [12], HPV-16/18 type distribution in cancer [13], and age-specific cancer incidence (Kyadondo registry, 2003–2007) [14]; details of the model parameterization process, including calibration and adaptation to the Ugandan setting have been published previously [15], [16]. The HPV incidence reductions from the vaccination scenarios generated in Harvard-HPV were used as inputs into Harvard-CC in order to project HPV-16 and HPV-18 cervical cancer incidence reductions by age associated with alternative HPV vaccination scenarios. Outputs from Harvard-CC then served as inputs into the companion population-based model, Harvard-Scale Up.
As previously described [17], Harvard-Scale Up is a multi-cohort, Excel-based companion model used to capture the health and economic benefits at the population level taking into account changing demographics (e.g., population size, mortality rates) over time. Unlike Harvard-CC described above, Harvard-Scale Up does not track the complex natural history of cervical cancer; rather, the model uses country- or region-specific data on the age-specific incidence of cervical cancer [18] and HPV-16/18 type distribution (i.e. 73%) in cancer [13]. Projected cervical cancer cases and deaths are adjusted for population growth over time. We applied the age-specific cancer incidence reductions over time projected from Harvard-CC to the baseline age-specific cancer incidence rates in Harvard-Scale Up [18] in order to estimate the number of cancer cases and deaths averted, disability-adjusted life years (DALYs) averted, and total economic costs at the population level associated with the alternative HPV vaccination scenarios.
2.3. Strategies
We conducted analyses to evaluate the impact of one-dose HPV vaccination in the context of settings that have not considered an HPV vaccination program (“Scenario A”), and settings that are considering two-dose HPV vaccination (“Scenario B”). In Scenario A, we compared routine one-dose HPV vaccination of 9-year-old girls starting in 2017 to no HPV vaccination. In Scenario B, we compared routine one-dose HPV vaccination to two-dose HPV vaccination of 9-year-old girls.
2.4. Coverage, efficacy and cost inputs
We assumed 70% coverage of the target population of girls only for all vaccination scenarios, but also considered increased coverage to 90% for one-dose vaccination in sensitivity analysis. For all one-dose scenarios, we assumed 80% efficacy against incident HPV-16 and -18 infections, based on the lower-bound target efficacy for one-dose HPV vaccination in an upcoming randomized control trial (ClinicalTrials.gov Identifier: NCT 03180034). Given the unknown duration of one-dose vaccine protection, we assumed 15 years of full protection followed by waning protection at a constant rate over an additional 20 years, but explored waning beginning earlier at 10 years as well as lifetime protection. For two-dose vaccination, we assumed 100% protection against HPV-16 and -18 infections over the lifetime.
We assumed a baseline HPV vaccine cost of $4.50 per dose (for tradeable goods, one international dollar [I$] is equivalent to one US$) [19] as well as a vaccine delivery cost of I$2.40 per dose, which assumes delivery costs are attributed evenly by dose [20]. Cancer costs (in 2011 I$) included the direct (e.g., treatment) and non-direct medical (e.g., transportation) costs, as well as women’s time costs associated with treating invasive cervical cancer in Uganda (i.e., stage I: I$888; stages II-IV: I$1176) [15].
2.5. Outcomes
The primary outcomes of the analysis included the cost of the vaccine program (including dosage and delivery costs), the cost of cervical cancer treatment, and total societal costs in 2011 I$. Health outcomes included the number of cervical cancer cases and DALYs. Costs and DALYs were discounted at 3% per year. Model outcomes were aggregated over multiple birth cohorts to capture the lifetime costs and benefits of women aged 9–50 years in year 2017, as well as nine incoming birth cohorts of 9-year-old girls from years 2018–2026 (i.e., the entire live population of Ugandan women up to age 50 in 2017). Routine vaccination of 9-year-old girls with either dose schedule was assumed to continue indefinitely. Disease costs reflect all costs related to treatment of cervical cancer, as well as cost offsets due to cancer prevention following HPV vaccination.
We calculated the incremental cost-effectiveness ratio (ICER) defined as the additional cost of a particular strategy divided by the additional health benefits (i.e., DALYs averted). Strategies that were either more costly and less effective (‘dominated’), or less costly and less cost-effective (‘extended dominated’), than an alternative strategy were considered inefficient, and removed from further consideration. Remaining strategies were identified as efficient, and those with an ICER below the per-capita gross domestic product (GDP) (Uganda: 2011 I$1603) [21] were considered ‘very cost-effective’ [22].
3. Results
3.1. Health benefits
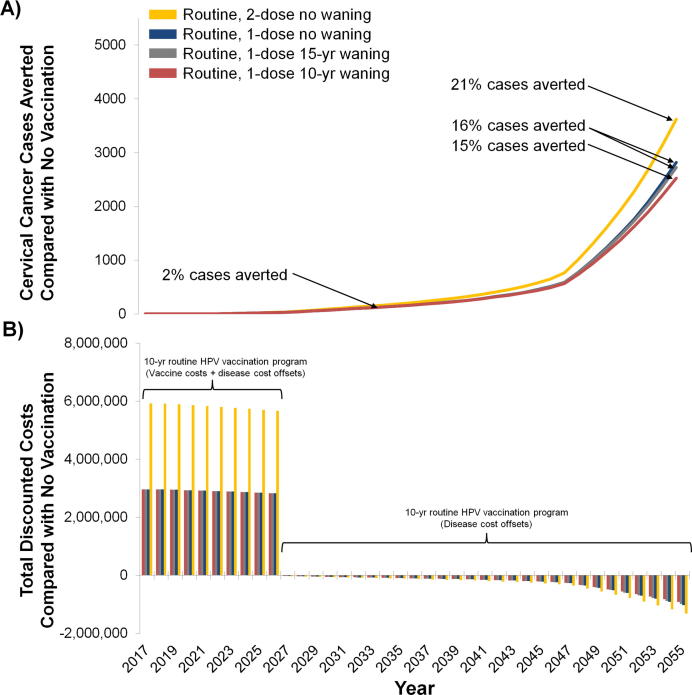
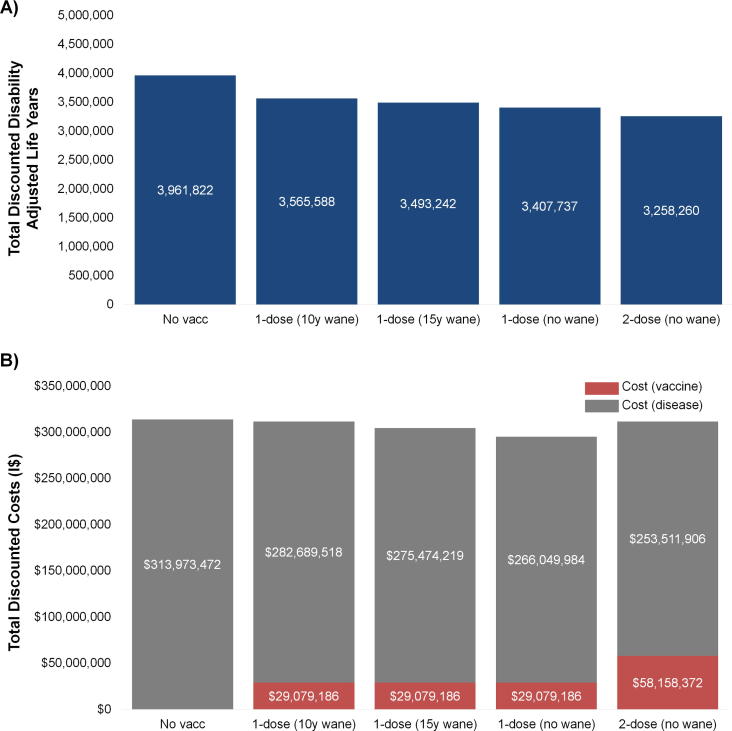
Compared with a scenario of no HPV vaccination, the number of cervical cancer cases averted with routine one-dose HPV vaccination (assuming 80% efficacy and 70% coverage) was projected to increase over time and was greater at longer duration of protection associated with a one-dose schedule (Fig. 2a). Even when including herd immunity benefits to unvaccinated cohorts of men and women, there was a considerable time delay (∼30 years) between initiation of routine 9-year-old HPV vaccination and impact on cervical cancer cases averted at the population level (Fig. 2a). For example, routine one-dose vaccination was projected to avert 2% of cancer cases (i.e. 146–147 cases) in 2035 compared with no vaccination, increasing to 15–16% of cancer cases in 2055 (depending on one-dose waning assumption). In contrast, two-dose HPV vaccination assuming 100% lifelong efficacy was projected to avert 21% of cervical cancer cases in 2055 compared with no vaccination. Compared with routine one-dose HPV vaccination, two-dose vaccination with higher efficacy was projected to result in approximately 150,000–310,000 more DALYs averted (i.e. 5–9% more DALYs averted compared with one-dose) over the lifetime of the analytic cohorts, depending on assumptions regarding the duration of the one-dose vaccine protection (Fig. 3a).
Fig. 2.
Annual number of cervical cancer cases averted (Panel A) and total discounted economic costs (Panel B) associated with one-dose human papillomavirus (HPV) vaccination programs over time, assuming 70% vaccination coverage under alternative waning scenarios compared with no HPV vaccination and two-dose HPV vaccination. Percentage values (Panel A) represent the change in averted cancer cases in the population in years 2035 and 2055 following ten years of routine HPV vaccination, including indirect herd immunity benefits, compared with no HPV vaccination. For each calendar year, cases and costs are aggregated over multiple birth cohorts (i.e., the population of Ugandan women aged <50 years alive in 2017).
Fig. 3.
Total discounted disability-adjusted life years (DALYs) (Panel A) and total discounted economic costs (Panel B) associated with one-dose human papillomavirus (HPV) vaccination at 70% vaccination coverage, cumulative over the lifetimes of women aged <50 years alive in year 2017. Vaccine program costs (red bars) reflect 10 birth cohorts of 9-year-old girls from years 2017–2026 (total 6,893,994 girls) at 70% vaccination coverage. Disease costs (blue bars) reflect disease offsets over the lifetimes of women aged <50 years alive in year 2017.
3.2. Economic outcomes
Ten years of routine one- and two-dose HPV vaccination required substantial upfront investments in vaccine procurement and delivery but resulted in long-term cost offsets from future averted cancer cases (Fig. 2b). For example, the total vaccine-related cost associated with one-dose vaccination with 70% coverage exceeded I$29 million over the first 10-year period, whereas two-dose vaccination cost over I$58 million (Fig. 3b). Compared with no HPV vaccination, the total disease-specific costs were lower under all vaccination programs in Scenarios A and B due to prevented cancer cases, which accrued over time. Of note, routine two-dose HPV vaccination required twice the initial investment of one-dose vaccination, while the cost offsets due to cancer prevention was only marginally higher (an additional 5–10%).
Routine one-dose HPV vaccination of 9-year-old girls provided greater health benefit for less money (i.e., was cost-saving), compared with no vaccination regardless of waning scenario (Table 1, top panel). In contrast, while two-dose vaccination was always more effective than one-dose vaccination, the incremental cost of two doses versus one dose depended on the duration of one-dose protection (Table 1, bottom panel). For example, two-dose vaccination was more costly than one-dose vaccination when we assumed one-dose protection did not wane or began to wane at 15 years; despite the higher cost, two-dose vaccination had an ICER below Uganda’s per-capita GDP. When one-dose waning was assumed to begin earlier at 10 years, two-dose vaccination was less costly and more beneficial (i.e., was cost-saving) compared to one-dose vaccination.
Table 1.
Discounteda incremental costs, disability-adjusted life years (DALYs) averted and cost-effectiveness of one-doseb HPV vaccination versus no HPV vaccination (Scenario A) and two-dosec HPV vaccination versus one-dose HPV vaccination (Scenario B), assuming 70% vaccination coverage and alternative one-dose waning scenarios.
| Scenario A | Incremental costd | Incremental DALYs avertede | Cost (I$) per DALY averted (1-dose vs no vaccination) |
|---|---|---|---|
| – 1-dose (no wane) vs. No vaccination | −$18,844,302 | 554,085 | Cost-savingf |
| – 1-dose (15y wane) vs. No vaccination | −$9,420,067 | 468,581 | Cost-savingf |
| – 1-dose (10y wane) vs. No vaccination | −$2,204,767 | 396,234 | Cost-savingf |
| Scenario B | Incremental costd | DALYs avertede | Cost (I$) per DALY averted (2-dose vs 1-dose) |
| – 2-dose (no wane) vs. 1-dose (no wane) | $16,541,108 | 149,477 | $111 g |
| – 2-dose (no wane) vs. 1-dose (15y wane) | $7,116,873 | 234,982 | $30 g |
| – 2-dose (no wane) vs. 1-dose (10y wane) | −$98,427 | 307,328 | Cost-savingf |
Costs and DALYs discounted at 3% per year.
One-dose HPV vaccine efficacy of 80%.
Two-dose HPV vaccine efficacy of 100% and lifelong durability.
Incremental costs reflect vaccine program costs associated with 10 incoming birth cohorts of 9-year-old girls from years 2017–2026 (total 6,893,944 girls) at 70% vaccination coverage, and disease cost offsets over the lifetimes of women aged <50 years alive in year 2017.
Incremental DALYs averted are aggregated over multiple birth cohorts and capture the benefit over the lifetimes of women aged <50 years alive in year 2017.
Cost-saving interventions provide greater health benefit for less money than the comparator.
Considered ‘very cost-effective’ as the incremental cost-effectiveness ratio is less than the gross domestic product per capita in Uganda (i.e., I$1603). Abbreviations. HPV: Human papillomavirus; ICER: Incremental cost-effectiveness ratio.
3.3. Sensitivity analysis
When we explored potential improvements in coverage associated with one-dose vaccination (i.e., 90% coverage) compared with two-dose vaccination at base case (i.e., 70%) coverage, we found that a higher vaccination coverage with the less efficacious one-dose vaccination achieved near-equivalent (i.e., 99%) health benefits in terms of DALYs averted relative to two-dose vaccination, if one-dose vaccine protection did not wane (Table 2). Under these assumptions, one-dose vaccination was cost-saving, and two-dose vaccination exceeded the Ugandan per-capita GDP threshold. However, although one-dose vaccination with higher coverage remained cost-saving regardless of waning assumption, two-dose vaccination was considered ‘very cost-effective’ when one-dose protection waned at 10 or 15 years.
Table 2.
Sensitivity analysis evaluating one-dose routine HPV vaccination programs with higher coverage (90%) compared with two-dose lower vaccination coverage (70%) by one-dose waning scenarios.
| Discounted costs (I$)a | Discounted DALYsa | ||||
|---|---|---|---|---|---|
| No waning (One-dose) | Total cost | Incremental costs | Total DALYs | Incremental DALYs averted | Cost (I$) per DALY averted |
| No vaccination | $313,973,472 | – | 3,961,822 | – | – |
| 1-dose vaccination (90% cov) | $291,639,143 | −$22,334,328 | 3,268,629 | 693,193 | Cost-savingb |
| 2-dose vaccination (70% cov) | $311,670,277 | $20,031,134 | 3,258,260 | 10,369 | $1932 |
| 15-year waning (One-dose) | |||||
| No vaccination | $313,973,472 | – | 3,961,822 | – | – |
| 1-dose vaccination (90% cov) | $301,497,401 | −$12,476,071 | 3,358,907 | 602,916 | Cost-savingb |
| 2-dose vaccination (70% cov) | $311,670,277 | $10,172,877 | 3,258,260 | 100,647 | $101c |
| 10-year waning (One-dose) | |||||
| No vaccination | $313,973,472 | – | 3,961,822 | – | – |
| 1-dose vaccination (90% cov) | $310,297,455 | −$3,676,016 | 3,447,321 | 514,502 | Cost-savingb |
| 2-dose vaccination (70% cov) | $311,670,277 | $1,372,822 | 3,258,260 | 189,060 | $7c |
Costs and DALYs discounted at 3% per year.
Cost-saving interventions provide greater health benefit for less money than the comparator.
Considered ‘very cost-effective’ as the incremental cost-effectiveness ratio is less than the gross domestic product per capita in Uganda (i.e., I$1603). Abbreviations: HPV: Human papillomavirus.
4. Discussion
Using a model-based approach that incorporates HPV transmission dynamics, cervical cancer disease natural history, and population demographics, we projected that one-dose HPV vaccination assuming 80% efficacy against HPV-16/18 infections would provide substantial population health benefits compared with no vaccination, averting between 400,000 and 550,000 DALYs over the lifetimes of the current population of Ugandan women under age 50. In settings where two-dose HPV vaccination may be feasible, considering a one-dose schedule would require explicit tradeoffs between health and economic consequences (i.e., lower costs but also lower health benefit). However, we found that the lower efficacy of a one-dose schedule could nearly be compensated for by improved coverage if one-dose vaccination does not wane. To our knowledge, our study is the first to assess the value of one-dose HPV vaccination exploring the effects of reduced efficacy, under scenarios of vaccine coverage and durability in a low-income setting. Consistent with previous analyses that explored reduced-dose regimens of HPV vaccination [23], [24], [25], [26], this analysis highlights the importance of the duration of one-dose vaccine protection, particularly compared with a scenario of a highly efficacious two-dose vaccination program. Notably, we found that despite using a simulation model that captures the clearance and progression of HPV as a function of time since infection rather than age, health benefits and cost-effectiveness diminished considerably with decreased duration of vaccine protection, which is a major source of uncertainty given the lack of empirical data currently available.
Although both one- and two-dose schedules would provide ‘good value for money’ (i.e., were cost-saving or very cost-effective) under the majority of scenarios we considered, the cost-effectiveness results do not account for the budget impact and the ability of countries to pay for vaccine procurement and delivery. Even high-value interventions, such as HPV vaccination, may not be affordable [27]. Even under favorable vaccine cost assumptions (i.e., I$6.90 per delivered vaccine dose), significant upfront investments would be required for a one-dose vaccination program, which would be even greater for a two-dose program (Fig. 2b).
There are several limitations to this analysis. Due to limited data on sexual behavior in Uganda, we used a dynamic model that reflects HPV transmission according to sexual behavior patterns in the U.S. population. To the extent that sexual behaviors are different across settings, the herd immunity benefits we projected may not be generalizable to Uganda or other settings. However, initial explorations indicate that the error introduced by not including any herd immunity was greater than when including herd immunity under various scenarios of sexual mixing; we therefore elected to include some measure of herd immunity even using data from a different setting. Exploring the implications of these assumptions is a priority for ongoing work.
This analysis captured the costs of the vaccination program for only the first ten years and captured the disease costs and health benefits over the lifetimes of women alive up to age 50 years in 2017. Costs and population-level impact of HPV vaccination will likely continue to increase as additional adolescent girls are vaccinated; however, we only captured a limited time horizon of vaccination costs and health effects given likely changes in available technologies and policies, and assumed that cervical cancer incidence rates were stable over this time period (although population dynamics were included).
We did not explore alternative levels of efficacy for one-dose vaccination other than our assumption of 80%, considered a lower-bound estimate. We also did not include efficacy against high-risk HPV types other than HPV-16/18 (i.e., cross-protection). Our assumption of immediate roll-out to 70% coverage is optimistic, and slower implementation and scale-up would delay the expected benefits projected in the current analysis.
Due to limited data, we did not include program costs for increasing coverage. As a result, the findings for these scenarios should be interpreted with caution, particularly if the delivery costs associated with expanding the vaccination program increase with higher coverage or if costs for delivering a second dose are different than delivering the first. We also assumed that any ongoing screening programs did not change under the HPV vaccination scenarios. We note that scale-up of screening programs may represent a synergistic and high-value approach to cervical cancer prevention. Finally, we did not evaluate the impact HPV vaccination may have on non-cervical cancers in women and men, which would increase the value of all HPV vaccination programs; however, there is even greater uncertainty of one-dose efficacy on these cancers. For similar reasons, we did not include scenarios of male vaccination or take into explicit consideration the effect of HIV co-infection. Analyses will be updated once more information is available.
5. Conclusions
For countries that have yet to adopt an HPV vaccination program, our models predicted that one-dose vaccination results in cost-savings compared with no HPV vaccination given the downstream cost offsets from averting cervical cancer; however, even this high-value program would require significant upfront investments. For countries considering a two-dose schedule, two-dose vaccination represented ‘good value for money’, unless one-dose vaccination could achieve higher coverage and provide lifelong protection. Continued exploration of the potential differences in vaccine properties, as well as delivery and scale-up strategies, between one and two doses will be important as data continue to emerge. Ultimately, the type of HPV vaccination program a country selects will depend on multiple factors, including feasibility, acceptability, affordability, and value for money.
Funding
This work was based on research funded in part by the Bill & Melinda Gates Foundation. The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of the Bill & Melinda Gates Foundation. The funders had no role in study design; data collection, analysis, and interpretation; preparation of the manuscript; or decision to submit the article for publication.
Conflicts
The authors have no conflicts to declare.
Author contributions
EAB, NC and JJK contributed to the conception and design of the study; all authors contributed to the analysis and interpretation of the data. EAB drafted the manuscript and all authors contributed to revising the manuscript for critically important content. All authors have approved the final article.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.vaccine.2018.04.061.
Appendix A. Supplementary material
References
- 1.Schiffman M., Doorbar J., Wentzensen N., de Sanjose S., Fakhry C., Monk B.J. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers. 2016;2:16086. doi: 10.1038/nrdp.2016.86. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization & International Agency for Research on Cancer. IARC Monographs on the evaluation of carcinogenic risks to humans; 2007.
- 3.World Health Organization. Weekly epidemiological record; 2017. p. 241–68.
- 4.Safaeian M., Sampson J., Pan Y. Durability of protection afforded by fewer doses of the HPV16/18 vaccine: the CVT trial. J Natl Cancer Inst. 2018;110(2) doi: 10.1093/jnci/djx158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sankaranarayanan R., Prabhu P.R., Pawlita M., Gheit T., Bhatla N., Muwonge R. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncol. 2016;17:67–77. doi: 10.1016/S1470-2045(15)00414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kreimer A.R., Struyf F., Del Rosario-Raymundo M.R., Hildesheim A., Skinner S.R., Wacholder S. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA trials. Lancet Oncol. 2015;16:775–786. doi: 10.1016/S1470-2045(15)00047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J.J., Goldie S.J. Health and economic implications of HPV vaccination in the United States. New England J Med. 2008;359:821–832. doi: 10.1056/NEJMsa0707052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munoz N., Mendez F., Posso H., Molano M., van den Brule A.J., Ronderos M. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis. 2004;190:2077–2087. doi: 10.1086/425907. [DOI] [PubMed] [Google Scholar]
- 9.Herrero R., Hildesheim A., Rodriguez A.C., Wacholder S., Bratti C., Solomon D. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine. 2008;26:4795–4808. doi: 10.1016/j.vaccine.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCredie M.R., Sharples K.J., Paul C., Baranyai J., Medley G., Jones R.W. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9:425–434. doi: 10.1016/S1470-2045(08)70103-7. [DOI] [PubMed] [Google Scholar]
- 11.Sankaranarayanan R., Swaminathan R., Brenner H., Chen K., Chia K.S., Chen J.G. Cancer survival in Africa, Asia, and Central America: a population-based study. Lancet Oncol. 2010;11:165–173. doi: 10.1016/S1470-2045(09)70335-3. [DOI] [PubMed] [Google Scholar]
- 12.Jeronimo J., Bansil P., Lim J., Peck R., Paul P., Amador J.J. A multicountry evaluation of careHPV testing, visual inspection with acetic acid, and papanicolaou testing for the detection of cervical cancer. Int J Gynecol Cancer: Off J Int Gynecol Cancer Soc. 2014;24:576–585. doi: 10.1097/IGC.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan P., Howell-Jones R., Li N., Bruni L., de Sanjose S., Franceschi S. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer. 2012;131:2349–2359. doi: 10.1002/ijc.27485. [DOI] [PubMed] [Google Scholar]
- 14.Forman D., Bray F., Brewster D., Gombe Mbalawa C., Kohler B., Piñeros M. Vol IX. International Agency for Research on Cancer; Lyon, France: 2013. (Cancer Incidence in Five Continents). [Google Scholar]
- 15.Campos N.G., Tsu V., Jeronimo J., Mvundura M., Lee K., Kim J.J. When and how often to screen for cervical cancer in three low- and middle-income countries: a cost-effectiveness analysis. Papillomavirus Res. 2015;1:38–58. [Google Scholar]
- 16.Campos N.G., Burger E.A., Sy S., Sharma M., Schiffman M., Rodriguez A.C. An updated natural history model of cervical cancer: derivation of model parameters. Am J Epidemiol. 2014;180:545–555. doi: 10.1093/aje/kwu159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldie S.J., O'Shea M., Campos N.G., Diaz M., Sweet S., Kim S.Y. Health and economic outcomes of HPV 16,18 vaccination in 72 GAVI-eligible countries. Vaccine. 2008;26:4080–4093. doi: 10.1016/j.vaccine.2008.04.053. [DOI] [PubMed] [Google Scholar]
- 18.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 cancer incidence and mortality worldwide: IARC cancerbase No. 11. Lyon, France: International Agency for Research on Cancer; 2013.
- 19.Gavi TVA. Human Papillomavirus (HPV) Vaccine Documents: Detailed Product Profiles; 2017.
- 20.Botwright S., Holroyd T., Nanda S., Bloem P., Griffiths U.K., Sidibe A. Experiences of operational costs of HPV vaccine delivery strategies in Gavi-supported demonstration projects. PloS One. 2017;12:e0182663. doi: 10.1371/journal.pone.0182663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Bank. World development indicators. http://databank.worldbank.org/data/reports.aspx?source=world-development-indicators; 2011 [accessed 08.02.18].
- 22.World Health Organization . World Health Organization; Geneva: 2001. Macroeconomics and health: investing in health for economic development: report of the commission on macroeconomics and health. [Google Scholar]
- 23.Jit M, Brisson M, Laprise J-F, Choi YH. Comparison of two dose and three dose human papillomavirus vaccine schedules: cost effectiveness analysis based on transmission model. BMJ: British Med J 2015; 350. [DOI] [PMC free article] [PubMed]
- 24.Laprise J.F., Markowitz L.E., Chesson H.W., Drolet M., Brisson M. Comparison of 2-dose and 3-dose 9-valent human papillomavirus vaccine schedules in the United States: a cost-effectiveness analysis. J Infect Dis. 2016;214:685–688. doi: 10.1093/infdis/jiw227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laprise J.F., Drolet M., Boily M.C., Jit M., Sauvageau C., Franco E.L. Comparing the cost-effectiveness of two- and three-dose schedules of human papillomavirus vaccination: a transmission-dynamic modelling study. Vaccine. 2014;32:5845–5853. doi: 10.1016/j.vaccine.2014.07.099. [DOI] [PubMed] [Google Scholar]
- 26.Jit M., Choi Y.H., Laprise J.F., Boily M.C., Drolet M., Brisson M. Two-dose strategies for human papillomavirus vaccination: how well do they need to protect? Vaccine. 2014;32:3237–3242. doi: 10.1016/j.vaccine.2014.03.098. [DOI] [PubMed] [Google Scholar]
- 27.Marseille E., Larson B., Kazi D.S., Kahn J.G., Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93:118–124. doi: 10.2471/BLT.14.138206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.