Abstract
BACKGROUND
Worsening renal function (WRF) in the setting of aggressive diuresis for acute heart failure treatment may reflect renal tubular injury or simply indicate a hemodynamic or functional change in glomerular filtration. Well-validated tubular injury biomarkers, N-acetyl-β-D-glucosaminidase, neutrophil gelatinase-associated lipocalin, and kidney injury molecule 1, are now available that can quantify the degree of renal tubular injury. The ROSE-AHF trial (Renal Optimization Strategies Evaluation–Acute Heart Failure) provides an experimental platform for the study of mechanisms of WRF during aggressive diuresis for acute heart failure because the ROSE-AHF protocol dictated high-dose loop diuretic therapy in all patients. We sought to determine whether tubular injury biomarkers are associated with WRF in the setting of aggressive diuresis and its association with prognosis.
METHODS
Patients in the multicenter ROSE-AHF trial with baseline and 72-hour urine tubular injury biomarkers were analyzed (n=283). WRF was defined as a ≥ 20% decrease in glomerular filtration rate estimated with cystatin C.
RESULTS
Consistent with protocol-driven aggressive dosing of loop diuretics, participants received a median 560 mg IV furosemide equivalents (interquartile range, 300–815 mg), which induced a urine output of 8425 mL (interquartile range, 6341–10 528 mL) over the 72-hour intervention period. Levels of N-acetyl-β-D-glucosaminidase and kidney injury molecule 1 did not change with aggressive diuresis (both P>0.59), whereas levels of neutrophil gelatinase-associated lipocalin decreased slightly (−8.7 ng/mg; interquartile range, −169 to 35 ng/mg; P<0.001). WRF occurred in 21.2% of the population and was not associated with an increase in any marker of renal tubular injury: neutrophil gelatinase-associated lipocalin (P=0.21), N-acetyl-β-D-glucosaminidase (P=0.46), or kidney injury molecule 1 (P=0.22). Increases in neutrophil gelatinase-associated lipocalin, N-acetyl-β-D-glucosaminidase, and kidney injury molecule 1 were paradoxically associated with improved survival (adjusted hazard ratio, 0.80 per 10 percentile increase; 95% confidence interval, 0.69–0.91; P=0.001).
CONCLUSIONS
Kidney tubular injury does not appear to have an association with WRF in the context of aggressive diuresis of patients with acute heart failure. These findings reinforce the notion that the small to moderate deteriorations in renal function commonly encountered with aggressive diuresis are dissimilar from traditional causes of acute kidney injury.
Keywords: acute kidney injury, biomarkers, heart failure, renal insufficiency
Worsening renal function (WRF) commonly complicates decongestion of patients hospitalized with acute heart failure (AHF) and affects key therapeutic decisions such as continued attempts at aggressive diuresis and neurohormonal blockade.1 Furthermore, WRF has been associated with adverse long-term outcomes; several recent clinical trials have even used it as a primary end point.2–4 Whereas acute tubular injury/necrosis is a prominent mechanism in settings of insults such as exposure to iodinated contrast, severe sepsis, and nephrotoxic medications, the mechanistic underpinnings of WRF in the setting of aggressive diuresis remain incompletely understood.5–7
There is growing consensus that understanding the pathogenesis and mechanisms driving increases in creatinine, which practice guidelines have lumped into a syndrome called acute kidney injury, is critically important in defining its clinical significance and treatment approach.8,9 Paradoxically, despite having “injury” in its name, true renal injury is not a requisite mechanism for a change in creatinine to be called acute kidney injury. This distinction may be of practical importance because changes in creatinine induced by aggressive decongestion may be largely functional or hemodynamic and thus, by inference, clinically benign. Well-validated kidney tubular injury biomarkers such as neutrophil gelatinase-associated lipocalin (NGAL), N-acetyl-β-D-glucosaminidase (NAG), and kidney injury molecule 1 (KIM-1) that can both detect and quantify the degree of tubular damage are now available.10–12 In animal models, NGAL, KIM-1, and NAG provide exquisite discrimination for histopathological severity of kidney tubular injury, whether induced by nephrotoxins or ischemia/reperfusion injury.11–15 Furthermore, compelling data exist in support of their ability to quantify the degree of tubular injury in patients with traditional stimuli for acute kidney injury such as contrast exposure or cardiopulmonary bypass.11,16,17
Thus far, several single-center studies have examined the role of kidney tubular injury biomarkers in AHF; however, because of their small size, lack of serial measurements, and absence of protocol-driven aggressive diuresis, a consistent signal has not emerged.18–20 The recently published multicenter ROSE-AHF clinical trial (Renal Optimization Strategies Evaluation–Acute Heart Failure), however, provides an ideal experimental platform for the study of the determinants of WRF during aggressive diuresis.4 First, the ROSE-AHF trial was unique in its use of very high diuretic doses to cause brisk decongestion: per protocol, all patients received 2.5 times their home dose of loop diuretics as background therapy, regardless of randomization. This resulted in 8.4 L urine during the 72-hour study period in the placebo arm. Of note, this dose of diuretics was in accordance with the “high-dose” intervention tested in the DOSE trial (Diuretic Optimization Strategies Evaluation), in which it was shown to induce a significantly greater incidence of WRF without worsening clinical outcomes.2,21,22 Second, the coprimary end points of the ROSE-AHF trial focused entirely on key cardiorenal parameters of interest, cumulative urine volume and change in serum cystatin C, making the trial uniquely suited to address our hypothesis. Third, the trial enrolled only patients with an estimated glomerular filtration rate (eGFR) <60 mL · min−1 · 1.73 m−2, a population that is at a high risk for kidney injury. Last, determination of urine levels of kidney injury biomarkers was an a priori ancillary study of the ROSE-AHF trial.
Therefore, using the ROSE-AHF trial as our experimental platform, we aimed in this investigation to determine whether kidney tubular injury is a predominant cause of WRF in the setting of aggressive diuresis and to examine whether the degree of injury can risk-stratify patients experiencing WRF.
METHODS
Data Availability Statement
The data, analytical methods, and study materials are available to other researchers for purposes of reproducing the results via the Biological Specimen and Data Repository Information Coordinating Center (BioLINCC).23
Patient Population
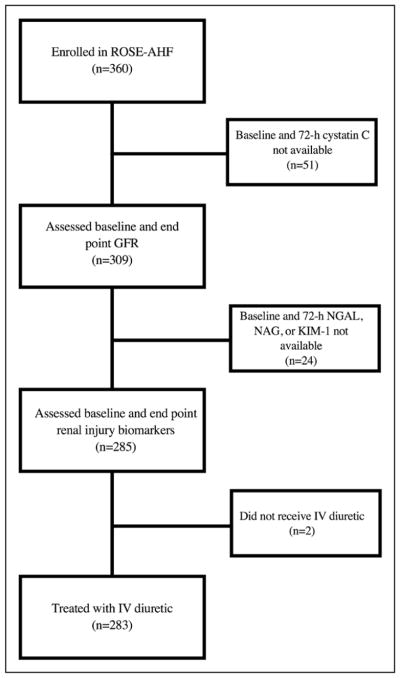
The rationale and design of the ROSE-AHF trial have been previously described.4 The overall study comprised 360 patients who were hospitalized for the treatment of AHF who had renal dysfunction, defined as an eGFR of 15 to 60 mL · min−1 · 1.73 m−2. The diagnosis of AHF was based on at least 1 symptom (dyspnea, orthopnea, or edema) and 1 sign of heart failure (HF) (rales, edema, ascites, or pulmonary vascular congestion on chest radiography), regardless of ejection fraction. All patients received open-label, intravenous loop diuretic treatment with a recommended total daily dose equal to 2.5 times the total daily oral outpatient furosemide (or equivalent) dose at 7 days before admission. Patients naive to outpatient loop diuretics received furosemide at 80 mg/d IV. Half the total daily diuretic dose was administered as a bolus twice daily for at least 24 hours. The 2 coprimary end points were the 72-hour cumulative urine volume as an index of diuresis and the change in cystatin C from randomization to 72 hours as a measure of renal function preservation. The study found no differences in the above end points among study drug groups (placebo, low-dose dopamine, low-dose nesiritide), and there were no differences in the change in NGAL, NAG, or KIM-1 between study groups (P>0.23 for all). Thus, the present study analyzed the cohort collectively. All patients had a telephone assessment of vital status and rehospitalization at 60 and 180 days from randomization. Figure 1 shows a consort diagram of the present study. We excluded patients without baseline or 72-hour cystatin C or urine kidney injury markers and those who did not receive intravenous diuretics. This left 283 patients whose data were used for the present analysis. The ROSE-AHF study was conducted within the National Heart, Lung, and Blood Institute–sponsored Heart Failure Clinical Trials Network. The protocol for the study was approved by the institutional review boards at each participating site, and written informed consent was obtained from all patients before randomization. This article was prepared with ROSE-AHF research materials obtained directly from the National Heart, Lung, and Blood Institute BioLINCC.
Figure 1. CONSORT (Consolidated Standards of Reporting Trials) diagram of patient selection into the study cohort.
GFR indicates glomerular filtration rate; KIM-1, kidney injury molecule 1; NAG, N-acetyl-β-D-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; and ROSE-AHF, Renal Optimization Strategies Evaluation.
Measurement of Biomarkers
As previously described, patients had plasma creatinine, cystatin C, and NT-proBNP (N-terminal pro-B-type natriuretic peptide) levels determined at a core laboratory (Heart Failure Clinical Research Network Core Biomarker Laboratory, University of Vermont).4 Twenty-four–hour urine collections for volume and sodium, and spot urine samples for kidney tubular injury biomarkers, were performed daily for the 72-hour study intervention period. Urinary KIM-1 and NGAL were measured with microbead-based assays at the Brigham and Women’s Hospital, as previously described.12,21,24 Specifically, urine samples were incubated with microbeads that were coupled with NGAL (Enzo Lifesciences) and KIM-1 (R&D Systems) antibodies and quantified with the Bio-Plex 200 system (Bio-Rad). Urinary NAG was measured with the NAG kit per the manufacturer’s (Roche Diagnostics) instructions. Because patients were receiving high doses of diuretics and urine dilution could be substantial, all urine biomarker levels were indexed to urine creatinine to address variability in urinary dilution.
Statistical Analysis and End Points
Baseline characteristics are presented as median (quartile 1–3) or percentiles. eGFR was calculated with the cystatin-based and creatinine-based Chronic Kidney Disease Epidemiology formulas.25 To maintain consistency with prior publications, WRF was defined as a ≥ 20% reduction in eGFR from baseline to 72 hours.26 Relationships between the absolute change in cystatin C/creatinine and change in biomarker levels are presented graphically. The changes in tubular injury biomarkers (KIM-1, NGAL, NAG) were not normally distributed, had many extreme outliers, and frequently had negative values. We therefore performed a rank-based correlation between the change (Δ) in eGFR and in renal tubular injury biomarkers and report them as the Spearman ρ. For survival analysis, we took a similar rank-based approach and generated a percentile rank for the change in each biomarker for which the patient with the largest improvement in biomarker levels had a rank of 0, and the largest worsening had a rank of 100. Linearity of the relationship between mortality and change in biomarker was confirmed by examining trends across deciles of the change in biomarkers. A composite biomarker score was generated that took the average of the percentile rank for each of the 3 biomarkers. Using Cox proportional hazards modeling, we evaluated the risk of death through 180 days in patients with combinations of WRF and change in kidney tubular injury biomarkers using the above percentile rank variables. Comprehensive analyses adjusted for the following risk factors: age, sex, race, heart rate, systolic blood pressure, atrial arrhythmias, diabetes mellitus, ischemic HF pathogenesis, left ventricular ejection fraction, angiotensin-converting enzyme/angiotensin receptor blocker use, β-blocker use, aldosterone antagonist use, baseline chloride, sodium, blood urea nitrogen, eGFR, NT-proBNP, ΔNT-proBNP, 72-hour urine output, weight change, cumulative diuretic dose, and baseline kidney tubular injury biomarker levels. Statistical analysis was performed with IBM SPSS Statistics version 23 (IBM Corp, Armonk, NY), and statistical significance was defined as 2-tailed value of P<0.05 for all analyses except tests for interaction, for which P<0.01 was considered significant.
RESULTS
Baseline characteristics of the analyzed study population with complete data on renal tubular injury biomarkers (n=283) are described in Table 1. This subgroup mirrored the overall ROSE-AHF trial in terms of key HF characteristics: the patients tended to be white men with ischemic cardiomyopathy, multiple comorbid conditions, and a high prevalence of physical examination findings consistent with volume overload.
Table 1.
Baseline Characteristics of the Study Population
| Characteristics | Study Cohort (n=283) | WRF (n=60) | No WRF (n=223) | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 70 (62, 79) | 73 (63, 82) | 69 (62, 79) | 0.14 |
| Male sex, % | 75 | 73 | 75 | 0.81 |
| White, % | 76 | 83 | 74 | 0.13 |
| Clinical variables | ||||
| SBP, mmHg | 114 (103, 126) | 119 (109, 134) | 113 (102, 125) | 0.02* |
| Edema ≥ 2+, % | 71 | 77 | 69 | 0.26 |
| Orthopnea, % | 89 | 95 | 88 | 0.13 |
| JVP ≥ 8 cm H2O, % | 96 | 97 | 95 | 0.68 |
| Rales, % | 55 | 60 | 54 | 0.38 |
| HF hospitalization, % | 67 | 61 | 69 | 0.25 |
| LVEF, % | 33 (20, 51) | 34 (25, 53) | 30 (20, 51) | 0.40 |
| LVEF <50%, % | 71 | 75 | 70 | 0.43 |
| IHD, % | 58 | 65 | 57 | 0.24 |
| DM type 2, % | 55 | 58 | 54 | 0.57 |
| AF/AFL, % | 58 | 63 | 57 | 0.34 |
| ICD, % | 45 | 37 | 47 | 0.15 |
| Baseline medications | ||||
| ACE-I/ARB, % | 50 | 57 | 48 | 0.26 |
| β-Blocker, % | 85 | 83 | 85 | 0.79 |
| Hydralazine, % | 20 | 28 | 18 | 0.08 |
| Nitrates, % | 25 | 38 | 22 | 0.01* |
| Aldosterone antagonist, % | 28 | 18 | 30 | 0.07 |
| Digoxin, % | 26 | 22 | 27 | 0.41 |
| Loop diuretic, % | 95 | 88 | 96 | 0.02* |
| Diuretic dose, mg | 100 (60, 160) | 120 (80, 190) | 100 (60, 160) | 0.70 |
| Laboratory values | ||||
| Cystatin C, mg/L | 1.70 (1.41, 2.15) | 1.59 (1.41, 2.03) | 1.73 (1.41, 2.17) | 0.15 |
| Creatinine, mg/dL | 1.63 (1.32,1.97) | 1.48 (1.26, 1.85) | 1.67 (1.38, 1.98) | 0.05* |
| eGFR, mL · min−1 · 1.73 m−2 | 44 (33, 56) | 47 (37, 58) | 44 (33, 54) | 0.18 |
| BUN, mg/dL | 37 (28, 50) | 33 (25, 44) | 38 (28, 54) | 0.02* |
| NT-proBNP, pg/mL | 5268 (230, 10 348) | 5918 (1800, 9855) | 5249 (2371, 10 422) | 0.57 |
| NGAL, ng/mg · uCR | 68 (16, 443) | 64 (20, 252) | 73 (15, 555) | 0.69 |
| NAG, mU/mg · uCR | 8.9 (5.2, 17.4) | 8.1 (4.7, 12.3) | 9.0 (5.5, 18.1) | 0.31 |
| KIM-1, pg/mg · uCR | 960 (334, 3181) | 1118 (324, 2721) | 872 (368, 3220) | 0.54 |
Values are median (interquartile range) or n (%). ACE-I indicates angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; AFL, atrial flutter; ARB, angiotensin receptor blocker; BUN, blood urea nitrogen; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter-defibrillator; IHD, ischemic heart disease; JVP, jugular venous pressure; KIM-1, kidney injury molecule 1; LVEF, left ventricular ejection fraction; NAG, N-acetyl-β-D-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SBP, systolic blood pressure; and WRF, worsening renal function.
Significant.
Worsening Renal Function
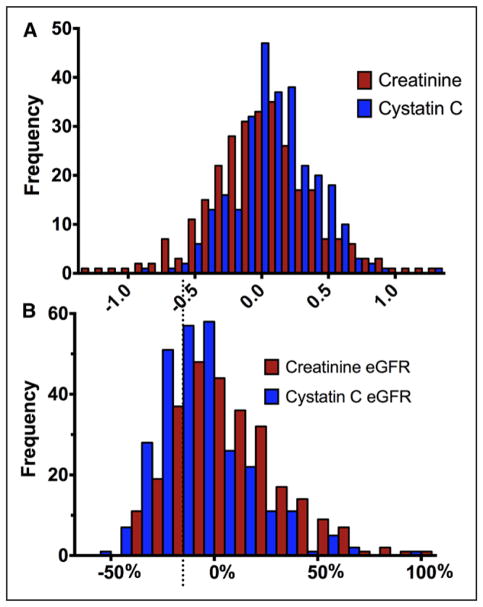
Baseline characteristics of patients with (n=60, 21.2%) and without (n=223, 78.8%) WRF from randomization to 72 hours are presented in Table 1. The distribution of change in renal function is depicted in Figure 2, which demonstrates that severe deterioration in renal function was uncommon, and most WRF events represented small to moderate-sized changes in renal function. Similar to the overall ROSE-AHF trial, there were no differences in the change in cystatin C (P=0.22) or incidence of WRF (P=0.42) between randomized interventions (dopamine, nesiritide, placebo) in the present subset. Overall, baseline characteristics of those with and without WRF were similar aside from a tendency for somewhat better kidney function in patients who would ultimately experience WRF. Consistent with protocol-driven aggressive dosing of loop diuretics in the trial, patients received a median 560 mg furosemide equivalents (interquartile range, 300–815 mg), which induced a median urine output of 8425 mL (interquartile range, 6341–10 528 mL) over the 72-hour intervention period. Both diuretic dose and fluid output were similar between patients with and without WRF (P>0.18 for both).
Figure 2. Absolute or relative changes in kidney function.
Change in kidney function assessed from baseline to 72 hours. A, Absolute change in renal function with units of milligrams per deciliter (creatinine) and milligrams per liter (cystatin C). B, Relative change in estimated glomerular filtration rate (eGFR) calculated with either cystatin C or creatinine. Dotted line represents those patients with 20% increase in eGFR.
Changes in Kidney Tubular Injury Biomarkers
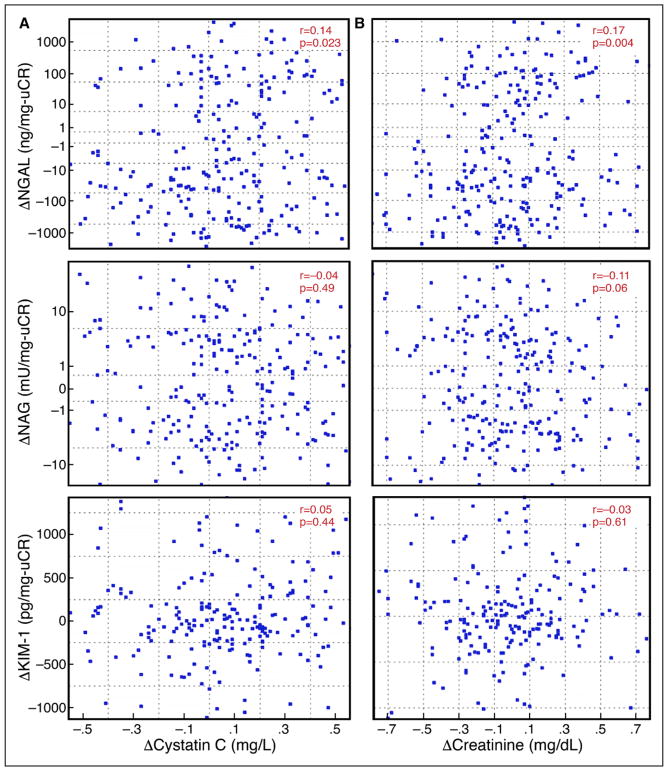
Baseline levels of KIM-1, NGAL, and NAG were similar among patients with and without WRF (Table 1). Furthermore, in-hospital and treatment-related parameters were largely similar between patients with and without increases in tubular injury biomarkers (Table 2). Similarly, from randomization to 72 hours, levels of KIM-1 and NAG did not change in the overall population (P>0.59 for both), but levels of NGAL tended to decrease (−8.7 ng/mg; interquartile range, −169 to 35 ng/mg; P<0.001). NGAL demonstrated a statistically significant but very small-magnitude correlation with the change in cystatin C (r=0.14, P=0.02; Figure 3A). There was no correlation between the change in NAG (r=−0.04, P=0.49; Figure 3A) or KIM-1 (r=0.05, P=0.42; Figure 3A) with the change in cystatin C. Similar results were found for the change in creatinine (rather than cystatin C) with the above biomarkers (Figure 3B). There were no correlations between the change in tubular injury biomarkers and metrics of diuresis and decongestion (Table 3).
Table 2.
Day 3 Characteristics of the Study Population by Number of Elevated Renal Tubular Injury Biomarkers
| Characteristics | Overall Cohort (n=283) | 0 ↑ Biomarkers (n=65) | 1 ↑ Biomarkers (n=92) | 2 ↑ Biomarkers (n=82) | 3 ↑ Biomarkers (n=44) | P for Trend |
|---|---|---|---|---|---|---|
| Diuresis | ||||||
| ΔWeight, lb | −6.8 (−11.7, −3.1) | −6.8 (−10.4, −2.4) | −8.4 (−13.1, −3.6) | −6.2 (−12.0, −2.8) | −6.2 (−10.4, −3.1) | 0.89 |
| Cumulative urine sodium, mmol | 475 (308, 699) | 472 (300, 648) | 511 (309, 770) | 515 (308, 744) | 409 (305, 551) | 0.79 |
| Cumulative urine output, mL | 8425 (6340, 10528) | 7865 (6584, 9905) | 8750 (6300, 10641) | 8750 (6635, 11019) | 8500 (6069, 10758) | 0.55 |
| Cumulative diuretic dose, mg | 560 (300, 815) | 435 (240, 748) | 560 (320, 795) | 539 (240, 914) | 738 (499, 884) | 0.005* |
| Diuretic efficiency | −360 (−590, −183) | −402 (−765, −249) | −366 (−611, −251) | −364 (−728, −174) | −217 (−388, −128) | 0.001* |
| Clinical variables | ||||||
| SBP, mmHg | 107 (96, 120) | 106 (99, 122) | 105 (94, 120) | 108 (97, 120) | 107 (97, 121) | 0.79 |
| ΔSBP | −7 (−19, 4) | −9 (−17, 3) | −7 (−18, 3) | −7 (−20, 3) | −6 (−16, 8) | 0.48 |
| Edema ≥ 2+, % | 31 | 29 | 36 | 26 | 30 | 0.70 |
| Improved edema | 67 | 66 | 69 | 70 | 61 | 0.72 |
| Orthopnea, % | 70 | 66 | 70 | 66 | 81 | 0.28 |
| Improved orthopnea | 53 | 57 | 50 | 53 | 51 | 0.64 |
| JVP ≥ 8 cm H2O, % | 65 | 62 | 66 | 59 | 77 | 0.30 |
| Improved JVP | 72 | 73 | 72 | 72 | 71 | 0.78 |
| Dyspnea VAS | 80 (64, 90) | 77 (67, 90) | 85 (58, 93) | 80 (64, 91) | 74 (64, 89) | 0.61 |
| Global VAS | 78 (60, 90) | 79 (65, 89) | 79 (57, 90) | 78 (60, 87) | 75 (56, 84) | 0.14 |
| Body swelling VAS | 75 (57, 86) | 73 (54, 82) | 75 (55, 90) | 88 (56, 93) | 75 (46, 83) | 0.59 |
| Laboratory values | ||||||
| Cystatin C, mg/L | 1.79 (1.46, 2.27) | 1.78 (1.47, 2.28) | 1.81 (1.46, 2.23) | 1.71 (1.37, 2.18) | 1.93 (1.54, 2.52) | 0.68 |
| Creatinine, mg/dL | 1.61 (1.30, 2.00) | 1.67 (1.22, 2.03) | 1.59 (1.24, 1.93) | 1.58 (1.27, 2.03) | 1.68 (1.46,1.99) | 0.23 |
| eGFR, mL · min−1 · 1.73 m−2 | 44.4 (34.2, 55.9) | 44.5 (35.1–60.6) | 45.0 (35.0–58.3) | 44.4 (31.5–55.5) | 42.4 (34.2–51.6) | 0.30 |
| BUN, mg/dL | 39 (29, 55) | 35 (29, 51) | 41 (29, 54) | 36 (27, 57) | 47 (36, 59) | 0.14 |
| NT-proBNP, pg/mL | 3391 (1375, 7477) | 3220 (1031, 8452) | 4181 (2185, 7794) | 2650 (1352, 6954) | 2913 (1028, 8284) | 0.69 |
| ΔNT-proBNP, pg/mL | −1382 (−3839, −192) | −1860 (−4319, −740) | −1302 (−3448, −124) | −1333 (−4933, −179) | −738 (−3536, −59) | 0.12 |
| ΔNGAL, ng/mg · uCR | −8.7 (−169, 35) | −79.9 (−616.2, −19.6) | −19.1 (−169.1, 2.0) | 0.1 (−64.1, 68.8) | 89.1 (18.4, 381.4) | <0.001* |
| ΔNAG, mU/mg · uCR | −0.07 (−3.2, 4.0) | −3.9 (−10.2, −2.0) | −0.5 (−3.6, 3.8) | 2.1 (−0.4, 5.7) | 3.5 (1.3, 11.4) | <0.001* |
| ΔKIM-1, pg/mg · uCR | −36 (−399, 406) | −402 (−1671, −180) | −125 (−601, 67) | 188 (−1, 886) | 802 (288, 3863) | <0.001* |
BUN indicates blood urea nitrogen; eGFR, estimated glomerular filtration rate; JVP, jugular venous pressure; KIM-1, kidney injury molecule 1; NAG, N-acetyl-β-D-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SBP, systolic blood pressure; and VAS, visual analog scale.
Significant.
Figure 3. Scatterplots of changes in kidney tubular injury biomarkers.
A, Scatterplots of changes in kidney tubular injury biomarkers by change in cystatin C. Left, The 72-hour changes in cystatin C vs log changes in neutrophil gelatinase-associated lipocalin (NGAL) and N-acetyl-β-D-glucosaminidase (NAG). The 72-hour changes in cystatin C vs absolute changes in kidney injury molecule 1 (KIM-1) (Bottom). B, Scatterplots of changes in kidney tubular injury biomarkers by change in creatinine. Right, the 72-hour changes in creatinine vs log changes in NGAL and NAG. The 72-hour changes in creatinine vs absolute changes in KIM-1 (Bottom).
Table 3.
Correlations Between Renal Tubular Injury Biomarkers Changes and Measures of Decongestion
| 72-h ΔNGAL,ng/mg uCR | P Value | 72-h ΔNAG,mU/mg uCR | P Value | 72-h ΔKIM-1,pg/mg uCR | P Value | |
|---|---|---|---|---|---|---|
| ΔWeight, lb | −0.041 | 0.49 | −0.094 | 0.12 | 0.079 | 0.19 |
| Total urinary Na output, mmol | −0.034 | 0.59 | 0.090 | 0.16 | −0.017 | 0.79 |
| ΔNT-proBNP, pg/mL | 0.015 | 0.81 | 0.024 | 0.69 | 0.110 | 0.07 |
| Cumulative urine output, mL | 0.071 | 0.23 | 0.087 | 0.14 | −0.047 | 0.43 |
KIM-1 indicates kidney injury molecule 1; NAG, N-acetyl-β-D-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; and NT-proBNP, N-terminal pro-B-type natriuretic peptide.
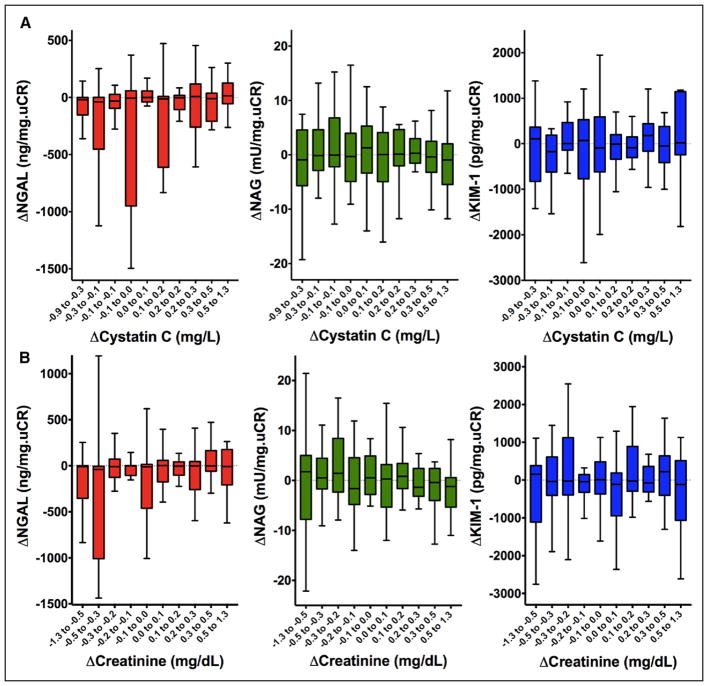
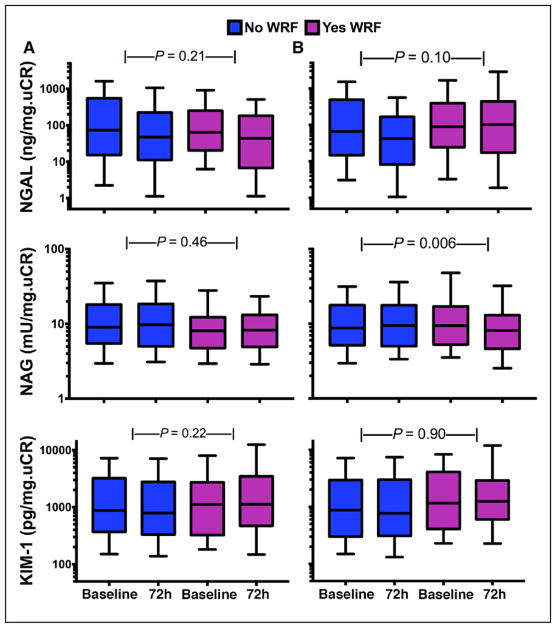
To capture any correlations that may have gone undetected because of differences in timing of peak levels of the different tubular injury and glomerular filtration markers queried (as is well described in biomarkers of myocardial injury), we performed a sensitivity analysis examining all combinations of tubular injury and glomerular filtration biomarkers at 24, 48, and 72 hours. We did not identify any meaningful correlations with any combination of biomarker and time point examined (Table 4). To determine whether there were any threshold or nonlinear effects that were masking a relationship between tubular injury markers and change in renal function, we assessed the change in biomarkers across deciles of absolute change in renal function. As shown in Figure 4A, there was no clear threshold or nonlinear relationship with any of the biomarkers at the range of change in cystatin C observed in this cohort. Similar findings were present with the change in creatinine (Figure 4B). Finally, the change in tubular injury biomarker levels did not differ between patients with and without WRF defined by cystatin C (Figure 5A). Similarly, changes in NGAL and KIM-1 levels did not differ on the basis of WRF when defined by creatinine (Figures 5B, top and middle). However, there was a statistically significant improvement in NAG among patients with creatinine-based WRF compared with those without WRF (P=0.006; Figure 5B, middle).
Table 4.
Correlations Between Serial Changes in Renal Tubular Injury Biomarkers and Cystatin C
| 24-h ΔCystatin C, mg/L | P Value | 48-h ΔCystatin C, mg/L | P Value | 72-h ΔCystatin C, mg/L | P Value | |
|---|---|---|---|---|---|---|
| ΔNGAL, ng/mg · uCR | ||||||
| 24 h | −0.030 | 0.62 | −0.058 | 0.34 | −0.002 | 0.97 |
| 48 h | 0.006 | 0.92 | 0.017 | 0.79 | 0.064 | 0.29 |
| 72 h | 0.029 | 0.62 | 0.073 | 0.22 | 0.135* | 0.023* |
| ΔNAG, mU/mg · uCR | ||||||
| 24 h | −0.106 | 0.08 | −0.001 | 0.99 | 0.030 | 0.62 |
| 48 h | −0.018 | 0.77 | 0.013 | 0.83 | 0.009 | 0.88 |
| 72 h | −0.094 | 0.12 | −0.069 | 0.25 | −0.041 | 0.49 |
| ΔKIM-1, pg/mg · uCR | ||||||
| 24 h | 0.025 | 0.67 | 0.102 | 0.09 | 0.032 | 0.59 |
| 48 h | 0.029 | 0.63 | 0.140* | 0.022* | 0.083 | 0.17 |
| 72 h | 0.014 | 0.81 | 0.051 | 0.40 | 0.048 | 0.43 |
KIM-1 indicates kidney injury molecule 1; NAG, N-acetyl-β-D-glucosaminidase; and NGAL, neutrophil gelatinase-associated lipocalin.
Significant.
Figure 4. Changes in kidney tubular injury biomarkers by decile change.
A, Changes in kidney tubular injury biomarkers by decile change in cystatin C. B, Changes in kidney tubular injury biomarkers by decile change in creatinine. (Left to right): Tukey box plots of 72-hour changes in neutrophil gelatinase-associated lipocalin (NGAL), N-acetyl-β-D-glucosaminidase (NAG), and kidney injury molecule 1 (KIM-1) across each decile change in cystatin C (top) and creatinine (bottom).
Figure 5. Baseline and 72-hour biomarkers of kidney tubular injury according to cystatin C–based worsening renal function (WRF) status.
A (Top to bottom), Box plots (whiskers represent 10th and 90th percentiles) of 72-hour changes in neutrophil gelatinase-associated lipocalin (NGAL), N-acetyl-β-D-glucosaminidase (NAG), and kidney injury molecule 1 (KIM-1) between patients with and without WRF. B, Baseline and 72-hour biomarkers of kidney tubular injury according to creatinine-based WRF status. (Top to bottom), Box plots (whiskers represent 10th and 90th percentiles) of 72-hour changes in NGAL, NAG, and KIM-1 between patients with and without WRF, as gauged by creatinine changes.
Associations With Survival
Over a median follow-up of 178 days, 55 patients (19.4%) died. Univariate trends were noted between the baseline and 72-hour change in NGAL (P=0.06), NAG (P=0.06), and KIM-1 (P=0.16), with increases in kidney tubular injury biomarker levels associated with improved survival. Notably, after extensive adjustment for 22 covariates (age, sex, race, heart rate, systolic blood pressure, atrial arrhythmias, diabetes mellitus, ischemic HF pathogenesis, left ventricular ejection fraction, angiotensin-converting enzyme/angiotensin receptor blocker use, β-blocker use, aldosterone antagonist use, baseline chloride, sodium, blood urea nitrogen, eGFR, NT-proB-NP, 72-hour urine output, weight change, change in NT-proBNP, cumulative diuretic dose, and baseline tubular injury biomarker levels), this association became statistically significant for all 3 biomarkers (NGAL: adjusted hazard ratio [HR], 0.88 per 10 percentile increase; 95% confidence interval [CI], 0.80–0.98; P=0.014; NAG: adjusted HR, 0.90 per 10 percentile increase; 95% CI, 0.81–0.98; P=0.017; and KIM-1: adjusted HR, 0.88 per 10 percentile increase; 95% CI, 0.80–0.98; P=0.014). Evaluating a composite score of all 3 biomarkers revealed a highly significant effect whereby the larger the temporal increase was in the 3 biomarkers, the lower the risk of death at 180 days was (adjusted HR, 0.80 per 10 percentile increase; 95% CI, 0.69–0.91; P=0.001). Baseline levels of NGAL and KIM-1 were not associated with 180-day survival (adjusted P>0.42 for both). Higher baseline levels of NAG were associated with reduced survival (adjusted HR, 1.12 per 10 percentile increase; 95% CI, 1.0–1.2; P=0.026).
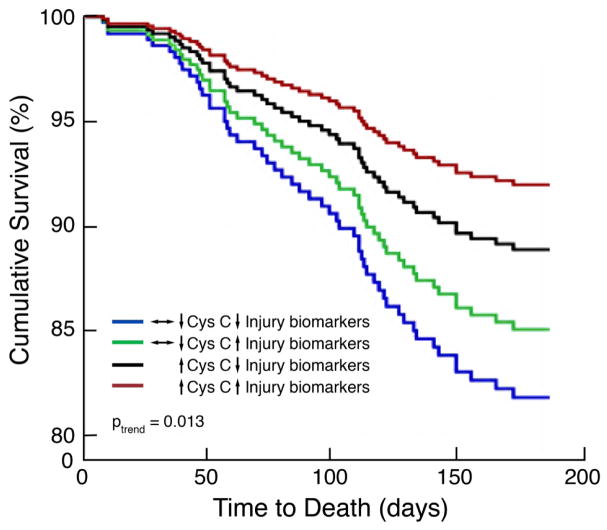
Although limited in power by the small number of events, WRF in this aggressively diuresed population was not associated with worsened 180-day survival (adjusted P=0.84), and the co-occurrence of an increase in tubular injury biomarkers could not differentiate low- from high-risk WRF (adjusted Pinteraction=0.17). In a sensitivity analysis to increase the number of patients in each subgroup, we further examined possible effect modification by looking at any increase in cystatin C (rather than requiring a 20% change in eGFR) and an increase in combined biomarker percentile above the median. Once again, we found no effect modification (adjusted Pinteraction=0.82); however, there was a linear trend (adjusted P=0.045) whereby patients with a decline in kidney function and increase in tubular injury markers had the best outcomes and patients with no change or improvement in kidney function/tubular injury biomarkers had the worst outcomes (Figure 6).
Figure 6. Associations between kidney tubular injury biomarkers and renal dysfunction with survival.
↑ Cys C indicates increase in cystatin C; ↔↓ Cys C, no change or decrease in cystatin C; ↑ Injury biomarkers, change in composite biomarker score >50th percentile; and ↓ Injury biomarkers, change in composite biomarker score <50th percentile. As shown, patients with a decline in kidney function and increase in tubular injury markers had the best outcomes and patients with no change or improvement in kidney function/tubular injury biomarkers had the worst outcomes; Ptrend indicates adjusted linear trend increasing from ↔↓ Cys C/↓ Injury biomarkers (high risk) to ↑ Cys C/↑ Injury biomarkers (low risk).
DISCUSSION
In a multicenter population of AHF patients, we found that changes in renal filtration markers with aggressive diuresis were not associated with changes in markers of renal tubular injury. Specifically, we found no support for serial changes in urine levels of 3 renal tubular injury biomarkers—NAG, NGAL, or KIM-1—being meaningfully related to changes in either cystatin C or creatinine. We also found no significant differences in levels of these tubular injury biomarker levels in patients with or without WRF. Furthermore, in this aggressively diuresed population, both WRF and increases in tubular injury biomarkers over the intervention period were not associated with adverse outcomes; rather, there was a paradoxical trend toward improved outcomes. Our data suggest that the small to moderate declines in GFR that commonly occur during aggressive diuresis, colloquially referred to as bumps in creatinine, may not primarily be a manifestation of tubular injury to the kidney; rather, they are likely to represent clinically benign changes in filtration.
Whereas WRF is common among patients hospitalized for AHF, its underlying mechanisms and clinical implications remain unclear.26 WRF in the setting of aggressive diuresis is often considered acute kidney injury, and thus, further diuresis or renin-angiotensin-aldosterone system blockade is suspended.27 This belief has been widely incorporated into clinical practice, and WRF is a commonly used outcome for multiple AHF clinical trials, both completed and planned.2,3,15 However, several lines of recent evidence challenge this paradigm, suggesting that it is the context by which WRF develops, rather than simply its presence, that is the principal determinant of adverse outcomes.21,28–32 For example, WRF in the setting of successful decongestion or titration of angiotensin-converting enzyme inhibitors may not have any negative prognostic implications.1,33,34 Furthermore, this notion of the importance of mechanism of WRF has been demonstrated in murine models of the disease in which the subtypes of kidney injury are biologically unrelated, and it is believed that molecular analysis rather than changes in markers of glomerular filtration such as creatinine or cystatin C should clarify our current definitions of acute changes in kidney excretory function.35 In clinical practice, however, it remains unclear whether treatment-induced WRF episodes are truly innocuous or whether there is meaningful renal damage that is offset by the overall gain from the therapeutic intervention. Our finding of no significant change in 3 well-established markers of tubular injury with treatment-induced WRF provides substantial reassurance that small to moderate treatment-induced bumps in creatinine that inevitably occur in the setting of aggressive diuresis should not carry negative connotations and trigger withdrawal of potentially beneficial therapy.27
Our a priori primary hypothesis was that renal tubular injury would be noted in a substantial subset of patients who underwent aggressive diuresis and experienced WRF. Second, we felt that injury would be much more common in patients with a larger magnitude of WRF and, when present, would identify a subset of patients who were at high risk for adverse outcomes. The findings presented here are especially intriguing because they entirely rebutted our hypotheses: not only was WRF of any degree unrelated to kidney injury, but there was a paradoxical trend toward improved outcomes in patients with WRF and increases in tubular injury biomarker levels. Although paradoxical, we suspect that our findings concerning WRF may parallel recent data showing the decreases in renal filtration occurring with treatments that benefit patients with HF might represent clinically benign changes rather than be a mediator of adverse outcomes. Although occurring via distinct molecular pathways, mild increases in renal tubular injury biomarkers might reflect intrarenal physiological changes that might be clinically benign as well. That said, whether WRF, as defined by increases in serum levels of filtration markers, or increases in renal tubular injury biomarkers are surrogates for clinically positive or negative outcomes in the setting of aggressive diuresis requires further study. Unfortunately, we cannot confirm the degree of decongestion via direct measures of hemoconcentration in the current data set, and this should be a focus of future studies. That said, the findings here support the contention that the clinical implications of WRF and increases in kidney tubular injury biomarkers in the setting of aggressive diuresis may not be detrimental; however, the current observations should serve primarily to motivate investigation of the above hypothesis and thus need to be confirmed with future study.
Before clinical application of this provocative concept, we must take into consideration a few caveats. First, ROSE-AHF was a clinical trial in which patients were selected for their stability and probability of successfully tolerating aggressive diuresis. Second, the participating clinical centers were largely HF centers of excellence with board-certified physicians experienced in advanced HF guiding care. Third, these observations were obtained in the context of physicians likely trying hard to avoid significant increases in creatinine. Thus, in the setting of less stable patients, with less highly trained caregivers driving therapy, and less deliberate avoidance of WRF, we are liable to see much larger bumps in creatinine, significant renal injury, and worse outcomes. Therefore, a prospective randomized trial is required to definitively understand how to incorporate changes in renal function directly into our clinical approach to patients with HF.
In addition, our results should be considered in light of several limitations. First and foremost, as previously mentioned, our results are primarily hypothesis generating and important preliminary data for a randomized clinical trial. Second, investigators adjusted HF therapies during the study, introducing unmeasured confounding into our analyses. Third, the results are most generalizable to patients with AHF and chronic renal dysfunction who would mirror the ROSE-AHF population. Fourth, given the low number of events, our study is underpowered to make any definitive statements about null mortality associations. To that end, although WRF has not universally been associated with worsened mortality in the recent literature, many larger studies have described this association, and thus, this null association should be viewed with appropriate skepticism. Fifth, given the small number of events and substantial number of covariates in the multivariable models, these models are likely overfit. As a result, although these results provide information on the qualitative independence of the observations within the ROSE-AHF data set, the quantitative strength of the association and the generalizability of this model to other data sets are likely limited. Finally, there is a paucity of data on expected levels of kidney injury biomarkers in HF; although the biomarkers of tubular injury have been proved sensitive and specific for acute tubular injury/necrosis in other clinical settings, they have been less rigorously studied in AHF. This may have contributed to the rarity of their clinical use.
CONCLUSIONS
We found that WRF in the setting of aggressive diuresis was not related to renal tubular injury as measured by validated urinary biomarkers. Furthermore, the clinical factors and therapeutic interventions driving these small to moderate decreases in renal function and increases in tubular injury markers appear to offset the risk of adverse outcomes associated with the WRF. In light of prior studies, our findings support the hypothesis that bumps in creatinine in the setting of aggressive diuresis may simply be the result of hemodynamic or functional alterations that need not trigger a withdrawal of potentially beneficial HF therapies.
Clinical Perspective.
What Is New?
There is pervasive concern that decreases in glomerular filtration rate (as measured by serum creatinine or cystatin C) in the setting of aggressive diuresis of heart failure patients reflect renal tubular injury.
We show, using validated biomarkers of tubular injury, that the small to moderate worsening of creatinine or cystatin C in the setting of aggressive dosing of loop diuretics was not associated with elevations in these markers of tubular injury.
We also show that worsening creatinine or cystatin C with aggressive diuresis is not associated with adverse clinical outcomes and that patients with mild elevations in markers of both glomerular filtration rate and tubular injury surprisingly tended to have the best outcomes.
What Are the Clinical Implications?
The small to moderate increases in creatinine that commonly complicate decongestion of patients hospitalized with acute heart failure may not be driven by renal tubular injury and thus may not indicate that continued attempts at adequate decongestion and uptitration of neurohormonal blockade are no longer indicated.
There is a need to further evaluate how to interpret changes in serum creatinine in the management of patients with heart failure and as an outcome measure in clinical trials.
Acknowledgments
This manuscript was prepared using ROSE-AHF research materials obtained from the National Heart, Lung, and Blood Institute BioLINCC and does not necessarily reflect the opinions or views of the study investigators or the National Heart, Lung, and Blood Institute.
Sources of Funding
This work was supported by National Institutes of Health grants K23HL114868 and L30HL115790 (Dr Testani), and K23DK097201 (Dr Wilson). Dr Ahmad was supported by a grant from the Heart Failure Society of America and startup funds from the Yale Section of Cardiovascular Medicine. The funding sources had no role in study design and data collection, analysis, or interpretation.
Footnotes
Disclosures
Dr Bonventre appears as coinventor on KIM-1 patents that have been licensed by Partners Healthcare to several companies. He has received royalty income from Partners Healthcare. The other authors report no conflicts.
ARTICLE INFORMATION
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
References
- 1.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–272. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O’Connor CM NHLBI Heart Failure Clinical Research Network. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bart BA, Goldsmith SR, Lee KL, Givertz MM, O’Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO, Stevenson LW, Semigran MJ, Felker GM, Chen HH, Hernandez AF, Anstrom KJ, McNulty SE, Velazquez EJ, Ibarra JC, Mascette AM, Braunwald E Heart Failure Clinical Research Network. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296–2304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, Bart BA, Bull DA, Stehlik J, LeWinter MM, Konstam MA, Huggins GS, Rouleau JL, O’Meara E, Tang WH, Starling RC, Butler J, Deswal A, Felker GM, O’Connor CM, Bonita RE, Margulies KB, Cappola TP, Ofili EO, Mann DL, Dávila-Román VG, McNulty SE, Borlaug BA, Velazquez EJ, Lee KL, Shah MR, Hernandez AF, Braunwald E, Redfield MM NHLBI Heart Failure Clinical Research Network. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA. 2013;310:2533–2543. doi: 10.1001/jama.2013.282190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metra M, Davison B, Bettari L, Sun H, Edwards C, Lazzarini V, Piovanelli B, Carubelli V, Bugatti S, Lombardi C, Cotter G, Dei Cas L. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail. 2012;5:54–62. doi: 10.1161/CIRCHEARTFAILURE.111.963413. [DOI] [PubMed] [Google Scholar]
- 6.Forman DE, Butler J, Wang Y, Abraham WT, O’Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–67. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 7.Ather S, Bavishi C, McCauley MD, Dhaliwal A, Deswal A, Johnson S, Chan W, Aguilar D, Pritchett AM, Ramasubbu K, Wehrens XH, Bozkurt B. Worsening renal function is not associated with response to treatment in acute heart failure. Int J Cardiol. 2013;167:1912–1917. doi: 10.1016/j.ijcard.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barasch J, Zager R, Bonventre JV. Acute kidney injury: a problem of definition. Lancet. 2017;389:779–781. doi: 10.1016/S0140-6736(17)30543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Guideline Clearinghouse (NGC) Guideline summary: KDIGO clinical practice guideline for acute kidney injury. Rockville, MD: Agency for Healthcare Research and Quality; Mar 1, 2012. [Accessed January 12, 2018]. https://www.guideline.gov. [Google Scholar]
- 10.Brisco MA, Testani JM. Novel renal biomarkers to assess cardiorenal syndrome. Curr Heart Fail Rep. 2014;11:485–499. doi: 10.1007/s11897-014-0226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, Perentes E, Cordier A, Vonderscher J, Maurer G, Goering PL, Sistare FD, Bonventre JV. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28:478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaidya VS, Ford GM, Waikar SS, Wang Y, Clement MB, Ramirez V, Glaab WE, Troth SP, Sistare FD, Prozialeck WC, Edwards JR, Bobadilla NA, Mefferd SC, Bonventre JV. A rapid urine test for early detection of kidney injury. Kidney Int. 2009;76:108–114. doi: 10.1038/ki.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park M, Hsu CY, Go AS, Feldman HI, Xie D, Zhang X, Mifflin T, Waikar SS, Sabbisetti VS, Bonventre JV, Coresh J, Nelson RG, Kimmel PL, Kusek JW, Rahman M, Schelling JR, Vasan RS, Liu KD. Chronic Renal Insufficiency Cohort (CRIC) Study Investigators; CKD Biomarkers Consortium. Urine kidney injury biomarkers and risks of cardiovascular disease events and all-cause death: the CRIC study. Clin J Am Soc Nephrol. 2017;12:761–771. doi: 10.2215/CJN.08560816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonventre JV. Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transplant. 2009;24:3265–3268. doi: 10.1093/ndt/gfp010. [DOI] [PubMed] [Google Scholar]
- 15.Parikh CR, Moledina DG, Coca SG, Thiessen-Philbrook HR, Garg AX. Application of new acute kidney injury biomarkers in human randomized controlled trials. Kidney Int. 2016;89:1372–1379. doi: 10.1016/j.kint.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasaki D, Yamada A, Umeno H, Kurihara H, Nakatsuji S, Fujihira S, Tsubota K, Ono M, Moriguchi A, Watanabe K, Seki J. Comparison of the course of biomarker changes and kidney injury in a rat model of drug-induced acute kidney injury. Biomarkers. 2011;16:553–566. doi: 10.3109/1354750X.2011.613123. [DOI] [PubMed] [Google Scholar]
- 17.Emmens JE, Ter Maaten JM, Matsue Y, Metra M, O’Connor CM, Ponikowski P, Teerlink JR, Cotter G, Davison B, Cleland JG, Givertz MM, Bloomfield DM, Dittrich HC, Todd J, van Veldhuisen DJ, Hillege HL, Damman K, van der Meer P, Voors AA. Plasma kidney injury molecule-1 in heart failure: renal mechanisms and clinical outcome. Eur J Heart Fail. 2016;18:641–649. doi: 10.1002/ejhf.426. [DOI] [PubMed] [Google Scholar]
- 18.Maisel AS, Wettersten N, van Veldhuisen DJ, Mueller C, Filippatos G, Nowak R, Hogan C, Kontos MC, Cannon CM, Müller GA, Birkhahn R, Clopton P, Taub P, Vilke GM, McDonald K, Mahon N, Nuñez J, Briguori C, Passino C, Murray PT. Neutrophil gelatinase-associated lipocalin for acute kidney injury during acute heart failure hospitalizations: the AKI-NESIS study. J Am Coll Cardiol. 2016;68:1420–1431. doi: 10.1016/j.jacc.2016.06.055. [DOI] [PubMed] [Google Scholar]
- 19.Sokolski M, Zymliński R, Biegus J, Siwolowski P, Nawrocka-Millward S, Todd J, Yerramilli MR, Estis J, Jankowska EA, Banasiak W, Ponikowski P. Urinary levels of novel kidney biomarkers and risk of true worsening renal function and mortality in patients with acute heart failure. Eur J Heart Fail. 2017;19:760–767. doi: 10.1002/ejhf.746. [DOI] [PubMed] [Google Scholar]
- 20.Legrand M, De Berardinis B, Gaggin HK, Magrini L, Belcher A, Zancla B, Femia A, Simon M, Motiwala S, Sambhare R, Di Somma S, Mebazaa A, Vaidya VS, Januzzi JL., Jr Global Research on Acute Conditions Team (GREAT). Evidence of uncoupling between renal dysfunction and injury in cardiorenal syndrome: insights from the BIONICS study. PLoS One. 2014;9:e112313. doi: 10.1371/journal.pone.0112313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verbrugge FH, Dupont M, Shao Z, Shrestha K, Singh D, Finucan M, Mullens W, Tang WH. Novel urinary biomarkers in detecting acute kidney injury, persistent renal impairment, and all-cause mortality following decongestive therapy in acute decompensated heart failure. J Card Fail. 2013;19:621–628. doi: 10.1016/j.cardfail.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanberg JS, Tang WHW, Wilson FP, Coca SG, Ahmad T, Brisco MA, Testani JM. An exploratory analysis of the competing effects of aggressive decongestion and high-dose loop diuretic therapy in the DOSE trial. Int J Cardiol. 2017;241:277–282. doi: 10.1016/j.ijcard.2017.03.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coady SA, Mensah GA, Wagner EL, Goldfarb ME, Hitchcock DM, Giffen CA. Use of the National Heart, Lung, and Blood Institute Data Repository. N Engl J Med. 2017;376:1849–1858. doi: 10.1056/NEJMsa1603542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McWilliam SJ, Antoine DJ, Sabbisetti V, Pearce RE, Jorgensen AL, Lin Y, Leeder JS, Bonventre JV, Smyth RL, Pirmohamed M. Reference intervals for urinary renal injury biomarkers KIM-1 and NGAL in healthy children. Biomark Med. 2014;8:1189–1197. doi: 10.2217/bmm.14.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Testani JM, McCauley BD, Chen J, Shumski M, Shannon RP. Worsening renal function defined as an absolute increase in serum creatinine is a biased metric for the study of cardiorenal interactions. Cardiology. 2010;116:206–212. doi: 10.1159/000316038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Testani JM, Brisco-Bacik MA. Worsening renal function and mortality in heart failure: causality or confounding? Circ Heart Fail. 2017;10:e003835. doi: 10.1161/CIRCHEARTFAILURE.117.003835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felker GM, O’Connor CM, Braunwald E Heart Failure Clinical Research Network Investigators. Loop diuretics in acute decompensated heart failure: necessary? Evil? A necessary evil? Circ Heart Fail. 2009;2:56–62. doi: 10.1161/CIRCHEARTFAILURE.108.821785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail. 2011;4:685–691. doi: 10.1161/CIRCHEARTFAILURE.111.963256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Testani JM, Brisco MA, Chen J, McCauley BD, Parikh CR, Tang WH. Timing of hemoconcentration during treatment of acute decompensated heart failure and subsequent survival: importance of sustained decongestion. J Am Coll Cardiol. 2013;62:516–524. doi: 10.1016/j.jacc.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metra M, Nodari S, Parrinello G, Bordonali T, Bugatti S, Danesi R, Fontanella B, Lombardi C, Milani P, Verzura G, Cotter G, Dittrich H, Massie BM, Dei Cas L. Worsening renal function in patients hospitalised for acute heart failure: clinical implications and prognostic significance. Eur J Heart Fail. 2008;10:188–195. doi: 10.1016/j.ejheart.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Testani JM, Damman K, Brisco MA, Chen S, Laur O, Kula AJ, Tang WH, Parikh C. A combined-biomarker approach to clinical phenotyping renal dysfunction in heart failure. J Card Fail. 2014;20:912–919. doi: 10.1016/j.cardfail.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kula AJ, Hanberg JS, Wilson FP, Brisco MA, Bellumkonda L, Jacoby D, Coca SG, Parikh CR, Tang WHW, Testani JM. Influence of titration of neurohormonal antagonists and blood pressure reduction on renal function and decongestion in decompensated heart failure. Circ Heart Fail. 2016;9:e002333. doi: 10.1161/CIRCHEARTFAILURE.115.002333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Testani JM, McCauley BD, Chen J, Coca SG, Cappola TP, Kimmel SE. Clinical characteristics and outcomes of patients with improvement in renal function during the treatment of decompensated heart failure. J Card Fail. 2011;17:993–1000. doi: 10.1016/j.cardfail.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu K, Rosenstiel P, Paragas N, Hinze C, Gao X, Huai Shen T, Werth M, Forster C, Deng R, Bruck E, Boles RW, Tornato A, Gopal T, Jones M, Konig J, Stauber J, D’Agati V, Erdjument-Bromage H, Saggi S, Wagener G, Schmidt-Ott KM, Tatonetti N, Tempst P, Oliver JA, Guarnieri P, Barasch J. Unique transcriptional programs identify subtypes of AKI. J Am Soc Nephrol. 2017;28:1729–1740. doi: 10.1681/ASN.2016090974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data, analytical methods, and study materials are available to other researchers for purposes of reproducing the results via the Biological Specimen and Data Repository Information Coordinating Center (BioLINCC).23