Abstract
In response to microbial infection, the human host deploys metal-sequestering host-defense proteins, which reduce nutrient availability and thereby inhibit microbial growth and virulence. Calprotectin (CP) is an abundant antimicrobial protein released from neutrophils and epithelial cells at sites of infection. CP sequesters divalent first-row transition metal ions to limit the availability of essential metal nutrients in the extracellular space. While functional and clinical studies of CP have been pursued for decades, advances in our understanding of its biological coordination chemistry, which is central to its role in the host–microbe interaction, have been made in more recent years. In this review, we focus on the coordination chemistry of CP and highlight studies of its metal-binding properties and contributions to the metal-withholding innate immune response. Taken together, these recent studies inform our current model of how CP participates in metal homeostasis and immunity, and they provide a foundation for further investigations of a remarkable metal-chelating protein at the host–microbe interface and beyond.
Keywords: nutritional immunity, metal homeostasis, host–microbe interaction, S100 protein, antimicrobial activity
INTRODUCTION TO CALPROTECTIN
Nutritional Immunity
Transition metal ions are essential for life and are utilized by all organisms for functions that include enzymatic catalysis, electron transfer, signaling, and structure (1). Transition metals can also be toxic, and all organisms strive to maintain appropriate levels of metal ions by using regulatory pathways, metal import and efflux machineries, and depots for metal storage. In the context of infection, a microbial pathogen faces the challenge of acquiring nutrients, including essential transition metals, from the host. During microbial infection, the host innate immune system mounts a metal-withholding response; this process is often termed nutritional immunity (2, 3). Host cells release metal-chelating proteins into the extracellular space to sequester metal nutrients and starve the pathogen. Canonical examples of metal-chelating host-defense proteins sequester one nutrient metal. For example, lactoferrin and lipocalin-2 limit iron availability by chelating Fe(III) and capturing ferric siderophores, respectively (4, 5). Calprotectin (CP), the focus of this review, is a unique player in nutritional immunity because it sequesters multiple divalent first-row transition metal ions.
Discovery
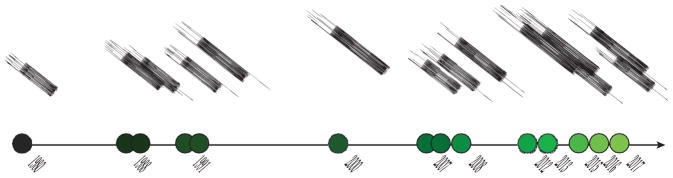
During the 1980s, CP was independently discovered three times in different contexts and given several names, including the leukocyte-derived protein (L1 antigen), the cystic fibrosis antigen, calgranulins A and B, and migration inhibitory factor–related proteins 8 and 14 (MRP-8 and MRP-14) (Figure 1) (6–12). In each case, the protein was associated with inflammation in human tissues, and each report indicated that the protein binds Ca(II). In 1988, the common identity of the L1 antigen, the cystic fibrosis antigen, and MRP-8/MRP-14 was recognized (13). Shortly thereafter, the function of this protein was illuminated with the observation of its growth inhibitory activity against the opportunistic fungal pathogen Candida albicans, and the protein was renamed calprotectin (14, 15). This name highlights two properties of the protein that were appreciated at the time: Ca(II) binding and antimicrobial activity.
Figure 1.
Timeline of notable events during the discovery and evaluation of transition metal binding of calprotectin (CP). A number of investigations of the role of CP in infectious disease dating back to 1980 built upon each other to elucidate the structure and function of CP. While the involvement of metal ions in the activity of CP was noted as early as 1991, the metal-binding characteristics of CP were not thoroughly evaluated until approximately 20 years later.
The discovery of the antifungal activity of CP motivated additional explorations of its antimicrobial activity, which established that CP exhibits broad-spectrum growth inhibitory activity against bacteria and fungi (14–21). Some of this work also provided the first indication that CP is a metal-chelating protein. One study described the growth inhibitory activity of neutrophil lysates, which was later attributed to CP, as reversible with the addition of Zn(II) (17). Subsequent investigations with purified CP demonstrated that its antimicrobial activity was markedly attenuated upon Zn(II) addition and independent of microbial contact (18–20, 22–24). Based on these findings and given the abundance of CP during the inflammatory response, it was hypothesized that CP functions in host defense by chelating Zn(II) and depriving microbes of this essential nutrient (17–24).
Structure, Calcium Binding, and Oligomerization
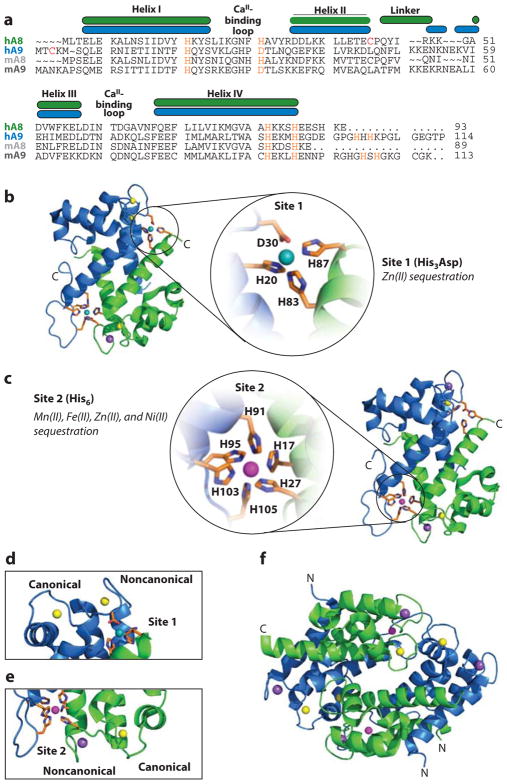
Human CP is a heteroligomer of two S100 proteins: S100A8 and S100A9 (Figure 2) (25–27). Both S100A8 (93 amino acids, 10.8 kDa) and S100A9 (114 amino acids, 13.2 kDa) possess two EF-hand Ca(II)-binding domains. The C-terminal EF-hand of each polypeptide provides a calmodulin-like, canonical seven-coordinate Ca(II)-binding site, whereas the N-terminal EF-hands are noncanonical and exhibit five- or six-coordinate Ca(II)-binding sites (Figure 2a,d,e) (28, 29). Apo CP, which we define as the species without bound divalent cations, is a S100A8/S100A9 heterodimer. Calcium binding causes two heterodimers to self-associate and form a (S100A8/S100A9)2 heterotetramer (Figure 2f) (30–33). As described below, Ca(II) binding and heterotetramerization are important components of the current working model for how CP functions to sequester transition metals in the extracellular space.
Figure 2.
Structure of human calprotectin (CP). S100A8 subunits are green, and S100A9 subunits are blue. (a) Amino acid sequence alignment of human (h) and murine (m) CP. The secondary structural elements are presented above the alignment for the human form. The transition metal–binding residues are presented in orange. For the murine S100A9 subunit, His105 and His107 are predicted to contribute to the His6 site. (b) The heterodimer bound to Ni(II) (teal ), Ca(II) ( yellow), and Na(I) ( purple) [Protein Data Bank (PDB) 5W1F]. Site 1 is expanded to show the Ni(II)-bound His3Asp motif. (c) The dimer bound to Mn(II) (magenta), Ca(II) ( yellow), and Na(I) ( purple) (PDB 4XJK). Site 2 is expanded to show the Mn(II)-bound His6 motif. (d ) S100A9 canonical and noncanonical EF-hands and proximity to the Ni(II)-bound His3Asp site (site 1) (PDB 5W1F). Both EF-hands are Ca(II) bound. (e) S100A8 canonical and noncanonical EF-hands and proximity to the Mn(II)-bound His6 site (site 2) (PDB 4XJK). The canonical EF-hand is Ca(II) bound, and the noncanonical EF-hand is Na(I) bound. ( f ) The Ca(II)-, Na(I)-, and Mn(II)-bound (S100A8/S100A9)2 tetramer (PDB 4XJK). Panel a modified from Reference 50.
Current Working Model
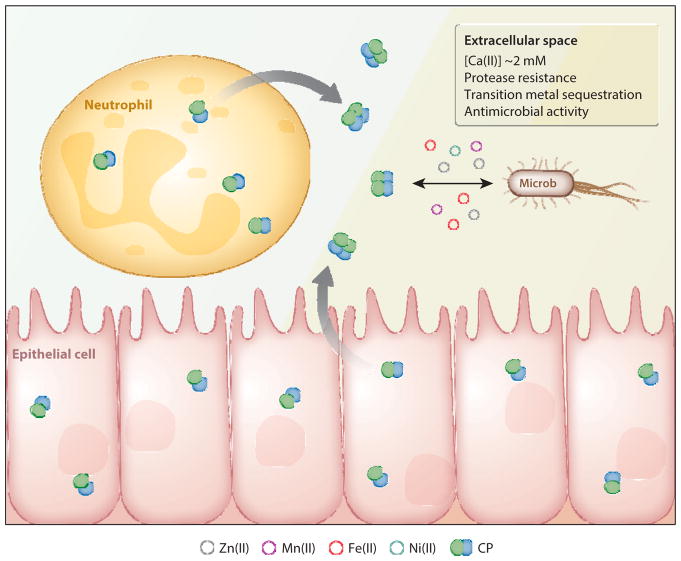
The current working model for metal sequestration by CP in the extracellular space is based on the biological, biophysical, and biochemical studies presented herein (Figure 3). CP is a cytoplasmic protein expressed in myeloid cell types that include neutrophils, monocytes, and macrophages (8, 34). CP is most abundant and constitutively expressed in neutrophils, composing ~40% of the total cytoplasmic protein. It is also inducibly expressed in epithelial cells and keratinocytes (8, 35). At infection sites, these cell types release CP into the extracellular space. Neutrophils release CP during the formation of neutrophil extracellular traps (NETs) (36). CP experiences relatively low Ca(II) ion levels in the cytoplasm (e.g., nanomolar concentrations in a resting cell) as well as high Ca(II) levels in the extracellular environment (~2 mM) (37). Thus, the CP heterodimer is likely to be an abundant intracellular species and Ca(II) binding during or after release results in heterotetramerization. Ca(II) binding and heterotetramerization provide CP with protease resistance, enhanced transition metal affinities, and enhanced growth inhibitory activity (Figure 3) (16, 32, 38, 39). In the following subsections, we provide an overview of our current understanding of these properties.
Figure 3.
Model for the extracellular role of calprotectin (CP) in metal sequestration. CP is released from neutrophils or epithelial cells and encounters high concentrations of Ca(II) ( 2 mM) in the extracellular space, causing the protein to form the (S100A8/S100A9)2 heterotetramer. Ca(II)-induced tetramerization affords protease resistance, enhanced transition metal affinities, and enhanced antimicrobial activity. In the extracellular space, CP competes with microbes for bioavailable metals in the 2 oxidation state to impart its growth inhibitory activity.
Protease Resistance
The extracellular milieu and sites of neutrophil influx present challenging environments, and CP must retain its bound metal ions to exert its host-defense function in these harsh locales. Extracellular proteases produced by both the host and the pathogen can be abundant at infection sites, and the structures of many host-defense peptides confer protease resistance to circumvent this threat (40, 41). Indeed, an early report of CP indicated its protease resistance to human matrix metalloproteinases (Figure 1) (42). Recent biochemical investigations informed the molecular basis for the proteolytic stability of CP by demonstrating that heterotetramerization provides resistance to extracellular host proteases that include trypsin and human neutrophil elastase (32). In the presence of a protease, a tetramer-deficient variant of CP that binds Ca(II) but cannot undergo Ca(II)-dependent tetramerization was rapidly degraded, whereas metal-bound heterotetramers were not. Thus, heterotetramerization likely provides an element of temporal control, modulating the lifetime of CP at a biological locale (Figure 3).
Transition Metal Binding
The first crystal structure of human CP was reported in 2007 and provided a snapshot of the Ca(II)-bound CP heterotetramer as well as a guide for studies of transition metal binding (Figure 1) (43). This structure revealed two transition metal–binding sites in each heterodimer. Each of these sites forms at the S100A8/S100A9 heterodimer interface. One site is a His3Asp motif (site 1) comprising H83 and H87 of S100A8 and H20 and D30 of S100A9 (Figure 2a,b). The second site was identified as a His4 motif (site 2) formed by H17 and H27 of S100A8 and H91 and H95 of S100A9. These crystallographic data were consistent with (a) the observation that the antimicrobial activity of CP is abolished upon addition of the histidine-modifying compound diethylpy-rocarbonate (23) and (b) early secondary structure predictions of CP, which proposed the importance of histidine-containing sequences in S100A9 (residues 91–95) and S100A8 (residues 83–87) for Zn(II) binding (44). Moreover, this structure highlights that each CP heterodimer unit contains six unique sites for cation binding: two different EF-hand domains per S100 subunit (Figure 2d,e) and two different transition metal sites at the S100A8/S100A9 interface.
Four crystal structures of the human CP heterotetramer in different metal-bound forms have been reported: the Ca(II)-bound structure noted above (1XK4) (43), two Ca(II)- and Mn(II)-bound forms (4GGF and 4XJK, respectively) (45, 46), and a Ca(II)- and Ni(II)-bound structure (5W1F) (47). Overall, these four structures show similar structural attributes. In each structure, S100A8 and S100A9 of a heterodimer unit associate in an antiparallel fashion with helices 1 and 4 at the heterodimer interface (Figure 2b,c), and the heterotetramer interface is largely made of contacts between S100A8 subunits (Figure 2f). A comparison of these structures indicates that the absence or presence of bound transition metal does not cause significant structural changes in the overall protein scaffold. Nevertheless, a dramatic conformational change in the S100A9 C-terminal tail, defined as residues 96–114, occurs with transition metal binding at site 2. As a result, site 2 is an unusual His6 site comprising the His4 motif identified in the Ca(II)-only structure and H103 and H105 of the S100A9 C-terminal tail (Figure 2a,c) (45, 46). Whereas His3Asp motifs occur in other Zn(II)-binding proteins, including S100A7 and S100A12 (48, 49), CP is currently the only example of a metalloprotein containing a native His6 site.
Solution studies have revealed several important facets about the coordination chemistry of CP that we summarize here and consider on a metal-by-metal basis below. First, both apo and Ca(II)-bound CP coordinate divalent first-row transition metal ions at the His3Asp and His6 sites. For all metals examined to date, the Ca(II)-bound form coordinates M(II) (M = Mn, Fe, Zn, Ni) with markedly higher affinity than the apo protein (38, 50–51; T.G. Nakashige, S.E.J. Bowman, E.M. Zygiel, C.L. Drennan & E.M. Nolan, unpublished manuscript). Sites 1 and 2 exhibit different metal-binding properties. Site 1 has high affinity for Zn(II) and relatively low affinity for Mn(II), Fe(II), and Ni(II) (Figure 2b; Table 1). Thus, site 1 can be described as Zn(II)-selective because it only coordinates this metal with sufficiently high affinity to contribute to sequestration (52). In contrast, site 2 is functionally versatile because it can sequester Mn(II), Fe(II), Ni(II), and Zn(II) when Ca(II) levels are high (Figure 2c; Table 1). Metal substitution experiments have delineated the relative metal affinities at site 2 (Kd,Mn > Kd,Fe > Kd,Zn > Kd,Ni), which are in agreement with the Irving–Williams series (47, 50, 51, 53).
Table 1.
Reported apparent dissociation constant values (Kd) for calprotectin (CP) and experimental methods
| Metal | Protein | Dissociation constant (Kd) | Method | Buffer conditions | Reference |
|---|---|---|---|---|---|
| Zn(II) | CP-Sera |
Kd, site 1 = 133 ± 58 pM Kd, site 2 = 185 ± 219 nM |
Competitionb | 75 mM HEPES, 100 mM NaCl, pH 7.5 | 38 |
| Zn(II) | CP-Sera |
Kd, site 1 ≤ 10 pMj Kd, site 2 ≤ 240 pMj |
Competitionb | 75 mM HEPES, 100 mM NaCl, pH 7.5 20 equivalents Ca(II)/CP (αβ) |
38 |
| Zn(II) | CP |
Kd1 = 1.4 nMc Kd2 = 5.6 nMc |
ITCd | 20 mM Tris, 100 mM NaCl, pH 7.5 5 mM β-mercaptoethanol Stoichiometric Ca(II)e |
55 |
| Zn(II) | CP-Sera |
Kd1 = 90 ± 366 fMc,j Kd2 = 0.9 ± 1 pMc,j |
Competitionf | 75 mM HEPES, 100 mM NaCl, pH 7.0 50 equivalents Ca(II)/CP (αβ) |
52 |
| Zn(II) | L’His3Aspa | Kd, site 2 = 3.4 ± 1.2 nM | ITCg | 20 mM HEPES, 75 mM NaCl, pH 7.5 Stoichiometric Ca(II)e |
45 |
| Zn(II) | L’His4a | Kd, site 1 = 8.2 ± 1.5 nM | ITCg | 20 mM HEPES, 75 mM NaCl, pH 7.5 Stoichiometric Ca(II)e |
45 |
| Mn(II) | CP-Sera |
Kd, site 2 = 4.9 ± 1.0 μM Kd = 1.0 mM (n = 2) |
EPRh | 75 mM HEPES, 100 mM NaCl, pH 7.5 | 50 |
| Mn(II) | CP-Sera |
Kd, site 2 = 194 ± 203 nM Kd, site 1 = 21 ± 5 μM |
EPRh | 75 mM HEPES, 100 mM NaCl, pH 7.5 40 equivalents Ca(II)/CP (αβ) |
50 |
| Mn(II) | CP-Sera | Kd, site 2 > 550 nM | Competitioni | 75 mM HEPES, 100 mM NaCl, pH 7.5 | 50 |
| Mn(II) | CP-Sera | Kd, site 2 < 550 nMj | Competitioni | 75 mM HEPES, 100 mM NaCl, pH 7.5 40 equivalents Ca(II)/CP (αβ) |
50 |
| Mn(II) | CP |
Kd1 = 1.3 nMc Kd2 = 3.7 μMc |
ITCd | 20 mM Tris, 100 mM NaCl, pH 7.5 Stoichiometric Ca(II)e 5 mM β-mercaptoethanol |
55 |
| Mn(II) | L’His3Aspa | Kd, site 2 = 5.8 ± 1.6 nM | ITCg | 20 mM HEPES, 75 mM NaCl, pH 7.5 Stoichiometric Ca(II)e |
45 |
| Fe(II) | CP-Sera |
Kd, site 2 = <2.2 ± 0.3 pMj,k |
Competitioni | 75 mM HEPES, 100 mM NaCl, pH 7.5 50 equivalents Ca(II)/CP (αβ) |
51 |
| Fe(II) | CP | Kd, site 2 = <2.2 ± 0.3 pMj,k | Competitioni | 75 mM HEPES, 100 mM NaCl, pH 7.5 50 equivalents Ca(II)/CP (αβ) |
51 |
| Ni(II) | L’His3Aspa | Kd, site 2 = <0.9 pMj | Metal substitution | 75 mM HEPES, 100 mM NaCl, pH 7.0 2 mM Ca(II) |
47 |
Abbreviations: EPR, electron paramagnetic resonance; HEPES, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; ITC, isothermal titration calorimetry.
CP contains two native cysteine residues that were mutated to serine for these metal-binding studies.
Competition titrations were performed at 25°C with the Zn(II) sensor Zinpyr-4 (ZP4). This sensor is based on an asymmetric fluorescein platform and contains an aniline-derivatized DPA chelator (di-(2-picolyl)amine).
The Kd values were not assigned to the metal-binding sites.
Direct ITC titrations were performed at 30°C. Stoichiometric Mn(II)/Zn(II) binding was observed.
The definition of stoichiometric Ca(II) in terms of molar equivalents per CP unit or Ca(II)-binding site is unclear.
Competition titrations were performed with the fluorescent Zn(II) sensor HNBO-DPA. This sensor is based on the HNBO fluorophore (2-(2thydroxy-3tnaphthyl)benzoxazole) and the DPA chelator at room temperature.
Direct ITC titrations were performed at 25°C. Stoichiometric Zn(II)/Mn(II) binding was observed.
Direct EPR titrations were performed at room temperature. The +Ca(II) titrations are limited by the concentrations required for detectable Mn(II).
Competition titrations were performed with the fluorescent Zn(II) sensor Zinpyr-1 (ZP1) at 25°C. This sensor is based on the 2t,7t-dichlorofluorescein fluorophore and contains two DPA moieties.
All Kd values in the presence of excess Ca(II) are upper limits. The relative affinity for metal binding at site 2 is as follows: Kd,Mn > Kd,Fe > Kd,Zn > Kd,Ni.
Competition titrations with Fe(II) were performed anaerobically.
A number of factors must be considered when conceptualizing metal sequestration by CP in the complex biological milieu. Both the thermodynamics and kinetics of metal binding are important parameters that contribute to which metal ion is coordinated by the promiscuous His6 site. Although no rigorous kinetic investigations of CP have been reported to date, all experimental observations indicate that the His6 site binds divalent metal ions rapidly and with slow exchange. From a kinetic perspective, CP likely binds the first metal it encounters, favoring metals that are available and in relatively high abundance. If present for a sufficient amount of time, the metal-binding thermodynamics indicate that metal speciation of CP favors metals bound with the highest affinities. Nevertheless, it has been proposed that the His6 site kinetically traps the bound metal ion, suggesting that thermodynamic considerations may have lesser importance on the biological timescale (46). Moreover, the relative concentrations of CP and metals must be taken into account. Given that the extracellular levels of CP can reach up to 1 mg/mL (~40 μM heterodimer) (35), CP is likely in excess of the concentration of bioavailable metal ions at sites of infection. Thus, all bioavailable divalent transition metal ions present at an infection site may be captured by CP. Lastly, it is important to acknowledge that an infection site is an open system where CP and bioavailable metal concentrations undoubtedly vary over time.
Antimicrobial Activity
A link between metal chelation and the antimicrobial activity of CP was established during the early discoveries of CP (17–24). This work and subsequent reports showed that supplementation of microbial cultures with Zn(II) attenuates the antimicrobial activity of CP (39, 52). Moreover, Zn(II)-bound CP or CP variants that lack both metal-binding sites do not inhibit microbial growth, demonstrating that sites 1 and 2 are essential for antimicrobial activity. Studies with CP variants that have only one transition metal–binding site (e.g., .6.
 His3Asp and .6.
His3Asp and .6.
 His4 variants) revealed that each site contributes to the antimicrobial activity of CP, albeit to different extents (38, 45, 51, 52, 54). Over the years, several studies have pointed to a role for Ca(II) in the antimicrobial activity of CP (16, 38, 39). The enhanced transition metal affinities observed in the presence of Ca(II) correlated well with observations of enhanced growth inhibitory activity in the presence of this cation (38).
His4 variants) revealed that each site contributes to the antimicrobial activity of CP, albeit to different extents (38, 45, 51, 52, 54). Over the years, several studies have pointed to a role for Ca(II) in the antimicrobial activity of CP (16, 38, 39). The enhanced transition metal affinities observed in the presence of Ca(II) correlated well with observations of enhanced growth inhibitory activity in the presence of this cation (38).
Purpose of Review
In the following sections, we present recent advances in our fundamental understanding of the coordination chemistry and biological function of CP. We first consider manganese and zinc sequestration, which serve as paradigms, and then describe studies that have uncovered the interplay between CP and iron as well as nickel.
MANGANESE SEQUESTRATION
In 2008, the discovery of a role for CP in manganese withholding was reported (39). Metal analyses of liver abscesses in a murine model of Staphylococcus aureus infection revealed that the abscesses of mice expressing murine CP were devoid of Mn, whereas abscesses in CP knockout mice exhibited Mn levels comparable with those of healthy tissue. Moreover, antimicrobial activity assays that employed S. aureus mutant strains deficient in an ABC transporter (ATP-binding cassette transporter) named MntABC that imports Mn(II) revealed increased sensitivity to CP, and strains lacking the Mn(II)-dependent transcriptional repressor MntR were less sensitive to Mn(II) toxicity when cultured with CP. This work presented preliminary metal-binding analyses, which showed that human CP coordinates Mn(II). Although the Zn(II)-reversible antimicrobial activity of CP was the hallmark of its proposed function for decades, few studies of its metal-binding properties had been reported. This seminal contribution motivated studies of Mn(II) chelation by CP, which uncovered several key insights and provided a guide for studies of its interactions with other first-row transition metal ions.
Coordination Chemistry
With evidence that CP coordinates Mn(II), biophysical and biochemical studies were launched to identify the Mn(II)-binding site(s) and ascertain the Mn(II) affinities (45, 46, 50, 54–56). To define the contributions of sites 1 and 2 to Mn(II) chelation, CP variants lacking one or more metal-binding residues were evaluated (50). In this work, both biochemical and electron paramagnetic resonance (EPR) spectroscopic studies demonstrated that site 2 is the high-affinity Mn(II) site. These studies also showed that the EF-hand domains do not coordinate Mn(II) to an appreciable extent and that site 1 coordinates Mn(II) with relatively low affinity. Moreover, the presence of excess Ca(II) ions markedly enhanced the Mn(II) affinity of CP. Regarding the latter point, current available data indicate that Ca(II) binding to CP lowers the Kd,Mn value of site 2 by at least three orders of magnitude (Table 1) (45, 50, 56).
EPR spectroscopic studies of Mn(II)-bound CP also demonstrated that the Mn(II) ion at site 2 is bound in a nearly idealized, slightly rhombically distorted octahedral coordination sphere (50). This result indicated that the His4 site observed in the Ca(II)-bound CP crystal structure is actually a His4X2 site with two unidentified ligands. Shortly thereafter, a crystal structure of Ca(II)- and Mn(II)-bound CP revealed that the Mn(II) coordination sphere at site 2 comprises the His4 motif, with H103 and H105 of the A9 C-terminal tail providing an unusual His6 site (45). The use of six histidine ligands to create a markedly high-affinity Mn(II) site is unprecedented among structurally characterized Mn-binding proteins. The vast majority of Mn coordination spheres in proteins contain oxygen ligands, and the reported dissociation constants for Mn-binding enzymes and transcription factors are typically in the low micromolar range (57, 58). Moreover, Mn(II) is a hard metal ion, and the hard–soft acid–base theory predicts that coordination of Mn(II) by hard oxygen ligands results in a more stable complex than coordination by softer nitrogen ligands. Thus, it was initially surprising that the His6 site of CP binds Mn(II) with higher affinity than the His3Asp site as well as many other octahedral Mn(II) coordination spheres found in metalloproteins. It was hypothesized that the protein scaffold, and in particular the C-terminal tail of S100A9, contributes to high-affinity Mn(II) binding by encapsulating the Mn(II) ion within the protein, blocking solvent access, and overcoming the kinetic lability of this 3d5 metal ion (54). This notion was supported by the crystal structures of Mn(II)-bound CP as well as by EPR spectroscopic studies (46). Both methods revealed a remarkably dry Mn(II) coordination sphere, and hydrogen-bonding interactions between Mn(II)-coordinating His residues and neighboring amino acids likely contribute to charge neutralization and restrict solvent access to the metal center (Figure 4) (46). Moreover, substitution of H103 or H105 of the C-terminal tail with noncoordinating alanine residues reduced the Mn(II) affinity of site 2 and allowed solvent access to the Mn(II) center (46, 54). Taken together, these studies describe how the C-terminal tail of S100A9 enables CP to entrap Mn(II) at an unprecedented biological His6 site.
Figure 4.
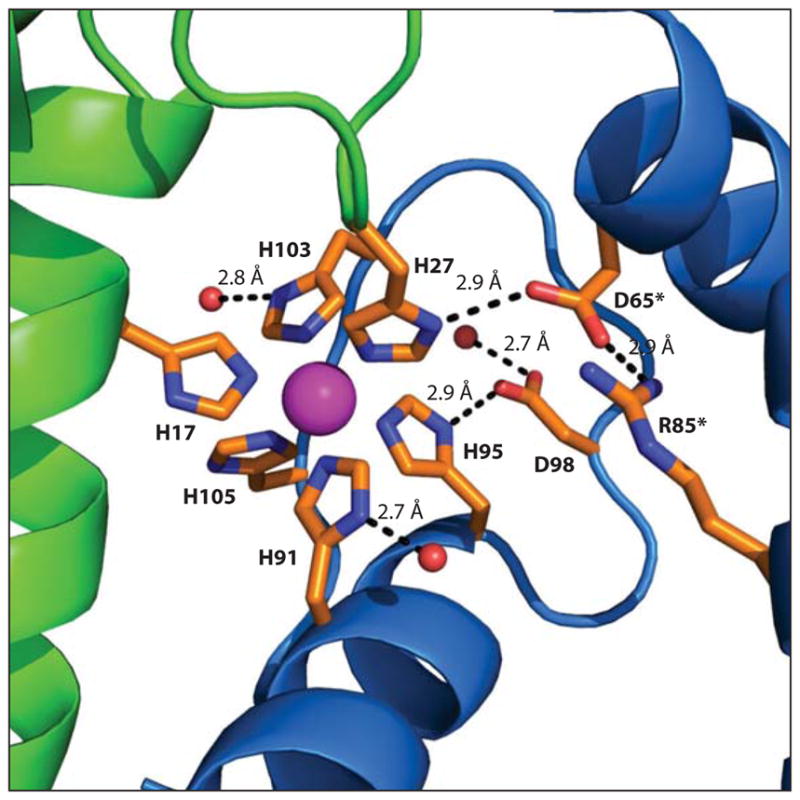
The S100A9 C-terminal tail and encapsulation of a Mn(II) ion at site 2 [Protein Data Bank (PDB) 4XJK]. S100A8 is shown in green, S100A9 is shown in blue, and the Mn(II) ion is shown in magenta. Primary and secondary coordination sphere residues are shown in orange. Water molecules are shown as red spheres. Hydrogen-bonding interactions are shown as black dashed lines and labeled with the internuclear distance. An asterisk (*) indicates residues from the S100A9 subunit in the other heterodimer of the heterotetramer.
Roles in Innate Immunity
Manganese is an essential nutrient and serves catalytic roles for a number of microbial enzymes involved in central metabolism and the oxidative stress response. Some microbial pathogens require manganese-containing enzymes such as superoxide dismutase (SOD) and ribonucleotide reductase (RNR) for viability and virulence in the host (56, 59–61). To illustrate the biological consequences of Mn(II) sequestration by CP, we present case studies that evaluate the battle between CP and microbes for Mn(II) and provide evidence of microbial adaptation to this stress.
Seminal investigations of CP-mediated Mn(II) starvation focused on S. aureus, a gram-positive opportunistic human pathogen that is sensitive to Mn(II) limitation (39, 62). S. aureus utilizes the high-affinity Mn(II) uptake system MntABC as well as the NRAMP (natural resistance-associated macrophage protein) transporter MntH to compete with CP for Mn(II) (39, 62). When S. aureus is challenged with CP, a Mn(II)-starvation response occurs, and expression of mntA and mntH increases 10- to 10,000-fold (62). Production of reactive oxygen species (ROS) is a mechanism of neutrophil-mediated host defense (63), and S. aureus utilizes SODs to protect itself from this oxidative burst. CP deprives S. aureus of Mn and inhibits its SOD activity, suggesting that CP attenuates S. aureus virulence by increasing its susceptibility to oxidative stress (45, 55). Nevertheless, further investigation revealed that one of the two Mn-dependent SODs expressed by S. aureus is cambialistic and exhibits comparable enzymatic activity with a Mn or an Fe cofactor, enabling S. aureus to adapt to Mn(II) starvation and resist oxidative stress by using the Fe-bound form of this SOD (64).
The use of high-affinity Mn(II) uptake systems to evade CP-mediated Mn(II) starvation and support ROS detoxifying enzymes has also been observed for the gram-negative gastrointestinal pathogen Salmonella enterica serovar Typhimurium (65). This pathogen uses Mn as a cofactor for an SOD and a catalase that provide resistance to the neutrophil-mediated oxidative burst. A murine model of infection showed that Salmonella Typhimurium utilizes the Mn(II)-uptake systems MntH and SitABCD to compete with CP and ultimately thrive in the inflamed gut.
To date, CP is the only known host-defense protein to sequester Mn(II), and many additional opportunities exist for examining this function. Evaluating the interplay between CP and other pathogens that require Mn(II) during infection and identifying additional cellular targets affected by Mn(II) limitation will be informative. Along these lines, Streptococcus pneumoniae may present an interesting case study. This human pathogen is sensitive to Mn(II) limitation, which perturbs metalation of a Mn phosphatase involved in cell division (66). The effect of CP on Mn phosphatases and other important Mn-requiring enzymes, including those employed by pathogens for deoxynucleotide biosynthesis and antibiotic resistance, warrants evaluation (56). Lastly, two recent reports demonstrated that Mn(II) sequestration by CP alters primary metabolism in S. aureus and Acinetobacter baumannii (67, 68), and future investigations will likely illuminate additional and currently unappreciated links between metal starvation and microbial central metabolism.
ZINC SEQUESTRATION
As noted above, the first insight into the function of CP arose from observations of an antimicrobial activity attributed to zinc limitation (Figure 1), and there is a rich history for consideration of the involvement of CP in Zn(II) homeostasis at the host–pathogen interface. In 2007, the crystal structure of Ca(II)-bound CP revealed the His3Asp and His4 motifs, and both of these sites were proposed to be Zn(II)-binding sites (43). Support for Zn(II) coordination at the His3Asp motif came from other Zn(II)-binding proteins, including S100A7 (49). In contrast, the notion of a native Zn(II)-His4 site in a protein lacked precedence (43). This crystal structure provided an initial guide for elucidating the Zn(II)-binding properties of CP in solution (38).
Coordination Chemistry
Solution studies established a 2:1 Zn(II):CP (heterodimer) stoichiometry in the absence and presence of Ca(II) and established that both sites 1 and 2 are high-affinity Zn(II) sites (38). The modulatory role of Ca(II) ions in transition metal binding was first recognized during these studies because Zn(II) competition titrations performed in the absence and presence of Ca(II) revealed that Ca(II) enhances the Zn(II)-binding affinities at both sites 1 and 2 (Table 1) (38). This initial investigation of Zn(II) binding was reported prior to the discovery of the Mn(II)-His6 site, and the coordination number of Zn(II) at site 2 was not elucidated yet. However, studies with Co(II) indicated that site 2 binds Co(II) in a six-coordinate geometry, which raised the possibility that unidentified protein residues contribute to the Zn(II) coordination sphere at this site (38). After the discovery of the Mn(II)-His6 motif and a serendipitous observation that Zn(II) binding protected the S100A9 C-terminal tail from proteolysis by proteinase K, further evaluation of Zn(II) binding at site 2 revealed the first example of a biological Zn(II)-His6 site (52). In particular, X-ray absorption spectroscopic data were consistent with Zn(II) in a His6 coordination environment at site 2. Moreover, Zn(II) competition titrations, designed to evaluate the contributions of H103 and H105 in the S100A9 C-terminal tail to Zn(II) binding, provided an improved assessment of the Zn(II) affinities of sites 1 and 2 (Table 1) and the first hint of cooperativity between the His3Asp and His6 sites of CP (52).
Roles in Innate Immunity
Zinc is the most abundant nonredox transition metal in biology, and it plays essential structural, catalytic, and signaling roles (69). Bacterial cells maintain a zinc quota of 0.1–0.5 mM and express ABC transporters to import Zn(II) and to maintain intracellular Zn(II) homeostasis (70–72). Thus, the interplay between CP and microbial Zn(II) acquisition machinery has been a topic of interest. Moreover, although reports of Zn(II) sequestration as an antimicrobial mechanism have populated the literature for decades, several notable examples of bacteria evading Zn(II) starvation by CP have been described recently. We consider these examples below.
The gram-negative pathogens Salmonella Typhimurium and A. baumannii express the high-affinity Zn(II) transporter ZnuABC to compete with CP for Zn(II) (73, 74). For both organisms, mutants defective in ZnuABC exhibit increased susceptibility to CP. A. baumannii possesses a second mechanism to resist Zn(II) limitation, which was uncovered during evaluation of the transcriptional response of A. baumannii to CP (74–76). This analysis revealed upregulation of ZnuABC and the Zn uptake repressor (Zur) as well as a gene that had been annotated as a GTPase and was named zigA (Zur-induced GTPase A) (74–76). Further investigations demonstrated that ZigA is a Zn(II)-responsive GTPase required for full growth in Zn(II)-limited conditions and afforded a model in which ZigA liberates a labile Zn(II) pool by promoting histidine degradation (76). Together with upregulation of ZnuABC, expression of ZigA promotes survival of A. baumannii when challenged with CP by increasing intracellular Zn(II) availability.
Helicobacter pylori, a pathogen known for its persistent colonization in the gastrointestinal tract, provides another example of how a pathogen adapts to CP (77). CP-deficient mice exhibit decreased H. pylori bacterial burdens in the stomach, suggesting that the fitness of H. pylori is enhanced in the presence of CP (77). Treatment of H. pylori with CP or the Zn(II)-sequestering protein S100A12 reduced the activity of the cag type 4 secretion system (cag T4SS), which promotes inflammation in gastric tissues (77, 78). These observations indicate that CP and Zn(II) availability affect the regulation of the H. pylori cag T4SS–mediated host inflammatory response.
The literature highlights additional strategies used by microbes to overcome Zn(II) sequestration by CP (79, 80). Two striking examples come from studies of pathogenic Neisseria species. Neisseria meningitidis and Neisseria gonorrhoeae express an outer membrane protein named CbpA (calprotectin-binding protein A) that captures metal-bound CP and extracts the bound metal (79, 80). Deciphering the molecular basis for CP capture and metal extraction by CbpA will undoubtedly set the stage for a broader consideration of this adaptation at the host–pathogen interface.
In closing, over the past three decades, many studies have pointed to the interplay between CP, microbes, and Zn(II), and a strong case can be made for the contributions of CP to Zn(II) with-holding and the ability of various pathogens to evade this host-defense mechanism. Nevertheless, it is important to consider that additional Zn(II)-sequestering host-defense factors exist and that the host-defense protein repertoires differ between mammals. Two other human S100 proteins, S100A7 and S100A12, house His3Asp motifs for Zn(II) sequestration and have been implicated in Zn(II) limitation during infection (81–84). The same cell types that produce and release CP also express and release S100A7 (epithelial cells) and S100A12 (neutrophils and epithelial cells) (81, 85). Thus, the human Zn(II)-withholding response is likely a combined effort that cannot be fully recapitulated by evaluating the role of each protein in isolation. Moreover, the presence of S100A7 or S100A12 may affect the availability of other metals. By lowering the bioavailable Zn(II) pool at an infection site, these proteins may boost the ability of CP to sequester other divalent metal ions like Mn(II) and Fe(II) at the His6 site (82). A thorough evaluation of the combined, and likely complex, effects of host-defense factors is an important area for future exploration.
IRON SEQUESTRATION
In 2015, the discovery that human CP sequesters Fe(II) at the His6 site was reported (51). Prior to this work, all studies addressing metal sequestration by CP had focused on Zn(II) and Mn(II). The possibility of a contribution to iron homeostasis was unappreciated at least in part because prior reports concluded that CP does not bind iron (39, 45). The Fe(II)-sequestering properties of CP were uncovered because two lines of reasoning motivated a reassessment of metal binding by CP (51). Guided by the Irving–Williams series as well as the Mn(II)- and Zn(II)-binding studies described above, it was reasoned that CP binds first-row transition metal ions that fall between Mn and Zn in the periodic table. From the perspective of microbial metal metabolism, microbes can be described as being more manganese centric or more iron centric (86), and CP has antimicrobial activity against microbes with very different manganese and iron requirements. Experiments that probed what metal ions CP depletes from a complex microbial growth medium uncovered that CP also binds iron, nickel, and copper with high affinity (Figure 1) (51). This observation motivated recent investigations of the interplay between CP and nutrient metals beyond Mn(II) and Zn(II).
Coordination Chemistry
CP sequesters iron in the ferrous (+2) oxidation state, and metal-binding studies have largely focused on its interactions with Fe(II) (51, 87). Moreover, a recent investigation demonstrated that the His6 site of CP causes a shift in the redox speciation of iron from Fe(III) to Fe(II) in solution under aerobic conditions (88). This behavior may have consequences for the redox speciation of iron at infection sites as well as iron availability.
The metal depletion experiments noted above indicated that site 2 is the high-affinity Fe(II) site, and a Mössbauer spectroscopy study established that site 2 houses a high-spin Fe(II) ion in an octahedral coordination sphere (51). Subsequent magnetic circular dichroism (MCD) spectroscopy provided further insight into the Fe(II)-His6 site, which expands the known coordination motifs of nonheme iron proteins (87). CP variants that lack H103 and H105 of the S100A9 C-terminal tail were evaluated, and the MCD spectroscopic analysis revealed that loss of a His residue resulted in a six-coordinate Fe(II) center with a bound hydroxide or water molecule in place of the missing histidine residue. This result is reminiscent of the EPR spectroscopic studies of Mn(II)-bound CP variants H103A and H105A that revealed water molecules in the vicinity of the Mn(II) center (46), providing further support for the notion that the S100A9 C-terminal tail blocks solvent access to the bound metal ion. Lastly, this work established that site 1 also binds Fe(II), albeit with lower affinity than site 2, and in a five-coordinate geometry.
The Fe(II) affinities are Ca(II) dependent, and the His6 site coordinates Fe(II) with subpicomolar affinity when excess Ca(II) ions are present (Table 1) (51). This affinity is remarkably high for a nonheme iron protein and contrasts with the reported dissociation constant values for other Fe(II)-binding proteins, which span the nanomolar to micromolar range (58). Moreover, metal substitution experiments showed that site 2 has a thermodynamic preference for Fe(II) over Mn(II). As observed for Mn(II) binding, mutation of H103 or H105 of the S100A9 C-terminal tail to alanine lowered the Fe(II) affinity of site 2, supporting the role of the tail in Fe(II) encapsulation (51).
Roles in Innate Immunity
Iron is the most abundant metal ion in biology and is an essential nutrient for virtually all microbial pathogens. Iron limitation by the host was the first recognized form of nutritional immunity (2). Established iron-chelating host-defense proteins such as lactoferrin and lipocalin-2 intercept iron in the ferric (+3) oxidation state (4, 5). The discovery that CP coordinates Fe(II) suggested that the host may use this protein to restrict the availability of ferrous iron, which persists in anaerobic or reducing environments. There is growing appreciation that infection sites can be reducing and anoxic and that microbial Fe(II) transport machineries contribute to host colonization (89–93). These considerations motivated exploratory biological studies that probed whether CP can function as an Fe(II)-sequestering protein at the host–microbe interface (51, 88). An initial evaluation of CP-mediated Fe(II) restriction under reducing conditions monitored radiolabeled 55Fe uptake and revealed that CP can block iron uptake into Escherichia coli and Pseudomonas aeruginosa (51).
Pathogens have clever ways to obtain iron, and several of these strategies involve the biosynthesis and deployment of secondary metabolites. Siderophores are Fe(III)-chelating small molecules that bacteria biosynthesize during periods of iron limitation and export to the extracellular space. These molecules sequester Fe(III) from the host environment and are ultimately internalized by bacteria that express the requisite ferric siderophore transport machinery (94). Phenazines are redox-active secondary metabolites that can affect iron speciation and availability (95). These redox-cycling molecules reduce Fe(III) to Fe(II) in the extracellular space and are predicted to facilitate Fe(II) uptake by the ferrous iron transporter FeoB (96). Thus, siderophores and phenazines provide case studies for examining the interplay between CP and microbial metabolites that influence metal availability and exist at infection sites. A recent investigation demonstrated that, under aerobic conditions, siderophores prevent CP-mediated reduction of Fe(III) to Fe(II) and hence the ability of CP to sequester iron (88). In contrast, the presence of pyocyanin, a phenazine employed by P. aeruginosa (97), enhances the ability of CP to capture Fe(II) under aerobic conditions (88).
In summary, these investigations provide a foundation for evaluating CP in Fe(II) homeostasis at the host–pathogen interface. Along these lines, most studies of CP have focused on acute infection models, which are associated with the rapid growth of planktonic bacteria and an environment where Fe(III) is expected to be the dominant oxidation state. In contrast, chronic infection sites are characterized by persistent biofilms and anoxic microenvironments. Fe(II) has been identified as a significant component of bioavailable iron in sputum from cystic fibrosis patients with chronic P. aeruginosa infections (91). CP was once named the cystic fibrosis antigen, and the chemical and redox environment in the cystic fibrosis lung may promote Fe(II) chelation by CP (88). Lastly, this work highlights the complexity of metal sequestration at the His6 site. Because CP is functionally versatile and can sequester multiple metal ions at the His6 site, this behavior must be considered when designing experiments and evaluating the antimicrobial activity of CP and microbial response to this stress.
NICKEL SEQUESTRATION
Although no Ni-requiring enzyme in humans has been identified, microbial pathogens use Ni as a cofactor for metalloenzymes implicated in virulence (98). Thus, the possibility that human CP captures Ni(II) presents an intriguing topic at the host–microbe interface and has motivated recent investigations of its Ni(II) coordination chemistry as well as the functional implications of Ni(II) sequestration (47).
Coordination Chemistry
A crystal structure of Ni(II)- and Ca(II)-bound CP revealed that both site 1 and site 2 coordinate Ni(II) (47). The Ni(II)- and Ca(II)-bound protein crystallized as a heterotetramer, and three Ni(II) ions were observed in the heterotetramer. Both His6 sites of the heterotetramer contained Ni(II), whereas only one of the His3Asp sites had electron density corresponding to bound Ni(II), which indicated that site 2 coordinates Ni(II) with greater affinity than site 1. Although no other naturally occurring Ni(II)-His6 site is known, this nickel site was expected because many nickel proteins contain six-coordinate Ni(II) sites with multiple histidine residues (58), and prior studies of CP showed that the His6 motif coordinates Mn(II), Fe(II), and Zn(II). In this structure, site 1 coordinates Ni(II) in a distorted tetrahedral geometry.
Some additional insights have emerged from solution studies of Ni(II) binding to CP (T.G. Nakashige, S.E.J. Bowman, E.M. Zygiel, C.L. Drennan, & E.M. Nolan, unpublished manuscript). In particular, the presence of Ca(II) ions enhances the Ni(II) affinity of CP. Although the Kd,Ni values for site 1 and site 2 have not been evaluated, metal substitution experiments revealed that (a) site 1 has a thermodynamic preference for Zn(II) over Ni(II) and (b) site 2 has a thermodynamic preference for Ni(II) over Zn(II) (47). The latter result supports the notion that CP can coordinate Ni(II) in the biological milieu.
Roles in Innate Immunity
To date, only one investigation has examined whether CP sequesters Ni(II) from microbes. This work focused on urease, a nickel enzyme that has been highlighted in the contexts of microbial virulence and infection (99). Urease catalyzes the hydrolysis of urea to produce ammonia and carbon dioxide, which buffers any acidic microenvironment encountered by a urease-positive organism. This enzyme has been shown to contribute to the virulence of the gastrointestinal pathogens H. pylori and Klebsiella pneumoniae (100–102). Furthermore, studies of S. aureus indicated that metal uptake systems involved in Ni acquisition, the NikABC and CntABC transporters, were required for urease activity in vitro and full virulence in a murine urinary tract infection model (103, 104). Treatment of S. aureus with CP reduced Ni uptake and urease activity (47). Attenuation of urease activity was also observed for K. pneumoniae when treated with CP. These results point to urease as a cellular target of CP-imposed Ni(II) deprivation.
Despite the established roles of Ni in microbial biology and pathogenesis, Ni(II) homeostasis in the context of the host–microbe interaction is virtually unexplored (105). The exploration of Ni(II) in nutritional immunity is particularly interesting given the scarcity of Ni(II) in the human body and the microbial dependence on nickel enzymes that is not shared by the human host (106–108). The initial demonstration that CP can block microbial Ni(II) uptake provides a foundation for further studies of the effect of CP on microbial processes involving additional Ni(II)-utilizing enzymes. For example, [NiFe]-hydrogenase is utilized by gram-negative organisms that colonize the gastrointestinal tract (109). Lastly, recent work has highlighted the role of staphylopine, a broad-spectrum metallophore that is imported by the Cnt transport system, in Ni(II) uptake by S. aureus (103, 104, 110). Examining the interplay between CP and staphylopine for Ni(II) and other divalent metal ions will likely illuminate the tug-of-war for bioavailable metal ions in S. aureus infections.
CONCLUDING REMARKS
This review provides an overview of the biological coordination chemistry employed by CP and its role in nutritional immunity. Major advances over the past decade have improved our under-standing of how CP contributes to metal homeostasis at the host–pathogen interface, and many unanswered questions about the chemistry and biology of this remarkable protein warrant exploration. We believe that the studies highlighted in this review provide a foundation for continued efforts at elucidating how CP functions in human health and disease.
Biophysical Properties
Many outstanding questions regarding coordination chemistry and biophysical properties of CP remain. Our understanding of Ca(II) binding remains in its infancy compared with that of transition metal coordination. The conversion from a heterodimer to a heterotetramer as a result of Ca(II) binding has been documented since the late 1990s (30–33), but we lack a molecular picture that describes this process. Evaluation of Ca(II) binding and Ca(II)-dependent oligomerization is likely to illuminate why the Ca(II)-bound form of CP exhibits increased transition metal affinities compared with the apo protein. Regarding the transition metal sites, recent work has hinted at cooperativity between the His3Asp and His6 sites, and further investigation of allostery is warranted (52). Lastly, we know much less about the His3Asp site than the His6 site, and further consideration of its metal-chelating properties as well as its putative physiological roles will be informative.
Intracellular Roles
Our current understanding of CP is largely in the context of the extracellular environment, and a paucity of information is available about intracellular roles of CP in metal homeostasis and host defense. Although the current model suggests that CP exists as a heterodimer in the cytoplasm, to the best of our knowledge, little evidence for the oligomeric state(s) of intracellular CP exists. Evaluation of its predominant intracellular form(s) and whether it interconverts between forms will be an important step toward deciphering its behavior in the cytoplasm. How CP affects the lifestyle and virulence of intracellular pathogens is virtually unexplored. One report described the Ca(II)-dependent mobilization of CP to microtubules following cellular invasion by Listeria monocytogenes (111). This study hints at interplay between CP and an intracellular pathogen, which warrants further investigation. Looking beyond metal homeostasis at the host–microbe interface, functional roles of intracellular S100A8 and S100A9 have been documented and include the regulation of differentiation, telomerase activity, and inflammatory responses (112). Whether one or more of these processes are influenced by the interplay between CP and transition metal ions is intriguing to consider.
Polymicrobial and Microbiome Interactions
The majority of studies of metal sequestration by CP focus on a single pathogen and studies performed in monoculture. Although this reductionist approach provides many important insights, it fails to recapitulate the complex host environment in which multiple microbes exist and multiple host-defense factors work in concert. A recent study examined the consequences of metal limitation by CP on polymicrobial cultures of S. aureus and P. aeruginosa, two dominant pathogens in cystic fibrosis lung disease (113). This work sets a precedent for evaluating CP beyond a simple one-protein one-pathogen model and for considering how CP modulates interactions between different microbial species. In a similar vein, commensal microbes also populate infection sites, and whether CP alters the viability in a beneficial or destructive manner requires consideration.
Beyond the Current Working Model: Fates and Functions of Metal-Bound CP
The current working model for CP ends with a Ca(II)- and transition metal–bound protein (Figure 3). At this point, CP has completed its functional role in nutritional immunity as we currently understand it, but questions remain: What happens next? What are the functions and fates of the metal-bound forms? Whether these metal-bound species have particular roles in human (patho)physiology is as yet undetermined. Although not covered in this review, elevated levels of CP are hallmarks of inflammatory conditions that include irritable bowel disease, Crohn’s disease, rheumatoid arthritis, and inflammatory muscle disease (11, 35, 114–117). Whether insights from the bioinorganic investigations of CP apply to these inflammatory conditions warrants evaluation.
Outlook
CP has provided the scientific community with three decades of discovery, which has expanded the scope of biological coordination chemistry and nutritional immunity. Recent studies of microbial interactions with CP have elucidated mechanisms of pathogen adaptation to metal limitation and have identified microbial factors that contribute to virulence. Taken together, these advances may provide inspirations for the development of untraditional antibiotic strategies. In closing, we believe that CP has many more lessons to teach us. We look forward to seeing the outcomes of future endeavors focused on understanding how this remarkable metal-chelating protein contributes to the biology of metals as well as to human health and disease.
SUMMARY POINTS.
CP is an abundant host-defense protein that imparts growth inhibitory activity against bacteria and fungi by sequestering nutrient metals.
CP is a Ca(II)-binding protein. The apo protein is a heterodimer of S100A8 and S100A9, and the Ca(II)-bound form is a (S100A8/S100A9)2 heterotetramer. The Ca(II)-bound heterotetramer exhibits enhanced transition metal affinities, enhanced antimicrobial activity, and protease resistance.
CP houses two transition metal–binding sites at the S100A8/S100A9 interface, a His3Asp motif (site 1) and a His6 motif (site 2).
The His3Asp motif (site 1) binds Zn(II) with high affinity and contributes to Zn(II) sequestration.
The His6 motif (site 2) binds several divalent first-row transition metals with relative affinities in agreement with the Irving–Williams series (Kd,Mn > Kd,Fe > Kd,Zn > Kd,Ni).
The His6 site and the S100A9 C-terminal tail present a remarkable solution to overcoming the kinetic lability of divalent first-row transition metal ions.
CP can prevent microbial uptake of first-row transition metals and inhibit microbial enzymes that utilize these metals for catalytic activity.
In some cases, microbes resist CP-mediated metal starvation by expressing high-affinity metal-uptake systems, using an alternative metal cofactor in an important enzyme, or altering central metabolism.
Acknowledgments
Current research on metal-sequestering host-defense proteins is supported by the National Institutes of Health (R01 GM118695 and R01 GM126376) and the National Science Foundation (NSF) (CHE-1352132). E.M.Z. is a recipient of an NSF Graduate Research Fellowship.
Abbreviation
- Nutritional immunity
a term first coined in 1975 to describe iron limitation; now used to broadly describe metal nutrient limitation by a host
- S100 proteins
a class of low-molecular-weight, calcium-binding proteins found in vertebrates
- EF-hand
a common structural motif of calcium-binding proteins characterized by a helix-loop-helix conformation that forms a calcium-binding site
- Metal sequestration
high-affinity binding of metal ions that results in metal entrapment
- Neutrophil extracellular traps (NETs)
a product of neutrophil death that results in the release of chromatin, entrapping pathogens and preventing host cell damage
- Irving–Williams series
a tenet describing the relative affinities of a given octahedral coordination sphere for first-row transition metals, as Mn < Fe < Co < Ni < Cu > Zn
- ABC transporters (ATP-binding cassette transporters)
couple ATP hydrolysis to substrate translocation across the membrane
- Hard–soft acid–base theory
describes favorable interactions between ions and donors with similar electron density and polarizability
- Superoxide dismutase (SOD)
catalyzes the removal of superoxide (O2−•) to form oxygen (O2) and hydrogen peroxide (H2O2)
- Ribonucleotide reductase (RNR)
an essential enzyme that contributes to DNA synthesis by catalyzing the formation of deoxynucleotides from ribonucleotides
- Reactive oxygen species (ROS)
oxygen-containing compounds that contain radicals, many of which are highly reactive and can oxidize biomolecules
- Cooperativity
for a molecule with multiple binding sites, this term describes the altered affinity of a second binding event following a first
- [NiFe]-hydrogenase
a Ni- and Fe-requiring enzyme that catalyzes the reversible oxidation of molecular hydrogen (H2)
Footnotes
NOTE ADDED IN PROOF
Recent work has revealed further insight into the antimicrobial roles of CP in the context of Mn(II) and Zn(II) sequestration during S. aureus infection, presenting (a) a situation in which CP does not effectively starve S. aureus of Mn(II) during bacterial endocarditis (118), (b) the competition of CP with the S. aureus metallophore named staphylopine for Zn(II) (119), and (c) a biophysical investigation of Mn(II) competition between human CP and the solute-binding proteins MntC and PsaA of S. aureus and S. pneumoniae, respectively (120). Furthermore, a study of host-mediated metal withholding from C. albicans has provided further evidence for CP-mediated Zn(II) starvation as well as the first report of Cu(II) sequestration by CP (121). This work provides motivation for deciphering the Cu(II) coordination chemistry of CP, including whether the transition metal–binding sites have thermodynamic preference for Cu(II) over Zn(II) and other divalent first-row transition metal ions.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Waldron KJ, Rutherford JC, Ford D, Robinson NJ. Metalloproteins and metal sensing. Nature. 2009;460:823–30. doi: 10.1038/nature08300. [DOI] [PubMed] [Google Scholar]
- 2.Weinberg ED. Nutritional immunity. Host’s attempt to withhold iron from microbial invaders. JAMA. 1975;231:39–41. doi: 10.1001/jama.231.1.39. [DOI] [PubMed] [Google Scholar]
- 3.Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen–host interface. Nat Rev Microbiol. 2012;10:525–37. doi: 10.1038/nrmicro2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogel HJ. Lactoferrin, a bird’s eye view. Biochem Cell Biol. 2012;90:233–44. doi: 10.1139/o2012-016. [DOI] [PubMed] [Google Scholar]
- 5.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–43. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 6.Fagerhol MK, Dale I, Andersson T. Release and quantitation of a leucocyte derived protein (L1) Scand J Haematol. 1980;24:393–98. [Google Scholar]
- 7.Dale I, Fagerhol MK, Naesgaard I. Purification and partial characterization of a highly immunogenic human leucocyte protein, the L1 antigen. Eur J Biochem. 1983;134:1–6. doi: 10.1111/j.1432-1033.1983.tb07522.x. [DOI] [PubMed] [Google Scholar]
- 8.Brandtzaeg P, Dale I, Fagerhol MK. Distribution of a formalin-resistant myelomonocytic antigen (L1) in human tissues. Am J Clin Pathol. 1987;87:681–99. doi: 10.1093/ajcp/87.6.681. [DOI] [PubMed] [Google Scholar]
- 9.Bullock S, Hayward C, Manson J, Brock DJH, Raeburn JA. Quantitative immunoassays for diagnosis of carrier detection in cystic fibrosis. Clin Genet. 1982;21:336–41. doi: 10.1111/j.1399-0004.1982.tb01380.x. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson MM, Busuttil A, Hayward C, Brock DJH, Dorin JR, van Heyningen V. Expression pattern of two related cystic fibrosis-associated calcium binding proteins in normal and abnormal tissues. J Cell Sci. 1988;91:221–30. doi: 10.1242/jcs.91.2.221. [DOI] [PubMed] [Google Scholar]
- 11.Odink K, Cerletti N, Brüggen J, Clerc RG, Tarcsay L, et al. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature. 1987;330:80–82. doi: 10.1038/330080a0. [DOI] [PubMed] [Google Scholar]
- 12.Dorin JR, Novak M, Hill RE, Brock DJH, Secher DS, van Heyningen V. A clue to the basic defect from cloning the CF antigen gene. Nature. 1987;326:614–17. doi: 10.1038/326614a0. [DOI] [PubMed] [Google Scholar]
- 13.Andersson KB, Sletten K, Berntzen HB, Dale I, Brandtzaeg P, et al. The leucocyte L1 protein: identity with the cystic fibrosis antigen and the calcium-binding MRP-8 and MRP-14 macrophage components. Scand J Immunol. 1988;28:241–45. doi: 10.1111/j.1365-3083.1988.tb02437.x. [DOI] [PubMed] [Google Scholar]
- 14.McNamara MP, Wiessner JH, Collins-Lech C, Hahn BL, Sohnle PG. Neutrophil death as a defence mechanism against Candida albicans infections. Lancet. 1988;332:1163–65. doi: 10.1016/s0140-6736(88)90234-6. [DOI] [PubMed] [Google Scholar]
- 15.Steinbakk M, Naess-Andresen C-F, Lingaas E, Dale I, Brandtzaeg P, Fagerhol MK. Antimicrobial actions of calcium binding leukocyte L1 protein, calprotectin. Lancet. 1990;336:763–65. doi: 10.1016/0140-6736(90)93237-j. [DOI] [PubMed] [Google Scholar]
- 16.Sohnle PG, Collins-Lech C, Wiessner JH. Antimicrobial activity of an abundant calcium-binding protein in the cytoplasm of human neutrophils. J Infect Dis. 1991;163:187–92. doi: 10.1093/infdis/163.1.187. [DOI] [PubMed] [Google Scholar]
- 17.Sohnle PG, Collins-Lech C, Wiessner JH. The zinc-reversible antimicrobial activity of neutrophil lysates and abscess fluid supernatants. J Infect Dis. 1991;164:137–42. doi: 10.1093/infdis/164.1.137. [DOI] [PubMed] [Google Scholar]
- 18.Miyasaki KT, Bodeau AL, Murthy ARK, Lehrer RI. In vitro antimicrobial activity of the human neutrophil cytosolic S-100 protein complex, calprotectin, against Capnocytophaga sputigena. J Dent Res. 1993;72:517–23. doi: 10.1177/00220345930720020801. [DOI] [PubMed] [Google Scholar]
- 19.Murthy ARK, Lehrer RI, Harwig SSL, Miyasaki KT. In vitro candidastatic properties of the human neutrophil calprotectin complex. J Immunol. 1993;151:6291–301. [PubMed] [Google Scholar]
- 20.Lusitani D, Malawista SE, Montgomery RR. Calprotectin, an abundant cytosolic protein from human polymorphonuclear leukocytes, inhibits the growth of Borrelia burgdorferi. Infect Immun. 2003;71:4711–16. doi: 10.1128/IAI.71.8.4711-4716.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bianchi M, Niemiec MJ, Siler U, Urban CF, Reichenbach J. Restoration of anti-Aspergillus defense by neutrophil extracellular traps in human chronic granulomatous disease after gene therapy is calprotectin-dependent. J Allergy Clin Immunol. 2011;127:1243–52. doi: 10.1016/j.jaci.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Clohessy PA, Golden BE. Calprotectin-mediated zinc chelation as a biostatic mechanism in host defence. Scand J Immunol. 1995;42:551–56. doi: 10.1111/j.1365-3083.1995.tb03695.x. [DOI] [PubMed] [Google Scholar]
- 23.Loomans HJ, Hahn BL, Li Q-Q, Phadnis SH, Sohnle PG. Histidine-based zinc-binding sequences and the antimicrobial activity of calprotectin. J Infect Dis. 1998;177:812–14. doi: 10.1086/517816. [DOI] [PubMed] [Google Scholar]
- 24.Sohnle PG, Hunter MJ, Hahn B, Chazin WJ. Zinc-reversible antimicrobial activity of recombinant calprotectin (migration inhibitory factor-related proteins 8 and 14) J Infect Dis. 2000;182:1272–75. doi: 10.1086/315810. [DOI] [PubMed] [Google Scholar]
- 25.Leukert N, Sorg C, Roth J. Molecular basis of the complex formation between the two calcium-binding proteins S100A8 (MRP8) and S100A9 (MRP14) Biol Chem. 2005;386:429–34. doi: 10.1515/BC.2005.051. [DOI] [PubMed] [Google Scholar]
- 26.Pröpper C, Huang X, Roth J, Sorg C, Nacken W. Analysis of the MRP8-MRP14 protein-protein interaction by the two-hybrid system suggests a prominent role of the C-terminal domain of S100 proteins in dimer formation. J Biol Chem. 1999;274:183–88. doi: 10.1074/jbc.274.1.183. [DOI] [PubMed] [Google Scholar]
- 27.Hunter MJ, Chazin WJ. High level expression and dimer characterization of the S100 EF-hand proteins, migration inhibitory factor-related proteins 8 and 14. J Biol Chem. 1998;273:12427–35. doi: 10.1074/jbc.273.20.12427. [DOI] [PubMed] [Google Scholar]
- 28.Gifford JL, Walsh MP, Vogel HJ. Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. Biochem J. 2007;405:199–221. doi: 10.1042/BJ20070255. [DOI] [PubMed] [Google Scholar]
- 29.Chazin WJ. Relating form and function of EF-hand calcium binding proteins. Acc Chem Res. 2011;44:171–79. doi: 10.1021/ar100110d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strupat K, Rogniaux H, Van Dorsselaer A, Roth J, Vogl T. Calcium-induced noncovalently linked tetramers of MRP8 and MRP14 are confirmed by electrospray ionization-mass analysis. J Am Soc Mass Spectrom. 2000;11:780–88. doi: 10.1016/S1044-0305(00)00150-1. [DOI] [PubMed] [Google Scholar]
- 31.Leukert N, Vogl T, Strupat K, Reichelt R, Sorg C, Roth J. Calcium-dependent tetramer formation of S100A8 and S100A9 is essential for biological activity. J Mol Biol. 2006;359:961–72. doi: 10.1016/j.jmb.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Stephan JR, Nolan EM. Calcium-induced tetramerization and zinc chelation shield human calprotectin from degradation by host and bacterial extracellular proteases. Chem Sci. 2016;7:1962–75. doi: 10.1039/c5sc03287c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teigelkamp S, Bhardwaj RS, Roth J, Meinardus-Hager G, Karas M, Sorg C. Calcium-dependent complex assembly of the myeloic differentiation proteins MRP-8 and MRP-14. J Biol Chem. 1991;266:13462–67. [PubMed] [Google Scholar]
- 34.Dale I, Brandtzaeg P, Fagerhol MK, Scott H. Distribution of a new myelomonocytic antigen (L1) in human peripheral blood leukocytes: immunofluorescence and immunoperoxidase staining features in comparison with lysozyme and lactoferrin. Am J Clin Pathol. 1985;84:24–34. doi: 10.1093/ajcp/84.1.24. [DOI] [PubMed] [Google Scholar]
- 35.Johne B, Fagerhol MK, Lyberg T, Prydz H, Brandtzaeg P, et al. Functional and clinical aspects of the myelomonocyte protein calprotectin. J Clin Pathol Mol Pathol. 1997;50:113–23. doi: 10.1136/mp.50.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLOS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brini M, Ottolini D, Calì T, Carafoli E. Calcium in health and disease. Met Ions Life Sci. 2013;20:87–93. doi: 10.1007/978-94-007-7500-8_4. [DOI] [PubMed] [Google Scholar]
- 38.Brophy MB, Hayden JA, Nolan EM. Calcium ion gradients modulate the zinc affinity and antibacterial activity of human calprotectin. J Am Chem Soc. 2012;134:18089–100. doi: 10.1021/ja307974e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319:962–65. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- 40.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–57. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 41.Zhao L, Lu W. Defensins in innate immunity. Curr Opin Hematol. 2014;21:37–42. doi: 10.1097/MOH.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 42.Nacken W, Kerkhoff C. The hetero-oligomeric complex of the S100A8/S100A9 protein is extremely protease resistant. FEBS Lett. 2007;581:5127–30. doi: 10.1016/j.febslet.2007.09.060. [DOI] [PubMed] [Google Scholar]
- 43.Korndörfer IP, Brueckner F, Skerra A. The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting α-helices can determine specific association of two EF-hand proteins. J Mol Biol. 2007;370:887–98. doi: 10.1016/j.jmb.2007.04.065. [DOI] [PubMed] [Google Scholar]
- 44.Clohessy PA, Golden BE. His-X-X-X-His motif in S100 protein, calprotectin: relation to microbiostatic activity. J Leukoc Biol. 1996;60:674. doi: 10.1002/jlb.60.5.674. [DOI] [PubMed] [Google Scholar]
- 45.Damo SM, Kehl-Fie TE, Sugitani N, Holt ME, Rathi S, et al. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. PNAS. 2013;110:3841–46. doi: 10.1073/pnas.1220341110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gagnon DM, Brophy MB, Bowman SEJ, Stich TA, Drennan CL, et al. Manganese binding properties of human calprotectin under conditions of high and low calcium: X-ray crystallographic and advanced electron paramagnetic resonance spectroscopic analysis. J Am Chem Soc. 2015;137:3004–16. doi: 10.1021/ja512204s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakashige TG, Zygiel EM, Drennan CL, Nolan EM. Nickel sequestration by the host-defense protein human calprotectin. J Am Chem Soc. 2017;139:8828–36. doi: 10.1021/jacs.7b01212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brodersen DE, Nyborg J, Kjeldgaard M. Zinc-binding site of an S100 protein revealed. Two crystal structures of Ca2+-bound human psoriasin (S100A7) in the Zn2+-loaded and Zn2+-free states. Biochemistry. 1999;38:1695–704. doi: 10.1021/bi982483d. [DOI] [PubMed] [Google Scholar]
- 49.Moroz OV, Blagova EV, Wilkinson AJ, Wilson KS, Bronstein IB. The crystal structures of human S100A12 in apo form and in complex with zinc: new insights into S100A12 oligomerisation. J Mol Biol. 2009;391:536–51. doi: 10.1016/j.jmb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Hayden JA, Brophy MB, Cunden LS, Nolan EM. High-affinity manganese coordination by human calprotectin is calcium-dependent and requires the histidine-rich site formed at the dimer interface. J Am Chem Soc. 2013;135:775–87. doi: 10.1021/ja3096416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakashige TG, Zhang B, Krebs C, Nolan EM. Human calprotectin is an iron-sequestering host-defense protein. Nat Chem Biol. 2015;11:765–71. doi: 10.1038/nchembio.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakashige TG, Stephan JR, Cunden LS, Brophy MB, Wommack AJ, et al. The hexahistidine motif of host-defense protein human calprotectin contributes to zinc withholding and its functional versatility. J Am Chem Soc. 2016;138:12243–51. doi: 10.1021/jacs.6b06845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Irving I, Williams RJP. Irving Williams series. Nature. 1948;162:746–47. [Google Scholar]
- 54.Brophy MB, Nakashige TG, Gaillard A, Nolan EM. Contributions of the S100A9 C-terminal tail to high-affinity Mn(II) chelation by the host-defense protein human calprotectin. J Am Chem Soc. 2013;135:17804–17. doi: 10.1021/ja407147d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kehl-Fie TE, Chitayat S, Hood MI, Damo S, Restrepo N, et al. Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe. 2011;10:158–64. doi: 10.1016/j.chom.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brophy MB, Nolan EM. Manganese and microbial pathogenesis: sequestration by the mammalian immune system and utilization by microorganisms. ACS Chem Biol. 2015;10:641–51. doi: 10.1021/cb500792b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harding MM, Nowicki MW, Walkinshaw MD. Metals in protein structures: a review of their principal features. Crystallogr Rev. 2010;16:247–302. [Google Scholar]
- 58.Cotruvo JA, Jr, Stubbe J. Metallation and mismetallation of iron and manganese proteins in vitro and in vivo: the class I ribonucleotide reductases as a case study. Metallomics. 2012;4:1020–36. doi: 10.1039/c2mt20142a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schimpff-Weiland G, Follmann H, Auling G. A new manganese-activated ribonucleotide reductase found in Gram-positive bacteria. Biochem Biophys Res Commun. 1981;102:1276–82. doi: 10.1016/s0006-291x(81)80149-0. [DOI] [PubMed] [Google Scholar]
- 60.Cotruvo JA, Jr, Stubbe J. An active dimanganese(III)–tyrosyl radical cofactor in Escherichia coli class Ib ribonucleotide reductase. Biochemistry. 2010;49:1297–309. doi: 10.1021/bi902106n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gregory EM, Fridovich I. Oxygen toxicity and the superoxide dismutase. J Bacteriol. 1973;114:1193–97. doi: 10.1128/jb.114.3.1193-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kehl-Fie TE, Zhang Y, Moore JL, Farrand AJ, Hood MI, et al. MntABC and MntH contribute to systemic Staphylococcus aureus infection by competing with calprotectin for nutrient manganese. Infect Immun. 2013;81:3395–405. doi: 10.1128/IAI.00420-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winterbourn CC, Kettle AJ, Hampton MB. Reactive oxygen species and neutrophil function. Annu Rev Biochem. 2016;85:765–92. doi: 10.1146/annurev-biochem-060815-014442. [DOI] [PubMed] [Google Scholar]
- 64.Garcia YM, Barwinska-Sendra A, Tarrant E, Skaar EP, Waldron KJ, Kehl-Fie TE. A superoxide dismutase capable of functioning with iron or manganese promotes the resistance of Staphylococcus aureus to calprotectin and nutritional immunity. PLOS Pathog. 2017;13:e1006125. doi: 10.1371/journal.ppat.1006125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diaz-Ochoa VE, Lam D, Lee CS, Klaus S, Behnsen J, et al. Salmonella mitigates oxidative stress and thrives in the inflamed gut by evading calprotectin-mediated manganese sequestration. Cell Host Microbe. 2016;19:814–25. doi: 10.1016/j.chom.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin JE, Lisher JP, Winkler ME, Giedroc DP. Perturbation of manganese metabolism disrupts cell division in Streptococcus pneumoniae. Mol Microbiol. 2017;104:334–48. doi: 10.1111/mmi.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Radin JN, Kelliher JL, Párraga Solórzano PK, Kehl-Fie TE. The two-component system ArlRS and alterations in metabolism enable Staphylococcus aureus to resist calprotectin-induced manganese starvation. PLOS Pathog. 2016;12:e1006040. doi: 10.1371/journal.ppat.1006040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Juttukonda LJ, Chazin WJ, Skaar EP. Acinetobacter baumannii coordinates urea metabolism with metal import to resist host-mediated metal limitation. mBio. 2016;7:e01475–16. doi: 10.1128/mBio.01475-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 70.Eide DJ. Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta. 2006;1763:711–22. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 71.Outten CE, O’Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–92. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 72.Hantke K. Bacterial zinc uptake and regulators. Curr Opin Microbiol. 2005;8:196–202. doi: 10.1016/j.mib.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 73.Liu JZ, Jellbauer S, Poe AJ, Ton V, Pesciaroli M, et al. Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe. 2012;11:227–39. doi: 10.1016/j.chom.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hood MI, Mortensen BL, Moore JL, Zhang Y, Kehl-Fie TE, et al. Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLOS Pathog. 2012;8:e1003068. doi: 10.1371/journal.ppat.1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mortensen BL, Rathi S, Chazin WJ, Skaar EP. Acinetobacter baumannii response to host-mediated zinc limitation requires the transcriptional regulator Zur. J Bacteriol. 2014;196:2616–26. doi: 10.1128/JB.01650-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nairn BL, Lonergan ZR, Wang J, Braymer JJ, Zhang Y, et al. The response of Acinetobacter baumannii to zinc starvation. Cell Host Microbe. 2016;19:826–36. doi: 10.1016/j.chom.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gaddy JA, Radin JN, Loh JT, Piazuelo MB, Kehl-Fie TE, et al. The host protein calprotectin modulates the Helicobacter pylori cag type IV secretion system via zinc sequestration. PLOS Pathog. 2014;10:e1004450. doi: 10.1371/journal.ppat.1004450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haley KP, Delgado AG, Piazuelo MB, Mortensen BL, Correa P, et al. The human antimicrobial protein calgranulin C participates in control of Helicobacter pylori growth and regulation of virulence. Infect Immun. 2015;83:2944–56. doi: 10.1128/IAI.00544-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jean S, Juneau RA, Criss AK, Cornelissen CN. Neisseria gonorrhoeae evades calprotectin-mediated nutritional immunity and survives neutrophil extracellular traps by production of TdfH. Infect Immun. 2016;84:2982–94. doi: 10.1128/IAI.00319-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stork M, Grijpstra J, Bos MP, Torres CM, Devos N, et al. Zinc piracy as a mechanism of Neisseria meningitidis for evasion of nutritional immunity. PLOS Pathog. 2013;9:e1003733. doi: 10.1371/journal.ppat.1003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vogl T, Pröpper C, Hartmann M, Strey A, Strupat K, et al. S100A12 is expressed exclusively by granulocytes and acts independently from MRP8 and MRP14. J Biol Chem. 1999;274:25291–96. doi: 10.1074/jbc.274.36.25291. [DOI] [PubMed] [Google Scholar]
- 82.Cunden LS, Gaillard A, Nolan EM. Calcium ions tune the zinc-sequestering properties and antimicrobial activity of human S100A12. Chem Sci. 2016;7:1338–48. doi: 10.1039/c5sc03655k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gläser R, Harder J, Lange H, Bartels J, Christophers E, Schröder J-M. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat Immunol. 2005;6:57–64. doi: 10.1038/ni1142. [DOI] [PubMed] [Google Scholar]
- 84.Cunden LS, Brophy MB, Rodriguez GE, Flaxman HA, Nolan EM. Biochemical and functional evaluation of the intramolecular disulfide bonds in the zinc-chelating antimicrobial protein human S100A7 (psoriasin) Biochemistry. 2017;56:5726–38. doi: 10.1021/acs.biochem.7b00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Madsen P, Rasmussen HH, Leffers H, Honoré B, Celis JE. Molecular cloning and expression of a novel keratinocyte protein (psoriasis-associated fatty acid-binding protein [PA-FABP]) that is highly up-regulated in psoriatic skin and that shares similarity to fatty acid-binding proteins. J Invest Dermatol. 1992;99:299–305. doi: 10.1111/1523-1747.ep12616641. [DOI] [PubMed] [Google Scholar]
- 86.Lisher JP, Giedroc DP. Manganese acquisition and homeostasis at the host-pathogen interface. Front Cell Infect Microbiol. 2013;3:91. doi: 10.3389/fcimb.2013.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baker TM, Nakashige TG, Nolan EM, Neidig ML. Magnetic circular dichroism studies of iron(II) binding to human calprotectin. Chem Sci. 2017;8:1369–77. doi: 10.1039/c6sc03487j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakashige TG, Nolan EM. Human calprotectin affects the redox speciation of iron. Metallomics. 2017;9:1086–95. doi: 10.1039/c7mt00044h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lau CKY, Krewulak KD, Vogel HJ. Bacterial ferrous iron transport: the Feo system. FEMS Microbiol Rev. 2015;40:273–98. doi: 10.1093/femsre/fuv049. [DOI] [PubMed] [Google Scholar]
- 90.Konings AF, Martin LW, Sharples KJ, Roddam LF, Latham R, et al. Pseudomonas aeruginosa uses multiple pathways to acquire iron during chronic infection in cystic fibrosis lungs. Infect Immun. 2013;81:2697–704. doi: 10.1128/IAI.00418-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hunter RC, Asfour F, Dingemans J, Osuna BL, Samad T, et al. Ferrous iron is a significant component of bioavailable iron in cystic fibrosis airways. mBio. 2013;4:e00557–13. doi: 10.1128/mBio.00557-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hunter RC, Klepac-Ceraj V, Lorenzi MM, Grotzinger H, Martin TR, Newman DK. Phenazine content in the cystic fibrosis respiratory tract negatively correlates with lung function and microbial complexity. Am J Respir Cell Mol Biol. 2012;47:738–45. doi: 10.1165/rcmb.2012-0088OC. [DOI] [PubMed] [Google Scholar]
- 93.Cowley ES, Kopf SH, LaRiviere A, Ziebis W, Newman DK. Pediatric cystic fibrosis sputum can be chemically dynamic, anoxic, and extremely reduced due to hydrogen sulfide formation. mBio. 2015;6:e00767–15. doi: 10.1128/mBio.00767-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hider RC, Kong X. Chemistry and biology of siderophores. Nat Prod Rep. 2010;27:637–57. doi: 10.1039/b906679a. [DOI] [PubMed] [Google Scholar]
- 95.Glasser NR, Saunders SH, Newman DK. The colorful world of extracellular electron shuttles. Annu Rev Microbiol. 2017;71:731–51. doi: 10.1146/annurev-micro-090816-093913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Y, Wilks JC, Danhorn T, Ramos I, Croal L, Newman DK. Phenazine-1-carboxylic acid promotes bacterial biofilm development via ferrous iron acquisition. J Bacteriol. 2011;193:3606–17. doi: 10.1128/JB.00396-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lau GW, Hassett DJ, Ran H, Kong F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol Med. 2004;10:599–606. doi: 10.1016/j.molmed.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 98.Mulrooney SB, Hausinger RP. Nickel uptake and utilization by microorganisms. FEMS Microbiol Rev. 2003;27:239–61. doi: 10.1016/S0168-6445(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 99.Rutherford JC. The emerging role of urease as a general microbial virulence factor. PLOS Pathog. 2014;10:e1004062. doi: 10.1371/journal.ppat.1004062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maroncle N, Rich C, Forestier C. The role of Klebsiella pneumoniae urease in intestinal colonization and resistance to gastrointestinal stress. Res Microbiol. 2006;157:184–93. doi: 10.1016/j.resmic.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 101.Hedelin H. Uropathogens and urinary tract concretion formation and catheter encrustations. Int J Antimicrob Agents. 2002;19:484–87. doi: 10.1016/s0924-8579(02)00095-x. [DOI] [PubMed] [Google Scholar]
- 102.de Reuse H, Vinella D, Cavazza C. Common themes and unique proteins for the uptake and trafficking of nickel, a metal essential for the virulence of Helicobacter pylori. Front Cell Infect Microbiol. 2013;3:94. doi: 10.3389/fcimb.2013.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hiron A, Posteraro B, Carrière M, Remy L, Delporte C, et al. A nickel ABC-transporter of Staphylococcus aureus is involved in urinary tract infection. Mol Microbiol. 2010;77:1246–60. doi: 10.1111/j.1365-2958.2010.07287.x. [DOI] [PubMed] [Google Scholar]
- 104.Remy L, Carrière M, Derré-Bobillot A, Martini C, Sanguinetti M, Borezeé-Durant E. The Staphylococcus aureus Opp1 ABC transporter imports nickel and cobalt in zinc-depleted conditions and contributes to virulence. Mol Microbiol. 2013;87:730–43. doi: 10.1111/mmi.12126. [DOI] [PubMed] [Google Scholar]
- 105.Zeer-Wanklyn CJ, Zamble DB. Microbial nickel: cellular uptake and delivery to enzyme centers. Curr Opin Chem Biol. 2017;37:80–88. doi: 10.1016/j.cbpa.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 106.Mushak P. Metabolism and systemic toxicity of nickel. In: Nriagu JO, editor. Nickel in the Environment. New York: Wiley; 1980. pp. 499–523. [Google Scholar]
- 107.Herber RFM. Review of trace element concentrations in biological specimens according to the TRACY protocol. Int Arch Occup Environ Health. 1999;72:279–83. doi: 10.1007/s004200050374. [DOI] [PubMed] [Google Scholar]
- 108.Ragsdale SW. Nickel-based enzyme systems. J Biol Chem. 2009;284:18571–75. doi: 10.1074/jbc.R900020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang G, Romero-Gallo J, Benoit SL, Piazuelo MB, Dominguez RL, et al. Hydrogen metabolism in Helicobacter pylori plays a role in gastric carcinogenesis through facilitating CagA translocation. mBio. 2016;7:e01022–16. doi: 10.1128/mBio.01022-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ghssein G, Brutesco C, Ouerdane L, Fojcik C, Izaute A, et al. Biosynthesis of a broad-spectrum nicotianamine-like metallophore in Staphylococcus aureus. Science. 2016;352:1105–9. doi: 10.1126/science.aaf1018. [DOI] [PubMed] [Google Scholar]
- 111.Zaia AA, Sappington KJ, Nisapakultorn K, Chazin WJ, Dietrich EA, et al. Subversion of antimicrobial calprotectin (S100A8/S100A9 complex) in the cytoplasm of TR146 epithelial cells after invasion by Listeria monocytogenes. Mucosal Immunol. 2009;2:43–53. doi: 10.1038/mi.2008.63. [DOI] [PubMed] [Google Scholar]
- 112.Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, et al. Functions of S100 proteins. Curr Mol Med. 2013;13:24–57. [PMC free article] [PubMed] [Google Scholar]
- 113.Wakeman CA, Moore JL, Noto MJ, Zhang Y, Singleton MD, et al. The innate immune protein calprotectin promotes Pseudomonas aeruginosa and Staphylococcus aureus interaction. Nat Commun. 2016;7:11951. doi: 10.1038/ncomms11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goyette J, Geczy CL. Inflammation-associated S100 proteins: new mechanisms that regulate function. Amino Acids. 2011;41:821–42. doi: 10.1007/s00726-010-0528-0. [DOI] [PubMed] [Google Scholar]
- 115.Foell D, Roth J. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum. 2004;50:3762–71. doi: 10.1002/art.20631. [DOI] [PubMed] [Google Scholar]
- 116.Stříž I, Trebichavský I. Calprotectin—a pleiotropic molecule in acute and chronic inflammation. Physiol Res. 2004;53:245–53. [PubMed] [Google Scholar]
- 117.Seeliger S, Vogl T, Engels IH, Schröder JM, Sorg C, et al. Expression of calcium-binding proteins MRP8 and MRP14 in inflammatory muscle disease. Am J Pathol. 2003;163:947–56. doi: 10.1016/S0002-9440(10)63454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Juttukonda LJ, Berends ETM, Zackular JP, Moore JL, Stier MT, et al. Dietary manganese promotes staphylococcal infection of the heart. Cell Host Microbe. 2017;22:531–42. doi: 10.1016/j.chom.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Grim KP, San Francisco B, Radin JN, Brazel EB, Kelliher JL, et al. The metallophore staphylopine enables Staphylococcus aureus to compete with the host for zinc and overcome nutritional immunity. mBio. 2017;8:e01281–17. doi: 10.1128/mBio.01281-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hadley RC, Gagnon DM, Brophy MB, Gu Y, Nakashige TG, et al. Biochemical and spectroscopic observation of Mn(II) sequestration from bacterial Mn(II) transport machinery by calprotectin. J Am Chem Soc. 2018;140:110–13. doi: 10.1021/jacs.7b11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Besold AN, Gilston BA, Radin JN, Ramsoomair C, Culbertson EM, et al. The role of calprotectin in withholding zinc and copper from Candida albicans. Infect Immun. 2017;86:e00779–17. doi: 10.1128/IAI.00779-17. [DOI] [PMC free article] [PubMed] [Google Scholar]