Abstract
Among the strategies targeting vector control, the exploitation of the endosymbiont Wolbachia to produce sterile males and/or invasive females with reduced vector competence seems to be promising. A new Aedes albopictus transinfection (ARwP-M) was generated by introducing wMel Wolbachia in the ARwP line which had been established previously by replacing wAlbA and wAlbB Wolbachia with the wPip strain. Various infection and fitness parameters were studied by comparing ARwP-M, ARwP and wild-type (SANG population) Ae. albopictus sharing the same genetic background. Moreover, the vector competence of ARwP-M related to chikungunya, dengue and zika viruses was evaluated in comparison with ARwP. ARwP-M showed a 100% rate of maternal inheritance of wMel and wPip Wolbachia. Survival, female fecundity and egg fertility did not show to differ between the three Ae. albopictus lines. Crosses between ARwP-M males and SANG females were fully unfertile regardless of male age while egg hatch in reverse crosses increased from 0 to about 17% with SANG males aging from 3 to 17 days. When competing with SANG males for SANG females, ARwP-M males induced a level of sterility significantly higher than that expected for an equal mating competitiveness (mean Fried index of 1.71 instead of 1). The overall Wolbachia density in ARwP-M females was about 15 fold higher than in ARwP, mostly due to the wMel infection. This feature corresponded to a strongly reduced vector competence for chikungunya and dengue viruses (in both cases, 5 and 0% rates of transmission at 14 and 21 days post infection) with respect to ARwP females. Results regarding Zika virus did not highlight significant differences between ARwP-M and ARwP. However, none of the tested ARwP-M females was capable at transmitting ZIKV. These findings are expected to promote the exploitation of Wolbachia to suppress the wild-type Ae. albopictus populations.
Author summary
Aedes albopictus is one of the major human disease vectors and, despite substantial control efforts, it is rapidly spreading worldwide and increasing its epidemiological role. Thus, innovative approaches to fight this mosquito are urgently needed. Among the available control strategies, the exploitation of the endosymbiotic bacterium Wolbachia seems to be promising. In nature, the infection by Wolbachia is generally not detrimental, instead, it causes a series of modifications in host physiology promoting the spread of the infection in uninfected populations. Herein, we report on the artificial transinfection of specific Wolbachia strains in Ae. albopictus to replace its native Wolbachia infection type. This manipulation aimed at exploiting the expected modifications in the reproductive biology and vector competence of the species to contribute to reduce its epidemiological role. Specifically, we found that the new double Wolbachia infection did not affect Ae. albopictus fitness. The males belonging to the manipulated line, ARwP-M, induced full egg infertility in the wild-type females they mate with and showed increased male mating competitiveness. Remarkably, the ARwP-M females demonstrated significantly reduced competence for chikungunya and dengue viruses while both tested Ae. albopictus lines showed a very low susceptibility for Zika virus. These findings may encourage the use of ARwP-M Ae. albopictus as a highly efficient and safe biocide to suppress the wild-type populations.
Introduction
Despite control measures applied worldwide over decades, arthropod-borne diseases continue to pose a constant threat to human and domestic animal health [1]. Human-induced changes in the environment, climate change, passive transportation and acquisition of resistance to insecticides by the vectors are contributing to a dramatic re-emergence of harmful viruses such as dengue (DENV) and yellow fever (YFV) (both Flavivirus, Flaviviridae) transmitted by mosquitoes [2,3,4]. As well, further pathogens are rapidly spreading in areas where suitable vectors and environmental conditions are present and are showing a day by day increasing status of pathogenic relevance. These are the cases of chikungunya (CHIKV; Alphavirus, Togaviridae) and Zika (ZIKV; Flavivirus, Flaviviridae) viruses [5,6,7].
Aedes spp. (Diptera: Culicidae) are considered the key vectors of DENV, YFV, CHIKV and ZIKV [8,9]. At present, Ae. aegypti seems to play a leading role as vector among all of the Aedes species, mainly due to its high anthropophily and preference for the urban areas of the tropical regions [10]. However, though generally considered a secondary vector when Ae. aegypti is present, Ae. albopictus, the Asian tiger mosquito, demonstrated a key epidemiological role when abundant [11,12]. Moreover, the species may be responsible of increased risks of epidemics in temperate climate areas [13], as demonstrated by the DENV [14,15] and CHIKV [16] outbreaks occurred in Europe in recent years. In fact, even if less adapted to survive in dry conditions compared to Ae. aegypti, Ae. albopictus eggs display a remarkable cold hardiness in the diapausing form [17] which is highly contributing to the impressive extension of the geographic distribution of the species [18]. In addition, a recent mutation in an envelope glycoprotein led to a significant increase in CHIKV infectivity for Ae. albopictus and enhanced dissemination in mosquito organs and transmission [19,20]. Ae. albopictus was also found susceptible to ZIKV [21,22,23] even if vector competence can be considered low [24]. At the time of writing, CHIKV outbreaks occurred in Lazio (Rome Province) and Calabria regions [25] are still recent, with nearly 300 confirmed cases [26], endorsing the urgency of renewed control approaches.
Besides insecticide spraying, various alternative mosquito control methods are being developed and experimented [27,28,29]. In particular, theoretical and experimental studies are showing that certain strategies targeting mosquito reproduction biology have the potential to significantly affect mosquito populations, leading to a diminished risk that they may support diseases [30,31]. Basically, these methods rely on the release of functionally sterile males produced by three main techniques, namely, the irradiation of pupae by γ- or x-rays [32,33], the introduction of lethal factors through genetic modification [34,35] and the manipulation of the insect microbiome by the transinfection of the symbiotic bacterium Wolbachia (Rickettsiales) [36]. A further control strategy once again involves Wolbachia and it is not based on the suppression of the vector population but instead on the gradual replacement of the wild-types with conspecifics displaying desired biological traits [37] as more thoroughly described below.
Wolbachia is a vertically transmitted endosymbiotic bacterium, quite common in arthropods and a few other invertebrate taxa [38], which mainly infects the germ line of both sexes and manipulates host reproduction promoting the spread of the infected individuals in uninfected populations [39]. Among the various Wolbachia-induced effects on host biology, Cytoplasmic Incompatibility (CI) occurs at early stages of embryonic development and characterizes unfertile crosses between individuals with different Wolbachia infection types [40]. Introducing artificially a CI-inducing strain of Wolbachia in a vector species may provide a tool to produce functionally sterile males to be used to compromise the fertility of wild-type females not infected by the above Wolbachia strain.
Wolbachia-based strategies for vector control started to encounter a significant record of success in recent years. This is mainly due to the property shown by certain Wolbachia strains to reduce the vector competence of newly infected mosquito species [41,42,43,44]. This principle has been applied with Ae. aegypti, which is not infected by Wolbachia in the wild, through the artificial introduction of a Wolbachia strain (wMel) caught from Drosophila melanogaster (Diptera: Drosophilidae) [43]. This manipulation proved to suppress the DENV replication in the infected individuals and is responsible for a 70% reduction of the vector competence of this Ae. aegypti line [45]. A specific ongoing program aims at fighting dengue through the replacement of the wild-type Ae. aegypti population with this manipulated line [46]. The replacement is made feasible by the CI phenomenon which favors the Wolbachia infected over the uninfected Ae. aegypti. The wMel infected Ae. aegypti also displayed reduced vector competence for ZIKV [47] and CHIKV [48].
Ae. albopictus is a competent vector for the above mentioned viruses despite being naturally infected with two Wolbachia strains (wAlbA and wAlbB). However, the introduction of the wMel Wolbachia strain in a Wolbachia-cured line of Aedes albopictus induced resistance to DENV and CHIKV [49,50].
wMel Wolbachia had been previously introduced in wild-type Ae. albopictus, obtaining a triple infection which showed detrimental effects on female fitness leading to the early loss of the transinfected line [51]. Shortly after, ARwP Ae. albopictus was produced through the introduction of wPip Wolbachia belonging to the IV Incompatibility group [52] from Culex pipiens in a Wolbachia-cured population from Central Italy [53]. The obtained line showed a bidirectional incompatibility pattern with wild-type Ae. albopictus and was found highly efficient in suppressing this vector under laboratory [54,55] and semi-field settings [56]. Remarkably, compared to wild-type individuals belonging to the same genetic background, ARwP males displayed a significantly better male mating competitiveness under semi-field conditions in large enclosures [56]. Differently from wMel, wPip Wolbachia was proved to not significantly reduce Ae. albopictus capability to transmit CHIKV compared to wild-type females (Calvitti and Failloux, previously unpublished data, 2011; S1 Fig).
Herein, we report on the transinfection of wMel Wolbachia in ARwP to combine the remarkable suitability to the mass rearing protocols and male mating competitiveness, shown by this Ae. albopictus line over more than 100 generations, with a reduction in the vector competence, as expected by the introduction of wMel Wolbachia. This research aims to obtain an innovative and safe tool to suppress and/or replace Ae. albopictus wild-type populations based on considerations and conditions discussed below.
Materials and methods
Mosquito lines and rearing
Mosquito lines used in the experiments shared the same genetic background. SANG is a wild-type strain of Ae. albopictus colonized by using ovitraps in Anguillara Sabazia (Rome) in 2006 and since then reared under laboratory conditions at ENEA-Casaccia Research-Center (Rome). ARwP is a CI-inducing line, established at ENEA in 2008 through the transinfection of Wolbachia-cured SANG individuals with wPip Wolbachia from Culex pipiens [53] and reared for about 100 generations under rearing settings described below. Both the lines described above were periodically outcrossed with wild-type individuals from the same area to preserve the genetic variability according to methods reported previously [55]. Specifically, virgin ARwP and SANG females were crossed every five generations with the same number of two weeks old males obtained from Anguillara wild-caught females. ARwP-M has been obtained through the transinfection of ARwP with wMel Wolbachia from D. melanogaster as reported in a further paragraph.
Larvae were brought to adulthood inside 0.5 litre larval trays at the density of 1 larva/1 ml, augmented with a powder obtained by crushing dry cat food (Friskies Adults) at a fixed dose of 4 mg/larva of which 10% was given on day 1, 45% on day 2 and 45% on day 5. Adult mosquitoes were kept inside 40x40x40 cm cages at T = 28±1 C°, RH = 70±10%, L:D = 14:10 hours and were supplied with water and sucrose. Blood meals were provided through the use of anesthetized mice in agreement with the Bioethics Committee for Animal Experimentation in Biomedical Research and following procedures approved by the ENEA Bioethical Committee according to the EU directive 2010/63/EU. Used mice belonged to a colony housed at CR ENEA Casaccia and maintained for experimentation based on the authorization N. 80/2017-PR released on February the 2nd 2017 by Italian Ministry of Health.
wMel Wolbachia transinfection in ARwP Aedes albopictus and vertical transmission
ARwP Ae. albopictus embryos were transinfected according to techniques already used for mosquito transinfection [53,57,58]. D. melanogaster belonging to the yw67C23 genotype [59] was kindly furnished by Luis Teixeira (Instituto Gulbenkian de Ciência, Oeiras, Portugal) to be used as wMel Wolbachia donor. Cytoplasm was withdrawn from the posterior pole of donor eggs by borosilicate needles (Sutter Instrument; Novato, CA, USA) and then injected into the posterior of the recipient embryos using MN-151/MMO-202ND micromanipulators and an IM300 microinjector (Narishige Scientific; Tokyo, Japan).
After 5 days of development, the eggs were hatched by using a nutrient broth medium [60] and larvae were reared to the adult stage. G0 females, isolated as pupae to assure virginity, were mated with ARwP males and then provided with a blood meal. After oviposition, the infection status of G0 females and males was ascertained by PCR analysis using the wMel-wsp loci primers [61]. In the case of a positive result, the obtained amplicons were sequenced to confirm the Wolbachia infection type. The progeny produced by infected females were selected to establish a new transinfected Ae. albopictus line, ARwP-M. To reduce the inbreeding effects, ARwP-M females were outcrossed with ARwP males for five generations. During the ARwP line establishment, the first 6 generations were monitored for transmission efficiency of Wolbachia infection. All the G1 adults were PCR assayed for presence of wPip Wolbachia and infected offspring were chosen to start a new generation. Starting from G2, the maternal inheritance rate was estimated by assaying 5 daughters and 5 sons for each of three isolated females (mothers), randomly chosen.
Fitness parameters
Adult survival, female fecundity and egg fertility of the ARwP-M line were measured in comparison with SANG and ARwP Ae. albopictus. Namely, each treatment consisted of 50:50 females:males in 40x40x40 cages furnished with 10% sugar solution and under climatic conditions reported above. Dead mosquitoes were counted and removed every four days to assess longevity until the test was stopped at 60 days.
At 1-week intervals and starting with 3±1 days-old females, a blood meal was provided and mosquito eggs were collected on wet germination paper until 7th day after feeding. The eggs produced by the 3±1 days old females were counted and then hatched to measure female fecundity and mean egg fertility in the three lines. Each treatment was replicated three times.
Cytoplasmic incompatibility and male mating competitiveness
Three different series of crossing experiments were set up to evaluate the CI pattern between ARwP-M and SANG and to measure the male mating competitiveness of the ARwP-M males in comparison with the SANG males in 100×50×50 cm cages. For this purpose, respectively, 2±1 and 3±1 days-old females and males were used: i) 20:20 SANG males:females were allowed to mate in control crosses; ii) CI crosses consisted of populations of 20:20 ARwP-M males:SANG females; iii) populations of 20:20 SANG males:ARwP-M females were used to measure CI in the reciprocal cross; iv) competition crosses involved 20:20:20 ARwP males:SANG males:SANG females respectively. After 24 h, males were retrieved and females were provided with a blood meal. On the day of oviposition, females were isolated into plastic tubes furnished with wet paper for individualized egg laying. Produced eggs were counted and then allowed to hatch to measure CI. In the case of no hatching egg, females were checked for the presence of spermatozoa to ascertain the occurrence of a mating and virgins were excluded from the counts. CI crosses (ii and iii) were also repeated with males aged 10±1 and 17±1 days to investigate age-dependant changes in the incompatibility level. The degree of CI was computed using the corrected index of cytoplasmic incompatibility (CIcorr) and the Fried competitiveness index, as described previously [55].
DNA purification and quantitative qPCR to evaluate wMel and wPip Wolbachia density
Ten male and ten female individuals belonging to the ARwP-M line were aged 5–10 days and then analyzed for wPip and wMel Wolbachia titer in comparison with the ARwP line.
Total DNA was extracted from whole body of individual mosquitoes, using the ZR Tissue & Insect DNA Kit MicroPrep (Zymo Research, Irvine, CA, USA), according to manufacturer instructions. Strain-specific primers were used to amplify the wPip-wsp and wMel-wsp loci (Zhou et al., 1998), using previously described oligonucleotides: wPF (CGACGTTAGTGGTGCAACATTTA) and wPR (AATAACGAGCACCAGCAAAGAGT) [54] to obtain a 272 bp fragment of the wPip-wsp gene; 308F (TTA AAG ATG TAA CAT TTG) [61] and QArev2 (CAC CAG CTT TTA CTT GAC C) [62] leading to a 219 bp fragment of the wMel-wsp gene.
Aedes albopictus actin gene was used as a nuclear reference and amplified with the primers pair actAlbqPCRsense (CCCACACAGTCCCCATCTAC) and actAlbqPCRantisense (CGAGTAGCCACGTTCAGTCA), leading to a 119 bp amplification product.
Amplification reaction was prepared using the FluoCycle II SYBR Master Mix (Euroclone, Milano, Italy) in 20 μl final volume. Each mosquito extract was analyzed in triplicate using 2 μl total DNA extract as a reaction template. PCR was performed on ABI Prism 7100 (Applied Biosystems, Foster City, CA, USA) thermal cycler, optimizing the elongation temperature for each primer pair. Hence, the following amplification programs were applied: 5 min at 95°C, followed by 40 cycles of 15 sec at 95°C and 1 min at 60°C/52°C/62°C, for primer pair wPF-wPR/308F-QArev2/ actAlbqPCRsense-actAlbqPCRantisense, respectively. The presence of specific amplification products was verified with dissociation curves.
A plasmid (named pBS-M-P-act) containing single copy of wPip-wsp, wMel-wsp and actin was constructed to obtain a quantitative reference in qPCR amplifications. To this aim, specific DNA sequences encoding for wPip-wsp, wMel-wsp and actin, were cloned from total DNA extracts. The actin fragment (119 bp) was obtained by PCR using field-caught Ae. albopictus total DNA as a template and the primers pair actAlbqPCRsense/actAlbqPCRantisense. A 404 bp fragment of wPip-wsp locus was amplified using field-caught Culex pipiens total DNA extract as a template and the primers pair 183F/wPF [54,61], while a 405 bp fragment of wMel-wsp locus was amplified using D. melanogaster total DNA extract and primers pair 308F/691R [61]. All amplicons were then cloned in pCR 2.1 (TA Cloning Kit, Invitrogen, Carlsbad, CA) plasmid vector.
The amplified sequences were then assembled in a single plasmid, according to the following procedure. Actin gene fragment was transferred from pCR 2.1 into BamHI-NotI sites of pBluescript II SK (+) vector, resulting in pBS-act plasmid. Then, wPip-wsp fragment was cloned from pCR 2.1 into NotI-SacI sites of pBS-act, obtaining pBS-P-act plasmid. Finally, wMel-wsp fragment was cloned from pCR 2.1 into KpnI-XhoI sites of pBS-P-act, resulting in pBS-M-P-act plasmid. All obtained constructs were sequenced to assess the correct assembling and the absence of unwanted sequence variations.
For qPCR quantitation the pBS-M-P-act plasmid was serially diluted to build a standard curve with all three loci present at an equimolar concentration. The same standard dilutions were used in each qPCR, in order to standardize the signal with the nuclear actin reference. Quantitative PCR amplification was performed in triplicate for each mosquito extract and mean genome number of wPip-wsp and wMel-wsp was obtained per nuclear actin copy number.
Accession numbers for the genes mentioned in the paragraph are reported in S1 Table.
Vector competence tests for chikungunya, dengue and zika viruses
ARwP-M vector competence for CHIKV, DENV and ZIKV viruses was evaluated in comparison with ARwP Ae. albopictus to ascertain whether the introduction of the wMel Wolbachia infection may affect this biological trait.
Viruses
CHIKV (CHIKV 06.21; accession number AM258992) was isolated in 2005 from a newborn male from La Reunion presenting meningo-encephalitis symptoms [63]. This strain belongs to the East-Central-South African (ECSA) lineage known to be better adapted to Ae. albopictus due to the E1-A226V mutation [19,20] and this genotype was involved in the 2007 outbreak in Emilia-Romagna Region (Italy) [64]. We assumed that the widespread of this CHIKV strain was a valid argument to chose it over others as more suitable to be involved in severe epidemics. DENV (DENV-1 1806; accession number EU482591) was obtained in 2010 from an autochthonous case in Nice, France [14]. ZIKV (ZIKV PE243; accession number KX197192) was isolated from a patient in Recife (Brazil) in 2015 [65]. Viral stocks were prepared after several passages of the isolate onto Ae. albopictus C6/36 cells for CHIKV and DENV, and Vero cells for ZIKV.
Experimental infections and viral titrations
One-week-old mosquitoes were isolated in boxes (60 females/box) and starved for 24 h before infection. The blood meal was composed of two parts of washed rabbit erythrocytes, one part of the viral suspension and a phagostimulant (ATP) at 5 mM. The infectious blood-meal at a viral titer of 107FFU/mL for CHIKV and DENV-1 and, 107PFU/mL for ZIKV was placed in capsules (Hemotek, Lancashire, UK) wrapped with a piece of pork intestine maintained at 37°C. After 15–20 min of feeding, engorged females were sorted on ice and incubated at 28°C, 80% RH and 16h:8h L:D cycle, with free access to 10% sucrose. Batches of 20–24 mosquitoes were examined at 7 and 14 days post-infection (dpi) for CHIKV, and 14 and 21 dpi for DENV-1 and ZIKV. Mosquitoes were processed as follows: abdomen and thorax (referred to as body) were examined to determine infection, head for dissemination and saliva for transmission. Infection rate (IR) corresponds to the proportion of mosquitoes with infected midgut, dissemination efficiency (DE) to the percentage of mosquitoes with virus detected in heads suggesting a successful viral dissemination from the midgut, and transmission efficiency (TE) to the proportion of mosquitoes with infectious saliva. IR, DE and TE were calculated by titrating body, head homogenates, and saliva, respectively.
To determine viral infection and dissemination rates, each mosquito body and head were ground in 300 μL of medium (Leibovitz L15 medium for CHIKV and DENV, and Dulbecco’s Modified Eagle medium (DMEM) for ZIKV) supplemented with 2% fetal bovine serum (FBS), centrifuged at 10,000 × g for 5 min at +4°C and inoculated onto monolayers of Ae. albopictus C6/36 cell culture (for CHIKV and DENV) or Vero cells (for ZIKV) in 96-well plates. Vero cells were incubated for 7 days at 37°C then stained with a solution of crystal violet (0.2% in 10% formaldehyde and 20% ethanol). Presence of viral particles was assessed by detection of CPE. C6/36 cells were incubated for 3 days (CHIKV) or 5 days (DENV) at 28°C and then were fixed with 10% formaldehyde, washed, and revealed using hyper-immune ascetic fluid as the primary antibody and Alexa Fluor 488 goat anti-mouse IgG as the second antibody (Life Technologies).
To estimate viral transmission, mosquito saliva was collected in individual pipette tips containing 5 μL FBS for 30 min as previously described [66]. FBS containing mosquito saliva was expelled into 45 μL of L15 medium, inoculated on C6/36 cell culture or Vero cells stained as described above.
Cell cultures
C6/36 (Ae. albopictus) cells used for CHIKV and DENV titrations were maintained at 28°C in L-15 medium supplemented with non-essential amino-acids (1X), 10% fetal bovine serum (FBS), 100 units/mL penicillin and 100 μg/mL streptomycin. Vero (green monkey kidney, ATCC CCL-81) cells used for ZIKV titrations were maintained at 37°C, 5% CO2 in DMEM with 10% FBS, 100 units/mL penicillin and 100 μ g/mL streptomycin.
Data analysis
Survival curves of the three different lines (ARwP, SANG, and ARwP-M) were compared using Kaplan-Meier method and log-rank (Mantel-Cox) test. One-way repeated-measures ANOVA and Bonferroni mean separation were used to compare fecundity and egg hatch data between lines. Percent data was transformed to arcsin square root of proportions before the analysis. Normality of the experimental data was determined by the Shapiro–Wilk test. ANOVA was also used to compare the mean level of observed and expected CI in the male competitiveness trials.
Difference between lines in infection rate (IR) dissemination efficiency (DE) and transmission efficiency (TE) were analyzed by the Fisher’s exact test while Kruskal-Wallis test was used to compare the mean number of viral particles detected in bodies and saliva.
Statistical analysis was performed by PASW statistics (PASW Statistics for Windows, Version 18.0. SPSS Inc., Chicago, USA).
Results
Transinfection results and vertical transmission
More than 900 Ae. albopictus embryos were microinjected in total and 12 eggs were viable after the treatment and gave first instar larvae. Among obtained larvae, 8 emerged as adults, 4 of which were found infected with wMel Wolbachia. Two infected females were used to establish transinfected isofemale lines and one out of them transmitted the wMel infection to the progeny. All of the tested G1 individuals were confirmed as positive for wMel Wolbachia and vertical transmission accuracy always approached 100% over the following generations with few exceptions only among male progeny (98.89±1.01% in mean) (Table 1). The obtained line was named ARwP-M. The confirmation of the transinfected Wolbachia strain was achieved by sequencing the wsp gene [61] to perform a comparison with published wMel wsp sequence (Accession Number: AF020064.1; S2 Fig).
Table 1. Maternal inheritance efficiency of the wMel infection in the ARwP-M Ae. albopictus line.
The data sheet shows the number (N) of analyzed and the percentage of infected male and female individuals at each generation following the wMel transinfection.
| G1 | G2 | G3 | G4 | G5 | G6 | mean | SE | ||
|---|---|---|---|---|---|---|---|---|---|
| males | N | 4 | 15 | 15 | 15 | 15 | 15 | ||
| % infected | 100 | 100 | 93.33 | 100 | 100 | 100 | 98.89 | 1.01 | |
| females | N | 5 | 15 | 15 | 15 | 15 | 15 | ||
| % infected | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 0 |
Fitness parameters
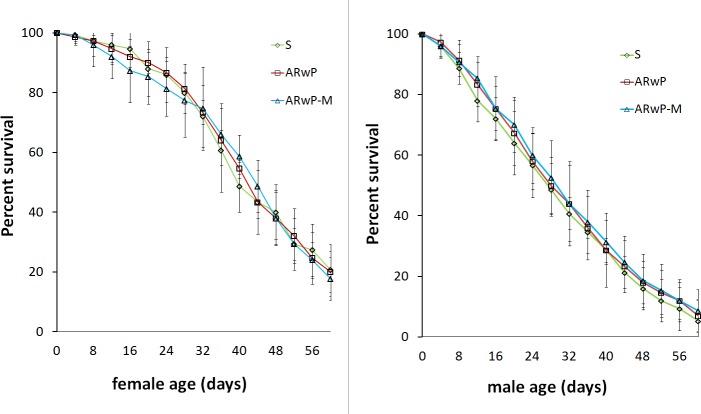
Regardless of the sex, survival did not show to significantly differ between SANG, ARwP and ARwP-M Ae albopictus (Fig 1). The average female life span was slightly higher than 38 days in all of the three Ae. albopictus lines under these experimental conditions (P = 0.984, log rank test). Average life expectancy for males was reduced to about 30 days in all of the tested lines (P = 0.984, log rank test).
Fig 1.
Survival of ARwP-M females (left) and males (right) in comparison with recipient ARwP and wild-type Ae. albopictus. S = SANG wild-type Ae. albopictus; ARwP = wPip infected Ae. albopictus; ARwP-M wPip + wMel infected Ae. albopictus. Error bars show the SEM of three biological replicates, each containing 50:50 females:males. In both cases, survival curves did not show to significantly differ by Kaplan-Meier method and log-rank (Mantel-Cox) test.
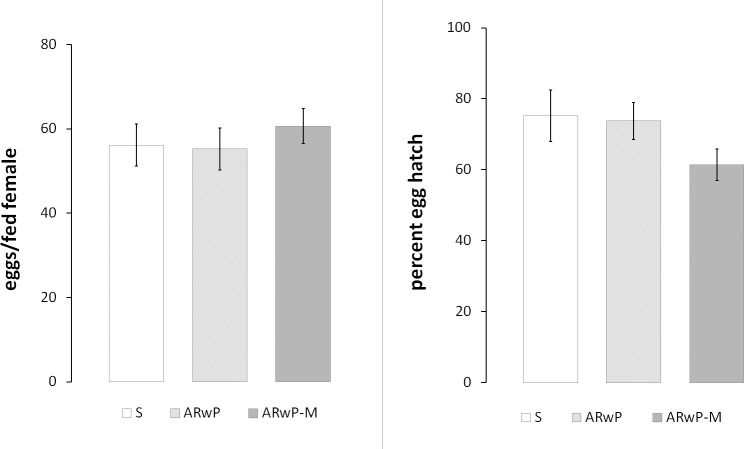
At ARwP-M G8, mean female fecundity did not significantly differ between tested Ae. albopictus lines (F(2,6) = 0.005; P = 0.995; Fig 2). As well, the wMel infection did not significantly affect ARwP-M fertility compared to both SANG and ARwP lines (F(2,6) = 1.395; P = 0.318).
Fig 2.
Female fecundity (left) and hatch rate (right) in ARwP-M Ae. albopictus in comparison with recipient ARwP and wild-type Ae. albopictus. S = SANG wild-type Ae. albopictus; ARwP = wPip infected Ae. albopictus; ARwP-M wPip + wMel infected Ae. albopictus. Error bars show the SEM of three biological replicates, each containing 17–20 fed females. In both cases, values are not significantly different by ANOVA-Bonferroni (P > 0.05).
CI and male mating competitiveness
Regardless of age, ARwP-M males compromised the hatchability of all of the eggs produced by the wild-type females they mated with (Table 2). Instead, the reverse crosses (SANG males × ARwP-M females) gave age-dependant results with egg fertility values gradually increasing from 0.09 ± 0.05 to 17.29 ± 2.32 when SANG males were, respectively, 3 and 17days (±1) old. ARwP-M males demonstrated higher mating competitiveness compared to the wild-types presenting the same genetic background as shown by the measured level of CIcorr and by the Fried competitiveness index (Table 2) which was significantly higher than 1 (F(1,4) = 11.24; P = 0.028).
Table 2. Crosses between ARwP-M and wild-type Ae. albopictus (SANG) to measure the level of induced cytoplasmic incompatibility and compare the male mating competitiveness.
In all of the crosses, females were 2±1 days old. The CIcorr level in the CI crosses was measured at three different male ages. Competition crosses consisted of young (3 ±1 days old) ARwP-M and SANG males at 1:1 ratio.
| crosses | N | percent egg hatch | CIcorr | Fried index | |
|---|---|---|---|---|---|
| females | males (*) | ||||
| SANG | SANG (3) | 2076 | 72.19 ± 3.12 | 0 | |
| SANG | ARwP-M (3) | 2152 | 0.00 ± 0.00 | 100 | |
| SANG | ARwP-M (10) | 2010 | 0.00 ± 0.00 | 100 | |
| SANG | ARwP-M (17) | 1962 | 0.00 ± 0.00 | 100 | |
| ARwP-M | SANG (3) | 2175 | 0.09 ± 0.05 | 99.87 ± 0.07 | |
| ARwP-M | SANG (10) | 1982 | 12.84 ± 1.50 | 82.22 ± 2.08 | |
| ARwP-M | SANG (17) | 1985 | 17.29 ± 2.32 | 76.06 ± 3.21 | |
| SANG | 1:1 SANG:ARwP-M | 2253 | 26.32 ± 2.25 | 62.07 ± 3.60 | 1.71 ± 0.24** |
*in brackets, male ages (days±1) are specified
N = total number of screened eggs; mean percent egg hatch and SE represent three biological replicates; CIcorr calculation derives from the equation: CIcorr(%) = [(CIobs − CCM)/(100 − CCM)] × 100, where CCM represents the natural egg mortality in SANG control; the Fried index of male competitiveness is obtained from the equation: (N/S)[(Hn-Ho)/(Ho-Hs)] where N/S stands for the ratio between the males belonging to the two lines (in this case 1), Hn the egg hatch in compatible crosses, Ho the egg hatch in competition trials and Hs the egg hatch in the CI crosses.
**The Fried index of competitiveness is significantly higher than that expected for an equal competitiveness between SANG and ARwP-M males (P < 0.05, ANOVA).
Wolbachia density in ARwP-M
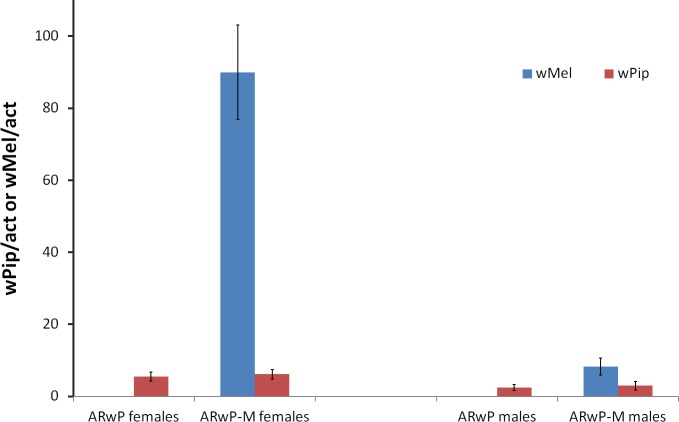
Adding wMel Wolbachia to the ARwP line (wPip-only infected) led to a significant increase in the overall Wolbachia titer (F(1,18) = 51.346; P < 0.005) which, specifically, was about 15 fold higher in the ARwP-M compared to the ARwP females (Fig 3). The increase in Wolbachia density in ARwP-M males was less evident but significant as well (F(1,18) = 12.673; P < 0.005). The titer of wPip Wolbachia seemed to be not affected by the introduction of the additional Wolbachia strain (females: F(1,18) = 0.133; P = 0.720; males: F(1,18) = 0.136; P = 0.716).
Fig 3. wMel and wPip Wolbachia density in ARwP and ARwP-M females and males measured by using Ae. albopictus actin gene as reference.
Vector competence
We experimentally infected mosquitoes with the three viruses, CHIKV, DENV and ZIKV provided to mosquitoes at a titer of 107 FFU(PFU)/mL.
When analyzing mosquitoes infected with CHIKV, significant differences were detected between the two Ae. albopictus lines at each dpi (7, 14) and parameters examined (IR, DE, TE) (Fig 4A). The ARwP line showed higher rates of infection, dissemination and transmission suggesting that ARwP was more susceptible to CHIKV than ARwP-M (Fisher exact test: P < 0.05). Previous results had shown that the vector competence for CHIKV was not significantly different comparing ARwP to SANG Ae. albopictus (Calvitti and Failloux, previously unpublished data, 2011; S1 Fig). Thus we can reasonably conclude that adding wMel to ARwP led to a reduced vector competence for CHIKV also compared to the wild-types. In fact, about 5 and 0% of the infected ARwP-M females were able to transmit the virus, respectively, at 14 and 21 dpi.
Fig 4. Rates of infection, dissemination efficiency and transmission efficiency for CHIKV, DENV and ZIKV in ARwP and ARwP-M Ae. Albopictus.
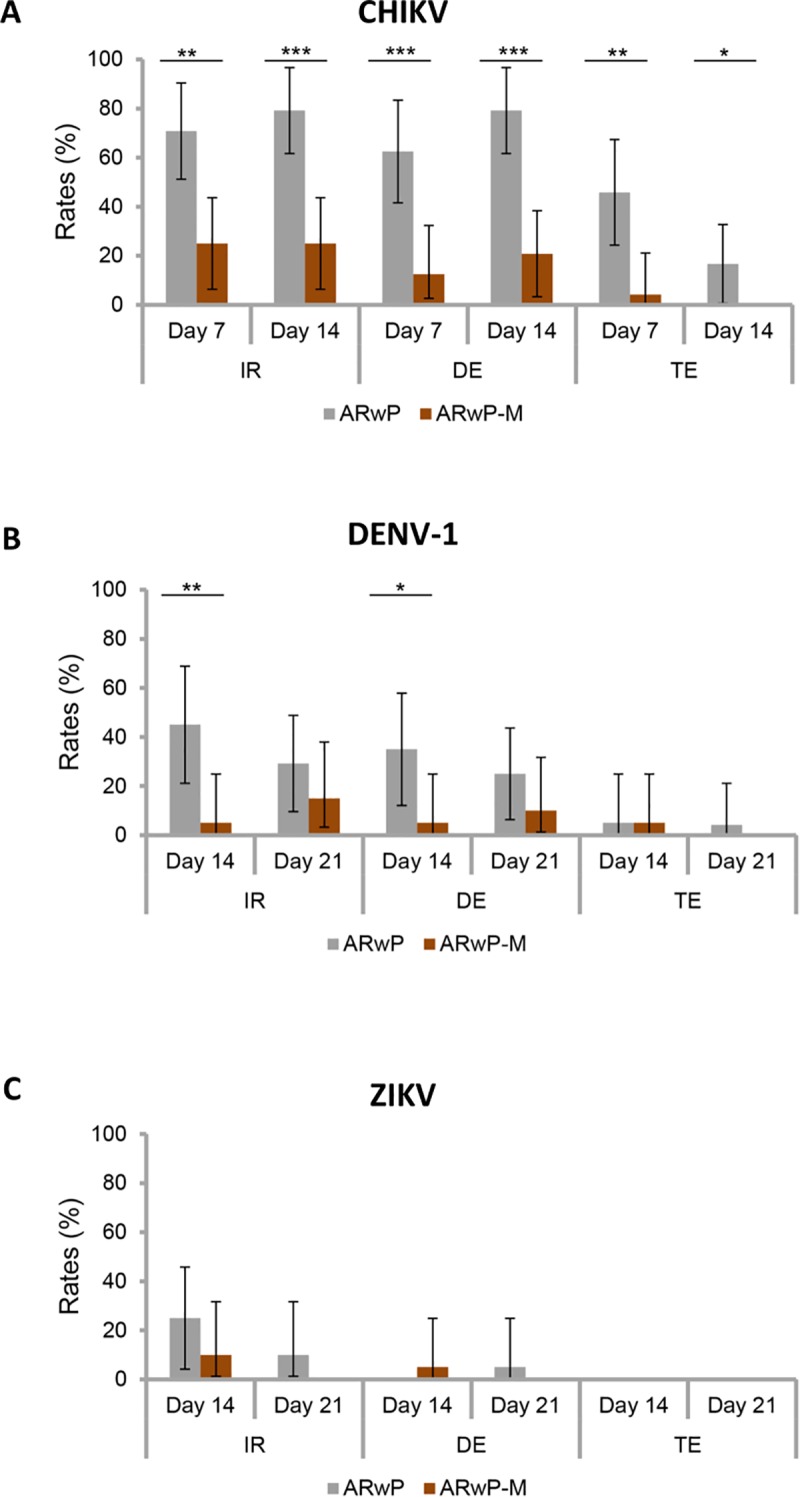
IR = Infection rate; DE = Dissemination rate; TE = transmission rate; A: the differences between Ae. albopictus lines are significant with respect to all of the three parameters and at both time intervals (7, 14 dpi) post the infection (Fisher exact test, P < 0.05); B: ARwP and ARwP-M significantly differed with regard to IR and DE at 14 dpi (Fisher exact test, P < 0.05); C: ARwP and ARwP-M did not significantly differ with regard to any of the evaluated parameters.
When examining mosquitoes infected with DENV-1, only IR and DE at 14 dpi were significantly different between the two mosquito lines (Fig 4B). Again, ARwP was better infected and better disseminated by DENV-1 at 14 dpi compared to ARwP-M (Fisher exact test: P < 0.05).
When comparing mosquitoes infected with ZIKV, no significant differences were detected between the two Ae. albopictus lines (Fig 4C) with very low rates at 14 and 21 dpi.
When examining the number of viral particles detected in bodies and saliva (Fig 5A–5C), no significant differences were found between ARwP and ARwP-M (Kruskal–Wallis test: P > 0.05). Regarding CHIKV, very low values of viral particles were found in ARwP-M saliva at 14 dpi and this value decreased to 0 at 21 dpi. Regarding DENV and ZIKV, viral particles were undetectable in the saliva of ARwP-M females at both dpi.
Fig 5. Titration of the viral particles of CHIKV, DENV and ZIKV in body and saliva of ARwP and ARwP-M Ae. Albopictus.
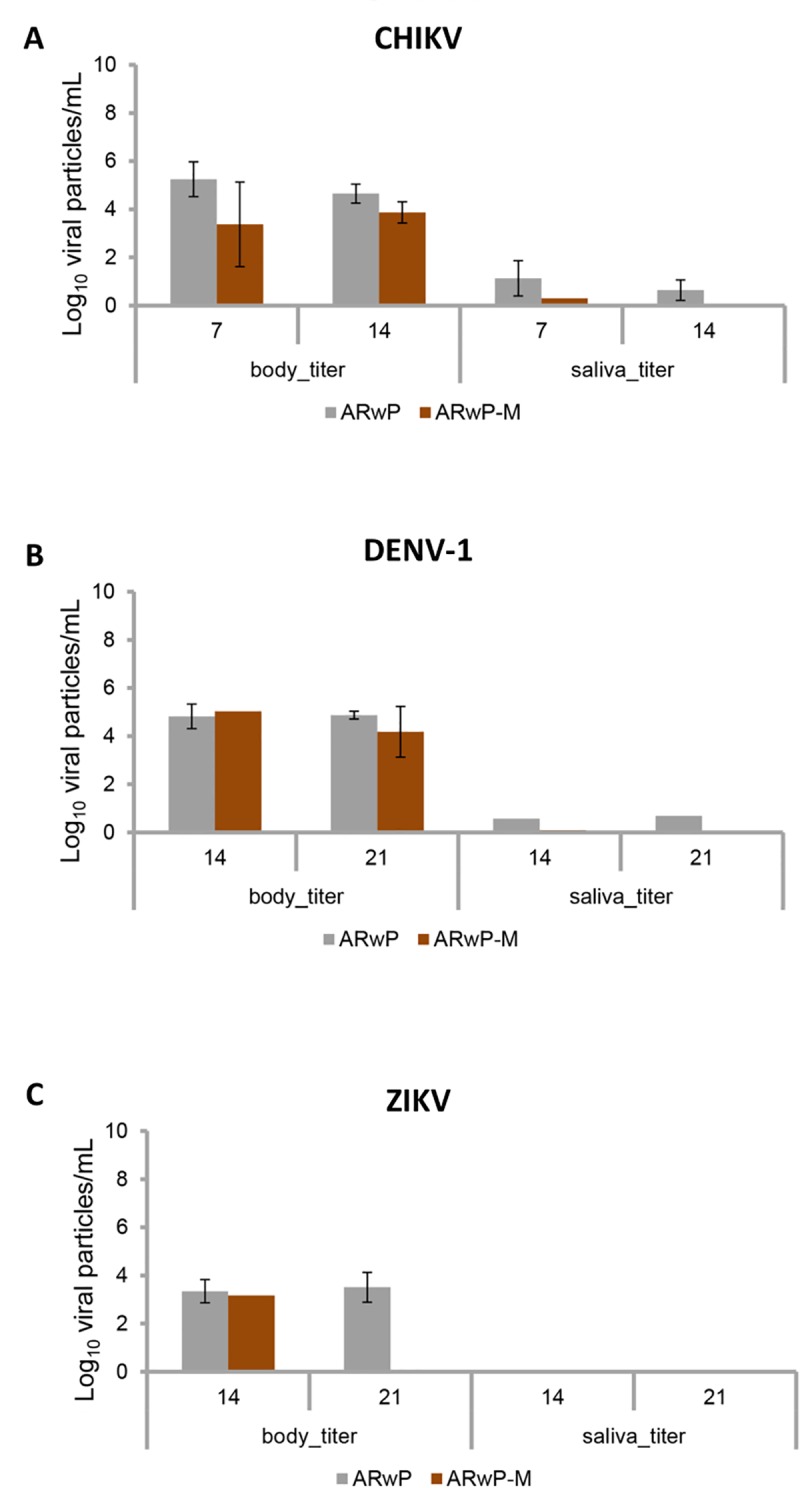
The number of viral particles in the body and saliva of both mosquito lines were titrated for evaluating the viral load in each mosquito line. A: the number of CHIKV viral particles in the body and saliva of ARwP and ARwP-M at 7 and 14 dpi; B and C: the number of DENV-1 (B) and ZIKV (C) viral particles in the body and saliva of ARwP and ARwP-M at 14 and 21 dpi. Differences between Ae. albopictus lines were not statistically significant (Kruskal–Wallis test: P > 0.05).
Discussion
The Wolbachia-based Incompatible Insect Technique (IIT) may offer a highly efficient approach to suppress mosquito vector populations because it can combine high efficacy with sustainable costs and negligible side-effects [67,68,69]. The efficiency of the approach has started to be demonstrated in the field with Ae. albopictus [70]. In this context, the introduction of different Wolbachia strains in a species may provide new resources among which to select the most suitable phenotypic effects for mosquito control purposes [71,72,73]. By introducing wMel Wolbachia in ARwP, we hoped to retain certain useful traits characterizing the line while adding further beneficial biological features to increase its potential as a control tool of Ae. albopictus-borne diseases. Based on the obtained results, these expectations were fulfilled.
As already reported for a wMel-only infected Ae. albopictus [49], the wMel infection was not found to affect Ae. albopictus fitness even in the case of coexistence with wPip Wolbachia. In addition, the CI trials demonstrated that ARwP-M maintained the notable male mating competitiveness already reported for ARwP in large enclosures under field conditions [56]. This advantage over the wild-types seemed to increase when moving from small cages to larger environments thus, we previously hypothesized that it could be due to ARwP male size [56,74]. However, this idea should be confirmed by more specific tests with regard to both ARwP and ARwP-M because changing environment could significantly affect the outcome of the trials. In any case, it is clear that releasing males with higher mating competitiveness compared to the wild-types may lead to induced infertility levels not reachable when using the same amount of irradiated males or males carrying dominant lethal mutations. This is because, using irradiation to obtain fully infertile Ae. albopictus males means reducing their mating competitiveness and survivorship, while preserving these latter traits by lowering the irradiation doses leads to a residual fertility which was found to increase with age [75]. Similarly, RIDL Ae. aegypti males showed reduced survivorship and mating competitiveness compared to the wild-types [76]. Furthermore, ARwP and, as confirmed herein, ARwP-M can be easily outcrossed, thanks to the partial fertility between the old wild-type males and the females belonging to these Ae. albopictus lines [77], allowing the preservation of the genetic variability and the transfer of the wPip Wolbachia infection into local Ae. albopictus genotypes by introgression. This possibility may consent ARwP/ARwP-M to be adapted to local environmental conditions and to acquire useful mutations from the wild-types of the target areas such as the ones responsible for the insecticide-resistance [29].
Compared to SIT, exploiting Wolbachia to produce functionally sterile males could also save costs (radiation sources would be not needed) and reduce logistic problems (it would not be necessary to manipulate and transport mosquito pupae as needed by the sterilization procedure).
Aside from these obvious advantages attributable to IIT, the opportunity to set up application protocols based on male-only releases or not is highly debated [77,78,79]. In fact, since 100% efficient sexing methods are not yet available for Aedes mosquitoes, applying the IIT would mean releasing in the wild fertile females harboring a new Wolbachia infection type. Due to bidirectional CI and immigration, small ARwP/ARwP-M populations are not expected to establish and invade much larger wild-type Ae. albopictus populations as the eventual replacement would not be self-sustaining [56,77,79]. However, it is certain that it would be preferable to avoid releasing vector females in areas subjected to epidemics.
As expected, the introduction of wMel in ARwP Ae. albopictus had a profound impact on the vector competence of this line. wAlbA and wAlbB Wolbachia were proved to not interfere with the transmission of CHIKV [80]. Also, we demonstrated previously (S1 Fig) that wPip Wolbachia was not capable of blocking this virus. Instead, when introducing wMel in ARwP Ae. albopictus, a blockade of CHIKV was detected lowering the potential of this mosquito to transmit the virus. This phenotype was shared with Wolbachia-cured Ae. albopictus transfected with wMel [49].
Mounting experimental evidence suggests that the low vector competence of wild-type Ae. albopictus for DENV is correlated with the presence of the natural of wAlbA and wAlbB Wolbachia strains [81,82]. While removing these strains canceled the inhibition exerted by Wolbachia on DENV [82], we demonstrated that adding wMel to wPip imposed a higher reduction of DENV-1 transmission by ARwP-M Ae. albopictus compared to ARwP. An even higher level of refractoriness to DENV transmission was previously obtained in a wMel-only infected Ae. albopictus [50], possibly due to a higher wMel Wolbachia titer compared to ARwP-M. However, the Wolbachia density data reported in the latter article is only expressed as a ratio compared to the wild-types thus, a direct comparison with the results reported herein is not feasible.
Lastly, the effect of exogenous Wolbachia strains in Ae. albopictus susceptibility to ZIKV is difficult to apprehend as the basic level of Ae. albopictus competence for ZIKV is already very low compared to CHIKV and DENV [83,84,85]. Our results confirmed the above results also in the Ae. albopictus lines infected with wPip alone or with wPip and wMel Wolbachia. However, the inhibition of ZIKV transmission seems to be significantly enhanced in both ARwP and ARwP-M Ae. albopictus compared to the wild-type Ae. albopictus from the same geographic area [83]. In fact, the above authors reported on ZIKV transmission rates of 29% in Ae. albopictus from Rome while, in this work, none of the tested ARwP-M females was capable of transmitting the virus.
Making available an Ae. albopictus line which couples high male mating competitiveness and suitability to the mass rearing protocols to a reduced vectorial competence would diminish the concerns associated with the possible escape of females among the released males in IIT programs. However, a series of issues will certainly need to be addressed before moving with ARwP-M to field testing. Further studies will have to evaluate ARwP-M vector competence in comparison with local wild-type populations and also testing other DENV and CHIKV serotypes. Moreover, the long-term stability of the new Wolbachia infection will be investigated because natural selection might gradually lead to reduced symbiont density and the loss of antiviral protection [86]. In particular, the suitability of the line to the stressing mass production protocols will be studied together with its response to the environmental conditions of the open field. In fact, wMel Wolbachia is known to be quite susceptible to heat stress as Ae. aegypti eggs and larvae maintained at temperatures higher than 30°C showed a dramatic reduction of the Wolbachia titer [87,88]. Such temperatures are common at low latitudes as well as during the summer in the Mediterranean basin and they might lead to a reduced pathogen inhibition and to a progressive diminution or even loss of the infection.
Supporting information
wPip = ARwP Ae. albopictus; wAlbA & wAlbB = SANG wild-type Ae. albopictus; w- = Wolbachia-cured SANG; dpi = days post infection. Mosquitoes were infected with CHIKV at a titer of 107 FFU(PFU)/mL. (A) Transmission rate was not significantly different between Ae. albopictus lines both at 7 and 14 dpi (Fisher exact test, P < 0.05). (B) Virus titer in female Ae. albopictus did not differ between lines at both 7 and 14 dpi (Kruskal–Wallis test: P < 0.05).
(PDF)
The wsp gene was initially amplified by PCR, using wsp generic primers 81F and 691R [61]. The obtained amplicon (ARwP Mel-amplicon) was then sequenced using the 308F and QArev2 primers specific for wMel. The grey box indicates the regions of sequence homology. wsp sequence of wPip Wolbachia (wsp wPip AF301010) was also reported to highlight sequence differences with the wMel wsp locus (wsp wMel AF020064.1). The perfect alignment of the obtained amplicon with the wMel wsp gene demonstrated the presence of wMel Wolbachia in the transinfected ARwP-M Ae. albopictus line.
(PDF)
The sequence analysis of the cloned sequences revealed a complete homology with the corresponding genes in database.
(PDF)
Acknowledgments
We thank Luis Teixeira (Instituto Gulbenkian de Ciência, Oeiras, Portugal) for gently furnishing the D. melanogaster strain used as wMel Wolbachia donor during the microinjections. We thank Marta Piscitelli (ENEA-Casaccia Research Centre, Division for Health Protection Technologies) as responsible of the facility for the housing and care of the mice used for blood feeding. We also thank Giuseppe Marzo (ENEA-Casaccia Research Centre, Division for Technologies and Facilities for Nuclear Fission and Nuclear Material Management) for statistical advice and Jason Cardone for his help with language editing.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This project has received resources funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement No 731060 (Infravec2, Research Infrastructures for the control of vector-borne diseases; http://infravec2.eu/). Funding followed the positive evaluation of a formal request describing the research project which was prepared and submitted to Infravec2 by RM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Marcondes CB, editor. Arthropod Borne Diseases. Cham: Springer International Publishing, Switzerland; 2017. [Google Scholar]
- 2.Gibbons R V. Dengue conundrums. Int J Antimicrob Agents. 2010;36: S36–S39. 10.1016/j.ijantimicag.2010.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanaway JD, Shepard DS, Undurraga EA, Halasa A, Coffeng LE, Brady OJ, et al. The global burden of dengue: an analysis from the global burden of disease study 2013. Lancet Infect Dis. 2016;16: 712–723. 10.1016/S1473-3099(16)00026-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couto-lima D, Madec Y, Bersot MI, Campos SS, Motta DA, Barreto F, et al. Potential risk of re-emergence of urban transmission of Yellow Fever virus in Brazil facilitated by competent Aedes populations. Sci Rep. 2017;7: 1–12. 10.1038/s41598-016-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeller H, Bortel W Van, Sudre B. Chikungunya: its history in Africa and Asia and its spread to new regions in 2013–2014. 2018;214: 2014–2018. 10.1093/infdis/jiw391 [DOI] [PubMed] [Google Scholar]
- 6.Shragai T, Tesla B, Murdock C, Harrington LC. Zika and chikungunya: mosquito-borne viruses in a changing world. Ann N Y Acad Sci. 2017;1399: 61–77. 10.1111/nyas.13306 [DOI] [PubMed] [Google Scholar]
- 7.Weaver SC, Costa F, Garcia-blanco MA, Ko AI, Ribeiro GS, Saade G, et al. Zika virus: history, emergence, biology, and prospects for control. Antiviral Res. 2016;130: 69–80. 10.1016/j.antiviral.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Z, Zhou T, Lai Z, Zhang Z, Jia Z, Zhou G, et al. Competence of Aedes aegypti, Ae. albopictus, and Culex quinquefasciatus mosquitoes as Zika virus vectors, China. Emerg Infect Dis. 2017;23: 1085–1091. 10.3201/eid2307.161528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vega-Rúa A, Zouache K, Girod R, Failloux A-B, Lourenço-de-Oliveira R. High Level of Vector Competence of Aedes aegypti and Aedes albopictus from Ten American Countries as a Crucial Factor in the Spread of Chikungunya Virus. J Virol. 2014;88: 6294–6306. 10.1128/JVI.00370-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraemer MUG, Sinka ME, Duda KA, Mylne AQN, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4: 1–18. 10.7554/eLife.08347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paupy C, Ollomo B, Kamgang B, Moutailler S, Rousset D, Demanou M, et al. Comparative role of Aedes albopictus and Aedes aegypti in the emergence of dengue and chikungunya in Central Africa. Vector Borne Zoonotic Dis. 2010;10: 259–266. 10.1089/vbz.2009.0005 [DOI] [PubMed] [Google Scholar]
- 12.Whitehorn J, Thi D, Kien H, Nguyen NM, Nguyen HL, Kyrylos PP, et al. Comparative susceptibility of Aedes albopictus and Aedes aegypti to dengue virus infection after feeding on blood of viremic humans: implications for public health. J Infect Dis. 2015;212: 1182–1190. 10.1093/infdis/jiv173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brady OJ, Golding N, Pigott DM, Kraemer MUG, Messina JP, Reiner RC, et al. Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasit Vectors. 2014;7: 338 10.1186/1756-3305-7-338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.La Ruche G, Souarès Y, Armengaud A, Peloux-Petiot F, Delaunay P, Desprès P, et al. First two autochthonous dengue virus infections in metropolitan France, September 2010. Euro Surveill. 2010;15: 19676 [PubMed] [Google Scholar]
- 15.Gjenero-Margan I, Aleraj B, Krajcar D, Lesnikar V, Klobučar A. Autochthonous dengue fever in Croatia, August–September 2010. Euro Surveill. 2011;16: 19805 [PubMed] [Google Scholar]
- 16.Angelini R, Finarelli A, Angelini P, Po C, Petropulacos K, Macini P, et al. An outbreak of chikungunya fever in the province of Ravenna, Italy. Euro Surveill. 2007;12: 3260. [DOI] [PubMed] [Google Scholar]
- 17.Kreß A, Kuch U, Oehlmann J, Müller R. Effects of diapause and cold acclimation on egg ultrastructure: new insights into the cold hardiness mechanisms of the Asian tiger mosquito Aedes (Stegomyia) albopictus. J Vector Ecol. 2016;41: 142–150. 10.1111/jvec.12206 [DOI] [PubMed] [Google Scholar]
- 18.Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector borne zoonotic Dis. 2016;7: 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsetsarkin KA, Vanlandingham DL, Mcgee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3: e201 10.1371/journal.ppat.0030201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vazeille M, Moutailler S, Coudrier D, Rousseaux C, Khun H, Huerre M, et al. Two Chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS One. 2007;2: e1168 10.1371/journal.pone.0001168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epelboin Y, Talaga S, Dusfour I. Zika virus: an updated review of competent or naturally infected mosquitoes. PLoS Negl Trop Dis. 2017;11: e0005933 10.1371/journal.pntd.0005933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan CH, Wong PJ, Li MI, Yang H, Ng C, Neill SLO. wMel limits Zika and chikungunya virus infection in a Singapore Wolbachia—introgressed Ae. aegypti strain, wMel-Sg. PLoS Negl Trop Dis. 2017;11: e0005496 10.1371/journal.pntd.0005496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Luca M, Severini F, Toma L, Boccolini D, Romi R, Remoli ME, et al. Experimental studies of susceptibility of Italian Aedes albopictus to Zika virus. Eurosurveillance. 2016;21: 30223 10.2807/1560-7917.ES.2016.21.18.30223 [DOI] [PubMed] [Google Scholar]
- 24.Chouin-Carneiro T, Vega-Rua A, Vazeille M, Yebakima A. Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus. PLoS Negl Trop Dis. 2016;10: e0004543 10.1371/journal.pntd.0004543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manica M, Guzzetta G, Poletti P, Filipponi F, Solimini A, Caputo B, et al. Transmission dynamics of the ongoing chikungunya outbreak in Central Italy: from coastal areas to the metropolitan city of Rome, summer 2017. Euro Surveill. 2017;22: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Centre for Disease Prevention and Control. Clusters of autochthonous chikungunya cases in Italy, first update—9 October 2017 [Internet]. 2017. Available: https://ecdc.europa.eu/sites/portal/files/documents/RRA-chikungunya-Italy-update-9-Oct-2017.pdf
- 27.Baldacchino F, Caputo B, Chandre F, Drago A, della Torre A, Montarsi F, et al. Control methods against invasive Aedes mosquitoes in Europe: a review. Pest Manag Sci. 2015;71: 1471–1485. 10.1002/ps.4044 [DOI] [PubMed] [Google Scholar]
- 28.Benelli G, Jeffries CL, Walker T. Biological control of mosquito vectors: past, present, and future. Insects. 2016;7: 52 10.3390/insects7040052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moyes CL, Vontas J, Martins AJ, Ng LC, Koou SY, Dusfour I, et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Med. 2017;11: e0005625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alphey L, Benedict M, Bellini R, Clark GG, Dame DA, Service MW, et al. Sterile-insect methods for control of mosquito-borne diseases: an analysis. Vector Borne Zoonotic Dis. 2010;10: 295–311. 10.1089/vbz.2009.0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gould F, Schliekelman P. Population genetics of autocidal control and strain replacement. Annu Rev Entomol. 2004;49: 193–217. 10.1146/annurev.ento.49.061802.123344 [DOI] [PubMed] [Google Scholar]
- 32.Balestrino F, Medici A, Candini G, Carrieri M, Maccagnani B, Calvitti M, et al. γ ray dosimetry and mating capacity studies in the laboratory on Aedes albopictus males. J Med Entomol. 2010;47: 581–591. 10.1093/jmedent/47.4.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamada H, Parker AG, Oliva CF, Balestrino F, Gilles JR. X-ray-induced sterility in Aedes albopictus (Diptera: Culicidae) and male longevity following irradiation. J Med Entomol. 2014;51: 811–816. 10.1603/ME13223 [DOI] [PubMed] [Google Scholar]
- 34.Phuc HK, Andreasen MH, Burton RS, Vass C, Epton MJ, Pape G, et al. Late-acting dominant lethal genetic systems and mosquito control. BMC Biol. 2007;5: 11 10.1186/1741-7007-5-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wise de Valdez MR, Nimmo D, Betz J, Gong H-F, James AA, Alphey L, et al. Genetic elimination of dengue vector mosquitoes. Proc Natl Acad Sci. 2011;108: 4772–4775. 10.1073/pnas.1019295108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bourtzis K, Dobson SL, Xi Z, Rasgon JL, Calvitti M, Moreira LA, et al. Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Trop. 2014;1325: 5150–5153. 10.1016/j.actatropica.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 37.Sinkins SP, O’ Neill SL. Wolbachia as a vehicle to modify insect populations In: Handler AM, James AA, editors. Insect Transgenesis: methods and applications. Boca Raton (FL): CRC Press; 2000. pp. 271–287. [Google Scholar]
- 38.Zug R, Hammerstein P. Still a host of hosts for Wolbachia: Analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS One. 2012;7: 7–9. 10.1371/journal.pone.0038544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6: 741–751. 10.1038/nrmicro1969 [DOI] [PubMed] [Google Scholar]
- 40.Sullivan W, O’Neill SL. Manipulation of the manipulators. Nature. 2017;543: 182–183. 10.1038/nature21509 [DOI] [PubMed] [Google Scholar]
- 41.Hughes GL, Koga R, Xue P, Fukatsu T, Rasgon JL. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 2011;7: 3–10. 10.1371/journal.ppat.1002043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinkins SP. Wolbachia and arbovirus inhibition in mosquitoes. Future Microbiol. 2013;8: 1249–1256. 10.2217/fmb.13.95 [DOI] [PubMed] [Google Scholar]
- 43.Walker T, Johnson PH, Moreira L a, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. Nature Publishing Group; 2011;476: 450–453. 10.1038/nature10355 [DOI] [PubMed] [Google Scholar]
- 44.Rainey SM, Shah P, Kohl A, Dietrich I. Understanding the Wolbachia-mediated inhibition of arboviruses in mosquitoes: Progress and challenges. J Gen Virol. 2014;95: 517–530. 10.1099/vir.0.057422-0 [DOI] [PubMed] [Google Scholar]
- 45.Ferguson NM, Kien DTH, Clapham H, Aguas R, Trung VT, Chau TNB, et al. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Transl Med. 2015;7: 279ra37 10.1126/scitranslmed.3010370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt TL, Barton NH, Rasic G, Turley AP, Montgomery BL, Iturbe-ormaetxe I, et al. Local introduction and heterogeneous spatial spread of dengue-suppressing Wolbachia through an urban population of Aedes aegypti. PLoS Biol. 2017;15: e2001894 10.1371/journal.pbio.2001894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dutra HLC, Rocha MN, Dias FBS, Mansur SB, Caragata EP, Moreira LA. Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe. The Author(s); 2016;19: 771–774. 10.1016/j.chom.2016.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aliota MT, Walker EC, Yepes AU, Velez ID, Christensen M, Osorio JE. The wMel strain of Wolbachia reduces transmission of chikungunya virus in Aedes aegypti. PLoS Negl Trop Dis. 2016;10: e0004677 10.1371/journal.pntd.0004677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blagrove MSC, Arias-Goeta C, Di Genua C, Failloux AB, Sinkins SP. A Wolbachia wMel Transinfection in Aedes albopictus Is Not Detrimental to Host Fitness and Inhibits Chikungunya Virus. PLoS Negl Trop Dis. 2013;7 10.1371/journal.pntd.0002152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blagrove MSC, Arias-Goeta C, Failloux A, Sinkins SP. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. PNAS. 2011;109: 255–260. 10.1073/pnas.1112021108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moretti R, Lampazzi E, Mastrobattista G, Calvitti M. Generazione di una nuova infezione di Wolbachia (ceppo “wMel”) in Aedes albopictus (Skuse) attraverso trasferimento interspecifico. XXX Congresso Nazionale Italiano di Entomologia, Campobasso, 11–16 giugno 2007. 2007. p. 380. Available: http://doczz.it/doc/623164/xxi-cnie—campobasso-2007—accademia-nazionale-italiana-di
- 52.Atyame CM, Delsuc F, Pasteur N, Weill M, Duron O. Diversification of Wolbachia endosymbiont in the Culex pipiens mosquito. Mol Biol Evol. 2011;28: 2761–2772. 10.1093/molbev/msr083 [DOI] [PubMed] [Google Scholar]
- 53.Calvitti M, Moretti R, Lampazzi E, Bellini R, Dobson SL. Characterization of a new Aedes albopictus (Diptera: Culicidae)-Wolbachia pipientis (Rickettsiales: Rickettsiaceae) symbiotic association generated by artificial transfer of the wPip strain from Culex pipiens (Diptera: Culicidae). J Med Entomol. 2010;47: 179–187. 10.1603/ME09140 [DOI] [PubMed] [Google Scholar]
- 54.Calvitti M, Moretti R, Skidmore AR, Dobson SL. Wolbachia strain wPip yields a pattern of cytoplasmic incompatibility enhancing a Wolbachia-based suppression strategy against the disease vector Aedes albopictus. Parasites and Vectors. 2012;5: 254 10.1186/1756-3305-5-254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moretti R, Calvitti M. Male mating performance and cytoplasmic incompatibility in a wPip Wolbachia trans-infected line of Aedes albopictus (Stegomyia albopicta). Med Vet Entomol. 2013;27: 377–386. 10.1111/j.1365-2915.2012.01061.x [DOI] [PubMed] [Google Scholar]
- 56.Puggioli A, Calvitti M, Moretti R, Bellini R. wPip Wolbachia contribution to Aedes albopictus SIT performance: advantages under intensive rearing. Acta Trop. Elsevier B.V.; 2016;164: 473–481. 10.1016/j.actatropica.2016.10.014 [DOI] [PubMed] [Google Scholar]
- 57.Xi Z, Khoo CCH, Dobson SL. Interspecific transfer of Wolbachia into the mosquito disease vector Aedes albopictus. Proc Biol Sci. 2006;273: 1317–22. 10.1098/rspb.2005.3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xi Z, Dobson SL. Characterization of Wolbachia transfection efficiency by using microinjection of embryonic cytoplasm and embryo homogenate. Appl Environ Microbiol. 2006;71: 3199–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chrostek E, Marialva MSP, Esteves SS, Weinert LA, Martinez J, Jiggins FM, et al. Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: a phenotypic and phylogenomic analysis. PLoS Genet. 2013;9: e1003896 10.1371/journal.pgen.1003896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bellini R, Calvitti M, Medici A, Carrieri M, Celli G, Maini S. Use of the sterile insect technique against Aedes albopictus in Italy: first results of a pilot trial In: Vreysen MJB, Robinson AS, Hendrichs J, editors. Area-Wide Control of Insect Pests. Springer, Dordrecht, The Netherlands; 2007. pp. 505–515. [Google Scholar]
- 61.Zhou W, Rousset F, O’Neill SL. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc Biol Sci. 1998;265: 509–515. 10.1098/rspb.1998.0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tortosa P, Courtiol A, Moutailler S, Failloux A-B, Weill M. Chikungunya-Wolbachia interplay in Aedes albopictus. Insect Mol Biol. 2008;17: 677–684. 10.1111/j.1365-2583.2008.00842.x [DOI] [PubMed] [Google Scholar]
- 63.Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, Vaney M-C, et al. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3: 1058–1070. 10.1371/journal.pmed.0030263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bordi L, Carletti F, Castilletti C, Chiappini R, Sambri V, Cavrini F, et al. Presence of the A226V mutation in autochthonous and imported Italian chikungunya virus strains. Clin Infect Dis. 2008;47: 428–429. 10.1086/589925 [DOI] [PubMed] [Google Scholar]
- 65.Donald CL, Brennan B, Cumberworth SL, Rezelj V, Clark JJ, Cordeiro MT, et al. Full genome sequence and sfRNA interferon antagonist activity of Zika virus from Recife, Brazil. PLoS Negl Trop Dis. 2016;10: e0005048 10.1371/journal.pntd.0005048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dubrulle M, Mousson L, Moutailler S, Vazeille M, Failloux A. Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PLoS One. 2009;4: e5895 10.1371/journal.pone.0005895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brelsfoard CL, Dobson SL. Wolbachia-based strategies to control insect pests and disease vectors. Asia-Pacific J Mol Biol Biotechnol. 2009;17: 55–63. [Google Scholar]
- 68.Hoffmann AA, Ross PA, Rašić G. Wolbachia strains for disease control: Ecological and evolutionary considerations. Evol Appl. 2015;8: 751–768. 10.1111/eva.12286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jeffries CL, Walker T, Walker T. Wolbachia biocontrol strategies for arboviral diseases and the potential influence of resident Wolbachia strains in mosquitoes. Curr Trop Med Reports. 2016;3: 20–25. 10.1007/s40475-016-0066-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mains JW, Brelsfoard CL, Rose RI, Dobson SL. Female adult Aedes albopictus suppression by Wolbachia-infected male mosquitoes. Sci Rep. Nature Publishing Group; 2016;6: 33846 10.1038/srep33846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Joubert DA, Walker T, Carrington LB, De Bruyne JT, Kien DHT, Hoang NLT, et al. Establishment of a Wolbachia superinfection in Aedes aegypti mosquitoes as a potential approach for future resistance management. PLoS Pathog. 2016;12: e1005434 10.1371/journal.ppat.1005434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ross PA, Endersby NM, Hoffmann AA. Costs of three Wolbachia infections on the survival of Aedes aegypti larvae under starvation conditions. PLoS Negl Trop Dis. 2016;10: e0004320 10.1371/journal.pntd.0004320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ant TH, Herd CS, Geoghegan V, Hoffmann AA, Sinkins SP. The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti. PLoS Pathog. 2018;14: e1006815 10.1371/journal.ppat.1006815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Atyame CM, Labbé P, Lebon C, Weill M, Moretti R, Marini F, et al. Comparison of irradiation and Wolbachia based approaches for sterile-male strategies targeting Aedes albopictus. PLoS One. 2016;11: e0146834 10.1371/journal.pone.0146834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bellini R, Balestrino F, Medici A, Gentile G, Veronesi R. Mating competitiveness of Aedes albopictus radio-sterilized males in large enclosures exposed to natural conditions. J Med Entomol. 2013;50: 94–102. [DOI] [PubMed] [Google Scholar]
- 76.Massonnet-Bruneel B, Corre-Catelin N, Lacroix R, Lees RS, Hoang KP, Nimmo D, et al. Fitness of transgenic mosquito Aedes aegypti males carrying a dominant lethal genetic system. PLoS One. 2013;8: e62711 10.1371/journal.pone.0062711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Calvitti M, Marini F, Desiderio A, Puggioli A, Moretti R. Wolbachia density and cytoplasmic incompatibility in Aedes albopictus: concerns with using artificial Wolbachia infection as a vector suppression tool. PLoS One. 2015;10 10.1371/journal.pone.0121813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang D, Lees RS, Xi Z, Gilles JRL, Bourtzis K. Combining the sterile insect technique with Wolbachia-based approaches: II—A safer approach to Aedes albopictus population suppression programmes, designed to minimize the consequences of inadvertent female release. PLoS One. 2015;10: e0135194 10.1371/journal.pone.0135194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dobson SL, Fox CW, Jiggins FM. The effect of Wolbachia-induced cytoplasmic incompatibility on host population size in natural and manipulated systems. Proc Biol Sci. 2002;269: 437–45. 10.1098/rspb.2001.1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mousson L, Martin E, Zouache K, Madec Y, Mavingui P, Failloux AB. Wolbachia modulates Chikungunya replication in Aedes albopictus. Mol Ecol. 2010;19: 1953–1964. 10.1111/j.1365-294X.2010.04606.x [DOI] [PubMed] [Google Scholar]
- 81.Lu P, Bian G, Pan X, Xi Z. Wolbachia induces density-dependent inhibition to dengue virus in mosquito cells. PLoS Negl Trop Dis. 2012;6: e1754 10.1371/journal.pntd.0001754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mousson L, Zouache K, Arias-goeta C, Raquin V, Mavingui P, Failloux A-B. The native Wolbachia symbionts limit transmission of dengue virus in Aedes albopictus. PLoS Negl Trop Dis. 2012;6: e1989 10.1371/journal.pntd.0001989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Di Luca M, Severini F, Toma L, Boccolini D, Romi R, Remoli ME, et al. Experimental studies of susceptibility of Italian Aedes albopictus to Zika virus. Euro Surveill. 2016;21: pii = 30223. [DOI] [PubMed] [Google Scholar]
- 84.Heitmann A, Jansen S, Luhken R, Leggewie M, Badusche M, Pluskota B, et al. Experimental transmission of Zika virus by mosquitoes from central Europe References. Euro Surveill. 2018;22: 30437 10.2807/1560-7917.ES.2017.22.2.30437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jupille H, Seixas G, Mousson L, Sousa CA. Zika virus, a new threat for Europe? PLoS Negl Trop Dis. 2016;10: e0004901 10.1371/journal.pntd.0004901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martinez J, Ok S, Smith S, Snoeck K, Day JP, Jiggins FM. Should symbionts be nice or selfish? Antiviral effects of Wolbachia are costly but reproductive parasitism is not. PLoS Pathog. 2015;11: e1005021 10.1371/journal.ppat.1005021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ulrich JN, Beier JC, Devine GJ, Hugo LE. Heat sensitivity of wMel Wolbachia during Aedes aegypti development. PLoS Negl Trop Dis. 2016;10: e0004873 10.1371/journal.pntd.0004873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ross PA, Wiwatanaratanabutr I, Axford JK, White VL, Endersby-Harshman NM, Hoffmann AA. Wolbachia Infections in Aedes aegypti Differ Markedly in Their Response to Cyclical Heat Stress. PLoS Pathog. 2017;13: e1006006 10.1371/journal.ppat.1006006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
wPip = ARwP Ae. albopictus; wAlbA & wAlbB = SANG wild-type Ae. albopictus; w- = Wolbachia-cured SANG; dpi = days post infection. Mosquitoes were infected with CHIKV at a titer of 107 FFU(PFU)/mL. (A) Transmission rate was not significantly different between Ae. albopictus lines both at 7 and 14 dpi (Fisher exact test, P < 0.05). (B) Virus titer in female Ae. albopictus did not differ between lines at both 7 and 14 dpi (Kruskal–Wallis test: P < 0.05).
(PDF)
The wsp gene was initially amplified by PCR, using wsp generic primers 81F and 691R [61]. The obtained amplicon (ARwP Mel-amplicon) was then sequenced using the 308F and QArev2 primers specific for wMel. The grey box indicates the regions of sequence homology. wsp sequence of wPip Wolbachia (wsp wPip AF301010) was also reported to highlight sequence differences with the wMel wsp locus (wsp wMel AF020064.1). The perfect alignment of the obtained amplicon with the wMel wsp gene demonstrated the presence of wMel Wolbachia in the transinfected ARwP-M Ae. albopictus line.
(PDF)
The sequence analysis of the cloned sequences revealed a complete homology with the corresponding genes in database.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.