Abstract
Disseminated human cytomegalovirus (CMV) disease occurs mainly as a congenital infection and among immunocompromised hosts. Patients with acquired immunodeficiency syndrome (AIDS) are at increased risk for CMV infection, and the most prevalent clinical manifestation is retinitis, followed by colitis, esophagitis, pneumonitis, and encephalitis. CMV oophoritis is poorly described in the literature with some cases reported in patients with hematological or solid malignancies, bone marrow or solid organ transplantation, immunosuppressive therapy, and advanced AIDS cases. We report the case of a 61-year-old woman with a recent diagnosis of AIDS, which was associated with a wasting syndrome. The patient presented with abdominal pain, headache, cutaneous vesicular lesions on the abdomen, anemia, lymphopenia, and hyponatremia; she died suddenly on the fourth day of hospitalization. The autopsy was performed and demonstrated disseminated CMV infection with hemorrhagic encephalitis as the immediate cause of death. Additionally, pneumonitis, extensive adrenalitis, ulcerated enteritis, focal hepatitis, and necrotizing oophoritis were found.
Keywords: Cytomegalovirus, Acquired Immunodeficiency Syndrome, Oophoritis, Autopsy
CASE REPORT
A previously healthy 61-year-old woman presented with a 2-week history of severe abdominal pain associated with cutaneous vesicular lesions on the abdomen, significant weight loss, decreased visual acuity, and pulsatile occipital headache. During the investigation at a primary care facility, human immunodeficiency virus (HIV) screening with enzyme-linked immunosorbent assay and Western blotting was performed and the diagnosis of HIV infection was established. The patient was referred to an infectious disease hospital. On admission, the patient was apparently well, with steady vital signs, and was afebrile. Physical examination disclosed painful diffuse abdominal palpation without signs for peritonitis and cutaneous vesicular lesions in the iliac fossa and right flank, which extended to the dorsal aspect. The patient had normal neurological and funduscopic examination. A laboratory work-up showed mild anemia (hemoglobin of 10.2g/dL, reference range (RR): 13.0-18.0g/dL), leukopenia (white blood cell of 3,400 cells/mm3; RR: 4,000-11,000 cells/mm3) with lymphopenia (700 cells/mm3; RR: 900-3,400 cells/mm3) and platelets of 215,000 cells/mm3 (RR: 140,000-450,000 cells/ mm3). The biochemical results called attention only to hyponatremia (127mEq/L; RR: 135-147mEq/L). Serology for syphilis, and hepatitis B and C were negative. The patient had no previous CD4+ T-lymphocyte count nor the HIV viral load. Treatment with Acyclovir (1500 mg/day) for herpes zoster was initiated. A brain computed tomography scan revealed cortical and subcortical atrophy, and the cerebrospinal fluid (CSF) analysis showed 2 cells/mm3 (95% lymphocytes and 5% monocytes), proteins of 138 mg/dL (RR: ≤ 40 mg/dL), and glucose of 106 mg/dL (RR: 50-70 mg/dL). CSF cultures failed to isolate bacteria, fungi, and mycobacteria. In addition, the Venereal Disease Research Laboratory, GeneXpert TB test, and latex agglutination for Cryptococcus neoformans were negative. Four days after hospitalization, the patient had an acute and severe headache, followed by sudden cardiorespiratory arrest, which was unresponsive to all maneuvers of advanced cardiac life support. An autopsy was performed with the consent of the family.
AUTOPSY FINDINGS
The main pathological finding at autopsy was disseminated cytomegalovirus (CMV) infection associated with AIDS, which affected the brain, lungs, small intestine, liver, adrenals, ovaries, and skin. This was confirmed by immunohistochemistry, which detected CMV antigens in all the lesions (Figure 1D and 3D). CMV hemorrhagic encephalitis was responsible for the immediate cause of death with subarachnoid hemorrhage at the base of the encephalon compressing the cerebellar lobes and brainstem, especially the midbrain, as well as diffuse congestion and edema (Figure 1A). The sections revealed deviation of the cerebral midline to the right at the expense of hemorrhage in the left lateral ventricle, with extension to the subarachnoid space and the third ventricle. Microscopic examination revealed endothelial and parenchymal cells with the cytopathic effect of CMV, producing hemorrhagic necrosis (Figure 1B-1D), besides arteriolosclerosis and lipohyalinosis in the small vessels.
Figure 1. Cytomegalovirus (CMV) encephalitis: A – Gross view showing subarachnoid hemorrhage in the base of the brain, with a clot compressing the cerebellum and brain stem; B – Cortical area with subarachnoid encephalitis, parenchymal cells with CMV cytopathic effect, and a focus of neutrophilic abscess (arrow) (H&E, 110X); C – Various cerebral cells with characteristic CMV cytopathic effect: cytomegaly associated with intranuclear inclusions surrounded by a clear halo. Note discrete neutrophilic reaction and a small cortical vessel with endothelial tumefaction and cytomegalic endothelial cells collapsing the lumen (arrow) (H&E, 30X); D – In the center, a liver parenchymal cell is shown with CMV nuclear and cytoplasmic inclusions, surrounded by few neutrophils (IPX, 400X).
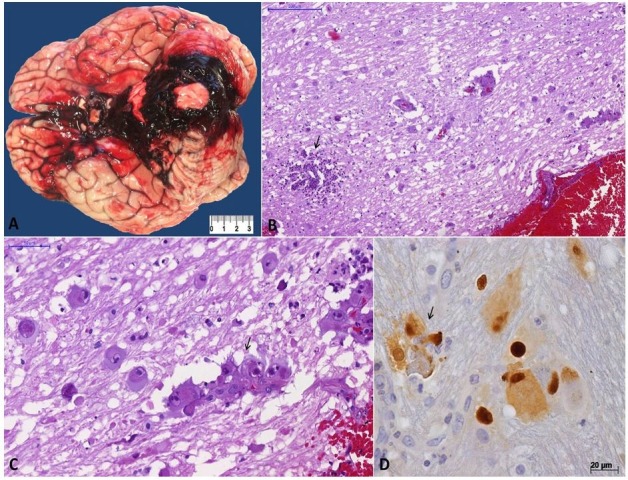
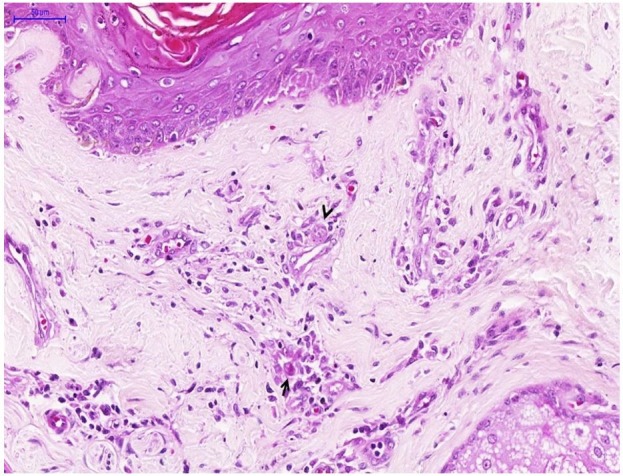
Figure 3. Micrograph of cytomegalovirus (CMV) oophoritis. A – Congestion, wedge cortical necrosis (arrow) and corpus albicans (H&E, 40X); B – Vessels in the base of wedge cortical necrosis showing fibrinoid necrosis (arrows) and various cytomegalic stromal cells (H&E, 100X); C – Endothelial cell with CMV-cytopathic effect (arrow and inset) near to necrotic areas with a discrete inflammatory reaction (H&E, 300X); D – Immunohistochemistry reaction showing ovarian stromal cells with CMV antigen in the nuclei and cytoplasm (IPX, 400X).
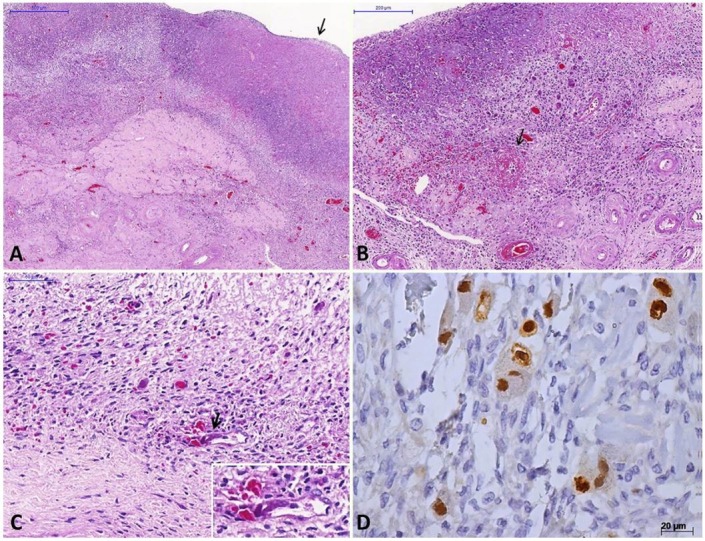
There was evidence of cytomegalic adrenalitis predominantly involving the medullary layer (Figure 2) with discrete and sparse inflammatory reaction. Both ovaries weighed 2.2 grams and exhibited ischemic oophoritis with wedge cortical necrosis. Stromal and endothelial cells showed typical CMV cytopathic effect (Figure 3), and there were foci of ischemic necrosis in the uterine tubes. The liver had sparse foci of CMV hepatitis (Figure 4A). The lungs had perivascular and septal inflammatory infiltrate, and rare alveolar macrophages with cytomegalic cytopathic effect (Figure 4B). The small intestine had a diffuse and ulcerated CMV-associated enteritis.
Figure 2. Micrograph of cytomegalovirus (CMV) adrenalitis. A – Medullary cells with CMV-cytopathic effect associated with moderate inflammatory reaction composed of histiocytes, lymphocytes, and neutrophils (H&E, 200X); B – Adrenal endothelial cells with CMV-cytopathic effect (arrows) (H&E, 400X); C – Adrenal cells with CMV-cytopathic effect with various aspects, one of them resembling a Reed–Stenberg cell or the “owl’s eyes” aspect (arrow) (H&E, 400X); D – A group of adrenal medullary cells with non-characteristic CMV-cytopathic effect (arrows) (H&E, 200X).
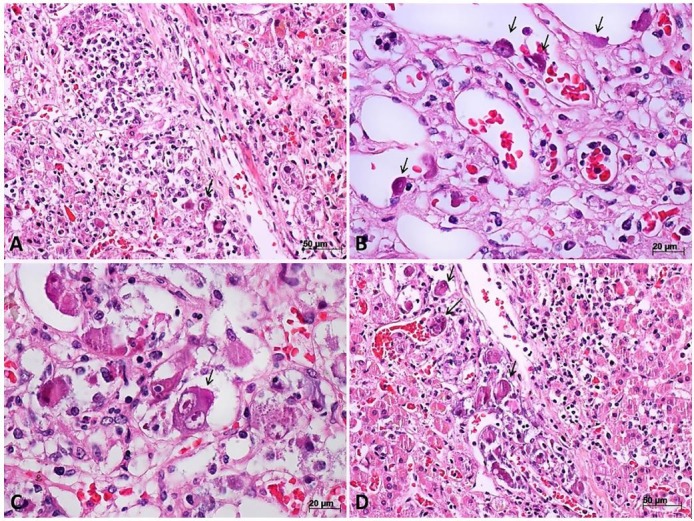
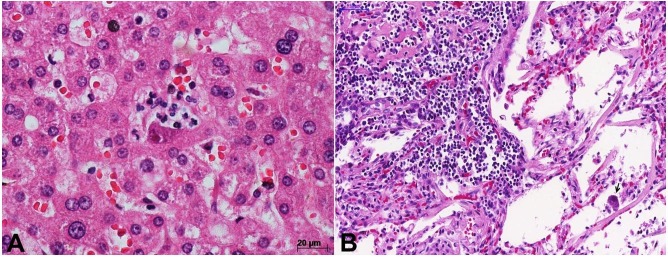
Figure 4. A – Micrograph of cytomegalovirus (CMV) hepatitis. In the center, a liver parenchymal cell with CMV nuclear and cytoplasmic inclusions, surrounded by few neutrophils (H&E, 400X); B – Micrograph of the lung in disseminated CMV infection, showing interstitial pneumonitis with a lymphomononuclear inflammatory infiltration in the interlobular septa and the alveolar septa. Note an intra-alveolar cell with CMV cytopathic effect (arrow) (H&E, 250X).
The skin, under microscopy, showed mixed inflammatory infiltrate in the reticular dermis, mainly perivascular, which was associated with endothelial cells with the cytopathic effect of CMV, besides mild spongiosis and pigment incontinence (Figure 5). No cytomegalic changes were observed in the bone marrow. Other autopsy findings include myocardial sclerosis, atherosclerotic plaques in the thoracic and abdominal aorta, lymphoid hypoplasia in the lymph nodes and spleen, and pulmonary anthracosis. The eyes were not examined during the autopsy.
Figure 5. Micrograph of skin in disseminated cytomegalovirus (CMV). Discrete spongiosis and a superficial perivascular dermatitis exhibiting endothelial tumefaction and cells with typical CMV cytopathic effect (arrow). Note a perivascular cell with cytomegaly and only granular cytoplasmic viral inclusions (arrowhead) (H&E, 250X).
DISCUSSION
CMV is a double-stranded DNA virus that belongs to the herpes virus family. The transmission of the disease can occur from person to person through close contact, sexual transmission, blood transfusions, transplacental infection, breastfeeding, and acquired infection from donated organs.1 Clinical syndromes caused by CMV range from oligosymptomatic cases in immunocompetent patients, congenital disease in newborns, and localized or severe disseminated disease in immunocompromised people due to HIV infection, neoplasms, or immunosuppressive therapy. In immunocompromised patients, the diseases caused by CMV occur predominantly in previously infected individuals; thus they represent the reactivation of a latent infection, or reinfection with a new viral strain.1
CMV infection in HIV-infected patients occurs with advanced immune dysfunction, typically with peripheral blood CD4+ T cell counts <50 cells/mm3. These patients are not receiving, or have not responded to, combined antiretroviral therapy (ART). Other risk factors include prior opportunistic infections, a high level of CMV viremia (through polymerase chain reaction), and elevated blood levels of HIV RNA (>100,000 copies/mL).1 Due to the short period of hospitalization, it was not possible to determine the patient’s HIV viral load or peripheral blood CD4+ T-cell count, but we can infer that she had low CD4+ T cells, based on her low blood lymphocyte count. In immunocompromised patients, including AIDS patients, CMV can affect several organs and systems, such as the lungs, retina, adrenals, brain, liver, esophagus, and colon.2 Infection of the female genital tract is poorly documented in the literature,3 and to date, only 18 cases of CMV oophoritis have been described. Some authors consider the actual frequency of genital involvement by CMV in immunocompromised women to be underestimated, either due to poor clinical suspicion or insufficient sampling of the organs in surgical or autopsy specimens.3-5
Through a search in the PubMed/Medline, SciELO, Lilacs, Scopus, Web of Science databases, and using the combination of words/mesh terms “oophoritis and cytomegalovirus and immunosuppressed” or “oophoritis and cytomegalovirus and AIDS or immunosuppressed” or “cytomegalovirus and oophoritis” or “oophoritis and AIDS,” 18 articles were selected in the PubMed/Medline database (no reports were found in the other sources). Three references were excluded in the first step (they cited female genital involvement by CMV but without reports of necrotizing oophoritis). Of the 15 remaining articles, only 5 were associated with AIDS. Originally described by Subietas et al.3 in 1977, bilateral CMV oophoritis is a rare pathological finding, which is usually associated with a systemic infection that may affect premenopausal and postmenopausal immunocompromised women. Case reports of CMV oophoritis are recorded in patients with hematologic malignancies, carcinomas, post-bone-marrow transplantation or solid organ transplantation, in patients subjected to immunosuppressive treatment and those with AIDS (Table 1).
Table 1. Clinical characteristics of reports of CMV oophoritis in the literature.
| First author & year of publication | Age | Menopausal status | Underlying disease |
Prior immunosuppressive treatment/state | Documented systemic CMV infection | Other associated opportunistic infections | Treatment of CMV | Diagnostic procedure | Presentation as mass lesion |
|---|---|---|---|---|---|---|---|---|---|
| Subietas et al.3 (1977) | 62 | Postmenopausal | Astrocytoma | Radiation | Yes (post-mortem) | No | No | Autopsy | No |
| 40 | Postmenopausal | Hodgkin lymphoma | Chemotherapy, steroids, radiation | Yes (post-mortem) | Pulmonary aspergillosis/ Pneumocystosis |
No | Autopsy | No | |
| 67 | Postmenopausal | Breast CA | Testosterone, Steroids | No | No | No | Autopsy | No | |
| Evans & Lampert6 (1978) | 37 | Premenopausal | SLE, glomerulonefritis | Steroids | No | Cryptococcosis/peritoneal candidiasis | No | Autopsy | No |
| LíVolsi & Merino7 (1979) | 61 | Postmenopausal | Non-Hodgkin lymphoma | Chemotherapy, Steroids | Yes (post-mortem) | Invasive gastrointestinal candidiasis | No | Autopsy | Yes* |
| Iwasaki et al.8 (1988) | 11 | Pre-pubertal | ALL | Chemotherapy, Steroids | Yes (pre-mortem) | Disseminated Candidiasis | N/A | Autopsy | No |
| Ribaux & Gloor9 (1988) | 54 | Postmenopausal | Lymphoma | N/A | Yes (pre-mortem) | Disseminated aspergillosis | No | Autopsy | No |
| Williams et al.10 (1990) | 40 | Premenopausal | Cholangio-CA | Liver transplant | Yes (post-mortem) | No | No | Autopsy | Yes |
| Familiari et al.11 (1991) | 33 | Premenopausal | AIDS | Antiretrovirals | Yes (pre-mortem) | Pulmonary aspergillosis/ neurotoxoplasmosis |
Yes | Autopsy | No |
| Sharma et al.12 (1994) | 50 | Postmenopausal | Breast CA | Autologous BMT | Yes (post-mortem) | Candidemia | No | Autopsy | No |
| Albert & Amstey2 (1995) | 39 | Premenopausal | AIDS | Antiretrovirals | Yes (pre-mortem) | No | N/A | Autopsy | Yes |
| Wales et al.13 (1996) | 31 | Premenopausal | AIDS, PID | Antiretrovirals | No | No | Yes | Surgical Resection | Yes (cystic and limited to left ovary) |
| Ortiz-Rey et al.14 (1997) | 31 | Premenopausal | AIDS | Antiretrovirals | Yes (post-mortem) | Pneumocystosis/ Pulmonary Tuberculosis | No | Autopsy | Yes |
| Nieto et al.15 (1999) | 50 | Postmenopausal | Breast CA | Allogenic BMT | Yes (pre-mortem) | No | Yes | Autopsy | No |
| Manfredi et al.4 (2000) | 36 | Premenopausal | AIDS | Presumed CNS toxoplasmosis & lymphoma | Yes (post-mortem) | Pulmonary aspergillosis | No | Autopsy | Yes |
| Yu et al.16 (2007) | 63 | Postmenopausal | Lung CA | Radiation, steroids | No | No | No | Surgical Resection |
Yes |
| Irsai et al.17 (2012) | 29 | Premenopausal | Hodgkin disease | Chemotherapy, allogeneic stem cell transplantation | Yes (post-mortem) | No | No | Autopsy | No |
| Current case report (2018) | 61 | Postmenopausal | AIDS | No | Yes (post-mortem) | Recent treatment for herpes zoster | No | Autopsy | No |
AIDS = acquired immunodeficiency syndrome; ALL = acute lymphocytic leukemia; BMT = bone marrow transplantation; CA = carcinoma; CMV = cytomegalovirus; CNS = central nervous system; N/A = not available; PID = pelvic infectious disease; SLE = systemic lupus erythematosus;
CMV ovarian infection, in this case, was restricted to a 1 cm fibrothecoma, located in the right ovary.
Primarily, oophoritis is presumably associated with systemic CMV, but some authors suggest that it may occur as a localized disease, due to reactivation of CMV in the genital tract or by reinfection through sexual transmission.13,18 We considered that the ovarian involvement in the present case was secondary to the systemic infection and was not a primary CMV infection of the genital tract of the patient due to local reactivation or sexual transmission, since (i) there were no complaints and alterations in the physical examination related to the genitalia; (ii) in the post-mortem exam, the uterus had macroscopic and microscopic normal aspects compatible with the patient’s age; and (iii) the CMV lesions were more severe in the adrenal, brain, and intestines, which suggested systemic disease.
Analyzing the cases reported in the literature, the median age of the patients was 40 years, and 50% occurred in postmenopausal women. All cases had associated immunosuppression: five cases (27.7%) were related to hematological malignancies3,7-9,17; one (5.5%) was associated with glial neoplas3; five (27.7%) was associated with carcinomas3,10,12,15,16; one (5.5%) was associated with lupus glomerulonephritis6; and six (33.6%; including the present report) were associated with AIDS.2,4,11,13,14 In one case, CMV ovarian infection affected a fibrothecoma, which was present in one of the ovaries of a patient with refractory angioimmunoblastic non-Hodgkin lymphoma.7 Fourteen of the 18 cases (77.7%) presented pre-mortem and post-mortem documentation of systemic involvement by CMV; 50% had an association with other opportunistic disease; and three cases (16.6%) received the treatment with ganciclovir. The diagnosis of oophoritis was made by autopsy in 16 of them (88.9%) and by surgical resection in 2 (11.1%). In the case of CMV-associated oophoritis, the ovaries—usually at macroscopy—are of normal size and weight with scattered hemorrhagic foci, as in the present report.3 In the review, 7 of 18 (38.8%) reported cases of CMV oophoritis had a clinical or macroscopic presentation of an ovarian mass lesion (Table 1).
The first case of CMV oophoritis in an HIV-infected patient was reported by Familiari et al.11 in a 33-year-old female patient diagnosed with AIDS, with laboratory evidence of pancreatitis, hepatitis, and pneumonitis, who died within a few days of hospitalization. The patient’s autopsy revealed a CMV-disseminated disease affecting the pancreas, liver, lungs, and both ovaries, which showed large areas of cortical necrosis and hemorrhagic foci. The diagnosis was confirmed by immunohistochemistry and in situ hybridization.11 In 1995, Albert et al.2 described the case of a 39-year-old woman with AIDS who developed severe and persistent metrorrhagia refractory to treatment with progesterone. A hysterectomy was performed and microscopy showed CMV endometritis. The virus was subsequently identified in the patient’s blood, sputum, and urine. The patient’s condition evolved with several abdominal complications, and she died a few months later. At autopsy, pneumonitis, colitis, and proctitis were also described, and there was extensive involvement of the ovaries.2 Wales et al.13 reported the case of a 31-year-old woman who was severely immunocompromised by HIV; she developed lower abdominal pain and dyspareunia. A diagnostic laparoscopy was performed with multiple biopsies, which revealed the involvement of the salpinges, myometrium, endometrium, and cervix, together with extensive oophoritis. Ortiz-Rey et al.14 described AIDS-associated CMV oophoritis in a 31-year-old intravenous drug user who had pneumocystosis and associated pulmonary tuberculosis.14 Manfredi et al.4 reported the case of a 36-year-old woman with advanced AIDS (CD4 lymphocyte count: 1 cell/mm3), who suffered from multiple opportunistic diseases and died after 9 weeks in hospital. The autopsy evaluation demonstrated adrenal and ovarian involvement by CMV.
The infection and disease caused by CMV represent an often-unexpected occurrence in histopathological analysis of samples obtained from immunocompromised patients. In the literature review, 16 cases (88.9%) of CMV oophoritis were diagnosed at autopsy, corroborating the importance of post-mortem examination—not only to the diagnosis of the disease, but also to other associated opportunistic infections and complications, as well as a better understanding of the physiopathology of this condition.19 The macroscopy of CMV-associated ovarian lesions are non-specific. However, the microscopy is diagnostic, and shows numerous stromal and endothelial cells with the typical CMV cytopathic effect (cytomegaly and nuclear inclusion with peripheral halo and granular cytoplasmic inclusions), variable tissue inflammatory reaction, and foci of necrosis of the ovarian cortex (Figure 3).3 Ancillary techniques, such as immunohistochemistry or in situ hybridization, are necessary for a more precise diagnosis.4 Similar to other reports in the literature, in the present case, we observed visceral involvement. The hemorrhagic encephalitis was responsible for the sudden death of the patient (Figure 1) with microscopic findings similar to a case described by Hall et al.20 Despite its sudden and fatal outcome, the CMV-encephalitis described here had a subacute course, which was manifested by headache but without neurological abnormalities on the physical examination, and with a slight increase in the CSF protein. The finding of superficial perivascular dermatitis associated with some cytomegalic endothelial cells (Figure 5) may explain the diffuse rash observed in the physical exam at hospital admission. Bournérias et al.21 described CMV-associated skin lesions in advanced AIDS, in the scenario of disseminated CMV, with affected endothelial and epithelial dermal cells at the microscopic exam, which we observed in our case. The diffuse and severe adrenalitis (Figure 2) was probably responsible for the adrenal insufficiency that lead to hyponatremia. Unfortunately, the definitive diagnosis with serum ACTH and cortisol dosage was not made. CMV adrenalitis was commonly described in early autopsy studies at the beginning of AIDS pandemic.22 The clinical course and microscopic aspects found in our case are compatible with studies described by other authors.22,23 The oophoritis (Figure 3) and enteritis were likely responsible for our patient’s diffuse abdominal pain. Finally, the liver (Figure 4A) and lungs (Figure 4B) had signs of CMV infection. However, the inflammatory reaction and tissue damage were discrete, and did not produce clinical repercussions.
CONCLUSION
CMV in AIDS patients should be considered in the differential diagnosis of nonspecific insidious signs and symptoms, such as chronic anemia, weight loss, hyponatremia (which may suggest adrenal insufficiency), and gastrointestinal complaints. Although rarely described in the medical literature, the ovaries may be affected as part of a disseminated CMV infection, and CMV oophoritis should be considered in the differential diagnosis of unexplained pelvic pain in women with AIDS, especially in a scenario where there is an increasing number of new cases of AIDS among women. The early diagnosis and treatment of CMV infection in the immunocompromised host certainly prevent dissemination of the virus and the consequent fatal outcome, which is similar to case reported by Albert et al.2 The most effective measure to reduce transmission, morbidity, and mortality related to HIV/AIDS infection and associated opportunistic diseases is prevention. Early diagnosis of HIV, the development of effective antiretroviral therapy (maintain CD4 count >100 cells/mm3), the negative viral load, and immune recovery are still the best measure for reducing the AIDS-related death rate.1
Acknowledgements
The authors would like to thank Mr. F. Santos for his technical assistance in the preparation of this manuscript.
Footnotes
How to cite: Soares LB, Buccheri R, Palhares RB, Duarte-Neto AN. Fatal disseminated cytomegalovirus infection with necrotizing oophoritis in a patient with acquired immunodeficiency syndrome. Autops Case Report [Internet]. 2018;8(3):e2018029. http://dx.doi.org/10.4322/acr.2018.029
Financial support: None
REFERENCES
- 1.U. S. Department of Health and Human Services Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. USA: U. S. Department of Health and Human Services; Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents [cited 2018 Mar 5]. Available from: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf [Google Scholar]
- 2.Albert MS, Amstey MS. CMV Oophoritis in an AIDS patient. Infect Dis Obstet Gynecol. 1995;3(5):202-4. 10.1155/S1064744995000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subietas A, Deppisch LM, Astarloa J. Cytomegalovirus oophoritis: ovarian cortical necrosis. Hum Pathol. 1977;8(3):285-92. 10.1016/S0046-8177(77)80025-7. [DOI] [PubMed] [Google Scholar]
- 4.Manfredi R, Alampi G, Talo S, Calza RML, Tadolini M, Martinelli GN. Silent oophoritis due to cytomegalovirus in a patient with advanced HIV disease. Int J STD AIDS. 2000;11(6):410-2. 10.1258/0956462001916001. [DOI] [PubMed] [Google Scholar]
- 5.Friedmann W, Schäfer A, Kretschmer R, Lobeck H. Disseminated Cytomegalovirus Infection of the female genital tract. Gynecol Obstet Invest. 1991;31(1):56-7. 10.1159/000293102. [DOI] [PubMed] [Google Scholar]
- 6.Evans DJ, Lampert IA. Ovarian involvement by cytomegalovirus. Hum Pathol. 1978;9(1):122. 10.1016/S0046-8177(78)80016-1. [DOI] [PubMed] [Google Scholar]
- 7.LiVolsi VA, Merino MJ. Cytomegalovirus infection of ovarian thecoma. Arch Pathol Lab Med. 1979;103(12):653-4. . [PubMed] [Google Scholar]
- 8.Iwasaki T, Sakuma T, Satodate R, Takano N, Sata T, Kurata T. Cytomegalovirus oophoritis with cortical necrosis during remission of acute lymphocytic leukemia. Acta Pathol Jpn. 1988;38(8):1069-76. [DOI] [PubMed] [Google Scholar]
- 9.Ribaux C, Gloor E. Necrotic oophoritis due to cytomegalovirus. Schweiz Med Wochenschr. 1988;119(5):160-3. [PubMed] [Google Scholar]
- 10.Williams DJ, Connor P, Ironside JW. Pre-menopausal cytomegalovirus oophoritis. Histopathology. 1990;16(4):405-7. 10.1111/j.1365-2559.1990.tb01150.x. [DOI] [PubMed] [Google Scholar]
- 11.Familiari U, Larocca LM, Tamburrini E, Antinori A, Ortona L, Capelll A. Premenopausal cytomegalovirus oophoritis in a patient with AIDS. AIDS. 1991;5(4):458-9. 10.1097/00002030-199104000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Sharma TM, Nadasdy T, Leech RW, Kingma DW, Johnson LD, Hanson-Painton O. In situ DNA hybridization study of ‘primary’ cytomegalovirus (CMV) oophoritis. Acta Obstet Gynecol Scand. 1994;73(5):429-31. 10.3109/00016349409006258. [DOI] [PubMed] [Google Scholar]
- 13.Wales NM, Nordin AJ, Newell AN, Smith JR, Barton SE, Nelson MR. Cytomegalovirus infection in the genital tract of HIV-seropositive women. AIDS. 1996;10(7):802-3. 10.1097/00002030-199606001-00023. [DOI] [PubMed] [Google Scholar]
- 14.Ortiz-Rey JA, Touza F, Perez-Valcarcel J, Pérez-Villanueva J. Oophoritis due to cytomegalovirus in a female AIDS patient. Med Clin (Barc). 1997;108(9):357-8. [PubMed] [Google Scholar]
- 15.Nieto Y, Ross M, Gianani R, et al. Post-mortem incidental finding of cytomegalovirus oophoritis after an allogeneic stem cell transplant. Bone Marrow Transplant. 1999;23(12):1323-4. 10.1038/sj.bmt.1701797. [DOI] [PubMed] [Google Scholar]
- 16.Yu J, Solano FX Jr, Seethala RR. Bilateral cytomegalovirus (CMV) oophoritis mimicking widely metastatic carcinoma: a case report and review of the literature. Diagn Pathol. 2007;2(1):50. 10.1186/1746-1596-2-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irsai G, Tampu-Kiss T, Dezso B, Miltényi Z, Illés A, Méhes G. Complications of systemic cytomegalovirus infection in therapy-resistant Hodgkin’s lymphoma. Orv Hetil. 2012;153(19):751-5. 10.1556/OH.2012.29364. [DOI] [PubMed] [Google Scholar]
- 18.Shen CY, Chang SF, Lin HJ, et al. Cervical cytomegalovirus infection in prostitutes and in women attending a sexually transmitted disease clinic. J Med Virol. 1994;43(4):362-6. 10.1002/jmv.1890430408. [DOI] [PubMed] [Google Scholar]
- 19.Fpathfu WAR, Nausheen Y, Fpathkfu RA, Khudairi AA, Nemenqani D. Histopathological features of na incidental case of cytomegalovirus salpingitis in a patient with inflammatory bowel disease. J Pak Med Assoc. 2013;63(6):780-3. [PubMed] [Google Scholar]
- 20.Hall WW, Farmer PM, Takahashi H, Tanaka S, Furuta Y, Nagashima K. Pathological features of virus infections of the central nervous system (CNS) in the acquired immunodeficiency syndrome (AIDS). Acta Pathol Jpn. 1991;41(3):172-81. [DOI] [PubMed] [Google Scholar]
- 21.Bournérias I, Boisnic S, Patey O, et al. Unusual cutaneous cytomegalovirus involvement in patients with acquired immunodeficiency syndrome. Arch Dermatol. 1989;125(9):1243-6. 10.1001/archderm.1989.01670210081012. [DOI] [PubMed] [Google Scholar]
- 22.Macher AM, Reichert CM, Straus SE, et al. Death in the AIDS patient: role of cytomegalovirus. N Engl J Med. 1983;309(23):1454. 10.1056/NEJM198312083092311. [DOI] [PubMed] [Google Scholar]
- 23.Pulakhandam U, Dincsoy HP. Cytomegaloviral adrenalitis and adrenal insufficiency in AIDS. Am J Clin Pathol. 1990;93(5):651-6. 10.1093/ajcp/93.5.651. [DOI] [PubMed] [Google Scholar]