Dear editor
We would like to report the case of a young lady with undiagnosed Wilson disease (WD) who presented with features of acute-on-chronic liver failure and died of its complications, which, if diagnosed in life, could have had a favorable evolution.
WD is a rare autosomal recessive disease, which is characterized by increased deposition of copper in the liver and the brain. The disease was first described in 1911 by Wilson in the monograph titled “Progressive lenticular degeneration: a familial nervous disease associated with cirrhosis of the liver.1 However, it is Bramwell who has been credited to have emphasized the importance of liver pathology in the progression of WD.1 By the end of the 1940s, multiple authors had published the demonstrable increased deposition of copper in the liver and other organs as being associated with WD.1 With further understanding of the disease pathogenesis, treatment for the chelation of copper was successfully introduced with the drug penicillamine in 1956 by Walshe.2 This discovery of successful chelation therapy makes WD one of the most satisfying genetic diseases to be diagnosed and treated.2
WD, as we understand today, is a genetic disease caused by a mutation in the gene coding for ATP7B, which is responsible for the copper-transporting ATPase.2 This protein is responsible for the elimination of copper into the bloodstream as well as its excretion in bile. The dysfunction of this protein, due to the mutated gene, results in an accumulation of copper within the hepatocytes. The spillage of the “free copper” results in the spread of copper into the various organs—principally the brain and cornea—resulting in oxidative damage and apoptosis of cells.
The manifestations of liver involvement have a varied spectrum depending on the stage of the disease. The earliest hepatic clinical features mirror those of non-alcoholic steatohepatitis (NASH) with histological features of micro and macrovesicular steatosis, and glycogenated nuclei.2 The distinction from NASH depends upon the demonstration of accumulated copper in the hepatocytes by histochemical stains. Ultrastructure shows mitochondrial changes, such as widened intercristal space, vacuolization, and increased granularity of the matrix.2 The intermediate stage of the disease shows histological features similar to those of autoimmune hepatitis, with the arrival of the portal and periportal inflammation composed of lymphocytes and plasma cells, which results in the destruction of the limiting plate, and parenchymal necrosis followed by bridging fibrosis. More than 50% of cases may show the presence of intra-cytoplasmic eosinophilic Mallory bodies.2 The cirrhotic stage may manifest as micronodular or mixed micro–macro nodular cirrhosis. The affected individual may also present with fulminant hepatic failure with histology showing features of necrosis and collapse against a background of cirrhosis.2
Estimations of serum ceruloplasmin, urinary excretion of copper and liver copper content are important primary aids in the diagnosis of WD.3 However, the only specific diagnostic test remains the genetic analysis for the mutated ATP7B gene. However, the disease can manifest due to multiple mutations in both coding and regulatory genes in the non-coding areas. The gene spans nearly 80kB, making the tests expensive and beyond the reach of many patients.2
Interpretation of simple biochemical tests have been shown to be both sensitive and fairly specific for WD. Two such indices include a ratio of alanine aminotransferase (ALT) by aspartate aminotransferase (AST), and a ratio of alkaline phosphatase (ALP) by total bilirubin (TB). An ALT/AST ratio of more than 2.2 has a sensitivity of 94% and a specificity of 86%; the ALP/TB ratio of less than 4 has a sensitivity of 94% and a specificity of 96%.4 These are important indices for the fact that serum ceruloplasmin—being an acute phase reactant—may be falsely high in patients with accompanying systemic inflammatory response syndrome or sepsis.
In keeping with a non-specific histomorphology of WD in the liver, the demonstration of accumulated copper remains an important tool in arriving at a diagnosis in a poor-resource setting.5 Orcein stain for copper-associated protein (CAP) is a useful histochemical stain. The cytoplasmic CAP on orcein stain gives a cola-colored, perinuclear, coarsely granular appearance. However, orcein positive CAP granules can be seen in chronic cholestatic disorders and are not specific for WD.6 The demonstration of elemental copper is possible using stains such as rubeanic acid or rhodanine. Rubeanic acid gives a greenish–black color to copper, while rhodanine stain gives a red to orange–red cytoplasmic granular positivity to elemental copper.6
The images referred to an autopsied female, aged 25 years, who had an undiagnosed case of WD and presented to our hospital as a case of acute-on-chronic liver failure, following a miscarriage. The patient had no history of alcohol or any drug intake, did not have the diagnosis of diabetes mellitus, had no family history of any hepatopathy, and had a body mass index of 21. On admission, the laboratory work-up showed a normocytic normochromic anemia with a hemoglobin level of 7.6 g/dL (reference range [RR]: 12–15.5 g/dL); mild thrombocytopenia (platelets of 104,000/mm3 [RR: 150,000–450,000]); AST 130 UI/L (RR: 5–40 UI/L); ALT 43.5 UI/L (RR: 7–56 UI/L); akaline phosphatse (ALP) 51UI/L (RR: 40–140 UI/L); total bilirrubin (TB) 8.9 mg/dL (RR: 0.1–1.2 mg/dL) at the expenses of indirect bilirubin; albumin 1.2 g/dL (RR: 3.5–5.5 g/dL); and prothrombin time enlarged with the international normalized ratio (INR) of 4.1 (RR: 1). Abdominal ultrasonography showed a coarse echotexture with nodular surface, without any focal lesion, and the portal and splenic veins were patent and measured 12 mm and 10 mm, respectively. Few collaterals were seen in the gastro hepatic and splenic hilum. Gross ascites was present—the analysis of which ruled out infection—and was consistent with cirrhosis. The patient’s viral serology, which included testing for hepatitis A, B, C, and E viruses, was negative; and autoimmune markers (anti-nuclear, anti-smooth muscle, anti-liver-kidney microsome-1, and anti-liver-cytosol-1 antibodies) were tested, with negative results. She had a low serum ceruloplasmin (11 mg/dL; RR: 20–35 mg/dL). An eye examination did not reveal the presence of the Kayser–Fleischer ring. The patient slipped into grade III hepatic encephalopathy and developed terminal acute kidney injury. She finally succumbed to refractory shock.
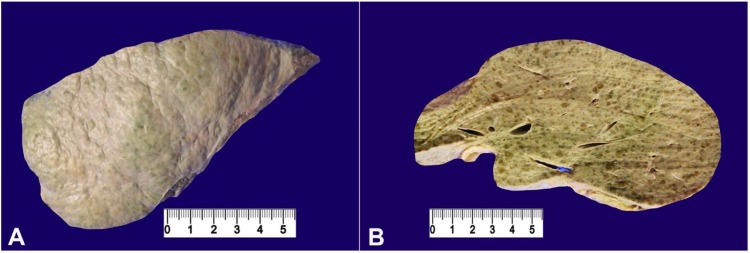
The autopsy revealed a liver weighing 1280 g (RR: 968-1860 g). The capsular surface was nodular (Figure 1A). The cut surface showed both micro and macro nodules, which were bile stained with intervening whitish bands corresponding to the fibrosis between the regenerative nodules (Figure 1B).
Figure 1. Gross view of the liver. A – The nodular capsular surface; B – The cut surface with micro and macro nodules with intervening fibrosis.
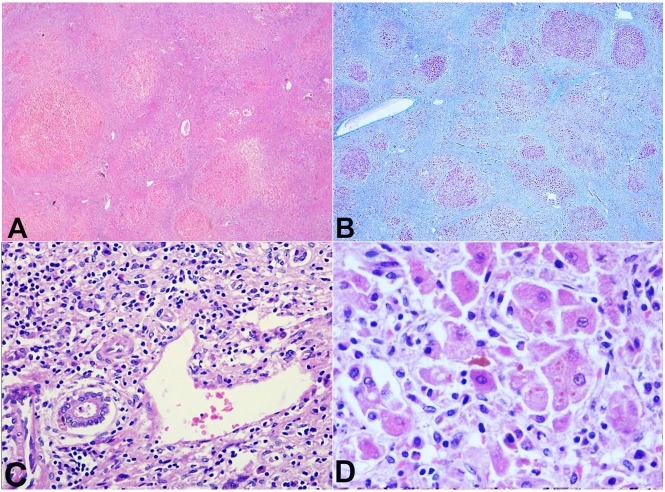
The histology confirmed the mixed cirrhotic pattern with inflamed intervening fibrosis (Figure 2A, 2B). There was moderate to dense portal inflammation composed of lymphocytes and plasma cells (Figure 2C). The nodules showed occasional Mallory hyaline bodies (Figure 2D).
Figure 2. Photomicrography of the liver. A – The cirrhotic pattern (H&E, 20X), which is better highlighted on Masson trichrome stain in B (20X); C – Portal tract with moderate lymphoplasmacytic cell infiltrate (H&E, 100X); D – Hepatocytes showing the presence of dense eosinophilic cytoplasmic Mallory–Denk hyaline bodies (H&E, 400X).
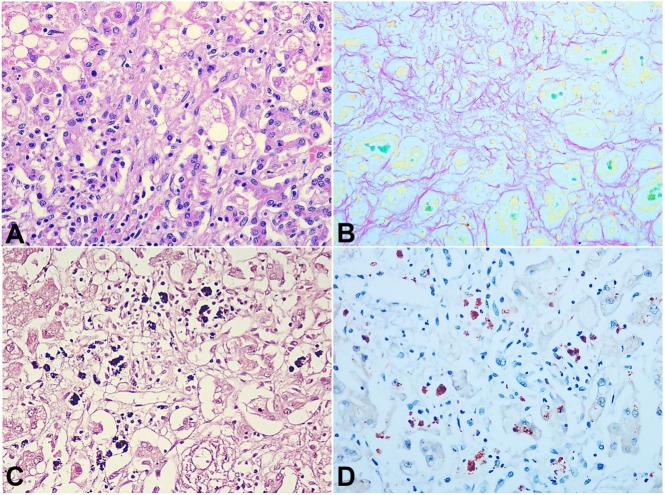
The hepatic parenchyma showed both micro and macrovesicular steatosis (Figure 3A). There was a marked intra-cellular and canalicular cholestasis as highlighted on Fouchet stain (Figure 3B) There was a marked increase in copper-associated protein as shown by the extensive cytoplasmic coarsely granular cola-colored granules on orcein stain (Figure 3C). The rhodanine stain accentuated the accumulated elemental copper within the hepatocytes in the form of red cytoplasmic granules (Figure 3D). These changes were consistent with the histopathological changes of WD.
Figure 3. Photomicrography of the liver. A – Micro and macro-vesicular steatosis (H&A, 100X); B – Intracellular and ductular cholestasis, better highlighted by emerald green staining on Fouchet stain (100X); C – Orcein stain highlighting copper-associated protein by the cytoplasmic coarsely granular cola-colored granules (100X); D – Rhodanine stain highlighting elemental copper by red cytoplasmic granules (100X).
This case is a reminder that an undiagnosed WD can present in patients, especially young females, as acute-on-chronic liver failure. In the absence of positive autoimmune and viral markers, clues should be drawn from biochemical markers, such as ALP and bilirubin in making a diagnosis. The pattern of the histopathology and staining pattern of histochemical stains, such as orcein and rhodamine, demonstrated in the index case provides an overview of what to expect in a classic case of WD.
The authors retain an informed consent for the autopsy performance and the manuscript is in accordance with the Institutional Ethics committee requirements.
ACKNOWLEDGEMENTS
Authors thank Mr. Charan Singh for the histopathological stains.
Footnotes
How to cite: Madakshira MG, Das A, Umair M, Dutta U. Liver histology and histochemistry in Wilson disease. Autops Case Report [Internet]. 2018;8(3):e2018026. http://dx.doi.org/10.4322/acr.2018.026
Financial support: None
REFERENCES
- 1.Barbosa ER, Machado AAC, Cançado ELR, Deguti MM, Scaff M. Wilson’s disease: a case report and a historical review. Arq Neuropsiquiatr. 2009;67(2b):539-43. 10.1590/S0004-282X2009000300036. [DOI] [PubMed] [Google Scholar]
- 2.Mak CM, Lam C-W. Diagnosis of Wilson’s disease: a comprehensive review. Crit Rev Clin Lab Sci. 2008;45(3):263-90. 10.1080/10408360801991055. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Castro KI, Hevia-Urrutia FJ, Sturniolo GC. Wilson’s disease: a review of what we have learned. World J Hepatol. 2015;7(29):2859-70. 10.4254/wjh.v7.i29.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korman JD, Volenberg I, Balko J, et al. Screening for Wilson disease in acute liver failure: a comparison of currently available diagnostic tests. Hepatology. 2008;48(4):1167-74. 10.1002/hep.22446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pilloni L, Lecca S, Van Eyken P, et al. Value of histochemical stains for copper in the diagnosis of Wilson’s disease. Histopathology. 1998;33(1):28-33. 10.1046/j.1365-2559.1998.00455.x. [DOI] [PubMed] [Google Scholar]
- 6.Clark I, Torbenson MS. Immunohistochemistry and special stains in medical liver pathology. Adv Anat Pathol. 2017;24(2):99-109. [DOI] [PubMed] [Google Scholar]