Abstract
Objective: To explore the feasibility of disease-specific clinical pathways when used in primary care.
Design: A mixed-method sequential exploratory design was used. First, merging and exploring quality interview data across two cases of collaboration between the specialist care and primary care on the introduction of clinical pathways for four selected chronic diseases. Secondly, using quantitative data covering a population of 214,700 to validate and test hypothesis derived from the qualitative findings.
Setting: Primary care and specialist care collaborating to manage care coordination.
Results: Primary-care representatives expressed that their patients often have complex health and social needs that clinical pathways guidelines seldom consider. The representatives experienced that COPD, heart failure, stroke and hip fracture, frequently seen in hospitals, appear in low numbers in primary care. The quantitative study confirmed the extensive complexity among home healthcare nursing patients and demonstrated that, for each of the four selected diagnoses, a homecare nurse on average is responsible for preparing reception of the patient at home after discharge from hospital, less often than every other year.
Conclusions: The feasibility of disease-specific pathways in primary care is limited, both from a clinical and organisational perspective, for patients with complex needs. The low prevalence in primary care of patients with important chronic conditions, needing coordinated care after hospital discharge, constricts transferring tasks from specialist care. Generic clinical pathways are likely to be more feasible and efficient for patients in this setting.
Key points
Clinical pathways in hospitals apply to single-disease guidelines, while more than 90% of the patients discharged to community health care for follow-up have multimorbidity. Primary care has to manage the health care of the patient holistically, with all his or her complex needs.
Patients most frequently admitted to hospitals, i.e. patients with COPD, heart failure, stroke and hip fracture are infrequent in primary care and represent a minority among patients in need of coordinated community health care.
In primary care, the low rate of receiving patients discharged from hospitals of major chronic diseases hampers maintenance of required specific skills, thus constricting the transfer of tasks to primary care. Generic clinical pathways are suggested to be more feasible than disease-specific pathways for most patients with complex needs.
Keywords: Health service research, care coordination, integrated care, general practice, home health nursing, practice guideline, multimorbidity
Introduction
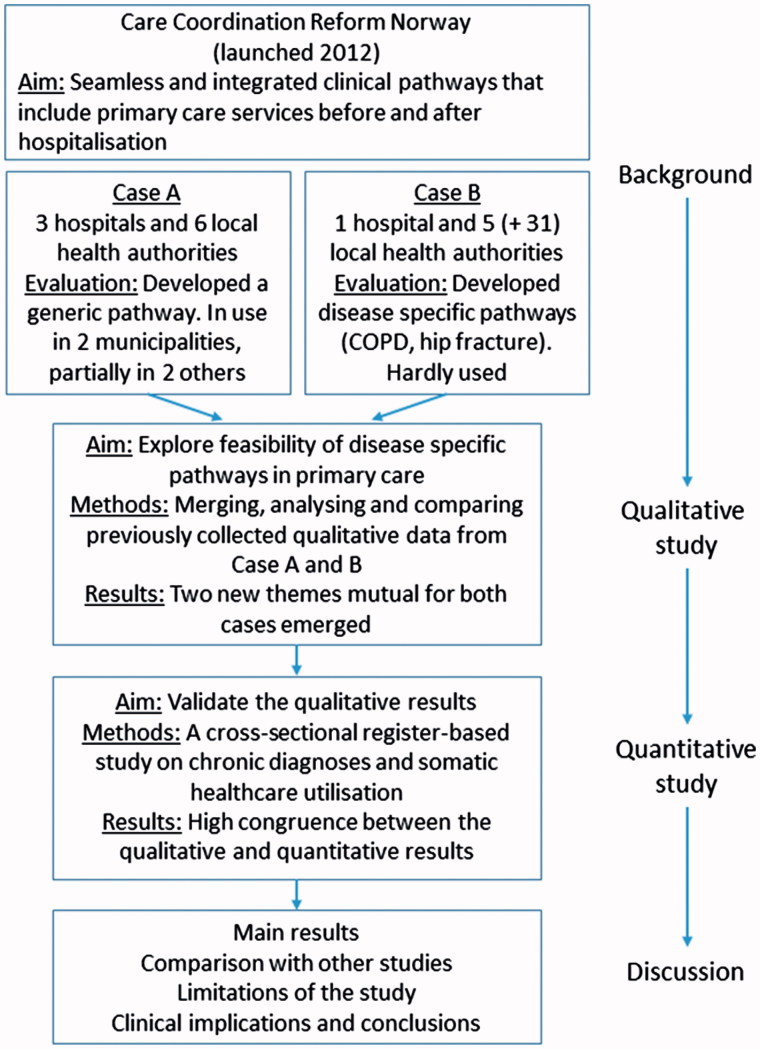
The main motive for implementing clinical pathways in hospitals has been quality improvement. Positive results have emerged in several projects [1]. There has also been a continuous effort to shorten hospital stays to increase capacity and cost-effectiveness. Facing an ageing population, health authorities in several countries are now looking for ways to shift more responsibility and tasks into primary care. One option at hand is to develop comprehensive clinical pathways that include the transfer of tasks to primary care before and after hospitalisation to both prevent admissions and facilitate earlier discharge [2]. This was one of the main goals of the Norwegian government’s Care Coordination Reform launched in 2012 (Figure 1) [3]. The study presented in this article originates from an evaluation research programme funded by the Research Council of Norway to track the implementation of the reform.
Figure 1.
Schematic overview of the sequences in the mixed-methods exploratory study and their presentation in this paper.
Although clinical pathways have several labels and definitions, they share some clear characteristics. Most are disease-specific; they facilitate care coordination and build on key elements from evidence-based medicine guidelines [4]. Relatively few projects have dealt with clinical pathways in primary care settings. Some studies have evaluated the transition from hospital to primary care [5], but most are concerned with implementing clinical pathways within specialist care settings [1].
Adherence to guidelines is strong in designing clinical pathways in specialist care. However, in primary care the average adherence to guidelines is less than 50% in treating patients [6]. The overall aim of this study was, therefore, to study the feasibility of disease-specific clinical pathways when extended from specialist care into primary care. Characteristics of disease-specific guidelines were first explored in a qualitative study and the findings were thereafter validated in a large quantitative study based on health-register data.
Setting
In Norway, there is a distinct two-part health service. The Ministry of Health and Care Services has supervisory responsibility for all hospitals and specialist care. About 9% of state spending on specialist care goes to private hospitals and institutions. The municipalities are responsible for providing all primary care services, including general practitioners (GPs), initial out-of-hours emergency care, homecare services, nursing homes and preventive services.
Two cases of collaboration between specialist and primary care in Mid-Norway had previously examined independently the development and implementation of comprehensive clinical pathways [7,8]. The researchers came from both social science and health service research, with and without basic health-professional education. However, these two studies were conducted at different sites and planned independently, and their organisation was apparently similar. Both projects had a three-level structure with a steering group, project group and local clinical pathway process groups. In addition, each had a project manager and external financial support, and engaged clinical pathways supervisors from the hospitals. They started with the intention of extending existing disease-specific clinical pathways from hospitals into primary care for patients with selected chronic diagnoses that required follow-up by both homecare nursing and the GP. Their objectives were identical: seamless and integrated clinical pathways that should include primary care services before and after hospitalisation. The progress and outcomes of the two studies were, however, quite different.
Case A
Three hospitals and six municipalities (covering 215,000 inhabitants) came together to develop clinical pathways for patients with chronic obstructive pulmonary disease (COPD), heart failure and stroke. As described in previous publications [7,9], the project was almost halted near its start because of disagreements between primary care and hospital representatives about the approach to patients’ needs and aims in developing clinical pathways. The two groups eventually agreed to develop one generic care pathway for transition into and follow-up in primary care, emphasising the common needs of most patients with chronic diseases. The generic pathway was implemented fully in two of the six participating municipalities and partially in another two.
Case B
One hospital and five adjacent municipalities (covering 37,000 inhabitants) developed two disease-specific clinical pathways, one for patients with COPD and one for patients with hip fracture. Both included transition from the hospital and follow-up in primary care [8,10]. The participants spent 2 years completing the description and guidelines for the pathways. The pathways were introduced to another 31 municipalities in the region, but this rollout was unsuccessful. A follow-up study conducted 18 months later found that the two disease-specific clinical pathways were not in use in any of the 36 municipalities. Even the five pilot municipalities hardly used either of them.
The results from Cases A and B were underpinned by empirical systematic reviews questioning the effects of disease-specific guidelines in primary care [11]. We wondered why the primary care staff in Case A [7] did not want to adopt an approach to developing clinical pathways that is accepted in hospitals, and in Case B [10], why the two disease-specific clinical pathways were not used. According to the UK Medical Research Council’s (MRC) updated version of the guidelines for complex interventions, an intervention may have limited success either because it is not properly implemented or because of weaknesses in its design in relation to the context in which it is implemented [12]. The first statement had been the main purpose of the process evaluations already done in Case A and Case B. The previous independent evaluations of the two cases had focused on processes, assessing fidelity, organisational aspects and quality of the implementation, using a qualitative inductive approach in order to clarify causal assumptions derived from relevant social science theories to explain the outcome [9,10]. However, no causes stood out explaining the success or non-success of the implementation process across the different sites. The picture emerging from both studies was rather that multiple factors were present.
This made us shift to the second statement of the MRC guidelines, weaknesses in its design in relation to the context. Based on recent theory [13], we questioned the feasibility of disease-specific clinical pathways in primary care and hypothesized that there might be inherent attributes in disease-specific pathways developed in specialist care that do not apply successfully to the context of primary care.
Thus, in line with the aim, we formulated the following main research question for the present study: Do characteristics of disease-specific clinical pathways provide incompatibility with contextual factors in primary care?
Overall design of the study
To answer the main research question, we used an exploratory sequential mixed-methods design [14]. We first merged and explored the former qualitative materials, and then, based on the new results, we designed a quantitative study to test the findings empirically.
Our idea for the qualitative study was that by studying the two cases, A and B, closer and comparing them, we would get greater insights than previously obtained by studying each case separately. We used a deductive approach assessing the qualitative data collected in Cases A and B to search for observations or experiences of the clinicians that could reveal problems related to attributes of the intervention itself. In this process, we searched for specific hypothesis that, afterwards, could be tested empirically in a quantitative study (Figure 1). We detected two themes mutually shared by the primary care representatives in both cases but not considered in the two projects previously. Then, to validate the qualitative findings we used an available dataset and designed and conducted a quantitative study on prevalence.
To reflect the sequential design and the progress, we have organised the paper as follows (Figure 1 to the right): We have already presented above the background and the two cases. Next, we describe the qualitative assessment and results from merging the two cases. Third, we describe the research questions derived from the qualitative analysis, the methods used in collecting quantitative data, and then the results thereof. Finally, in the discussion section, we review and discuss the qualitative and quantitative findings together and compare them with other studies.
The qualitative study
The qualitative study was based on secondary analysis of data collected in two previous studies on Cases A and B that have previously been published [7,8].
The participants were persons who had participated in the development and implementation of the clinical pathways in the two cases. They came from both hospitals and primary care, and they included clinicians and managers. A total of 155 persons were interviewed. The size of the participant municipalities varied from 4,000 to 180,000 inhabitants.
Data were collected from individual and focus group interviews. In addition, the researchers participated as observers at meetings, took reflexive notes or tape-recorded sessions, and had access to additional sources including minutes, notes, reports and other written materials. For the present study, 193 pages of transcribed text were made available.
The analysis was conducted by AL and AG and was presented and discussed with TR and IG, who had conducted the original studies. The analysis started with the transcriptions being read several times and discussed during a three-month period, writing comments on the sheets and letting reflections mature over time. In an iterative process, the text relevant for the aim was categorised, and themes were identified that could provide explanations for the experiences in implementing the care pathways [14].
Qualitative findings
We identified four categories of inexpediency experienced by the primary care health personnel using disease-specific clinical pathways. First, the concept of clinical pathways as a trajectory or timeline with a beginning and an end was unfamiliar to the home healthcare nurses in both cases. Their picture was more long-term and holistic: ‘The care is continuous, like in a circle around the patient' (home healthcare nurse, Case A). Second, their experience was that most patients needing home care have multiple chronic diseases and thus opposed the focus on single diagnoses: 'Older patients have many additional problems that the clinical guidelines don't take into consideration', and 'We have to take care of the whole patient – not only the disease treated by the hospital' (home healthcare nurses, Case B).
Third, in Case B, the process groups were loyal to the assignment and managed to develop two disease-specific pathways. In attempting to take into consideration patients’ additional problems, the pathway guidelines ended up becoming very lengthy: 'In our day-to-day work, we don't have time to consult these guidelines' (home healthcare nurse, Case B). In Case A, the dissension between primary care and specialists about the content of clinical pathways was resolved when they agreed to shift the focus to common problems of patients with chronic diseases. However, this move was met with scepticism by the specialists: 'We struggled hard to grasp how we could make a generic clinical pathway in primary care' (hospital supervisor, Case A).
Fourth, the selection of diseases in these studies was based on their high frequency of admissions to hospital. However, according to the observations reported in both cases, patients discharged with the selected diagnoses were seldom seen in home-care nursing: 'It was a good idea to include all patients over 70. Last year, we received no patients with any of the initially selected diagnoses' (home healthcare nurse, Case A); 'The clinical pathways have not been used much. We have not had any patients with COPD or hip fracture, but I am certain they will show up someday' (home healthcare nurse, Case B, after 18 months).
The quantitative study
To validate the four issues that the health personnel considered inexpedient using disease-specific clinical pathways in the qualitative study, we hypothesized that their capability of explaining the implementation problems in case A and B would depend on how widespread and frequent these obstacles are. We considered the second and fourth findings to be the most plausible explanations for why they in case A ended up developing and using a generic pathway, and in case B, hardly started using any of the two disease-specific pathways they had developed. Furthermore, both issues could be operationalised and empirically tested in a quantitative study. We developed the following research questions:
What are the numbers of inhabitants with COPD, heart failure, stroke or hip fracture in the total population?
What are the numbers of home healthcare nursing patients with each of the four diagnoses?
How many of these home healthcare nursing patients have more than one chronic disease, and on average, how many chronic diseases does each one have?
How many of these home healthcare nursing patients have emergency admissions to hospitals that initiate a clinical pathway programme, and how often do such admissions occur?
How often will each home healthcare nurse use clinical pathways designed for the four selected diagnoses?
Methods
We conducted a cross-sectional register-based study on somatic healthcare utilisation covering 214,722 inhabitants mainly overlapping the Case A area. Routine patient administrative data including diagnoses from St. Olav’s University Hospital, four adjacent municipalities and the Norwegian Health Economics Administration database (HELFO) from 2012 and 2013 were used. We linked the registers using a project ID based on the unique national personal-identification numbers assigned to each Norwegian citizen. We also collected demographic information and primary healthcare service statistics for the four municipalities from public registers provided by Statistics Norway. All data was de-identified before the analysis.
Inclusion criteria for the analysis were people with a diagnosis of COPD, heart failure, stroke or hip fracture registered in hospitals, general practices, private specialist and with physiotherapist, using the International Classification of Diseases (ICD-10) or International Classification of Primary Care (ICPC-2) codes:
COPD. ICD-10: J44/ICPC-2: R85;
Heart failure. ICD-10: I50/ICPC-2: K77;
Stroke. ICD-10: I61, I63 or I64/(ICPC-2: K89, K90); and
Hip fracture. ICD-10: S720, S721, S722 or S723/(ICPC-2: L75).
Persons under 18 years of age and anyone who had used only psychiatric services were excluded.
To identify people with multiple chronic diseases, we employed O’Halloran et al.’s definition, using 147 different ICPC-2 codes [15]. ICD-10 codes were mapped to ICPC-2 codes using a table provided by the WONCA International Classification Committee [16]. Multimorbidity was defined as having two or more chronic conditions in two or more different ICPC-2 organ chapters [17]. We used the conservative approach of counting only one code per organ chapter to identify those with multimorbidity. Thus, a person with five chronic codes in two different ICPC-2 organ chapters was recorded as having two different chronic diseases. Hospitalisation was measured as any registered inpatient stay at the university hospital. Emergency hospitalisation was defined as any non-elective inpatient stay.
The data are presented descriptively. The number of patients receiving home healthcare nursing and being hospitalised for the selected diagnoses was stable from 2012 to 2013. The number of new patients registered per year and the number of patients who had died or been discharged from home healthcare were very similar. We therefore chose to report the figures for 2013 (one year).
Quantitative results
Table 1 provides an overview of the study population. Approximately 4% were community-dwelling persons receiving home healthcare nursing; about half of them were over 80 years old, and most were women. Nearly all patients admitted (93%) were emergency admissions. A quarter (25%) had at least one elective admission, but most also had emergency admissions.
Table 1.
Characteristics of inhabitants ages 18 years and older in the four municipalities in 2013 (N = 214 722).
| Those receiving home healthcare nursing from municipalities |
Those ages 18 years and older |
|||
|---|---|---|---|---|
| n | % | n | % | |
| Number of people | 6 061 | 168 285 | ||
| - Female, n (%) | 3 570 | 60 | 83 681 | 50 |
| - Ages 80 years and older | 2 858 | 47 | 7 839 | 5 |
| Number of patients hospitalised, n (%) | ||||
| - All admissions | 2 914 | 48 | 16 920 | 10 |
| - Emergency admissions | 2 701 | 45 | 14 403 | 9 |
Table 2 shows the population rates of the four selected diagnoses. GPs in Norway have, on average, 1,200 patients on their list and cover 98% of the inhabitants. If their patients had the same gender and age distribution as the municipalities in this study, the GPs in 2013 had an average of 28 patients with COPD, 17 with heart failure, 4 with stroke and 3 with hip fracture.
Table 2.
Inhabitants ages 18 years and older with COPD, heart failure, stroke or hip fracture in four municipalities (N = 168 285).
| COPD | Heart failure | Stroke | Hip fracture | |
|---|---|---|---|---|
| No. of inhabitants (age ≥ 18) with four selected diagnoses | 3458 | 2005 | 491 | 348 |
| Standardised rate of patients per 10,000 inhabitants* | 232 | 141 | 34 | 25 |
| Average no. of chronic diseases per patient | 3.6 | 3.8 | 3.5 | 3.6 |
| Percentage enrolled in home healthcare nursing | 20 | 44 | 39 | 48 |
Year prevalence adjusted for age and gender among all inhabitants ages 18 years and older in the four municipalities.
Of the total 6,061 home healthcare nursing patients, 1,602 (26%) unique patients had one or more of the four selected diagnoses; 12% had COPD, 15% heart failure, 3% stroke and 3% hip fracture. Table 2 also shows the proportion of patients with each diagnosis enrolled in homecare nursing. The lower proportion of COPD patients receiving home healthcare nursing is likely to be because they tended to be younger on average.
Table 3 shows the number of patients receiving homecare nursing, their number of chronic diseases, and the number of patients discharged from hospital within a year. The latter group was the target for developing clinical pathways. Table 3 demonstrates that being a homecare patient with only a single diagnosis is rare. Home healthcare patients with at least one of the four selected diagnoses had more than four chronic diseases that required treatment, with COPD patients being the group most represented. In addition, the table shows that patients with the four diagnoses had different patterns of emergency care utilisation. Only about one-fifth of the COPD patients were admitted to the hospital during the year, but those who were admitted had the highest frequency of hospitalisations, with an average of two hospitalisations per year. All patients with hip fracture and nearly all with stroke were admitted to hospitals, but the majority for this diagnosis were admitted only once.
Table 3.
Home healthcare nursing patients, by chronic diseases and emergency hospitalisations, for four selected diagnoses.
| COPD | Heart failure | Stroke | Hip fracture | |
|---|---|---|---|---|
| Home healthcare nursing patients | ||||
| Number of patients with selected diagnosis | 704 | 879 | 190 | 167 |
| Standardised rate of patients per 10,000 inhabitants* | 49 | 64 | 13 | 12 |
| Average number of chronic diseases per patient | 4.8 | 4.4 | 4.0 | 4.2 |
| Patients having two or more chronic diseases (%) | 99 | 95 | 94 | 93 |
| Patients with emergency hospitalisations for main diagnosis | ||||
| Number of patients with emergency hospitalisations | 158 | 135 | 158 | 167 |
| Standardised rate per 10,000 inhabitants* | 23 | 15 | 12 | 15 |
| Proportion of patients hospitalised in each group (%) | 22 | 15 | 83 | 100 |
| Number of emergency hospitalisations during one year | ||||
| Number of hospitalisations for main diagnosis | 335 | 211 | 174 | 215 |
| Number of emergency hospitalisations per patient hospitalised | 2.1 | 1.6 | 1.1 | 1.3 |
Year prevalence adjusted for age and gender among all inhabitants ages 18 years and older in the four municipalities.
The standardised numbers of hospitalisations per 10,000 for each diagnosis also appear in Table 3. Patients with COPD, heart failure, stroke and hip fracture, taken together, account for 10% of the total number of home healthcare nursing patients admitted to hospitals as emergencies each year.
According to public statistics, an average 11.5 patients are seen in home care per full-time equivalent nurse. Because some nurses work part-time, the number of patients per employed nurse is 9.5. Using the frequency of hospitalisations per 10,000 inhabitants in Table 3, each home healthcare nurse has an annual average of 0.5 patients hospitalised with COPD, 0.3 with heart failure, 0.2 with stroke and 0.4 with hip fracture.
Discussion
The results from the quantitative study verified the comments made by the primary healthcare personnel in the qualitative study. First, they opposed the single-disease focus forwarded by the specialists. They stated that their responsibility is to manage care of all of their patients’ problems. The quantitative study revealed that home healthcare nursing patients are characterised by extensive multimorbidity, with few having only one disease. Second, home healthcare nurses said that they rarely had a chance to practice any disease-specific clinical pathway. The quantitative study showed that, for patients with one of the four selected diagnoses, a homecare nurse might be responsible for preparing reception at home at discharge from hospital, on average, less often than every other year.
Limitations of the study
Health care, and especially primary care, is organised quite differently in different countries. The delineation of responsibilities between specialist and primary care also differs [18], and this must be taken into consideration in reviewing and using the results from this study [19]. Moreover, there is no standard way to measure multimorbidity [17], so comparing different studies to validate the prevalence of multimorbidity has limitations. Unlike most studies, we added any diagnoses of chronic diseases found in the hospital record to those included in the GP’s record for each patient. This increased the number of chronic diagnoses per patient substantially, even when we allowed for only one chronic diagnosis per ICPC organ chapter.
Our experience is that extracting data from patient records in general practice covering two years is not sufficient to capture all chronic diseases registered by the GP. Hospitals, however, are paid more for patients with increasing number chronic diseases, so they take care to record all diagnoses. Studying the registrations closely, we also discovered that the municipalities had registered the use of home healthcare nursing in different ways. We were, however, able to trace and adjust for this.
We have examined the data regarding the prevalence of COPD, heart failure, stroke and hip fracture found in other studies, and they vary depending on the method used. For COPD, a clinical population screening shows a prevalence of 400 diagnoses per 10,000 people; however, according to the Norwegian Prescription Database, about 63 per 10,000 have COPD in Norway. Our number was 232 (Table 2). For heart failure, stroke and hip fracture, official national sources [20] state an average prevalence per 10,000 people of 150, 30 and 20, respectively. That corresponds fairly well to our numbers shown in Table 2.
We used quantitative data only from the area where Case A had been conducted. However, the prevalence numbers we acquired indicate that adding quantitative data from Case B would not make any significant difference regarding the issues studied. All of the participating hospitals belong to the same regional health authority, and there was a similar mixture of participating cities and smaller rural municipalities.
The quantitative results align well with the qualitative findings, thereby strengthening the validity of the study.
Disease-specific guidelines do not extend to the majority of patients in primary care
Home healthcare nursing patients with multimorbidity and emergency hospitalisations are usually involved with several healthcare stakeholders and are, accordingly, those most in need of coordinated services. Their needs are complex, not only because they are being treated for multiple diseases but also – and equally important in primary care – they need support aimed at helping them to manage their own lives at home for as long as possible. Thus, additional complexity emerges for following up patients with multimorbidity at home.
In that perspective, research supports the primary care view about the limitations of disease-specific guidelines [21]. Guidelines seldom give advice regarding accompanying diseases or discuss whether they are relevant, safe and applicable to patients with multimorbidity. Few guidelines mention short- and long-term goals or offer guidance for incorporating patient preferences in treatment plans [22]. Most disease-specific guidelines do not discuss any effects on quality of life or functional ability or burdens that treatment may cause for patients and their caregivers [23].
The change in strategy from disease-specific pathways to developing a generic pathway for the follow-up of discharged patients in Case A [7] was a bold step. The generic pathway that was developed balances condition-specific needs and those needs that apply to impairments, disabilities and social contexts encountered, and thus, it merges different perspectives including meeting patients’ preferences and enhancing their possibilities to continue living at home. This generic approach has little support in evidence-based guidelines, which are focused almost entirely on the medical treatment of single diseases [22]. However, tailoring treatment to address individuals’ needs and preferences is similarly described in guidelines for palliative care [24]. We also found that several recent editorials and commentaries have favoured a change such as in Case A [25,26]. Moreover, the National Institute for Health and Care Excellence (NICE) published for the first time ever in 2016 guidelines for the treatment of patients with multimorbidity in general practice [27].
The guidelines’ practical usability was also important to the home healthcare nurses in both cases. This became apparent in Case B. Attempting to include all the potential needs and ways to follow up patients with multimorbidity in primary care, the guidelines for COPD and hip fracture in Case B exceeded a hundred pages. For instance, for patients with hip fracture, approximately 30% have dementia, 10% heart failure, 20% diabetes, 25% frailty, etc. [20]. These wide-ranging guidelines turned out to be useless on a busy day. On the other hand, the primary care personnel argued that patients with chronic diseases have many common needs that do not necessarily have to be repeated in every guideline, and research supports their view. Broad and multidisciplinary approaches applied across diseases provide the best results in transitions and follow-up of patients with multiple chronic diseases [2,11,21,28].
Low prevalence of major chronic diseases constrains the transfer of tasks to primary care
One of the main aims in the Care Coordination Reform was to extend the clinical pathways already in use in hospitals to include primary care tasks both before and after hospital admission. The main motive was better collaboration, but there was also an expectation that this would form a basis for transferring more responsibility for the treatment of patients to primary care. The selected four groups of patients experience frequent hospital admissions. Hence, it was quite difficult to believe that many of the homecare nurses had no experience with patients having one of the four selected diagnoses being discharged from hospital in an entire year. However, the quantitative study confirmed that this was true.
Comparing Table 2 and Table 3, we can see one reason for the low number. Most patients with the four selected diagnoses were not enrolled in homecare nursing. They managed their needs and coordination of care themselves, most likely in collaboration with their GP. Nevertheless, recipients of homecare nursing are those patients most in need of clinical pathways and were given priority in both Case A and Case B. A nationwide survey after the introduction of the Care Coordination Reform found that 70% of the municipalities experienced increased pressure from hospitals to engage nurses trained to take greater responsibility for the transition from hospital for the most frequent specific diagnoses.
However, findings in our study do not support the idea of having specialised nurses in home healthcare nursing. Table 3 and the interviews reveal that the number of patients eligible for continuing disease-specific pathways into primary care is too low to justify specialisation of home healthcare nurses targeting these four diseases in spite of their frequent appearance in hospitals. Specialising is not only a matter of education; maintaining skills is also dependent upon sufficient training, experience on an on-going basis, and being part of a specialist environment. The infrequent discharge from hospital experienced by each nurse also indicates why he or she was unfamiliar with the concept of clinical pathways.
We have not found any earlier study that examines the prevalence of important chronic diseases in primary care as a constriction of what tasks are suitable for being transferred to primary care. Some may argue that there are enough patients in larger cities to support the idea of having disease-specialist nurses. However, almost all homecare patients have multiple chronic diseases and would therefore need several specialist nurses visiting them regularly at home. This would cause fragmentation of the care of individual patients, which is inconsistent with the aims of primary care; nor would it be an efficient use of resources. Together with extensive multimorbidity, this undermines the feasibility of disease-specific clinical pathways in primary care.
Implications and conclusion
We believe that the findings of this Norwegian study are relevant to other countries. The Western world is facing an increase in the number of patients with multimorbidity. A desired shift in tasks and responsibilities from specialist care to primary care, as in the Norwegian Coordination Reform, seems to recur in the initiatives and plans of several nations. Most countries also have in common a lack of research and guidelines for patients with multimorbidity. There is a large gap between the size of the group with multiple chronic diseases, their burden on health care and the resources allocated for research involving this group.
On the contrary, the last two decades have been characterised by an expanding array of disease-centred guidelines that do not fit the realities of the growth in clinical practice of people with multimorbidity [29]. The single-disease focus in most medical research has raised concerns [21,30]. Our findings have revealed that the feasibility of disease-specific guidelines for patients with complex needs in primary care is limited, both from a clinical and organisational perspective. The evidence base needed by policy makers and planners to support the deployment of generic clinical pathways in primary care is, however, scarce.
Acknowledgement
We want to thank the hospital staff, the primary care staff and the GPs who participated in this study.
Disclosure statement
The authors declared no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Funding
The Norwegian Research Council funded the work under Grant 220553.
Ethical approval
The Regional Committee for Medical and Health Research Ethics in Central Norway approved the study (2011/2047).
References
- 1. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;35:CD006632. [DOI] [PubMed] [Google Scholar]
- 2. Shepperd S, Lannin NA, Clemson LM, et al. Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;1:CD000313. [DOI] [PubMed] [Google Scholar]
- 3. St. meld. nr. 47 (2008–2009). The Coordination Reform. Proper treatment – at the right place and right time (English summary of the white paper). Oslo: Norwegian Ministry of Health and Care Services; 2009. [Google Scholar]
- 4. Kinsman L, Rotter T, James E, et al. What is a clinical pathway? Development of a definition to inform the debate. BMC Med. 2010;8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allen J, Hutchinson AM, Brown R, et al. Quality care outcomes following transitional care interventions for older people from hospital to home: a systematic review. BMC Health Serv Res. 2014;14:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mickan S, Burls A, Glasziou P.. Patterns of ‘leakage’ in the utilisation of clinical guidelines: a systematic review. Postgr Med J. 2011;87:670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosstad T, Garasen H, Steinsbekk A, et al. Development of a patient-centred care pathway across healthcare providers: a qualitative study. BMC Health Serv Res. 2013;13:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meese J, Rønhovde L.. Prosjektorganisering som strategi for kunnskapsoverføring og laering i forbindelse med samhandlingsreformen [Organizing projects as a strategy for transfer of knowldge and learning in the care coordination reform]. Nordiske Organisasjonsstudier. 2015;17:86–108. [Google Scholar]
- 9. Røsstad T, Garåsen H, Steinsbekk A, et al. Implementing a care pathway for elderly patients, a comparative qualitative process evaluation in primary care. BMC Health Serv Res. 2015;15:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gjerde I, Meese J, Aarseth T.. Helhetlige pasientforløp i utvikling, del 2 [Comprehensiv clinical pathways in progress. part 2]. Molde: Univerity of Molde; 2014. [Google Scholar]
- 11. Smith SM, Wallace E, O'Dowd T, et al. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2016; 3:CD006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. May CR, Johnson M, Finch T.. Implementation, context and complexity. Implement Sci. 2016;11:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Creswell JW. Research design: Qualitative, quantitative, and mixed methods approaches. 4th ed. Nebraska: University of Nebraska, London: SAGE; 2014. [Google Scholar]
- 15. O'Halloran J, Miller GC, Britt H.. Defining chronic conditions for primary care with ICPC-2. Fam Practice. 2004;21:381–386. [DOI] [PubMed] [Google Scholar]
- 16. WONCA An international glossary for general/family practice. ICPC-2e-v4.4 Trondheim: Wonca International Classification Committee; 2014. [cited 2015 Sept 24]. Available from: http://www.kith.no/templates/kith_WebPage____1111.aspx [Google Scholar]
- 17. Harrison C, Britt H, Miller G, et al. Examining different measures of multimorbidity, using a large prospective cross-sectional study in Australian general practice. BMJ Open. 2014;4:e004694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kringos D, Boerma W, Bourgueil Y, et al. The strength of primary care in Europe: an international comparative study. Br J Gen Pract. 2013;63:e742–e750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ashton T. Implementing integrated models of care: the importance of the macro-level context. Int J Integr Care. 2015;15:e019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Folkehelseinstiuttet Folkehelserapporten - Folkehelsen i Norge [Public Health Report - Health in Norway]. Rapport 2014:4 Oslo Folkehelseinstituttet;2014 [cited 2017 Jan 10]. Available from: https://www.fhi.no/nettpub/hin/ [Google Scholar]
- 21. Wallace E, Salisbury C, Guthrie B, et al. Managing patients with multimorbidity in primary care. BMJ. 2015;350:h176. [DOI] [PubMed] [Google Scholar]
- 22. Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294:716–724. [DOI] [PubMed] [Google Scholar]
- 23. Mair FS, May CR.. Thinking about the burden of treatment. BMJ. 2014;349:g6680. [DOI] [PubMed] [Google Scholar]
- 24. Murray SA, Kendall M, Mitchell G, et al. Palliative care from diagnosis to death. BMJ. 2017;356:j878. [DOI] [PubMed] [Google Scholar]
- 25. Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition-multimorbidity. JAMA. 2012;307:2493–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salisbury C. Multimorbidity: redesigning health care for people who use it. Lancet. 2012;380:7–9. [DOI] [PubMed] [Google Scholar]
- 27. National Institute for Health and Care Excellence Multimorbidity: clinical assessment and management. NICE Guideline NG56 London: NICE; 2016. [cited 2017 June 6]. Available from: https://www.nice.org.uk/guidance/ng56 [Google Scholar]
- 28. Coleman K, Austin BT, Brach C, et al. Evidence on the Chronic Care Model in the new millennium. Health Aff. 2009;28:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guthrie B, Payne K, Alderson P, et al. Adapting clinical guidelines to take account of multimorbidity. BMJ. 2012;345:e6341. [DOI] [PubMed] [Google Scholar]
- 30. Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. [DOI] [PubMed] [Google Scholar]