Abstract
Objective: The aim of this study is to describe patients with heart failure and an ejection fraction (EF) of more than or equal to 40%, managed in both Primary- and Hospital based outpatient clinics separately with their prognosis, comorbidities and risk factors. Further to compare the heart failure medication in the two groups.
Design: We used the prospective Swedish Heart Failure Registry to include 9654 out-patients who had HF and EF ≥40%, 1802 patients were registered in primary care and 7852 in hospital care. Descriptive statistical tests were used to analyze base line characteristics in the two groups and multivariate logistic regression analysis to assess mortality rate in the groups separately.
Setting: The prospective Swedish Heart Failure Registry.
Subjects: Patients with heart failure and an ejection fraction (EF) of more than or equal to 40%.
Main outcome measures: Comorbidities, risk factors and mortality.
Results: Mean-age was 77.5 (primary care) and 70.3 years (hospital care) p < 0.0001, 46.7 vs. 36.3% women respectively (p < 0.0001) and EF ≥50% 26.1 vs. 13.4% (p < 0.0001). Co-morbidities were common in both groups (97.2% vs. 92.3%), the primary care group having more atrial fibrillation, hypertension, ischemic heart disease and COPD. According to the multivariate logistic regression analysis smoking, COPD and diabetes were the most important independent risk factors in the primary care group and valvular disease in the hospital care group. All-cause mortality during mean follow-up of almost 4 years was 31.5% in primary care and 27.8% in hospital care. One year-mortality rates were 7.8%, and 7.0% respectively.
Conclusion: Any co-morbidity was noted in 97% of the HF-patients with an EF of more than or equal to 40% managed at primary care based out-patient clinics and these patients had partly other independent risk factors than those patients managed in hospital care based outpatients clinics. Our results indicate that more attention should be payed to manage COPD in the primary care group.
KEY POINTS
97% of heart failure patients with an ejection fraction of more than or equal to 40% managed at primary care based out-patient clinics had any comorbidity.
Patients in primary care had partly other independent risk factors than those in hospital care.
All-cause mortality during mean follow-up of almost 4 years was higher in primary care compared to hospital care.
In matched HF-patients RAS-antagonists, beta-blockers as well as the combination of the two drugs were more seldom prescribed when managed in primary care compared with hospital care.
Keywords: Heart failure, preserved ejection fraction, primary care, risk factors, outcome
Introduction
Heart failure (HF) is a common and serious disease. The estimated prevalence of HF in Sweden is 2.2% with an incidence of 3.8/1000 person-years [1]. Mortality is high with a mean five-year rate of 52%. Studies have shown that mortality and prevalence is essentially unchanged over the years [2,3]. Patients are managed both in Primary Care (PC) and Hospital Care (HC) but only a smaller portion (17%) is managed in PC only [1]. HF is a complex and heterogeneous condition; often divided into HF with reduced ejection fraction (HFrEF), defined as EF <40%, and HF with preserved ejection fraction (HFpEF), defined as EF ≥50% [4]. EF 40-49% is not normal but there is no evidence based therapy, and in the latest guidelines it has been considered to be an independent phenotype (HF with mid-range EF, HFmrEF) [4]. HFpEF is just as common as HFrEF but in contrast to HFrEF there is no evidence based therapy for HF-patients with an EF of more than or equal to 40% [4–6].
The role of comorbidities and risk factors in HF-patients with an EF of more than or equal to 40% is very important but the significance of this in a primary care setting has so far been poorly studied [7–12].
Studies indicate that HF-patients with an EF of more than or equal to 40% managed in PC are older, more often females and with a lower NYHA-class compared to patients managed in HC [7,13]. Previous studies also indicate non-compliance to recommendations of management of HF -patients in PC [5,14–16], even if adherence over time has increased [15]. Besides, individualized life-style counselling is also shown to reduce CVD risk [16].
To our knowledge no previous study describes the characteristics, co-morbidities, risk factors and mortality in a large group of outpatients with HF with an EF of more than or equal to 40% managed in PC.
Therefore, the aim of this study is to describe heart failure patients with an EF of more than or equal to 40% handled in PC and HC based outpatient clinics, prognosis, comorbidities and risk factors. Further to compare the heart failure medication in the two groups.
Methods
Study protocol
The Swedish Heart Failure Registry (SwedeHF) has been described previously [17]. Un-selected patients with HF are prospectively registered when hospitalized before discharge or in PC and in HC at an outpatient-visit. Inclusion criterion is clinician-judged HF. Approximately eighty variables are recorded and entered into a web-based database managed by Uppsala Clinical Research Center (Uppsala, Sweden). The protocol, registration form and annual reports are available at http://www.rikssvikt.se. Individual patient consent is not required but patients are informed of entry into the national registry and allowed to opt out. The registry and this study were approved by a multisite ethic committee and conform to the Declaration of Helsinki.
In this study we used data from the SwedeHF recorded between First of September 2001 and 15th of May 2014 after the database had been merged with the Swedish population registry and the Swedish patient registry of hospitalization. The two latter registries are governed by the Swedish Board of Health and Welfare. Sweden had 1156 PC units and 78 hospitals in year 2014. Of these 116 PC units and 67 hospitals participate in the registry. In total, 59075 unique patients, 6579 from PC and 52496 from HC were eligible for this study. We included only patients registered at an out-patient visit either in PC or in HC. We excluded patients without information about echocardiography (1041 = 15.8% in PC and 5938 = 11.3% in HC) and in the next step patients with an EF <40% as well as hospitalized HF-patients with an EF ≥40% who entered the registry before hospital discharge. There are no preregistration data in SwedeHF. Thus, 1802 patients registered at a PC-based outpatient clinic visit and 7852 patients registered at a HC-based outpatient clinic, all having an EF of more than or equal to 40%, remained in the study.
In Sweden, echocardiography is the recommended method for defining EF. Four categories are used in the SwedeHF, EF <30, 30–39, 40–49 and ≥50%. In this study we chose a cut-off value for EF ≥40% to define HFpEF since this has been used in a previous study [18]. However, we also conducted a sensitivity analysis with HFpEF defined as EF ≥50%.
S-creatinine was measured in µmol/l and based on this estimated glomerular filtration rate (e-GFR) was calculated according to the formula of MDRD and divided in four different classes, less than 30, 30–59, 60–89 and ≥90 µmol/l/min.
Statistical analysis
Baseline characteristics tables were constructed for PC patients and HC patients respectively. Tables were built for patients with EF ≥40%. We also studied the groups with EF 40–49 and EF ≥50% separately (data not shown). Mortality rate was calculated and chartered with the Kaplan-Meier method for the three EF-groups and mortality tables were constructed, showing 1, 3 and 5 years mortality rates for the three groups separately. Matching of patients in the overall cohort for renal function and blood pressure was performed since if abnormal these patients often may be admitted for specialized care before RAS-antagonists are prescribed. Thus, patients were matched for age (± 1 year), gender (same), systolic blood pressure (>110 mm Hg) and e-GFR–class (same). Matching was 1:1 yielding 1499 patients in each group. Baseline characteristics tables were constructed and mortality rates were calculated for the matched cohorts as for overall cohorts.
Multivariate logistic regression analyses were performed for time dependent different variables, calculating hazard ratios (HR) with 95-% CI for mortality, and presented in a Forest Plot figure. We included variables which all had a p-value of 0.1 or below at the univariate analyses. HR for medication was only analyzed for the matched cohorts since patients could be admitted for specialized care before Renin Angiotensin System (RAS)-antagonists were prescribed. We also calculated 1-, 3- and 5-year mortality rates for patients with no comorbidity in the PC and HC groups and for those with any comorbidity.
All statistical analyses were performed using SAS statistical software (version 9.4). Categorical variables were analyzed using the chi square-test and continuous variables using the t-test. Levels considered statistical significant were a p-value <0.05. All p-values and 95% CI are 2-sided.
Results
Characteristics at registration
Between first of September 2001 and 15th of May 2014 there were 59075 patients in SwedeHF. Hospitalized patients, patients without information about echocardiography (15.8% of PC patients and 11.3% of HC patients), hospitalized HF-patients with an EF≥ 40% and patients with an EF <40% were excluded from the study. Thereafter 1802 (18.7%) patients from 116 PC units and 7852 (81.3%) patients from 67 hospitals, all registered at an out-patient visit in either PC or HC remained in the study (Figure 1). Baseline characteristics are presented in Table 1.
Figure 1.
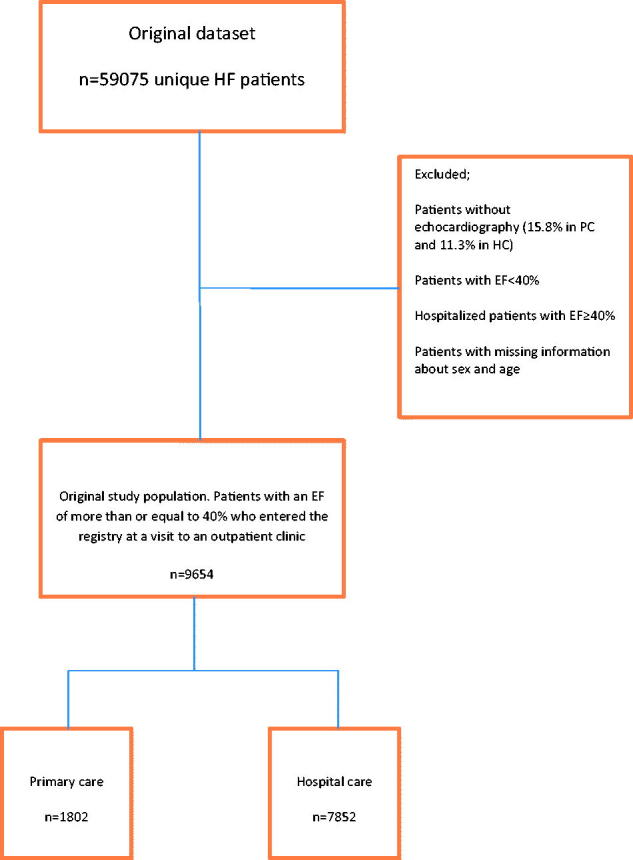
Schematic patient selection.
Table 1.
Characteristics in patients with an EF equal to or above 40%, overall and matched cohorts.
| Overall cohort |
Matched cohort |
|||
|---|---|---|---|---|
| Primary care, n = 1802 | Hospital care, n = 7852 | Primary care, n =1499 | Hospital care, n = 1499 | |
| Variabels | Mean ± SD or % | Mean ± SD or % | Mean ± SD or % | Mean ± SD or % |
| Dead during follow up, % | 31.5 | 27.8** | 29.7 | 34.6** |
| Age, years | 77.5 ± 8.9 | 70.3 ± 12.4*** | 77.4 ± 8.7 | 77.1 ± 8.7 |
| Follow up time, mean days | 1151.2 ± 752.5 | 1286.8 ± 925.5*** | 1189.9 ± 761.5 | 1212.0 ± 880.6 |
| Sex | ||||
| Male | 53.3 | 63.7*** | 53.8 | 53.8 |
| Female | 46.7 | 36.3*** | 46.2 | 46.2 |
| Smoking | ** | |||
| Current | 7.8 | 11.0 | 8.0 | 8.1 |
| Former | 43.0 | 42.4 | 42.5 | 38.0 |
| Never | 49.2 | 46.6 | 49.5 | 53.9 |
| Duration heart failure, months | *** | *** | ||
| >6 months | 73.0 | 50.2 | 71.6 | 48.7 |
| <6 months | 27.0 | 49.8 | 28.4 | 51.3 |
| Functional class NYHA | ** | *** | ||
| NYHA-class I | 15.4 | 17.8 | 16.1 | 12.3 |
| NYHA-class II | 57.3 | 51.3 | 58.0 | 52.3 |
| NYHA-class III | 26.0 | 29.7 | 24.5 | 34.2 |
| NYHA-class IV | 1.4 | 1.0 | 1.4 | 1.3 |
| QRS duration, mean ms | 103,3 ± 26.3 | 104,8 ± 25.1* | 103,8 ± 26.4 | 105,4 ± 26.9 |
| ECG-rhythm | *** | ** | ||
| Sinus | 53.2 | 58.2 | 55.0 | 53.4 |
| Atrial fibrillation/flutter | 40.5 | 33.1 | 38.9 | 37.7 |
| Systolic blood pressure, mean mmHg | 134,5 ± 20.3 | 129,5 ± 21.0*** | 139,0 ± 17.5 | 137,4 ± 17.3* |
| Diastolic blood pressure, mean mmHg | 75.1 ± 11.1 | 73.8 ± 11.7*** | 76.6 ± 10.7 | 74.8 ± 10.7*** |
| Co-morbidity | ||||
| IHD | 57.8 | 32.7*** | 40.2 | 42.5*** |
| IHD with coronar angiography | 14.6 | 25.1 | 14.5 | 20.6 |
| IHD without coronar angiography | 27.6 | 17.6 | 27.7 | 21.9 |
| Hypertension | 67.0 | 48.9*** | 70.2 | 56.2*** |
| Atrial fibrillation/flutter | 53.0 | 47.2*** | 51.6 | 51.5 |
| Valvular disease | 23.8 | 22.6 | 23.4 | 26.1 |
| COPD | 24.5 | 15.2*** | 24.1 | 14.5*** |
| Diabetes | 21.1 | 20.1 | 22.3 | 20.4* |
| Hemoglobin, mean g/L | 134,0 ± 15.5 | 134,8 ± 16.4* | 134,3 ± 15.4 | 132,6 ± 15.6** |
| Creatinine, micromol/L | 99.4 ± 35.1 | 100,3 ± 46.7 | 99.0 ± 35.0 | 100,5 ± 42.0 |
| eGFR, mean mL/min | 62.3 ± 21.6 | 67.3 ± 38.3*** | 62.6 ± 21.5 | 62.1 ± 21.0 |
| eGFR classes | *** | |||
| <30 | 4.8 | 4.4 | 4.7 | 4.7 |
| 30–59 | 43.3 | 37.1 | 43.2 | 43.2 |
| 60–89 | 41.8 | 44.2 | 42.0 | 42.0 |
| >90 | 10.1 | 14.3 | 10.1 | 10.1 |
| Medical treatment | ||||
| ACEi | 56.1 | 65.2*** | 56.2 | 62.6*** |
| ARB | 28.7 | 27.1 | 29.3 | 26.8 |
| ACEi or ARB | 83.2 | 90.2** | 83.7 | 87.6* |
| BB | 74.1 | 86.9*** | 74.2 | 85.7*** |
| ACEi or ARB + BB | 63.7 | 78.6*** | 63.8 | 75.2*** |
| MRA | 20.6 | 24.9*** | 19.5 | 21.0 |
| Diuretics | 32.7 | 34.2*** | 33.6 | 35.0** |
| Digoxin | 14.9 | 13.0* | 14.8 | 13.9 |
| Statins | 45.2 | 48.8** | 46.3 | 46.2 |
| Nitrates | 12.6 | 11.3 | 12.3 | 14.9* |
| ACEi or ARB + BB + MRA | 12.8 | 19.3*** | 12.4 | 14.8* |
| Oral anticoagulants | 39.6 | 42.0 | 38.7 | 42.8* |
| Aspirin or other trombocyte inhibitors | 44.6 | 44.4 | 45.2 | 47.4 |
| Oral antidiabetics | 5.8 | 6.0 | 5.6 | 6.5 |
| Insulin | 7.1 | 5.8* | 8.0 | 5.4** |
Continuous data are presented as mean + SD and statistically assessed by t-test and categorical data as proportions (%) assessed by chi2-test.
ACEi: ACE-inhibitors; ARB: Angiotensin receptor blockers; BB: Beta blockers; CABG: Coronary artery bypass grafting; COPD: Chronic obstructive pulmonary disease; CRT: Cardiac resynchronization therapy; DCM: Dilated cardiomyopathy; ICD: Implantable cardioverter defibrillator; IHD: Ischemic heart disease; MRA: Mineral corticoid receptor antagonist; NYHA: New York Heart Association; PCI: Percutaneous coronary intervention.
p-value <0.05; **p-value <0.01; BB: Beta blockers; ***p-value <0.0001.
The patients in the PC cohort were older, 77.5 vs. 70.3 years (p < 0.0001), with more women, 46.7% vs. 36.3% (p < 0.0001) and 26.1 vs. 13.4% had EF> 50% (p < 0.0001). Functional class by NYHA was often missing in both groups (45.4% missing in PC and 46.1% in HC), but when registered, 72.2% were in class I or II in the PC cohort and the corresponding figure for HC was 69.1%, (p < 0.01). Baseline characteristics are shown in Table 1.
Comorbidities
Comorbidities differed between the two groups, with more cases of atrial fibrillation, hypertension, ischemic heart disease and COPD in the PC cohort (p < 0.0001). However, this type of comorbidity was also found in the HC cohort. Only 50 patients (2.8%) in the PC cohort and 608 patients (7.7%) in HC had no comorbidities at all registered. The heart rate and the blood pressure were slightly higher, mean systolic BP 134 vs. 129 mm Hg (p < 0.0001) and mean diastolic BP 75.1 vs. 73.7 mm Hg (p < 0.0001), in the PC cohort. There was more renal dysfunction in the PC cohort vs. HC, 48, 1% vs. 41, 5% having eGFR-class <60ml/kg/min. There were more smokers in the HC cohort (Table 1).
Mortality
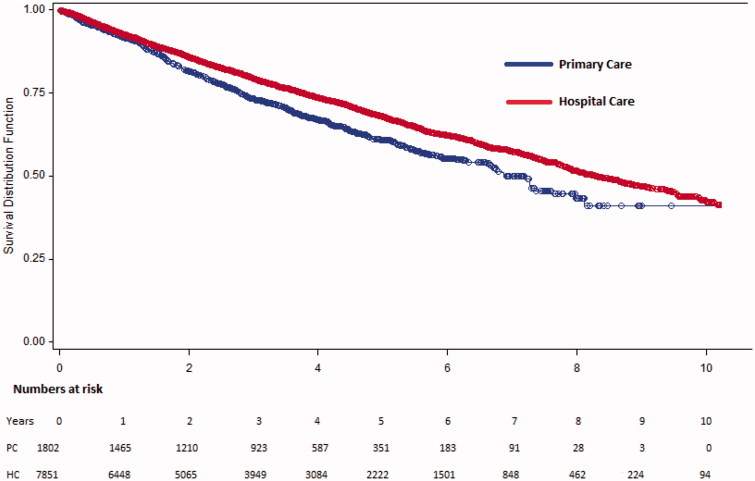
All-cause mortality in the PC cohort was 22.8% and 17.0% in the HC cohort during a 3-years period. The corresponding figures during a 5-years period were 28.9% and 23.0%). After a mean follow-up time of 1151 days in PC and 1286 days in HC cohort mortality rates were 31.5% in PC and 27.8% in the HC cohort (Figure 2). The 1-, 3- and 5-year mortality rates are shown in Table 2. When comparing the subgroups with EF 40–49% and EF> 50% the results were consistent and the difference seemed more pronounced in the group with EF 40–49% indicating higher mortality in the PC-based group compared to in the HC-based group (Table 2).
Figure 2.
Survival curves (Kaplan-Meier method) illustrating all-cause mortality in primary care based and hospital care based outpatients, the overall cohorts separately.
Table 2.
All cause mortality rates in out-patients with an EF of more than or equal to 40%.
| Patients with EF ≥40% PC and HC overall cohorts | ||||
| 1 year mortality rate | 3 years mortality rate | 5 years mortality rate | ||
| Primary care N = 1802 |
7.8 | 22.8 | 28.9 | |
| Hospital care N = 7852 |
7.0 | 17.0 | 23.0 | |
| Patients with EF ≥40–49% PC and HC overall cohorts | ||||
| 1 year mortality rate | 3 years mortality rate | 5 years mortality rate | ||
| Primary care N = 753 |
8.5 | 22.4 | 28.3 | |
| Hospital care N = 4881 |
6.8 | 16.0 | 21.7 | |
| Patients with EF ≥50% PC and HC overall cohorts | ||||
| 1 year mortality rate | 3 years mortality rate | 5 years mortality rate | ||
| Primary care N = 1049 |
7.3 | 23.0 | 29.3 | |
| Hospital care N = 2971 |
7.5 | 18.7 | 25.3 | |
| Patients with EF ≥40% PC and HC matched cohorts | ||||
| 1 year mortality rate | 3 years mortality rate | 5 years mortality rate | ||
| Primary care N = 1499 |
7.0 | 20.8 | 27.0 | |
| Hospital care N = 1499 |
7.7 | 20.3 | 27.9 | |
| Patients with EF ≥40% with any comorbidity | ||||
| 1 year | 3 years | 5 years | All follow-up | |
| Primary care N = 1671 |
8.0 | 23.0 | 29.1 | 31.5 |
| Hospital care N = 6750 |
7.4 | 17.8 | 24.0 | 28.9 |
| Patients with EF ≥40% with no comorbidity | ||||
| 1 year | 3 years | 5 years | All follow-up | |
| Primary care N = 50 |
8.0 | 20.0 | 24.0 | 30.0 |
| Hospital care N = 608 |
3.3 | 7.7 | 10.0 | 12.8 |
Mortality rate in the PC cohort with no comorbidities showed no difference after follow-up during the whole study (30.0% vs. 31.5% in the group with any comorbidity) whereas the difference was pronounced in the HC cohort (12.8% vs. 28.9%, p < 0.0001) (Table 2). Thus, the figures indicate higher mortality in the PC overall cohort. However, this is based on a descriptive comparison only since we had no intention to perform a complete analyzes which would have required another study design.
Risk factors
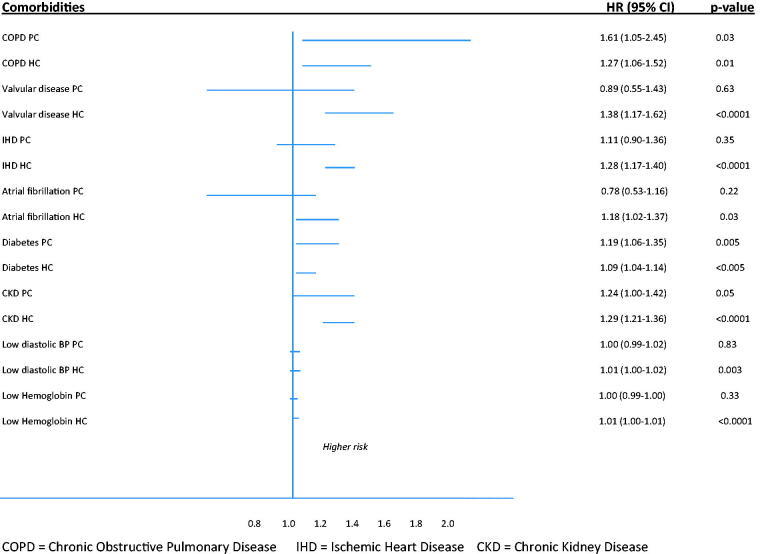
Despite there are statistically significances concerning co-morbidities, univariate analyses show almost the same type of co-morbidities to be associated with all-cause mortality in the two cohorts. In a multivariable logistic regression analyses smoking, COPD, diabetes, age and heart rate were shown to be independent risk factors for all-cause mortality in the PC cohort and valvular disease, eGFR-class, IHD, COPD, atrial fibrillation, low diastolic blood pressure and high heart rate and age in the HC cohort (Figure 3).
Figure 3.
Hazard ratios with 95% CI indicating potential impact of co-morbidities on all-cause mortality in PC and HC overall cohorts.
Medication
After matching there were more prescribed RAS-antagonists and beta blockers in the HC-based outpatient clinics (83.7 vs. 87.8% and 74.2 vs. 85.7%). The combination of RAS-antagonists and betablockers was also more used in the HC cohort (63.8% vs. 75.2%), p < 0.001. More diuretics were given in the PC cohort but there was no difference concerning MRAs. Almost the same medication pattern was seen in the unmatched cohorts (Table 1).
Characteristics and comorbidities EF 40–49 and EF ≥50%
When dividing the overall cohort for EF-groups, 40–49 or ≥50% (data not shown) there were more women (37.6% in PC EF 40–49% and 53.3% in PC ≥50 vs. 31.6% in HC EF 40–49% and 44.0% in HC ≥50%). There were more hypertension in the PC cohort (49.1% resp. 68.6% vs. 45.6% resp. 54.0%), COPD (22.5% resp. 25.6% vs. 14.0% resp. 17.1%) and ischemic heart disease (49.1% resp. 37.7% vs. 47.5% resp. 35.0%). In the EF group 40–49% there was no difference in NYHA class between PC and HC cohorts whereas there were more patients in NYHA II in PC and more patients in NYHA III in HC in the EF ≥50% group. Comorbidity was similar as in the overall cohort (97.1% in the EF-group 40–49% in PC and 97.3% in the EF-group ≥50% in PC vs. 93.5% and 91.5% in HC).
Discussion
We have studied outpatients with HF and an ejection fraction of more than or equal to 40% managed at a PC-based or at a HC-based outpatient clinic and we found several clinically significant differences between the cohorts. In both groups many patients could not be included in the study because of a missing echo-examination. There were more comorbidities in the PC cohort which also had higher all-cause mortality during the mean follow-up time of almost four years. COPD was the most important independent risk factor in the PC cohort and valvular disease in the HC group.
Co-morbidities and risk factors
In this study we found that the mortality for HF-patients with an ejection fraction of more than or equal to 40% managed in primary care based out-patient clinics is higher than for those managed in hospital care based outpatients clinics. This is found by descriptive analyze and is at least partly explaind by higher age. We also found that comorbidities are very common (97% vs. 93%) in both cohorts and that mortality is strongly associated with several of these conditions. In the light of this and since there today is no evidence-based therapy for this HF-population it is of vital importance to treat associated comorbidity as efficient as possible.
The PC cohort in our study had more cases of atrial fibrillation, hypertension, ischemic heart disease and COPD than the HC cohort. Other studies and systematic reviews show similar comorbidities in HC as in our study but these studies do not differentiate outpatients from inpatients [7,8,13,19].
We found COPD to have the strongest association and to be an independent risk factor for increased all-cause mortality in the PC cohort. COPD is a well-known comorbidity in heart failure and the diagnosis is easy to miss due to its stealthy course with often vague symptoms. Furthermore, the symptoms are often consonant with those of HFpEF which may strengthen the risk of wrong diagnosis. COPD is an important and disabling disease that often interacts with heart failure [20]. Also diabetes is appointed as an independent risk factor, but in both cohorts. In Sweden, almost all patients with diabetes type 2 are managed in PC and these patients often have multiple diseases and a high morbidity. Careful monitoring and treatment of their diabetes is of obvious need. In HC both valvular diseases and IHD turned out as independent risk factors. Both diagnoses must be carefully investigated with echocardiography or coronary angiography respectively since, if established, they may be treatable.
In our study the one-year mortality rate for patients managed in PC and HC were quite similar 7.8 vs. 7.0 but during the whole study the mortality was higher in PC than HC, 31.5% vs. 27.8% (p < 0.01). The annual mortality rates in outpatients with an EF of more than or equal to 40% treated by general practitioners (GPs) are less studied. In fact, despite thoroughly searching we could not find any study describing mortality in a population with an EF of more than or equal to 40% managed in a PC-based outpatient clinic setting.
Hospitalized HF-patients with an EF of more than or equal to 40% have as serious prognosis as patients with HFrEF. This is described in several previous studies and the mean annual mortality is as high as 20–25% [7,8,13,19].
In our study non-hospitalized HF-patients with an EF of more than or equal to 40%, if managed in PC, had a higher mortality then those managed in HC. This may, at least partly, be explained by less medication, higher age and more co-morbidities.
Many patients have cardiovascular comorbidities and RAS-blockade and betablockade are the most important drugs for these conditions However, it is difficult to determine whether these drugs are prescribed for IHD or for HF, which makes it even more difficult to draw conclusions on the influence of the medication on mortality rates.
Strengths and limitations
The SwedeHF is one of the largest heart failure registries in the world. The size of the registry, both in the number of patients and the amount of variables together with nationwide use, yields generalizability and unique possibilities to study large cohorts of HF patients. The opportunity to connect this register to other Swedish national registries as of death and hospitalization via the Swedish individual personal number system adds to the potential advantages.
Participating in SwedeHF is not mandatory in Sweden. Therefore there is a risk that PC units reporting to the registry often are more HF interested and also more dedicated to manage HF patients and follow the guidelines. Of Sweden’s 1156 PC units only 116 (10%) participate in SwedeHF which underlines this possibility. The corresponding rate for hospitals is 67 of 78 hospitals (86%). This may lead to a selection of PC units not being representative for Swedish primary care which could tend to show better results for the PC cohort than a study of PC units in general would do. Moreover it is a clear limitation not to have information on all morbidity, i.e. cancer and dementia. Moreover, there is no information on the seriousness of the comorbidities which is a limitation, since it is likely that cardiologists would keep patients with more serious comorbidities in hospital out-patient care. That might affect both medication and mortality.
We do also not have knowledge concerning hospitalizations and use of therapies before or after registration in the SwedeHF in this study.
Conclusions
Any co-morbidity was noted in 97. 2% of outpatients with HF and an ejection fraction of more than or equal to 40% managed at PC based clinics and these patients had other independent risk factors than those managed in HC based outpatients clinics. Our results indicate that more attention should be payed to manage COPD in the PC group. All comorbidity must be considered and carefully treated.
Funding Statement
The Swedish Heart Failure Registry is funded by the Swedish National Board of Health and Welfare, the Swedish Association of Local Authorities and Regions, and the Swedish Society of Cardiology. The work was funded by Stockholm County Council.
Acknowledgements
The authors thank all local center investigators and study personnel for data collection and entry.
Disclosure statement
There are no conflicts of interest related to the work submitted.
Outside the work submitted, there are the following potential conflicts of interest:
BE: none declared.
PW: none declared.
PN: none declared.
UD: research grants to author´s institution from AstraZeneca Inc. and speaker´s and consulting honoraria from Novartis Inc.
LHL: research grants to author’s institution, speakers and consulting fees, Astra-Zeneca, Inc.; Novartis, Inc.
ME: none declared.
No funding agency had any role in the design and conduct of the study, in the collection, management, analysis, or interpretation of the data, or in the preparation, review, or approval of the manuscript.
References
- 1. Zarrinkoub R, Wettermark B, Wändell P, et al. . The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur J Heart Fail. 2013;15:995–1002. [DOI] [PubMed] [Google Scholar]
- 2. Thorvaldsen T, Benson L, Dahlström U, et al. . Use of evidence-based therapy and survival in heart failure in Sweden 2003–2012. Eur J Heart Fail. 2016;18:503–511. [DOI] [PubMed] [Google Scholar]
- 3. Parén P, Schaufelberger M, Björck L, et al. . Trends in prevalence from 1990 to 2007 of patients hospitalized with heart failure in Sweden. Eur J Heart Fail. 2014;16:737–742. [DOI] [PubMed] [Google Scholar]
- 4. Ponikowski P, Voors AA, Anker SD, et al. . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 5. Cleland JG1, Swedberg K, Follath F, et al. . The EuroHeart Failure survey programme – a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24:442–463. [DOI] [PubMed] [Google Scholar]
- 6. Masoudi FA, Havranek EP, Smith G, et al. . Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41:217–223. [DOI] [PubMed] [Google Scholar]
- 7. Hogg K, Swedberg K, McMurray J, et al. . Heart failure with preserved left ventricular systolic function: epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–327. [DOI] [PubMed] [Google Scholar]
- 8. Lam CSP, Donal E, Kraigher-Krainer E, et al. . Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mentz RJ, Kelly JP, von Lueder TG, et al. . Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu M, Fang F, Yu CM.. Noncardiac comorbidities in heart failure with preserved ejection fraction – commonly ignored fact. Circ J. 2015;79:954–959. [DOI] [PubMed] [Google Scholar]
- 11. Lund LH, Donal E, Oger E, et al. . Association between cardiovascular vs. non-cardiovascular co-morbidities and outcomes in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16:992–1001. [DOI] [PubMed] [Google Scholar]
- 12. Campbell RT, McMurray JJ.. Comorbidities and differential diagnosis in heart failure with preserved ejection fraction. Heart Fail Clin. 2014;10:481–501. [DOI] [PubMed] [Google Scholar]
- 13. Bhatia RS, Tu JV, Lee DS, et al. . Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. [DOI] [PubMed] [Google Scholar]
- 14. Dahlstrom U, Hakansson J, Swedberg K, et al. . Adequacy of diagnosis and treatment of chronic heart failure in primary health care in Sweden. Eur J Heart Fail. 2009;11:92–98. [DOI] [PubMed] [Google Scholar]
- 15. Giezeman M, Arne M, Theander K, et al. . Adherence to guidelines in patients with chronic heart failure in primary health care. Scand J Prim Health Care. 2017;35:336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siren R, Eriksson JG, Vanhanen H.. Observed changes in cardiovascular risk factors among high-risk middle-aged men who received lifestyle counselling: a 5-year follow-up. Scand J Prim Health Care. 2016;34:336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jonsson Å, Edner M, Alehagen U, et al. . Heart failure registry: a valuable tool for improving the management of patients with heart failure. Eur J Heart Fail. 2010;12:25–31. [DOI] [PubMed] [Google Scholar]
- 18. Yusuf S, Pfeffer MA, Swedberg K, et al. . Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. [DOI] [PubMed] [Google Scholar]
- 19. Senni M, Gavazzi A, Oliva F, et al. . In-hospital and 1-year outcomes of acute heart failure patients according to presentation (de novo vs. worsening) and ejection fraction. Results from IN-HF Outcome Registry. Int J Cardiol. 2014;173:163–169. [DOI] [PubMed] [Google Scholar]
- 20. Kaszuba E, Odeberg H, Råstam L, et al. . Heart failure and levels of other comorbidities in patients with chronic obstructive pulmonary disease in a Swedish population: a register-based study. BMC Res Notes. 2016;9:215. [DOI] [PMC free article] [PubMed] [Google Scholar]