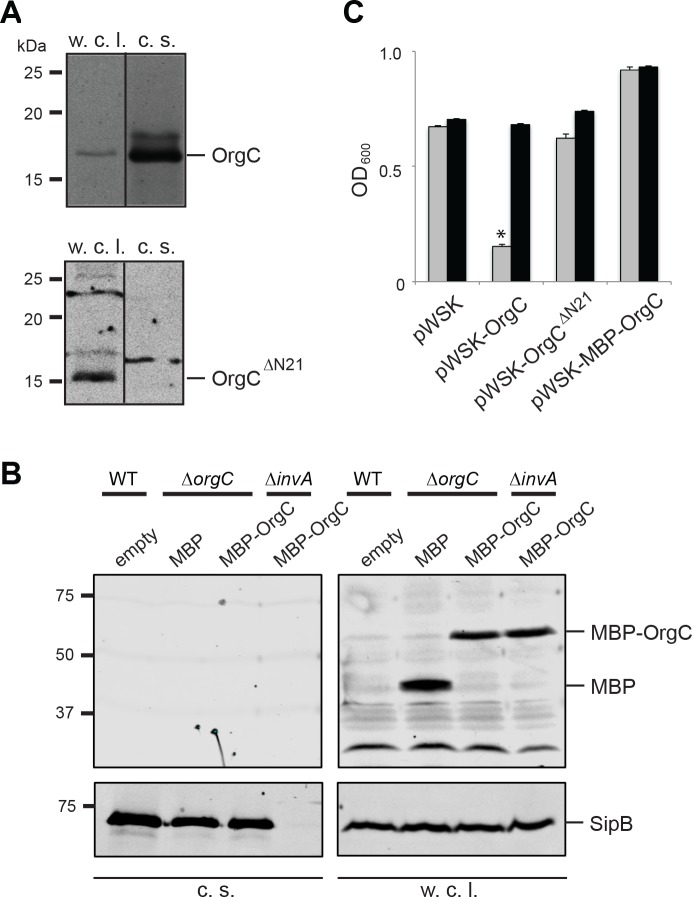
Figure 3. T3SS-mediated secretion of OrgC is required for its function.
(A and B) Removal of its first 21 amino acids (A) or N-terminal addition of MBP (B) prevents the secretion of OrgC. Whole cell lysates (w. c. l.) and culture supernatants (c. s.) of wild-type S. Typhimurium expressing, C-terminally FLAG-tagged full length OrgC or an equivalently tagged deletion mutant lacking its first 21 amino acids (N21) were analyzed by western immunoblot with a monoclonal antibody directed to the FLAG tag (A). Alternatively, whole cell lysates (w. c. l.) or culture supernatants of wild-type S. Typhimurium, or the indicated isogenic mutants carrying an empty pWSK129 plasmid or its derivatives expressing either maltose-binding protein (MBP) or MBP-OrgC fusion were analyzed by western-blot using specific antibodies directed to the MBP tag or the protein translocase SipB (as a secretion control) (B). (C) Non-secretable forms of OrgC are non-functional. The S. Typhimurium ∆invJ ∆orgC mutant strains carrying the indicated plasmids were analyzed by the clumping assay as indicated in Figure 2. Values represent OD600 before (grey bars) and after (black bars) vortexing and are the mean ± SEM of three independent measurements. Asterisks indicate statistically significant differences from the values of the vortexed sample (*p<0.001, Student t test).