Abstract
Background:
Exposure to ambient fine particulate matter () is associated with cardiovascular mortality, but underlying pathophysiologic mechanisms are not fully understood. Hypothalamic inflammation, characterized by the activation of Inhibitor kappaB kinase 2/Nuclear factor kappaB () signaling pathway, may play an important role in the pathogenesis of cardiovascular diseases. We recently demonstrated that hypothalamic inflammation is increased in mice exposed to concentrated ambient (CAP).
Objectives:
In the present study, we used a neuron-specific IKK2 knockout mouse model to examine the role of neural IKK2 expression and hypothalamic inflammation in the pathophysiologic effects of .
Methods:
We assessed inflammatory and vascular responses in () and littermate (control) mice after 4 mo of exposure to filtered air (FA) or CAP.
Results:
CAP exposure was associated with significantly higher tumor necrosis factor- and interleukin (IL)-6 mRNA in the hypothalamus of control mice, but not mice. In addition, CAP exposure–induced increases in bronchoalveolar lavage fluid (BALF) leukocytes, pulmonary macrophage infiltration and IL-6 expression, plasma and levels, adipose macrophage infiltration and expression, and endothelial dysfunction were reduced or absent in mice compared with controls.
Conclusions:
Our findings support a role of neural IKK2 in CAP exposure–induced local and systemic pro-inflammatory cytokine expression, pulmonary and adipose inflammation, and endothelial dysfunction, thus providing insight into pathophysiologic mechanisms that may mediate effects of exposure. https://doi.org/10.1289/EHP2311
Introduction
Exposure to ambient fine particulate matter () is associated with increased risk of cardiovascular morbidity and mortality (Brook et al. 2010). However, while the majority of inhaled are known to deposit in the airway and not enter the systemic circulation (U.S. EPA 2009), mechanisms that link with cardiovascular effects remain elusive. The putative mechanisms for this include a) egress from the lung of components, b) autonomic nervous system (ANS) dysfunction, and c) inflammation (Brook et al. 2010). Notably, despite that inhaled nanoparticles have been identified in remote organs, the pathophysiological role of these nanoparticles remain controversial, as their concentrations are low and inconsistent (Kreyling et al. 2009; Oberdörster et al. 2002; Oberdörster and Utell 2002; Semmler-Behnke et al. 2008). exposure is associated with decreased heart rate variability (Brook 2005; Chen and Hwang 2005; Godleski et al. 2000; Gong et al. 2008; Magari et al. 2001; Sivagangabalan et al. 2011; Tankersley et al. 2004), indicating sympathetic nervous system (SNS) activation and/or parasympathetic nervous system withdrawal (Brook 2008; Karemaker 1999). However, because the nervous system can adapt to chronic stimulation, ANS dysfunction has been thought to play a trivial role in the mediation of the long-term health effects of exposure (Brook et al. 2010). Evidence that cardiovascular effects of are mediated by inflammation is stronger, given the established role of in the of various cardiovascular diseases and evidence of inflammatory responses to exposure in humans (Peters et al. 2001; Pope 2000; Utell et al. 2002) and various animal models (Kennedy et al. 1998; Quay et al. 1998; van Eeden et al. 2001; Vogel et al. 2005).
Putative effect mechanisms that involve ANS dysfunction or inflammation are not mutually exclusive, but may affect the same or different stages of pathogenesis, and may have synergistic effects (Brook et al. 2010). For example, ANS dysfunction may impact various inflammatory responses (Lambert et al. 2015; Pavlov and Tracey 2017), and increases in circulating pro-inflammatory cytokines/mediators resulting from pulmonary or systemic inflammation may cause ANS dysfunction (Dunn 2000; Turnbull and Rivier 1999; Zhang et al. 2003). More importantly, there is emerging evidence that ANS dysfunction may also play a critical role in the long-term regulation of cardiovascular function (DiBona 2013; Froeschl et al. 2013; Simonds and Cowley 2013), which appears to be dependent on an inflammatory response in the hypothalamus. For example, rats and mice fed a high-fat diet (HFD) showed rapid increases in hypothalamic inflammation, which may contribute to HFD feeding-induced long-term effects, including obesity and insulin resistance (Thaler et al. 2012). Inhibition or deletion of IKK2 in some hypothalamic neurons was sufficient to abolish HFD feeding-induced ANS dysfunction, and subsequently, normalized blood pressure in a mouse model (Purkayastha et al. 2011). Collectively, these data have highlighted a crucial role of an IKK2-dependent hypothalamic inflammatory response in the pathophysiology of various cardiovascular diseases.
The signaling pathway is pivotal in both acute and chronic inflammatory responses (Pahl 1999). Previous mechanistic studies by our laboratory (Kampfrath et al. 2011; Wang et al. 2017) and others (Feng et al. 2017; Jeong et al. 2017; Song et al. 2017; Zhang et al. 2017) have demonstrated that exposure to ambient activates the pathway, which is believed to play a key role the pathophysiologic effects of (Brook et al. 2010) in various tissues. Notably, we recently reported that chronic exposure of C57Bl/6J mice to ambient resulted in activation of pathway in the hypothalamus, abnormal SNS activation, and hypertension (Ying et al. 2014), while in the KKay mouse model of type 2 diabetes, intracerebroventricular injection of an IKK2 inhibitor reduced exposure–induced abnormalities in glucose homeostasis (Liu et al. 2014). These findings suggest that an IKK2-dependent hypothalamic inflammatory response may be crucial in the development of adverse health effects in response to ambient exposure. In the present study, to verify the role of hypothalamic inflammation in the pathophysiologic effects of ambient , we exposed () mice and littermate controls () to filtered air (FA) or concentrated ambient (CAP), and assessed their inflammatory and vascular responses.
Methods
Animals
University of Maryland, Baltimore (UMB) is an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited institution. All procedures of this study were approved by the Institutional Animal Care and Use Committee at UMB, and all the animals were treated humanely and with regard for alleviation of suffering. Nestin-cre transgenic mice in the C57Bl/6J background were obtained from Jackson Laboratories (Stock No.: 003,771). mice were generated as previously described (Li et al. 2003) and back-crossed with C57Bl/6J for three generations. () and littermate (control) mice were generated through in-house crossing of and . The pups were genotyped at the age of 1 wk by polymerase chain reaction (PCR), and the genotyping results were verified before exposure to FA/CAP.
Concentrated Ambient PM2.5 Exposure
and control mice (male, 8–12 wk old) were grouped into four age-matched groups and randomly designated to be subjected to exposure to FA (six and seven controls) or CAP (seven and seven controls) from May 2016 to September 2016 for a total duration of 4 mo in a mobile trailer with a 12-h light/12-h dark cycle, temperatures of 18–25°C, and relative humidity of 40–60%. Because we failed to observe CAP exposure–induced impairment of glucose tolerance in female mice, male mice only were used in the present study. The mobile trailer was located on the campus of the UMB. Animal exposure and the monitoring of exposure atmosphere and ambient aerosol were performed as previously described using a versatile aerosol concentration enrichment system that was modified for long-term exposures (Geller et al. 2005; Maciejczyk et al. 2005). The exposure protocol comprised exposures for 6 h/d, 5 d/wk (no exposure took place during weekends). Ambient aerosol and exposure atmosphere were monitored as previously described (Maciejczyk et al. 2005). The elemental composition of CAP was determined by inductively coupled plasma mass spectroscopy (for trace element analysis as previously described (Mirowsky et al. 2013; Wang et al. 2017).
Bronchoalveolar Lavage and Lung Histopathology
After euthanasia by overdose of isoflurane, the mouse trachea was cannulated, and the right primary bronchus was closed off with a ligature. Through the tracheal cannula, sterile phosphate-buffered saline with ethylenediaminetetraacetic acid (EDTA) was instilled and withdrawn to recover bronchoalveolar lavage fluid (BALF). This was repeated twice. The total number of cells in the collected BALF (around ) was estimated using a hemocytometer. Cytospin slides were prepared using Shandon Cytospin 3™ (Shandon) and stained with Diff-Quik solution (EMS). Differential cell counts for neutrophils, eosinophils, macrophages/monocytes, and lymphocytes were assessed by a pathologist who was blinded to the grouping.
Following BALF collection, the right lung was harvested. About one-third were cut and fixed with 4% paraformaldehyde, and the rest were snap frozen in liquid nitrogen and then kept at . To assess the inflammation in the lung, tissue blocks were embedded in paraffin, -thick sections were cut, and the sections were subjected to hematoxylin and eosin staining. Three consecutive sections per sample were used for histopathology. Images covering all the tissue area were taken by a laboratory technician who was blinded to the grouping, and all images were then sent to and quantitated by the pathologist (blinded to the grouping, too).
Plasma Cytokine Analysis
To rapidly harvest blood samples for cytokine analysis, mouse retroorbital blood samples were collected in EDTA-treated tubes and subjected to centrifugation for 15 min at using a refrigerated centrifuge. The supernatants (plasma) were then transferred to new tubes and stored at until assessments of cytokine levels. Plasma cytokine levels were assessed using BD™ Cytometric Bead Array Kit (BD Biosciences) per manufacturer’s instructions. Briefly, plasma were incubated with the beads, and signaling was quantified by flow cytometry.
Real-Time Polymerase Chain Reactions
Mouse heads were removed before bronchoalveolar lavage. Hypothalamus, hippocampus, cortex, and olfactory bulb were immediately isolated. Epididymal adipose tissues were harvested after bronchoalveolar lavage. All these samples were snap frozen in liquid nitrogen and kept at . Total RNA was extracted and purified using the Trizol reagent (Invitrogen) from epididymal adipose tissue, lung, hypothalamus, hippocampus, cortex, and olfactory bulb. The quality of RNA was assessed by determination of the ratio of absorbance at to absorbance at by nanodrop. Two micrograms of total DNase-treated RNA were reverse transcribed into cDNA using High Capacity cDNA Reverse Transcription Kits (Applied Biosystems™) per manufacturer’s instruction. Real-time PCR was performed using LightCycler® 480 SYBR Green I Master in the LightCycler® (Roche). Reactions were performed in a total volume of containing cDNA, of each primer, and of the SYBR® Green reaction mix. The amplification protocol was as follows: (, , and . Following amplification, a dissociation curve analysis was performed to ensure purity of PCR product. The specific sense and antisense primers for tumor necrosis factor- , interleukin (IL)-6, , and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (the reference housekeeping gene) were previously described (Chen et al. 2017). In each sample, was calculated and used to represent its gene expression level.
Western Blotting
Standard techniques were performed with primary antibodies: mouse Anti-IKK2 (Millipore), mouse (Sigma-Aldrich), and mouse Anti-GAPDH (Cell Signaling Technology). Signals were detected by chemiluminescence and analyzed by densitometry.
Vascular Function Assay
After euthanasia, mouse thoracic aorta was quickly removed and cleaned in physiological salt solution (PSS) containing (mM): NaCl, 130; , 14.9; KCl, 4.7; , 1.18; –, 1.18; –, 1.56; EDTA, 0.026; and glucose, 5.5. The aorta was cut into 2-mm rings. The aortic rings were then mounted in a muscle bath containing PSS at 37°C and bubbled with 95% –5% . Isometric force generation was recorded with a Multi Myograph System (Danish Myo Technology). A resting tension of was imposed on each ring, and the rings were allowed to equilibrate for 1 h. To test vascular function, the contractile responses of aortic rings to phenylephrine (PE) in the absence or presence of an NOS inhibitor methyl ester (L-NAME; ) were assessed in an accumulative manner. To analyze the endothelial function, aortic rings were precontracted with PE (), and acetylcholine (ACh) or sodium nitroprusside (SNP) was added in an accumulative manner.
Statistics
All data are expressed as unless noted otherwise. Statistical tests were performed using one-way or two-way analysis of variance with Bonferroni post tests or unpaired t-tests using GraphPad Prism (version 4.1.2; GraphPad Software). The significance level was set at .
Results
Exposure Characterization
The average concentrations in the FA and CAP chambers were and , respectively. Table 1 shows the elemental composition of CAP and ambient . Although identifying emission sources is generally beyond the scope of this paper, the relatively high ratio of Na/Al reflects more contribution by the marine source than the crustal source, which is consistent with the geographic proximity of the study site to the ocean (Huang et al. 1999), whereas the relatively high sulfur suggests that the study site was most strongly affected by secondary aerosols, which are likely to include emissions from coal-fired utility boilers located regionally (Huang et al. 1999).
Table 1.
Ambient particulate matter and concentrated ambient (CAP) in the exposure chambers were collected to Teflon filters weekly, and their elemental compositions were determined by inductively coupled plasma mass spectroscopy (ICP-MS).
| Element | CAP | Ambient | ||
|---|---|---|---|---|
| Mean () | SD | Mean (%) | SD | |
| Na | 35.14 | 19.66 | 11.95 | 3.24 |
| Mg | 8.62 | 4.81 | 2.87 | 0.48 |
| Al | 25.93 | 12.67 | 2.56 | 2.41 |
| Si | 78.53 | 39.39 | 8.53 | 5.76 |
| P | 1.00 | 0.50 | 0.18 | 0.10 |
| S | 249.91 | 183.42 | 48.31 | 6.90 |
| K | 20.38 | 11.57 | 3.19 | 0.86 |
| Ca | 39.30 | 19.86 | 5.40 | 1.86 |
| Ti | 3.55 | 2.09 | 0.59 | 0.14 |
| V | 0.26 | 0.15 | 0.03 | 0.04 |
| Cr | 0.28 | 0.15 | 0.07 | 0.02 |
| Mn | 1.19 | 0.75 | 0.21 | 0.03 |
| Fe | 47.19 | 30.10 | 7.92 | 0.84 |
| Ni | 0.65 | 0.40 | 0.11 | 0.05 |
| Cu | 1.45 | 0.93 | 0.36 | 0.10 |
| Zn | 13.77 | 6.82 | 2.63 | 1.41 |
| As | 0.19 | 0.10 | 0.03 | 0.02 |
| Se | 0.13 | 0.06 | 0.01 | 0.02 |
| Br | 8.42 | 5.22 | 1.42 | 0.35 |
| Sr | 0.24 | 0.13 | 0.18 | 0.05 |
| Ag | 0.55 | 0.19 | 0.06 | 0.13 |
| Sn | 1.25 | 0.53 | 0.19 | 0.16 |
| Ba | 1.66 | 1.16 | 0.32 | 0.23 |
| Ce | 0.24 | 0.25 | 0.04 | 0.08 |
| Pr | 0.30 | 0.13 | 0.04 | 0.06 |
| Er | 0.98 | 0.75 | 0.20 | 0.06 |
| Lu | 1.33 | 1.19 | 0.32 | 0.12 |
| W | 0.41 | 0.26 | 0.12 | 0.08 |
| Ir | 0.11 | 0.04 | 0.01 | 0.02 |
| Pt | 0.20 | 0.09 | 0.02 | 0.03 |
| Au | 0.22 | 0.14 | 0.03 | 0.03 |
| Hg | 0.34 | 0.05 | 0.07 | 0.04 |
| Tl | 0.11 | 0.04 | 0.01 | 0.02 |
| Pb | 0.93 | 0.32 | 0.14 | 0.04 |
Note: CAP, concentrated ambient particulate matter ; SD, standard deviation.
Characterization of and Evaluation of Pro-Inflammatory Cytokines after Exposure to Filtered Air or Concentrated Ambient
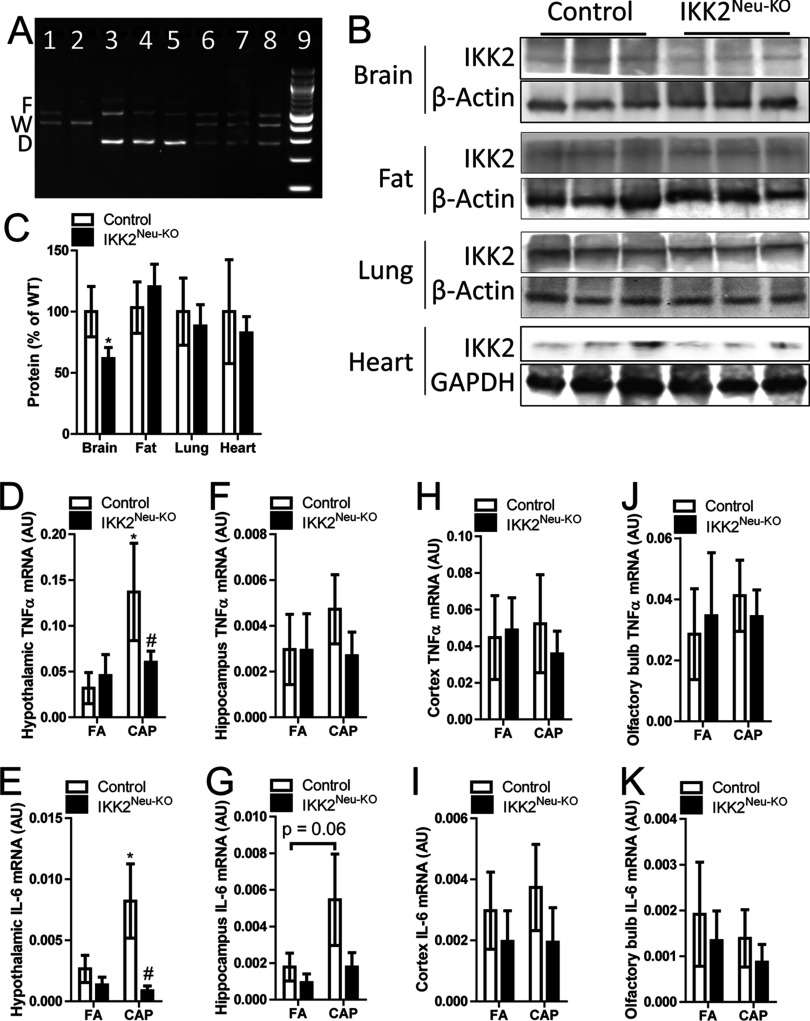
In order to examine the role of neural inflammation in the exposure–related pathophysiology, we generated () and littermate (control). Except for lower body weight at 12 wk of age (mean vs. for controls and , respectively, , student’s t-test ), adult mice were similar in appearance to adult control mice. Figure 1A shows the deletion of IKK2 in the brain of . To confirm the tissue-specific deletion of IKK2 in , we assessed IKK2 protein levels in different tissues. Figures 1B and C reveal that compared to control, had significantly decreased expression of IKK2 in the brain but not lung, heart, and epididymal adipose tissue, corroborating the neuron-specific deletion of IKK2 in these mice. Consistent with our previous study (Ying et al. 2014), Figures 1D and E show that CAP exposure significantly increased the hypothalamic expression of and IL-6 mRNAs. Neural IKK2 knockout did not significantly alter the hypothalamic expression levels of and IL-6 mRNAs in FA-exposed mice, but significantly reduced them in CAP-exposed mice. Given that recent studies have suggested that some components of ambient may enter the brain through the olfactory system (Maher et al. 2016), we isolated olfactory bulb, cortex, and hippocampus, and assessed their expression of and IL-6 mRNAs. Except for a marked trend of increase in the expression of IL-6 mRNA in the hippocampus of CAP-exposed control mice vs. that of FA-exposed control mice, no any other remarkable difference was observed (Figures 1F–K).
Figure 1.
Tissue-specific IKK2 expression and brain region–specific higher tumor necrosis factor- and interleukin (IL)-6 mRNA expression. (A) Polymerase chain reaction (PCR) assay of flox allele (F), wild-type allele (W), and deletion allele (D) according to genotype in 13-wk-old male mice. Lane 1: ; Lane 2, ; Lanes 3–5, (); Lanes 6–8, (control); Lane 9, marker. (B–C) Representative Western blot (B) and IKK2 protein expression in brain, fat, lung, and heart tissues from male control () and () mice at 10–16 wk of age. * vs. control, one-way analysis of variance (ANOVA). (D–K) and IL-6 mRNA expression in hypothalamus, hippocampus, cortex, and olfactory bulb tissues harvested from control and mice () at 8–12 wk of age following 4 mo of exposure to concentrated ambient particulate matter (CAP) or filtered air (FA). * vs. FA, # vs. control, ANOVA.
Evaluation of Pulmonary Inflammation after Exposure to Filtered Air or Concentrated Ambient
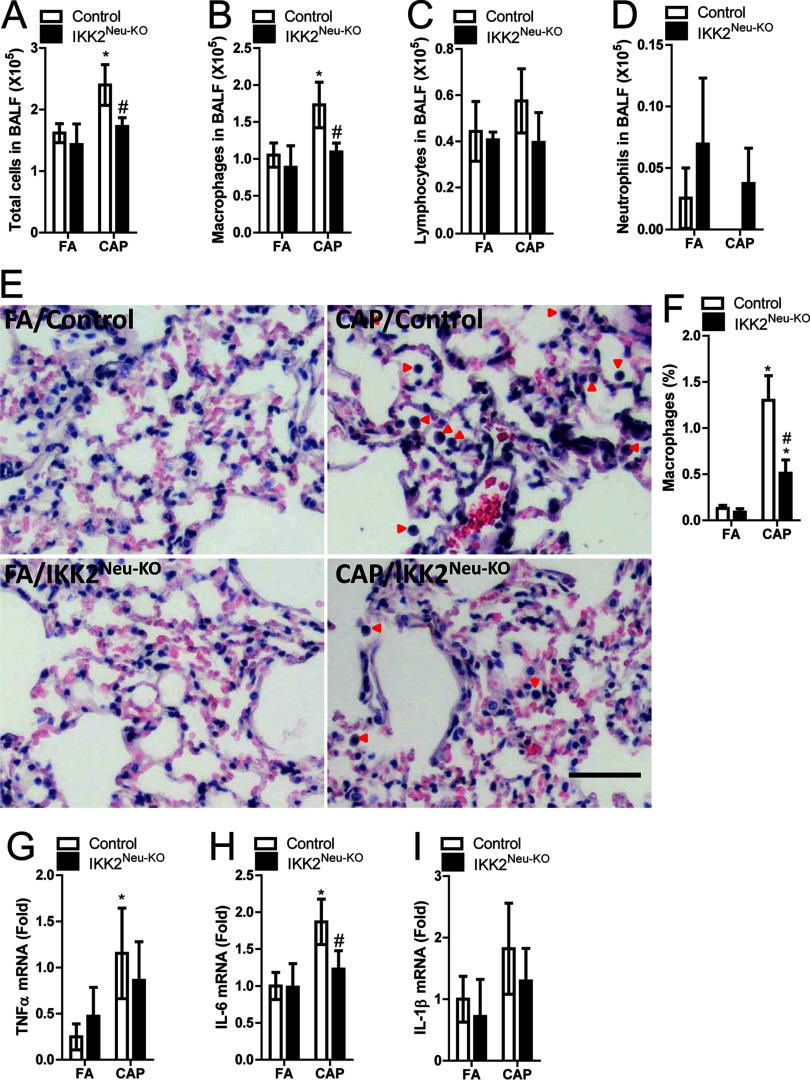
Central inflammation has been implicated in the regulation of peripheral inflammatory responses (Liu et al. 2014), and pulmonary inflammation is widely believed to be essential in the pathophysiology due to exposure to ambient (Brook et al. 2010). To determine whether central inflammation plays a role in the inflammatory response of the lung to inhalation, we performed BALF cell differentiation. Figures 2A–D reveal that exposure to CAP significantly increased BALF total cells and macrophages in control mice. Consistent with many previous studies (Filep et al. 2016; Yoshizaki et al. 2017), although neutrophil infiltration is the hallmark of inflammation, we did not observe significant increase in BALF neutrophils in CAP-exposed mice (Figure 2D). This may be a reflection of the crucial role of neutrophils in acute but not chronic inflammatory response. BALF cell numbers were not significantly different between CAP- vs. FA-exposed neural IKK2–deficient mice, which suggests that CAP exposure–induced pulmonary inflammation may be regulated by a central mechanism. To confirm this, we performed lung histological analysis. The percentage of alveolar macrophages was significantly higher in CAP-exposed vs. FA-exposed mice and control mice, but was significantly lower in CAP-exposed mice vs. CAP-exposed controls (Figure 2E–F). Pulmonary and IL-6 expression was higher in CAP-exposed vs. FA mice, though the difference was significant only for controls (Figure 2G–I). In addition, pulmonary IL-6 mRNA expression was significantly lower in CAP-exposed mice than in CAP-exposed controls (Figure 2H).
Figure 2.
Markers of pulmonary inflammation after 4 mo of exposure to concentrated ambient particulate matter (CAP) or filtered air (FA) in male control and () mice (). (A–D) Total cells, macrophages, lymphocyte, and neutrophil cell counts [] in bronchoalveolar lavage fluid (BALF). (E–F) Representative histologic sections (red arrows indicate macrophages), and % alveolar macrophages, respectively, according to exposure and genotype (). (G–I) Tumor necrosis factor- , interleukin (IL)-6, and mRNA expression in pulmonary tissue samples according to exposure and genotype (). * vs. FA, # vs. control, analysis of variance (ANOVA).
Evaluation of Systemic and Adipose Inflammation after Exposure to Filtered Air or Concentrated Ambient
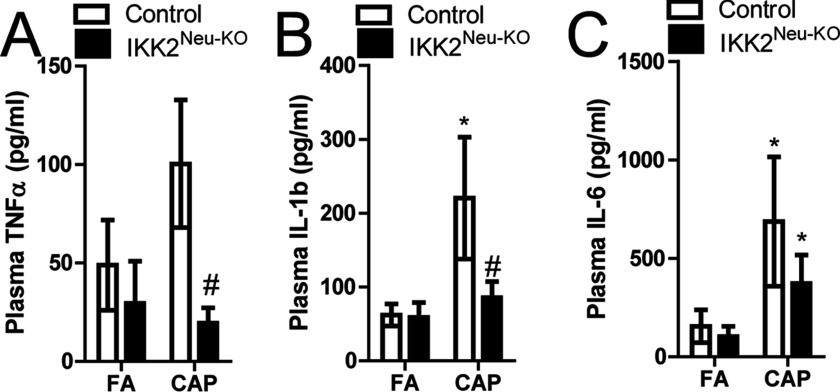
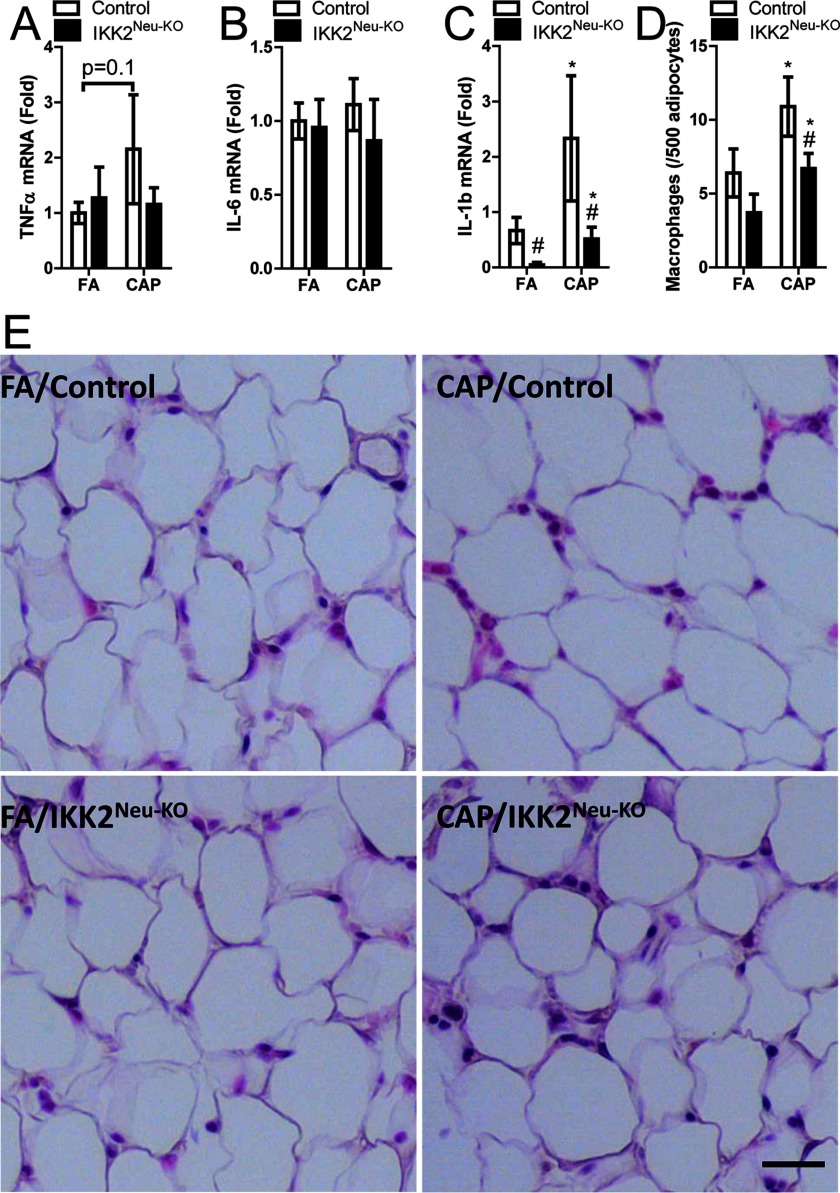
Systemic inflammation is one of the putative mechanisms that mediate the development of extrapulmonary abnormalities due to exposure to ambient (Brook et al. 2010). In control mice, CAP vs. FA exposure was associated with higher plasma , , and IL-6 levels (significant for and IL-6 only) (Figure 3A–C). In CAP-exposed mice, plasma , , and IL-6 were lower than in CAP-exposed controls (significant for and only). In addition, IL-6 was significantly higher in CAP- vs. FA-exposed mice. (Figure 3C). In addition, exposure to ambient has been shown to induce marked inflammation in adipose tissues, and the latter is believed to be subsequent to systemic inflammation and play a role in the exposure–related abnormalities in energy and glucose metabolism (Brook et al. 2010). CAP vs. FA exposure was associated with significantly higher mRNA expression in epididymal adipose tissue from both and control mice, but was not associated with significant differences in or IL-6 expression (Figure 4A–C). mRNA expression in adipose tissue was significantly lower in FA-exposed mice vs. FA controls and in CAP-exposed mice vs. CAP controls (Figure 4C). Macrophage infiltration in epididymal adipose tissue was significantly higher in CAP-exposed and control mice relative to FA-exposed and control mice, respectively, but was significantly lower in CAP-exposed mice than in CAP-exposed controls (Figure 4D–E).
Figure 3.
Plasma cytokine levels (BD™ Cytometric Bead Array Kit) following 4 wk of concentrated ambient particulate matter (CAP) or filtered air (FA) exposure in male control and () mice at 8–12 wk of age (). (A) Tumor necrosis factor- , (B) interleukin , (C) IL-6. * vs. FA, # vs. control, analysis of variance (ANOVA).
Figure 4.
Cytokine mRNA expression in epididymal adipose tissue samples following 4 wk of filtered air (FA) or concentrated ambient particulate matter (CAP) exposure in male control and () mice at 8–12 wk of age (). (A–C) Fold difference in mRNA [quantitative real-time polymerase chain reaction (qPCR), relative to control gene] of tumor necrosis factor- , interleukin (IL)-6, and , respectively, according to exposure and genotype []. (D) Macrophage infiltration (number/500 adipocytes) according to exposure and genotype (). (E) Representative histologic sections, scale bar, . * vs. FA, # vs. control, analysis of variance (ANOVA).
Evaluation of Endothelial Dysfunction after Exposure to Filtered Air or Concentrated Ambient
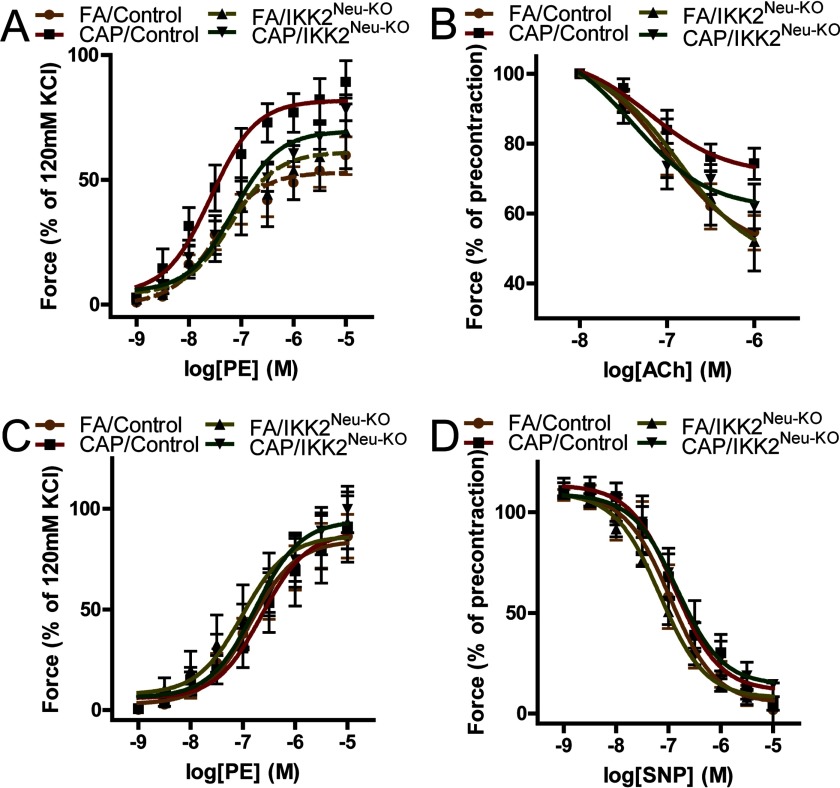
Endothelial dysfunction may contribute to exposure–related cardiometabolic abnormalities, such as insulin resistance and hypertension (Brook et al. 2010). CAP vs. FA exposure was associated with a significantly higher aortic contractile response to PE in both and control mice, though the maximal PE response was significantly lower in CAP-exposed mice than in CAP-exposed controls (Figure 5A and Table 2). As increased contractile response may result from increased contractility of vascular smooth muscle and/or decreased endothelium-dependent relaxation, we next assessed the aortic response to an endothelium-dependent vasodilator, Ach. CAP vs. FA exposure was associated with a significantly reduced Ach-induced aortic relaxation in both and control mice, though the maximal effect was significantly lower in CAP-exposed mice than in CAP-exposed controls (Figure 5B and Table 2). In contrast, we did not observe any significant effect of CAP exposure and neural IKK2 knockout on aortic contractile response to PE in the presence of a NOS inhibitor L-NAME (Figure 5C) and aortic relaxation by endothelium-independent vasodilator SNP (Figure 5D). Together, these findings support a role of central inflammation in the mediation of ambient exposure–induced endothelial dysfunction.
Figure 5.
Endothelial function following 4 wk of filtered air (FA) or concentrated ambient particulate matter (CAP) exposure in male control and () mice at 8–12 wk of age (). After sacrifice, aorta was immediately isolated and mounted onto myograph. (A) Aortic contractile response to phenylephrine (PE). (B) Aortic rings precontracted by PE () and then relaxed by acetylcholine (Ach). (C) Aortic contractile response to PE in the presence of the NOS inhibitor, L-NAME (). (D) Aortic rings precontracted by PE () and then relaxed by the NO donor, sodium nitroprusside (SNP). Values are . Numeric data for the maximum values are shown in Table 2.
Table 2.
Maximal effects and logEC50s [ from myograph analyses of endothelial function following 4 mo of filtered air (FA) or concentrated ambient particulate matter (CAP) exposure in male (control) and () mice at 8–12 weeks of age ().
| Experiment | FA/control | CAP/control | ||
|---|---|---|---|---|
| PE | ||||
| Maximal effect | * | *# | ||
| logEC50 | ||||
| Ach | ||||
| Maximal effect | * | *# | ||
| logEC50 | ||||
| L-NAME/PE | ||||
| Maximal effect | ||||
| logEC50 | ||||
| SNP | ||||
| Maximal effect | ||||
| logEC50 |
Note: Complete data are shown in Figure 5. Ach, acetylcholine; CAP, concentrated ambient particulate matter ; FA, filtered air; L-NAME, methyl ester; PE, phenylephrine; SNP, sodium nitroprusside.
vs. FA.
vs. control, analysis of variance (ANOVA).
Discussion
Findings from experimental studies of mice suggest that exposure to ambient may induce central inflammation characterized by activation of the signaling pathway in the hypothalamus (Liu et al. 2014; Ying et al. 2014). However, to our knowledge, the role of central inflammation in pathophysiologic responses to ambient exposure has not yet been systemically investigated. In the present study, we exploited a mouse model with reduced neuronal IKK2 expression to examine the role of central inflammation in exposure–induced peripheral inflammation and endothelial dysfunction. The main findings from the present study are that neural IKK2 reduction appears to inhibit CAP exposure–induced increases in a) hypothalamic and IL-6 mRNA expression; b) pulmonary, systemic, and adipose tissue inflammatory responses; and c) endothelial dysfunction. These findings provide further support for a role of central inflammation in the development of adverse cardiometabolic responses to ambient exposure.
Central inflammation, particularly in the hypothalamus, has been shown to play an important role in the pathogenesis of various cardiometabolic diseases such as obesity, hypertension, and diabetes (Han et al. 2016). We previously reported that exposure to ambient was associated with increased expression of pro-inflammatory cytokines and activation of IKK2 in the mouse hypothalamus (Ying et al. 2014). As in our previous study, we demonstrated that CAP exposure increased expression of pro-inflammatory cytokines in the hypothalamus. In addition, we demonstrated that these responses to CAP exposure were significantly inhibited or abolished in mice with reduced neural IKK2 expression. We found that, on average, IKK2 protein expression was only lower in the brains of mice compared with controls, consistent with neuron-specific knockout by Nestin-cre (Tronche et al. 1999) and the presence of cells other than neurons in brain, including astrocytes and microglia that may play a role in local inflammatory responses (Douglass et al. 2017; Jassam et al. 2017). Nonetheless, the partial reduction in brain IKK2 expression appeared to be sufficient to reduce or eliminate CAP exposure–induced expression of and IL-6 mRNA in the hypothalamus. A recent study reported hypothalamic inflammation induced by a single-day exposure to an HFD was inhibited in vagotomized mice, suggesting that the vagal afferent nerve may be responsible for transmitting gut-derived inflammatory signals to the hypothalamus (Waise et al. 2015). Further studies are needed to determine whether a similar mechanism might be involved in CAP-induced hypothalamic inflammation.
In addition to systemic circulation, foreign materials may also gain access to the brain via the olfactory mucosa, known as olfactory bulb transmission (Tjälve et al. 1996). Given the previous demonstration of ambient particles in the brain (Maher et al. 2016) and increasing evidence that exposure to ambient correlates to various brain insults (Underwood 2017), olfactory bulb transmission has been suspected to be an important mechanism for the access of ambient to the brain (Underwood 2017). In the present study, CAP vs. FA exposure was associated with significantly higher expression of the pro-inflammatory cytokines and IL-6 in the hypothalamus, whereas in the olfactory bulb, expression was higher in CAP- vs. FA-exposed control mice, but not significantly, and there was a nonsignificant decrease in IL-6 expression. Additional research is needed to clarify the potential for olfactory bulb transmission of to the brain, and its influence, if any, on central inflammation.
Another finding from the present study was that increases in plasma , , and IL-6 in CAP- vs. FA-exposed control mice were reduced or eliminated in mice. This suggests a potential role of the central nervous system in inflammatory responses to CAP, consistent with our previous finding that intracerebrovascular administration of an IKK2 inhibitor was associated with reduced systemic inflammatory responses to CAP exposure (Liu et al. 2014). Moreover, as hypothalamic inflammation has been shown to correlate to ANS dysfunction (Han et al. 2016), these present data are also consistent with increasing evidence that ANS regulates systemic inflammatory responses (Lambert et al. 2015; Pavlov and Tracey 2017). Our finding that CAP exposure–induced pulmonary inflammation was reduced in mice compared with controls (Figure 2), consistent with involvement of a central neural mechanism in pathophysiologic responses to exposure, may seem inconsistent with compelling evidence that pulmonary inflammation in response to ambient is induced through toll-like receptors (TLRs)- and/or pattern recognition receptors (PRRs)-dependent mechanisms (Bekki et al. 2016; Fuertes et al. 2013; Shoenfelt et al. 2009; Zhao et al. 2012). However, previous studies have also reported evidence of close cross-talk between the brain and lung (Davison et al. 2012; Engel et al. 2015; Winklewski et al. 2014).
Exposure to ambient has been associated with endothelial dysfunction in humans (O'Neill et al. 2005; Wauters et al. 2013) and various animal models (Davel et al. 2012; Lei et al. 2005; Ying et al. 2013), and effects of on endothelial function have been implicated in the pathogenesis of cardiometabolic diseases associated with exposure, such as atherosclerosis, hypertension, and type 2 diabetes mellitus (Corban et al. 2017). As such, endothelial dysfunction is widely believed to be central in the pathophysiology due to exposure to (Brook et al. 2010). In the present study, although CAP exposure was associated with evidence of endothelial dysfunction in both control and mice, the response was significantly less pronounced in the mice with reduced neural IKK2 expression compared with controls, consistent with a role of central inflammation in exposure–related effects. Many studies have demonstrated that systemic inflammation may lead to endothelial dysfunction (Piccardi et al. 2017).
Although the present study supports a role of neural IKK2 in pathophysiologic responses to ambient exposure, it has a number of important limitations. These include the fact that we did not evaluate whether specific types of neurons, or neurons in specific locations, are responsible for differences in responses to CAPs between control and mice. This will require additional experiments targeting different neurons, particularly pro-opiomelanocortin and agouti-related peptide neurons in the hypothalamus and those in the nucleus ambiguus and dorsal nucleus of the brainstem, and concomitantly measuring effects of exposure to CAP. In addition, we did not investigate specific mechanisms through which neural IKK2 might mediate systemic inflammation or other effects of exposure, or directly assess neural mechanisms linking ambient with central inflammation.
Conclusion
In summary, our findings support a role of neural IKK2 in CAP exposure–induced local and systemic pro-inflammatory cytokine expression, pulmonary and adipose inflammation, and endothelial dysfunction, and thus advance knowledge our understanding of the contribution of central inflammation to the pathophysiologic effects of ambient exposure.
Acknowledgments
This work was supported by the National Institutes of Health (R01ES024516 to Z. Ying), the American Heart Association (13SDG17070131 to Z. Ying), and the National Natural Science Foundation of China (81770805 to Z. Ying, 81500216 to M. Chen, and 81302452 to L. Qiu).
References
- Bekki K, Ito T, Yoshida Y, He C, Arashidani K, He M, et al. 2016. PM2.5 collected in China causes inflammatory and oxidative stress responses in macrophages through the multiple pathways. Environ Toxicol Pharmacol 45:362–369, PMID: 27393915, 10.1016/j.etap.2016.06.022. [DOI] [PubMed] [Google Scholar]
- Brook RD. 2005. You are what you breathe: evidence linking air pollution and blood pressure. Curr Hypertens Rep 7(6):427–434, PMID: 16386198, 10.1007/s11906-005-0037-9. [DOI] [PubMed] [Google Scholar]
- Brook RD. 2008. Cardiovascular effects of air pollution. Clin Sci 115(6):175–187, PMID: 18691154, 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA III, Brook JR, Bhatnagar A, Diez-Roux AV, et al. 2010. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 121(21):2331–2378, PMID: 20458016, 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Chen LC, Hwang JS. 2005. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. IV. Characterization of acute and chronic effects of ambient air fine particulate matter exposures on heart-rate variability. Inhal Toxicol 17(4-5):209–216, PMID: 15804938, 10.1080/08958370590912789. [DOI] [PubMed] [Google Scholar]
- Chen M, Liang S, Zhou H, Xu Y, Qin X, Hu Z, et al. 2017. Prenatal and postnatal mothering by diesel exhaust PM2.5-exposed dams differentially program mouse energy metabolism. Part Fibre Toxicol 14(1):3, PMID: 28100227, 10.1186/s12989-017-0183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corban MT, Duarte-Garcia A, McBane RD, Matteson EL, Lerman LO, Lerman A. 2017. Antiphospholipid syndrome: Role of vascular endothelial cells and implications for risk stratification and targeted therapeutics. J Am Coll Cardiol 69(18):2317–2330, PMID: 28473138, 10.1016/j.jacc.2017.02.058. [DOI] [PubMed] [Google Scholar]
- Davel AP, Lemos M, Pastro LM, Pedro SC, de André PA, Hebeda C, et al. 2012. Endothelial dysfunction in the pulmonary artery induced by concentrated fine particulate matter exposure is associated with local but not systemic inflammation. Toxicology 295(1-3):39–46, PMID: 22361244, 10.1016/j.tox.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Davison DL, Terek M, Chawla LS. 2012. Neurogenic pulmonary edema. Crit Care 16(2):212, PMID: 22429697, 10.1186/cc11226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBona GF. 2013. Sympathetic nervous system and hypertension. Hypertension 61(3):556–560, PMID: 23357181, 10.1161/HYPERTENSIONAHA.111.00633. [DOI] [PubMed] [Google Scholar]
- Douglass JD, Dorfman MD, Thaler JP. 2017. Glia: silent partners in energy homeostasis and obesity pathogenesis. Diabetologia 60(2):226–236, PMID: 27986987, 10.1007/s00125-016-4181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ. 2000. Cytokine activation of the HPA axis. Ann N Y Acad Sci 917:608–617, PMID: 11268389, 10.1111/j.1749-6632.2000.tb05426.x. [DOI] [PubMed] [Google Scholar]
- Engel O, Akyuz L, da Costa Goncalves AC, Winek K, Dames C, Thielke M, et al. 2015. Cholinergic pathway suppresses pulmonary innate immunity facilitating pneumonia after stroke. Stroke 46(11):3232–3240, PMID: 26451017, 10.1161/STROKEAHA.115.008989. [DOI] [PubMed] [Google Scholar]
- Feng L, Yang X, Asweto CO, Wu J, Zhang Y, Hu H, et al. 2017. Genome-wide transcriptional analysis of cardiovascular-related genes and pathways induced by in human myocardial cells. Environ Sci Pollut Res Int 24(12):11683–11693, PMID: 28326484, 10.1007/s11356-017-8773-3. [DOI] [PubMed] [Google Scholar]
- Filep Á, Fodor GH, Kun-Szabó F, Tiszlavicz L, Razga Z, Bozso G, et al. 2016. Exposure to urban PM1 in rats: development of bronchial inflammation and airway hyperresponsiveness. Respir Res 17:26, PMID: 26966003, 10.1186/s12931-016-0332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froeschl M, Hadziomerovic A, Ruzicka M. 2013. Renal sympathetic denervation for resistant hypertension. Can J Cardiol 29(5):636–638, PMID: 23541665, 10.1016/j.cjca.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Fuertes E, Brauer M, MacIntyre E, Bauer M, Bellander T, von Berg A, et al. 2013. Childhood allergic rhinitis, traffic-related air pollution, and variability in the GSTP1, TNF, TLR2, and TLR4 genes: results from the TAG study. J Allergy Clin Immunol 132(2):342–352.e2, PMID: 23639307, 10.1016/j.jaci.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Geller MD, Biswas S, Fine PM, Sioutas C. 2005. A new compact aerosol concentrator for use in conjunction with low flow-rate continuous aerosol instrumentation. J Aerosol Sci 36(8):1006–1022, 10.1016/j.jaerosci.2004.11.015. [DOI] [Google Scholar]
- Godleski JJ, Verrier RL, Koutrakis P, Catalano P, Coull B, Reinisch U, et al. 2000. Mechanisms of morbidity and mortality from exposure to ambient air particles. Res Rep Health Eff Inst 5–88; discussion 89–103, PMID: 10817681. [PubMed] [Google Scholar]
- Gong H Jr., Linn WS, Clark KW, Anderson KR, Sioutas C, Alexis NE, et al. 2008. Exposures of healthy and asthmatic volunteers to concentrated ambient ultrafine particles in Los Angeles. Inhal Toxicol 20(6):533–545, PMID: 18444007, 10.1080/08958370801911340. [DOI] [PubMed] [Google Scholar]
- Han C, Rice MW, Cai D. 2016. Neuroinflammatory and autonomic mechanisms in diabetes and hypertension. Am J Physiol Endocrinol Metab 311(1):E32–E41, PMID: 27166279, 10.1152/ajpendo.00012.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SL, Rahn KA, Arimoto R. 1999. Testing and optimizing two factor-analysis techniques on aerosol at Narragansett, Rhode Island. Atmos Environ 33:2169–2185, 10.1016/S1352-2310(98)00324-0. [DOI] [Google Scholar]
- Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J. 2017. Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron 95(6):1246–1265, PMID: 28910616, 10.1016/j.neuron.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SC, Cho Y, Song MK, Lee E, Ryu JC. 2017. Epidermal growth factor receptor (EGFR)-MAPK-nuclear factor(NF)--IL8: a possible mechanism of particulate matter(PM) 2.5-induced lung toxicity. Environ Toxicol 32(5):1628–1636, PMID: 28101945, 10.1002/tox.22390. [DOI] [PubMed] [Google Scholar]
- Kampfrath T, Maiseyeu A, Ying Z, Shah Z, Deiuliis JA, Xu X, et al. 2011. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ Res 108(6):716–726, PMID: 21273555, 10.1161/CIRCRESAHA.110.237560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karemaker JM. 1999. Autonomic integration: the physiological basis of cardiovascular variability. J Physiol 517 (Pt 2):316, PMID: 10332083, 10.1111/j.1469-7793.1999.0316t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy T, Ghio AJ, Reed W, Samet J, Zagorski J, Quay J, et al. 1998. Copper-dependent inflammation and nuclear factor- activation by particulate air pollution. Am J Respir Cell Mol Biol 19(3):366–378, PMID: 9730864, 10.1165/ajrcmb.19.3.3042. [DOI] [PubMed] [Google Scholar]
- Kreyling WG, Semmler-Behnke M, Seitz J, Scymczak W, Wenk A, Mayer P, et al. 2009. Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal Toxicol 21(suppl 1):55–60, PMID: 19558234, 10.1080/08958370902942517. [DOI] [PubMed] [Google Scholar]
- Lambert EA, Straznicky NE, Dixon JB, Lambert GW. 2015. Should the sympathetic nervous system be a target to improve cardiometabolic risk in obesity?. Am J Physiol Heart Circ Physiol 309(2):H244–H258, PMID: 25980020, 10.1152/ajpheart.00096.2015. [DOI] [PubMed] [Google Scholar]
- Lei YC, Hwang JS, Chan CC, Lee CT, Cheng TJ. 2005. Enhanced oxidative stress and endothelial dysfunction in streptozotocin-diabetic rats exposed to fine particles. Environ Res 99(3):335–343, PMID: 16307975, 10.1016/j.envres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Li ZW, Omori SA, Labuda T, Karin M, Rickert RC. 2003. IKK beta is required for peripheral B cell survival and proliferation. J Immunol 170(9):4630–4637, PMID: 12707341, 10.4049/jimmunol.170.9.4630. [DOI] [PubMed] [Google Scholar]
- Liu C, Fonken LK, Wang A, Maiseyeu A, Bai Y, Wang TY, et al. 2014. Central IKK inhibition prevents air pollution mediated peripheral inflammation and exaggeration of type II diabetes. Part Fibre Toxicol 11:53, PMID: 25358444, 10.1186/s12989-014-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejczyk P, Zhong M, Li Q, Xiong J, Nadziejko C, Chen LC. 2005. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. Ii. The design of a caps exposure system for biometric telemetry monitoring. Inhal Toxicol 17(4-5):189–197, PMID: 15804936, 10.1080/08958370590912743. [DOI] [PubMed] [Google Scholar]
- Magari SR, Hauser R, Schwartz J, Williams PL, Smith TJ, Christiani DC. 2001. Association of heart rate variability with occupational and environmental exposure to particulate air pollution. Circulation 104(9):986–991, PMID: 11524390, 10.1161/hc3401.095038. [DOI] [PubMed] [Google Scholar]
- Maher BA, Ahmed IA, Karloukovski V, MacLaren DA, Foulds PG, Allsop D, et al. 2016. Magnetite pollution nanoparticles in the human brain. Proc Natl Acad Sci USA 113(39):10797–10801, PMID: 27601646, 10.1073/pnas.1605941113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirowsky J, Hickey C, Horton L, Blaustein M, Galdanes K, Peltier RE, et al. 2013. The effect of particle size, location and season on the toxicity of urban and rural particulate matter. Inhal Toxicol 25(13):747–757, PMID: 24255952, 10.3109/08958378.2013.846443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, et al. 2002. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A 65(20):1531–1543, PMID: 12396867, 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, Utell MJ. 2002. Ultrafine particles in the urban air: to the respiratory tract–and beyond? Environ Health Perspect 110(8):A440–A441, PMID: 12153769, 10.1289/ehp.110-a440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill MS, Veves A, Zanobetti A, Sarnat JA, Gold DR, Economides PA, et al. 2005. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation 111:2913–2920, PMID: 15927967, 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- Pahl HL. 1999. Activators and target genes of Rel/NF- transcription factors. Oncogene 18(49):6853–6866, PMID: 10602461, 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. 2017. Neural regulation of immunity: molecular mechanisms and clinical translation. Nat Neurosci 20(2):156–166, PMID: 28092663, 10.1038/nn.4477. [DOI] [PubMed] [Google Scholar]
- Peters A, Frohlich M, Döring A, Immervoll T, Wichmann HE, Hutchinson WL, et al. 2001. Particulate air pollution is associated with an acute phase response in men; results from the MONICA-Augsburg study. Eur Heart J 22(14):1198–1204, PMID: 11440492, 10.1053/euhj.2000.2483. [DOI] [PubMed] [Google Scholar]
- Piccardi B, Giralt D, Bustamante A, Llombart V, Garcia-Berrocoso T, Inzitari D, et al. 2017. Blood markers of inflammation and endothelial dysfunction in cardioembolic stroke: Systematic review and meta-analysis. Biomarkers 22(3-4):200–209, PMID: 28117601, 10.1080/1354750X.2017.1286689. [DOI] [PubMed] [Google Scholar]
- Pope CA., III 2000. Epidemiology of fine particulate air pollution and human health: biologic mechanisms and who's at risk? Environ Health Perspect 108(suppl 4):713–723, PMID: 10931790, 10.2307/3454408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkayastha S, Zhang G, Cai D. 2011. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-β and NF-κB. Nat Med 17(7):883–887, PMID: 21642978, 10.1038/nm.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quay JL, Reed W, Samet J, Devlin RB. 1998. Air pollution particles induce Il-6 gene expression in human airway epithelial cells via NF- activation. Am J Respir Cell Mol Biol 19(1):98–106, PMID: 9651185, 10.1165/ajrcmb.19.1.3132. [DOI] [PubMed] [Google Scholar]
- Semmler-Behnke M, Kreyling WG, Lipka J, Fertsch S, Wenk A, Takenaka S, et al. 2008. Biodistribution of 1.4- and 18-nm gold particles in rats. Small 4(12):2108–2111, PMID: 19031432, 10.1002/smll.200800922. [DOI] [PubMed] [Google Scholar]
- Shoenfelt J, Mitkus RJ, Zeisler R, Spatz RO, Powell J, Fenton MJ, et al. 2009. Involvement of TLR2 and TLR4 in inflammatory immune responses induced by fine and coarse ambient air particulate matter. J Leukoc Biol 86(2):303–312, PMID: 19406832, 10.1189/jlb.1008587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds SE, Cowley MA. 2013. Hypertension in obesity: is leptin the culprit? Trends Neurosci 36(2):121–132, PMID: 23333346, 10.1016/j.tins.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Sivagangabalan G, Spears D, Masse S, Urch B, Brook RD, Silverman F, et al. 2011. The effect of air pollution on spatial dispersion of myocardial repolarization in healthy human volunteers. J Am Coll Cardiol 57(2):198–206, PMID: 21211691, 10.1016/j.jacc.2010.08.625. [DOI] [PubMed] [Google Scholar]
- Song L, Li D, Li X, Ma L, Bai X, Wen Z, et al. 2017. Exposure to PM2.5 induces aberrant activation of NF- in human airway epithelial cells by downregulating miR-331 expression. Environ Toxicol Pharmacol 50:192–199, PMID: 28192748, 10.1016/j.etap.2017.02.011. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Campen M, Bierman A, Flanders SE, Broman KW, Rabold R. 2004. Particle effects on heart-rate regulation in senescent mice. Inhal Toxicol 16(6-7):381–390, PMID: 15204754, 10.1080/08958370490439551. [DOI] [PubMed] [Google Scholar]
- Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, et al. 2012. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122(1):153–162, PMID: 22201683, 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjälve H, Henriksson J, Tallkvist J, Larsson BS, Lindquist NG. 1996. Uptake of manganese and cadmium from the nasal mucosa into the central nervous system via olfactory pathways in rats. Pharmacol Toxicol 79(6):347–356, PMID: 9000264, 10.1111/j.1600-0773.1996.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, et al. 1999. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 23(1):99–103, PMID: 10471508, 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier CL. 1999. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev 79(1):1–71, PMID: 9922367, 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (Environmental Protection Agency). 2009. Integrated Science Assessment (ISA) for Particulate Matter. Final Report. https://cfpub.epa.gov/ncea/isa/recordisplay.cfm?deid=216546 [accessed 5 May 2017].
- Underwood E. 2017. The polluted brain. Science 355(6323):342–345, PMID: 28126768, 10.1126/science.355.6323.342. [DOI] [PubMed] [Google Scholar]
- Utell MJ, Frampton MW, Zareba W, Devlin RB, Cascio WE. 2002. Cardiovascular effects associated with air pollution: potential mechanisms and methods of testing. Inhal Toxicol 14(12):1231–1247, PMID: 12454788, 10.1080/08958370290084881. [DOI] [PubMed] [Google Scholar]
- van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, et al. 2001. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (). Am J Respir Crit Care Med 164(5):826–830, PMID: 11549540, 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- Vogel CF, Sciullo E, Wong P, Kuzmicky P, Kado N, Matsumura F. 2005. Induction of proinflammatory cytokines and C-reactive protein in human macrophage cell line U937 exposed to air pollution particulates. Environ Health Perspect 113(11):1536–1541, PMID: 16263508, 10.1289/ehp.8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waise TM, Toshinai K, Naznin F, NamKoong C, Md Moin AS, Sakoda H, et al. 2015. One-day high-fat diet induces inflammation in the nodose ganglion and hypothalamus of mice. Biochem Biophys Res Commun 464(4):1157–1162, PMID: 26208455, 10.1016/j.bbrc.2015.07.097. [DOI] [PubMed] [Google Scholar]
- Wang X, Chen M, Zhong M, Hu Z, Qiu L, Rajagopalan S, et al. 2017. Exposure to concentrated ambient PM2.5 shortens lifespan and induces inflammation-associated signaling and oxidative stress in drosophila. Toxicol Sci 156(1):199–207, PMID: 28069988, 10.1093/toxsci/kfw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauters A, Dreyfuss C, Pochet S, Hendrick P, Berkenboom G, van de Borne P, et al. 2013. Acute exposure to diesel exhaust impairs nitric oxide-mediated endothelial vasomotor function by increasing endothelial oxidative stress. Hypertension 62(2):352–358, PMID: 23798345, 10.1161/HYPERTENSIONAHA.111.00991. [DOI] [PubMed] [Google Scholar]
- Winklewski PJ, Radkowski M, Demkow U. 2014. Cross-talk between the inflammatory response, sympathetic activation and pulmonary infection in the ischemic stroke. J Neuroinflammation 11:213, PMID: 25539803, 10.1186/s12974-014-0213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z, Xu X, Bai Y, Zhong J, Chen M, Liang Y, et al. 2014. Long-term exposure to concentrated ambient increases mouse blood pressure through abnormal activation of the sympathetic nervous system: a role for hypothalamic inflammation. Environ Health Perspect 122(1):79–86, PMID: 24240275, 10.1289/ehp.1307151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z, Xu X, Chen M, Liu D, Zhong M, Chen LC, et al. 2013. A synergistic vascular effect of airborne particulate matter and nickel in a mouse model. Toxicol Sci 135(1):72–80, PMID: 23788629, 10.1093/toxsci/kft136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki K, Brito JM, Silva LF, Lino-Dos-Santos-Franco A, Frias DP, E Silva ES, et al. 2017. The effects of particulate matter on inflammation of respiratory system: Differences between male and female. Sci Total Environ 586:284–295, PMID: 28174048, 10.1016/j.scitotenv.2017.01.221. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zheng L, Tuo J, Liu Q, Zhang X, Xu Z, et al. 2017. Analysis of -induced cytotoxicity in human HaCaT cells based on a microfluidic system. Toxicol In Vitro 43:1–8, PMID: 28431925, 10.1016/j.tiv.2017.04.018. [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Wei SG, Francis J, Felder RB. 2003. Cardiovascular and renal sympathetic activation by blood-borne TNF- in rat: the role of central prostaglandins. Am J Physiol Regul Integr Comp Physiol 284(4):R916–R927, PMID: 12626358, 10.1152/ajpregu.00406.2002. [DOI] [PubMed] [Google Scholar]
- Zhao C, Liao J, Chu W, Wang S, Yang T, Tao Y, et al. 2012. Involvement of TLR2 and TLR4 and TH1/TH2 shift in inflammatory responses induced by fine ambient particulate matter in mice. Inhal Toxicol 24(13):918–927, PMID: 23121301, 10.3109/08958378.2012.731093. [DOI] [PubMed] [Google Scholar]