Scientists have long known that when it comes to harm from environmental exposures, the youngest children often face the greatest risk.1 Chemicals and pollutants that pass through a woman’s placenta into her fetus can interfere with the child’s normal development and cause health effects lasting into adulthood.2 Newer research is examining the role a woman’s prepregnancy exposures may have on the fetus. What has gotten far less attention, however, is how the biological consequences of a father’s environmental exposures before conception might affect his unborn children.
A woman’s environmental exposures during the prenatal and even preconception periods are well known to potentially affect her unborn child’s child. Now studies are showing that a man’s exposures also can influence the health of his future children. Image: © PeopleImages/iStockphoto.
“We’ve been hyperfocused on how the mother’s environment shapes the health of a developing kid while ignoring the other half of the equation,” says Richard Pilsner, an associate professor in the School of Public Health at the University of Massachusetts Amherst. “But the father’s preconception exposures matter, too.”
Scientists have linked paternal exposures to certain chemicals with higher risks of cancer and genital malformations in children, and there is evidence that children may have a higher risk of metabolic diseases and type 2 diabetes if their father ate a poor diet prior to conception.3,4 Animal studies, too, are providing glimpses into how paternal environmental experiences can affect the health of offspring.5
According to Oliver Rando, a professor at the University of Massachusetts Medical School in Worcester, all these emerging findings, while inconclusive, bolster recommendations made by the World Health Organization and other public health groups that men should safeguard their health in anticipation of trying to conceive a baby.6,7 “If it turns out that by your own lifestyle you are setting your kids up for failure, then that should impact on how you treat yourself,” Rando says.
Early Observations on Sperm-Mediated Health Effects
The idea that environmental effects on sperm might affect child health was first proposed decades ago. One early study, published in 1974, suggested higher rates of cancer in children whose fathers were exposed to hydrocarbons on the job—for instance, auto mechanics, machinists, miners, and painters.8 The authors speculated that high cancer rates might be the result of children coming in contact with hydrocarbons brought home from the workplace. But the authors also raised the possibility of “a direct effect of some hydrocarbon contained in petrol and oil on spermatogenesis … [that] would then be transmitted to the child.”8
Later studies found evidence of higher rates of Wilm’s tumor, leukemia, and brain cancer in children whose fathers worked with paint products, in addition to higher rates of lymphoma and leukemia in the children of men who worked with pesticides and herbicides.9 Studies on fathers’ occupational exposures suggest that male babies born to men who worked with polychlorinated compounds, heavy metals, or phthalates are at increased risk of cryptorchidism, or the failure of one or both testicles to descend, compared with babies of unexposed men.10,11
The first evidence that men’s environmental exposures may be linked to their children’s health was published in 1974. That first study reported an increased risk of cancer in the children of auto mechanics, machinists, and other men exposed to hydrocarbons on the job. The authors suggested that the children with cancer may have been exposed to chemicals their fathers brought home from work—or that hydrocarbon exposure had somehow produced a “carcinogenic defect” in the men’s sperm before conception. Image: U.S. Environmental Protection Agency.
Other human studies suggest a connection between a father’s diet and his descendants’ health. A Swedish study published in 2002 found that the grandsons of men who suffered from food shortages at 9–12 years of age were at higher risk of death from diabetes.12 But the evidence did not follow any clear-cut pattern. For instance, if the grandfathers had gone hungry in early adulthood—and not as young adolescents—then their grandchildren were paradoxically less likely to become diabetic.
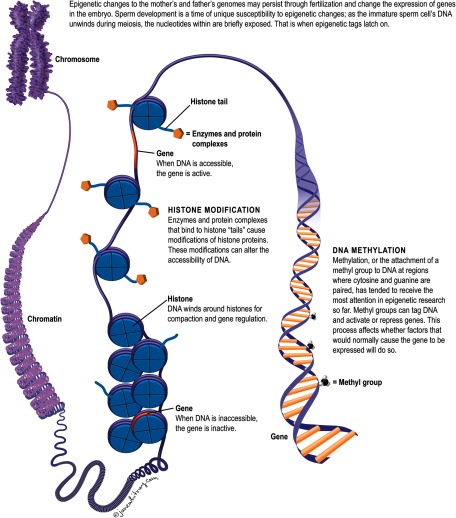
Scientists used to think that only the genetic information contained in parental DNA could dictate inherited traits in children. But that long-held assumption was upended when studies started to show that epigenetic changes to the mother’s and father’s genomes may persist through fertilization and change how genes are expressed in the embryo without altering the genes themselves.13 The two major epigenetic changes include methylation (in which methyl groups attach to DNA and alter gene function) and modifications to the histone proteins that package and order DNA on the chromosome. These processes may involve small RNA molecules that affect the synthesis, stability, and translation of messenger RNA.14
According to Stephen Krawetz, a professor at the Center for Molecular Medicine and Genetics at Wayne State University School of Medicine, sperm development is a time of unique susceptibility to epigenetic changes. Mature sperm cells are created when immature germ cells in the testicles divide during a process called meiosis. Each germ cell has 46 chromosomes, which are halved during meiosis, leaving each mature sperm cell with 23 chromosomes. (The egg also has 23 chromosomes, and during fertilization, the sperm and egg cells unite to create a zygote, or embryo precursor, with 46 chromosomes.) As the immature sperm cell’s DNA unwinds during meiosis, the nucleotides within are briefly exposed, and that is when epigenetic tags latch on.15,16,17,18 “It’s a vulnerable time,” Krawetz says.
It is still not known precisely at what point in their development from germ cell to maturation that sperm cells are most sensitive to environmental factors. Pilsner and Krawetz propose there could be six windows of vulnerability to external threats: during fetal development, immediately after birth, during the onset of puberty, during the first stages of sperm production, during final maturation of sperm in the epididymis, and shortly after the sperm fertilizes an egg (just before the embryonic genome becomes demethylated).19 The RNA that a man delivers at fertilization, Krawetz says, could have a marked effect on very early development of his offspring, setting the stage for effects later in life.20
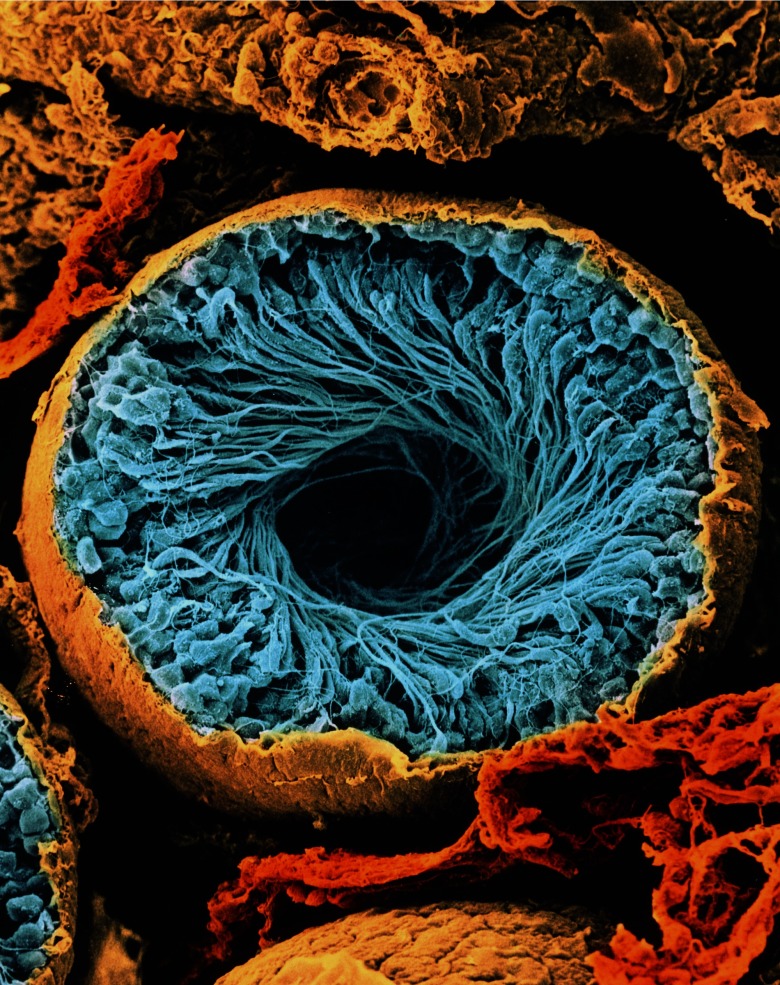
This scanning electron micrograph shows a cross-section of a seminiferous tubule. These tubules are the structures in the testis where sperm are produced. They are lined by Sertoli cells, which nourish developing sperm, and spermatogenic cells, which eventually develop into sperm. The sperm are released into the cavity of the tubule to migrate to the epididymis, where they mature. This tubule contains a swirl of forming sperm cells (blue). Image: © CNRI/Science Source.
Potential Mechanisms for Passing along Epigenetic Traits
Because it takes decades to study multiple generations from the same family, evidence of transgenerational effects passing through a man’s lineage accumulates very slowly. But animal studies are generating a wealth of supporting data and allowing scientists to drill down to the mechanisms by which not just genetic but epigenetic traits pass from one generation to the next.
Tracy Bale, a professor at the University of Maryland School of Medicine, published one such study in 2013.21 Bale reported that if male mice were deliberately stressed in the laboratory, their offspring responded less vigorously to stressful experiences as adults. When she analyzed the sires’ sperm cells, Bale found changes in a category of small RNA molecules called microRNAs; nine of them were expressed at unusually high levels compared with nonstressed control animals’ sperm. Bale injected these nine molecules into mouse control embryos that she then implanted into surrogate dams. The resulting offspring had the same reduced reactions to stress as the offspring of stressed sires.
“What that’s telling us is that mild changes to dad’s environment reprogram sperm DNA and shift brain development in the offspring,” Bale says. The sire’s sperm carry that epigenetic signal, which persists even after the stress has ended. But how that might affect the offspring’s health is unknown. “For now,” says Bale, “we just want to understand what’s happening to the sperm and how these changes get passed down to subsequent generations.”
Other small RNAs are thought to play important roles in paternal epigenetics, too. For instance, Rando investigates a category of the molecules known as transfer RNAs (tRNAs). Fully intact tRNAs play crucial roles in cell biology by helping to build proteins one amino acid at a time. But Rando focuses specifically on shorter tRNA fragments that he believes have other functions.22
After feeding mice a low-protein diet, he measured changes in the levels of specific tRNA fragments. These fragments were highly abundant in the epididymis, the duct where sperm complete their final 10–12 days of maturation. Rando speculates that tRNAs latch on to sperm in the epididymis and then hitch a ride into the developing embryo, where they alter its gene expression.22
In one study,23 Rando found that the offspring of protein-deprived mice had altered cholesterol synthesis in the liver, a finding he attributes to tRNA fragment activity. He says he does not know why a low-protein diet would have that effect or whether it signifies an adaptive response that the sire is trying to pass on to his children. But in a subsequent experiment Rando encountered a paternal effect response that he believes is adaptive: He exposed male mice to dissolved nicotine in drinking water and found that their offspring survived what would otherwise have been lethal doses of not just nicotine but also cocaine, two drugs that have entirely different effects on the body.24 However, the adaptive benefits of that parental exposure were offset by a negative consequence, in that the young animals cleared glucose poorly from the body. In humans, this would put an individual at higher risk of diabetes.
Rando suspects tRNA fragments play a central role in both findings, and he is starting experiments now to test that hypothesis. He is also investigating why a sire’s nicotine exposure would simultaneously boost resistance to toxic chemicals and impair glucose metabolism—findings that he believes reflect tradeoffs in epigenetic inheritance.
“We don’t know if or how the two findings are mechanistically connected,” he says. “You’d expect that after eating something toxic in the environment, wild mice would want to ‘tell their offspring about it.’ But whether that turns out to be a positive adaptive message depends on offspring’s own ecological circumstances, and the glucose findings are showing us that toxic exposure in fathers may also have harmful consequences for their young.”
Image: © Jane Whitney.
Apart from microRNAs and tRNAs, methyl groups on sperm DNA also are cited as transgenerational carriers of environmental information, but the supporting evidence is mixed. For instance, scientists at the Chinese Academy of Sciences reported in 2014 that male mice exposed to high-fat diets for the purpose of inducing a prediabetic state (meaning the animals had altered glucose metabolism and insulin resistance) went on to sire prediabetic offspring.25 The researchers pointed to altered methylation patterns as the likely explanation for their findings. But when Rando and his colleagues looked at the effect of high-fat diets on mouse sperm methylation in their own laboratory, they could not replicate the Chinese results.26
According to Thad Schug, a health scientist administrator in the Population Health Branch at the National Institute of Environmental Health Sciences, methylation changes have long been a default mechanism in epigenetic research. “Altered methylation is relatively easy to measure, and most researchers assumed that if you see it, then something is happening epigenetically,” he says. “But other complex processes involving small RNAs and histone modifications might also be involved. These are harder to measure, but we’re getting there.”
Susan Murphy, an associate professor at Duke University Medical Center, points out that at least one important class of genes, called imprinted genes, manages to escape the widespread demethylation that occurs in a newly formed embryo. All embryonic genes have two copies—one from the mother and one from the father—but for an imprinted gene, one of those copies is silenced. Because imprinted genes are not demethylated after fertilization, they can capture environmentally induced epigenetic information and transmit it to the next generation. Murphy and her colleagues recently reported that organophosphate flame retardants altered the methylation of many imprinted genes in human sperm, although the potential consequences for children remain unknown.27
A Fuller Picture with Couple-Based Studies
Germaine Buck Louis, a senior investigator and director of the Division of Intramural Population Health Research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, says scientists continue to grapple with a significant shortage of population-based data on semen and sperm quality. There are very few registries in the world to which men supply semen samples voluntarily. Unlike women, who routinely visit doctors for examinations, Buck Louis says men tend to interact with health care systems only when they have a problem, and they generally provide semen samples only during exams at fertility clinics.
Furthermore, Buck Louis adds, “While the father is important, we really need couple-based designs to understand couple-dependent outcomes like pregnancy.” She directs one such couple-based project, the Longitudinal Investigation of Fertility and the Environment Study, which collected semen samples from male partners of couples trying for pregnancy. This study recruited couples to investigate whether chemical exposures in the context of lifestyle would have any influence on how long it took them to get pregnant. The men donated semen and other biological samples as part of this study.
According to results published in 2016, men’s preconception exposures to phthalates (monobutyl phthalate, monobenzyl phthalate, and monomethyl phthalate, specifically), polychlorinated biphenyls, and lead were all associated with longer times to pregnancy even after adjusting for the mother’s exposure to the same chemicals.28 However, the study did not reveal associations between those exposures and pregnancy outcomes or birth weights.
While it is important to isolate environmental exposures that alter a man’s sperm, that is only part of the picture when it comes to the health of the next generation. “We really need couple-based designs to understand couple-dependent outcomes like pregnancy,” says researcher Germaine Buck Louis. Image: © monkeybusinessimages/iStockphoto.
UMass’s Pilsner, however, reported that paternal exposure to phthalates before conception may predict the viability of newly formed embryos. Pilsner directs the Sperm Environmental Epigenetics and Development Study together with the fertility clinic at Baystate Medical Center in Springfield, Massachusetts. Investigators with the study are currently recruiting 250 couples seeking in vitro fertilization procedures. Last year, they reported preliminary findings with 50 couples showing that higher levels of phthalates in men’s urine were associated with lower-quality blastocysts (early embryos).29
More recently, Pilsner found these same paternal phthalate exposures were also associated with sperm DNA methylation profiles, specifically in regions that code for embryonic growth and development,30 suggesting a plausible epigenetic connection that could affect the developing child. However, larger studies are necessary to replicate these results among men from the general population, he cautions.
Efforts to understand how environmental effects on sperm predispose children to health problems are gaining momentum, with researchers calling for new and expanded human studies to address critical data gaps, including potential paternal effects on neurodevelopment, asthma, allergies, and cardiovascular function.4 Schug administers a new grant program at the National Institute of Environmental Health Sciences addressing parental chemical exposures in animals and their influence on offspring health. The program started funding studies in July 2017,31 and Schug says it has a heavy focus on epigenetic mechanisms. “We’re wary of anyone making grandiose statements about how preconception exposure to either parent—and fathers especially—could change offspring and increase their risk of disease,” Schug says. “We’re at the early stages of the research, and there’s still a long way to go.”
Biography
Charles W. Schmidt, MS, an award-winning science writer from Portland, Maine, writes for Scientific American, Science, various Nature publications, and many other magazines, research journals, and websites.
References
- 1.Gluckman PD, Hanson MA, Spencer HG, Bateson P. 2005. Environmental influences during development and their later consequences for health and disease: implications for the interpretation of empirical studies. Proc Biol Sci 272(1564):671–677, PMID: 15870029, 10.1098/rspb.2004.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wadhwa PD, Buss C, Entringer S, Swanson JM. 2009. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med 27(5):358–368, PMID: 19711246, 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferguson-Smith AC, Patti ME. 2011. You are what your dad ate. Cell Metab 13(2):115117, PMID: 21284975, 10.1016/j.cmet.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Braun JM, Messerlian C, Hauser R. 2017. Fathers matter: why it’s time to consider the impact of paternal environmental exposures on children’s health. Curr Epidemiol Rep 4(1):46–55, PMID: 28848695, 10.1007/s40471-017-0098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rando OJ. 2012. Daddy issues: paternal effects on phenotype. Cell 151(4):702–708, PMID: 23141533, 10.1016/j.cell.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. 2013. “Preconception Care to Reduce Maternal and Childhood Mortality and Morbidity.” Meeting Report and Packages of Interventions: WHO HQ, February 2012. Geneva, Switzerland:World Health Organization; http://www.who.int/maternal_child_adolescent/documents/concensus_preconception_care/en/ [accessed 10 October 2017]. [Google Scholar]
- 7.Kotelchuck M. 2013. Father’s Role in Preconception Health. Presented at: Paternal Involvement in Pregnancy Outcomes Conference, 26 September 2013, Pontiac, Maryland: Available: https://www.nichd.nih.gov/sites/default/files/about/meetings/2013/Documents/2_Kotelchuck_Men_Preconception.pdf [accessed 10 October 2017]. [Google Scholar]
- 8.Fabia J, Thuy TD. 1974. Occupation of father at time of birth of children dying of malignant diseases. Br J Prev Soc Med 28(2):98–100, PMID: 4853418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soubry A, Hoyo C, Jirtle RL, Murphy SK. 2014. A paternal environmental legacy: evidence for epigenetic inheritance through the male germ line. Bioessays 36(4):359–371, PMID: 24431278, 10.1002/bies.201300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierik FH, Burdorf A, Deddens JA, Juttmann RE, Weber RFA. 2004. Maternal and paternal risk factors for cryptorchidism and hypospadias: a case–control study in newborn boys. Environ Health Perspect 112(15):1570–1576, PMID: 15531444, 10.1289/ehp.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonde JP. 2010. Male reproductive organs are at risk from environmental hazards. Asian J Androl 12(2):152–156, PMID: 19966832, 10.1038/aja.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaati G, Bygren LO, Pembrey M, Sjöström M. 2007. Transgenerational response to nutrition, early life circumstances and longevity. Eur J Hum Genet 15(7):784–790, PMID: 17457370, 10.1038/sj.ejhg.5201832. [DOI] [PubMed] [Google Scholar]
- 13.Curley JP, Mashoodh R, Champagne FA. 2011. Epigenetics and the origins of paternal effects. Horm Behav 59(3):306–314, PMID: 20620140, 10.1016/j.yhbeh.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Day J, Savani S, Krempley BD, Nguyen M, Kitlinska JB. 2016. Influence of paternal preconception exposures on their offspring: through epigenetics to phenotype. Am J Stem Cells 5(1):11–18, PMID: 27335698, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4913293. [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson GD, Mackie P, Jodar M, Moskovtsev S, Krawetz SA. 2015. Chromatin and extracellular vesicle associated sperm RNAs. Nucleic Acids Res 43(14):6847–6859, PMID: 26071953, 10.1093/nar/gkv591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martins RP, Krawetz SA. 2007. Decondensing the protamine domain for transcription. Proc Natl Acad Sci USA 104(20):8340–8345, PMID: 17483471, 10.1073/pnas.0700076104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson GD, Lalancette C, Linnemann AK, Leduc F, Boissonneault G, Krawetz SA. 2011. The sperm nucleus: chromatin, RNA, and the nuclear matrix. Reproduction 141(1):21–36, PMID: 20876223, 10.1530/REP-10-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson GD, Jodar M, Pique-Regi R, Krawetz SA. 2016. Nuclease footprints in sperm project past and future chromatin regulatory events. Sci Rep 6:25864, PMID: 27184706, 10.1038/srep25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu H, Hauser R, Krawetz SA, Pilsner JR. 2015. Environmental susceptibility of the sperm epigenome during windows of male germ cell development. Curr Environ Health Rep 2(4):356–366, PMID: 26362467, 10.1007/s40572-015-0067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jodar M, Selvaraju S, Sendler E, Diamond MP, Krawetz SA; Reproductive Medicine Network. 2013. The presence, role and clinical use of spermatozoal RNAs. Hum Reprod Update 19(6):604–624, PMID: 23856356, 10.1093/humupd/dmt031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. 2013. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci 33(21):9003–9012, PMID: 23699511, 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma U, Conine CC, Shea JM, Boskovic A, Derr AG, Bing XY, et al. 2016. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in animals. Science 351(6271):391–396, PMID: 26721685, 10.1126/science.aad6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, et al. 2010. Paternally induced transgenerational environmental programming of metabolic gene expression in mammals. Cell 143(7):1084–1096, PMID: 21183072, 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vallaster MP, Kukreja S, Bing XY, Ngolab J, Zhao-Shea R, Gardner PD. 2017. Paternal nicotine exposure alters hepatic xenobiotic metabolism in offspring. eLife 6:e24771, 10.7554/eLife.24771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei Y, Yang CR, Wei YP, Zhao Z-A, Hou Y, Schatten H, et al. 2014. Paternally induced transgenerational inheritance of susceptibility to diabetes in mammals. Proc Natl Acad Sci USA 111(5):1873–1878, PMID: 24449870, 10.1073/pnas.1321195111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shea JM, Serra RW, Carone BR, Shulha HP, Kucukural A, Ziller MJ, et al. 2015. Genetic and epigenetic variation, but not diet, shape the sperm. Develop Cell 35(6):750–758, PMID: 26702833, 10.1016/j.devcel.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soubry A, Hoyo C, Butt CM, Fieuws S, Price TM, Murphy SK, et al. 2017. Human exposure to flame-retardants is associated with aberrant DNA methylation at imprinted genes in sperm. Environ Epigenet 3(1):dvx003, 10.1093/eep/dvx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buck Louis GM, Barr DB, Kannan K, Chen Z, Kim S, Sundaram R. 2016. Paternal exposures to environmental chemicals and time-to-pregnancy: overview of results from the LIFE Study. Andrology 4(4):639–647, PMID: 27061873, 10.1111/andr.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu H, Ashcraft L, Whitcomb BW, Rahil T, Tougias E, Sites CK, et al. 2016. Parental contributions to early embryo development: influences of urinary phthalate and phthalate alternatives among couples undergoing IVF treatment. Hum Reprod 32(1):65–75, PMID: 27927842, 10.1093/humrep/dew301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu H, Estill MS, Shershebnev A, Suvorov A, Krawetz SA, Whitcomb BW, et al. 2017. Preconception urinary phthalate concentrations and sperm DNA methylation profiles among men undergoing IVF treatment: a cross-sectional study. Hum Reprod 32(11):2159–2169, PMID: 29024969, 10.1093/humrep/dex283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Department of Health and Human Services: Part 1. Overview Information. 2017. The Preconception Exposure Window and Health of the Offspring (R01) [website]. https://grants.nih.gov/grants/guide/rfa-files/RFA-ES-16-007.html [accessed 1 November 2017].