Abstract
Background:
Few epidemiologic studies have investigated health effects of water-soluble fractions of metals, the more biologically accessible fractions of metals, in their attempt to identify health-relevant components of ambient .
Objectives:
In this study, we estimated acute cardiovascular effects of components in an urban population, including a suite of water-soluble metals that are not routinely measured at the ambient level.
Methods:
Ambient concentrations of criteria gases, , and components were measured at a central monitor in Atlanta, Georgia, during 1998–2013, with some components only measured during 2008–2013. In a time-series framework using Poisson regression, we estimated associations between these pollutants and daily counts of emergency department (ED) visits for cardiovascular diseases in the five-county Atlanta area.
Results:
Among the components we examined during 1998–2013, water-soluble iron had the strongest estimated effect on cardiovascular outcomes [ (95% CI: 1.005, 1.019), per interquartile range increase ()]. The associations for and other components were consistent with the null when controlling for water-soluble iron. Among components that were only measured during 2008–2013, water-soluble vanadium was associated with cardiovascular ED visits [ (95% CI: 1.000, 1.025), per interquartile range increase ()].
Conclusions:
Our study suggests cardiovascular effects of certain water-soluble metals, particularly water-soluble iron. The observed associations with water-soluble iron may also point to certain aspects of traffic pollution, when processed by acidifying sulfate, as a mixture harmful for cardiovascular health. https://doi.org/10.1289/EHP2182
Introduction
Epidemiologic studies have indicated acute cardiovascular effects of fine particulate matter (; particulate matter with aerodynamic diameter ) (Brook 2008; Dominici et al. 2006; Pope and Dockery 2006; Stafoggia et al. 2013). Because is a complex mixture of various chemical species, there is an ongoing effort to identify its health-relevant components. Nationwide multisite studies in the United States have examined whether the associations between and cardiovascular morbidity and mortality are modified by chemical composition (Bell et al. 2009; Franklin et al. 2008; Zanobetti et al. 2009). Other time-series studies have estimated associations between cardiovascular morbidity and mortality and individual components directly (Atkinson et al. 2015; Basagaña et al. 2015; Bell et al. 2014; Ito et al. 2011; Levy et al. 2012; Lippmann et al. 2013; Ostro et al. 2006; Peng et al. 2009; Sarnat et al. 2015; Suh et al. 2011). Although the specific components that are associated with health outcomes vary across studies, there is growing evidence on the acute cardiovascular effects of metals/metalloids and carbonaceous components of (Kelly and Fussell 2012; Lippmann 2014; Rohr and Wyzga 2012).
Metals/metalloids exist in in different forms, with some forms being more water soluble and thus more biologically accessible than others (Allen et al. 2001; Birmili et al. 2006; Fang et al. 2015a; Heal et al. 2005). However, most ambient air pollution monitoring networks only measure these components in total elemental concentrations, and not in water-soluble concentrations. As a result, few epidemiologic studies have estimated health associations with water-soluble metals in their attempts to identify health-relevant components of (Heal et al. 2009; Huang et al. 2003).
To advance our understanding of acute cardiovascular effects of and its components, we conducted a time-series study in Atlanta, Georgia, to estimate the associations between daily counts of emergency department (ED) visits for cardiovascular diseases and daily concentrations of components, including a suite of water-soluble metals/metalloids that are not routinely measured at the ambient level. This analysis utilized up to 15 y of data on ambient air pollution and ED visits obtained as part of our ongoing Study of Particles and Health in Atlanta (SOPHIA) (Metzger et al. 2004; Sarnat et al. 2008; Tolbert et al. 2000; Ye et al. 2017).
Methods
Air Pollution Data
Ambient concentrations of criteria gases, , and components were measured at the Jefferson Street ambient monitoring site during the period of 14 August 1998–15 December 2013 as part of the South Eastern Aerosol Research and Characterization (SEARCH) network and the Aerosol Research and Inhalation Epidemiology Study (ARIES) in Atlanta (Hansen et al. 2006). Criteria gases were measured daily, including 1-h maximum carbon monoxide (CO), 1-h maximum nitrogen dioxide (), 1-h maximum sulfur dioxide (), and 8-h maximum ozone (). and its major components—including organic carbon (OC), elemental carbon (EC), ammonium (), nitrate (), and sulfate ()—were measured daily using filter-based 24-h integrated Federal Reference Methods. Total elemental concentrations of metals and metalloids (henceforth all referred to as metals), including titanium (Ti), manganese (Mn), iron (Fe), copper (Cu), zinc (Zn), aluminum (Al), lead (Pb), silicon (Si), calcium (Ca), sodium (Na), and potassium (K), were analyzed from the daily filters using X-ray fluorescence. X-ray fluorescence analyses were conducted by Desert Research Institute (Reno, NV) on filters collected through 22 March 2008, and by Atmospheric Research & Analysis, Inc. (Cary, NC) on filters collected after 23 March 2008; different limits of detection (LOD) were reported before and after the laboratory change for each species. Water-soluble concentrations of metals, including water-soluble vanadium (WS V), water-soluble chromium (WS Cr), water-soluble manganese (WS Mn), water-soluble iron (WS Fe), water-soluble nickel (WS Ni), and water-soluble copper (WS Cu), were analyzed using inductively coupled plasma optical emission spectrometry (ICP-OES) during 14 August 1998–6 April 2008. Starting on 7 April 2008, these water-soluble fractions were analyzed using inductively coupled plasma mass spectrometry (ICP-MS); again, different LODs were reported before and after the analytical change for these species. Additional water-soluble species—including water-soluble zinc (WS Zn), water-soluble cadmium (WS Cd), water-soluble lead (WS Pb), water-soluble selenium (WS Se), water-soluble arsenic (WS As), water-soluble barium (WS Ba), and water-soluble lanthanum (WS La)—were reported starting on 7 April 2008 from ICP-MS analyses. All water-soluble measures were available daily before 2009 and on one-in-three days after 2009.
The LODs of all metals are listed in Table S1. We calculated the percentage of samples below the LOD over the full time period, and over the time periods before and after measurement/laboratory changes separately. For this analysis, we included metals whose concentrations were above the LOD on at least 85% of days.
Ultimately, six metals (Si, K, Ca, Fe, Zn, WS Fe) were included in the analysis over the full time period (14 August 1998–15 December 2013), along with criteria gases (CO, , , and ), mass, and major components (OC, EC, , and ). We did not include in epidemiologic analyses because this component mainly exists as or Fifteen additional metals were included in the analysis over the later time period (7 April 2008–15 December 2013): Al, Na, Cu, Ti, WS Cr, WS Cu, WS Mn, WS Ni, WS V, WS As, WS Ba, WS Se, WS Zn, WS Cd, and WS Pb. For species included in the analysis, any observations below the LOD were assigned a value of the LOD divided by 2.
Emergency Department Visits
We obtained daily counts of cardiovascular ED visits for patients living within the five-county Atlanta area (Clayton, Cobb, DeKalb, Fulton, and Gwinnett) during 14 August 1998–15 December 2013. Daily ED visit counts were aggregated from individual-level billing records from metropolitan Atlanta hospitals as part of SOPHIA (Metzger et al. 2004; Winquist et al. 2016). We identified cardiovascular ED visits as those billing records with primary International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes for ischemic heart disease (ICD-9 410–414), cardiac dysrhythmias (ICD-9 427), congestive heart failure (ICD-9 428), or peripheral vascular and cerebrovascular disease (ICD-9 433–437, 440, 443–445, 451–453).
Analytic Approach
In a time-series framework, we estimated the associations between daily levels of air pollutants and daily counts of cardiovascular ED visits using Poisson regression accounting for over-dispersion. Based on our previous research of ambient air pollution and cardiovascular ED visits in Atlanta, we used the same-day (lag 0) pollution level (Metzger et al. 2004; Sarnat et al. 2013; Tolbert et al. 2000; Ye et al. 2017).
All models included the same covariate control for temporal trends and meteorology: time splines with monthly knots, cubic function of same-day maximum temperature, cubic function of lag 1-2–d moving average minimum temperature, cubic function of lag 0-1-2–d moving average mean dew point temperature, day of week, indicators for holidays, seasons, season–maximum temperature interaction, season–day of week interaction, indicators for hospital participation periods, and indicator for changes in air pollution measurement. The estimated associations were reported as rate ratios (RR) with 95% confidence intervals (CI) per interquartile range (IQR) increase in pollutant concentrations.
Primary Analysis
We included criteria gases (CO, , , and ), mass, major components (OC, EC, , and ), and metals (Si, K, Ca, Fe, Zn, WS Fe) in the analysis over the full time period (14 August 1998–15 December 2013). Fifteen additional metals (Al, Na, Cu, Ti, WS Cr, WS Cu, WS Mn, WS Ni, WS V, WS As, WS Ba, WS Se, WS Zn, WS Cd, and WS Pb) were included in the analysis over the later time period (7 April 2008–15 December 2013).
We first estimated the associations between these pollutants and cardiovascular ED visits using single-pollutant models. Based on the results, we applied multipollutant models to assess copollutant confounding. Because previous studies have reported differing effects of particulate matter on cardiovascular outcomes in cold versus warm days (Ito et al. 2011; Lippmann et al. 2013), we performed analyses in the warm and cold seasons separately for pollutants available over the full time period to see if the patterns of associations across pollutants were similar. We defined the warm season as May to October and the cold season as November to April in Atlanta.
For comparability, we restricted the analyses in each time period to days on which all pollutants were available. Thus, over the full time period, year-round analyses included 3,303 d; warm-season analyses included 1,737 d; and cold-season analyses included 1,566 d. Over the later time period, year-round analyses included 628 d.
Sensitivity Analyses
We evaluated model misspecification by estimating the associations between tomorrow’s pollutant levels and today’s ED visits, controlling for today’s (lag 0) pollutant and covariate levels. Tomorrow’s pollutant levels should not be associated with today’s ED visits in the absence of confounding, measurement error, or other model misspecification, because cause must precede effect (Flanders et al. 2011). To accommodate pollutants with one-in-three–day measurements, we defined “tomorrow” as the third day after today ().
We restricted the primary analysis to days on which all pollutants were available so that the health associations of different pollutants were estimated on the same set of days ( for the 1998–2013 year-round analysis; for the 1998–2013 year-round analysis). However, this led to reduced statistical power. As a sensitivity analysis, we performed the same set of analyses without this restriction by using all available days to see if the estimated associations were similar to those in the primary analysis.
Results
We calculated descriptive statistics of the pollutants over all seasons (Table 1), in the warm season (see Table S2a), and in the cold season (see Table S2b). OC, EC, , , and together contributed about 80% of the mass, whereas the concentrations of metals were much lower. Among metals, Si and Fe were most abundant. Water-soluble Fe had the highest average concentration among water-soluble species [as commonly seen in other studies (Allen et al. 2001; Birmili et al. 2006; Duan et al. 2014; Fang et al. 2015a; Lough et al. 2005)]. Secondary pollutants such as and had higher concentrations in the warm than in the cold season, whereas primary pollutant such as CO had higher concentrations in the cold than in the warm season. The concentrations of metals were generally similar in the warm and cold seasons, whereas water-soluble Fe was higher in the warm than in the cold season.
Table 1.
Summary statistics of ambient air pollutants measured at the Atlanta Jefferson Street monitoring site.
| Pollutants | Unit | 50th (25th, 75th) percentiles | Interquartile range | |||||
|---|---|---|---|---|---|---|---|---|
| 14 August 1998–15 December 2013 | ||||||||
| Criteria gases | ||||||||
| CO | ppm | 5,458 | 0.56 (0.36, 1.02) | 0.66 | ||||
| ppb | 5,321 | 35.9 (26.4, 46.3) | 20.0 | |||||
| ppb | 5,465 | 8.1 (3.2, 18.7) | 15.5 | |||||
| ppb | 5,490 | 39.6 (27.2, 54.9) | 27.7 | |||||
| mass | 5,588 | 12.81 (8.93, 18.21) | 9.28 | |||||
| OC | 5,546 | 3.22 (2.31, 4.47) | 2.16 | |||||
| EC | 5,515 | 0.98 (0.63, 1.58) | 0.95 | |||||
| 5,563 | 1.10 (0.72, 1.73) | 1.01 | ||||||
| 5,569 | 0.55 (0.31, 1.06) | 0.75 | ||||||
| 5,572 | 2.94 (1.88, 4.87) | 2.99 | ||||||
| Si | 4,932 | 68.16 (39.78, 110.79) | 71.01 | |||||
| K | 4,932 | 50.80 (35.28, 75.54) | 40.26 | |||||
| Ca | 4,932 | 29.32 (18.29, 44.71) | 26.41 | |||||
| Fe | 4,921 | 60.29 (39.64, 95.10) | 55.47 | |||||
| Zn | 4,880 | 8.84 (5.73, 13.31) | 7.58 | |||||
| WS Fe | 4,085 | 18.67 (10.81, 31.28) | 20.46 | |||||
| 7 April 2008–15 December 2013 | ||||||||
| Na | 1,930 | 26.03 (14.66, 47.09) | 32.43 | |||||
| Al | 1,931 | 31.75 (17.37, 56.11) | 38.74 | |||||
| Ti | 1,931 | 3.62 (2.33, 5.36) | 3.03 | |||||
| Cu | 1,916 | 3.78 (2.44, 5.68) | 3.23 | |||||
| WS V | 805 | 0.14 (0.07, 0.26) | 0.19 | |||||
| WS Cr | 805 | 0.10 (0.06, 0.15) | 0.09 | |||||
| WS Mn | 796 | 0.94 (0.57, 1.54) | 0.96 | |||||
| WS Ni | 805 | 0.15 (0.09, 0.25) | 0.16 | |||||
| WS Cu | 790 | 1.84 (1.10, 3.06) | 1.96 | |||||
| WS Zn | 682 | 7.32 (4.69, 11.16) | 6.47 | |||||
| WS As | 805 | 0.56 (0.36, 0.80) | 0.44 | |||||
| WS Se | 805 | 0.55 (0.33, 0.92) | 0.59 | |||||
| WS Cd | 805 | 0.06 (0.04, 0.09) | 0.05 | |||||
| WS Ba | 805 | 2.45 (1.36, 4.10) | 2.74 | |||||
| WS Pb | 803 | 0.87 (0.56, 1.42) | 0.86 | |||||
Note: Criteria gases were measured daily, including 1-h maximum carbon monoxide (CO), 1-h maximum nitrogen dioxide (), 1-h maximum sulfur dioxide (), and 8-h maximum ozone (). and its major components, including organic carbon (OC), elemental carbon (EC), ammonium (), nitrate (), and sulfate (), were measured daily using filter-based 24-h integrated Federal Reference Methods. Total elemental concentrations of metals and metalloids, including titanium (Ti), manganese (Mn), iron (Fe), copper (Cu), zinc (Zn), aluminum (Al), lead (Pb), silicon (Si), calcium (Ca), sodium (Na), and potassium (K), were analyzed from the daily filters using X-ray fluorescence. Water-soluble concentrations of metals, including water-soluble vanadium (WS V), water-soluble chromium (WS Cr), water-soluble manganese (WS Mn), water-soluble iron (WS Fe), water-soluble nickel (WS Ni), and water-soluble copper (WS Cu), were analyzed using inductively coupled plasma optical emission spectrometry (ICP-OES) during 14 August 1998–6 April 2008 and using inductively coupled plasma mass spectrometry (ICP-MS) starting on 7 April 2008. Water-soluble zinc (WS Zn), water-soluble cadmium (WS Cd), water-soluble lead (WS Pb), water-soluble selenium (WS Se), water-soluble arsenic (WS As), water-soluble barium (WS Ba), and water-soluble lanthanum (WS La) were reported starting on 7 April 2008 from ICP-MS analyses. All water-soluble measures were available daily before 2009 and one-in-three days after 2009.
Pearson correlations of the pollutants were also calculated over all seasons (see Table S3), in the warm season (see Table S4a), and in the cold season (see Table S4b). Over all seasons, was most correlated with (), OC (), EC (), and WS Fe (). Water-soluble Fe was most correlated with () and Fe (). OC and EC were highly correlated with one another (), and their correlations with other components were weak to moderate (). was more strongly correlated with and in the warm season, and with EC, OC, and metals in the cold season.
Summary statistics of cardiovascular ED visits are listed in Table 2. Briefly, average daily counts of cardiovascular ED visits were 76 during the full time period and 96 during the later time period. The average daily counts were similar in the warm and cold seasons in Atlanta.
Table 2.
Summary statistics of emergency department visits for cardiovascular diseases.
| Time period | Total visits (n) | Average visits [n (SD)] | Min, max visits (, ) | |
|---|---|---|---|---|
| 14 August 1998–15 December 2013 | Year-round | 426,252 | 76 (22) | (21, 143) |
| Warm season | 210,020 | 74 (21) | (23, 136) | |
| Cold Season | 216,232 | 78 (23) | (21, 143) | |
| 7 April 2008–15 December 2013 | Year-round | 199,343 | 96 (14) | (55, 143) |
| Warm season | 102,793 | 93 (13) | (61, 136) | |
| Cold season | 96,550 | 99 (14) | (55, 143) | |
Note: Daily counts of emergency department visits were aggregated from individual-level billing records from metropolitan Atlanta hospitals for patients living within the five-county Atlanta area (Clayton, Cobb, DeKalb, Fulton, and Gwinnett). We identified emergency department visits for cardiovascular diseases as those billing records with primary International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes for ischemic heart disease (ICD-9 410–414), cardiac dysrhythmias (ICD-9 427), congestive heart failure (ICD-9 428), or peripheral vascular and cerebrovascular disease (ICD-9 433–437, 440, 443–445, 451–453). Warm season includes May to October, and cold season includes November to April. Max, maximum; min, minimum.
Primary Analysis
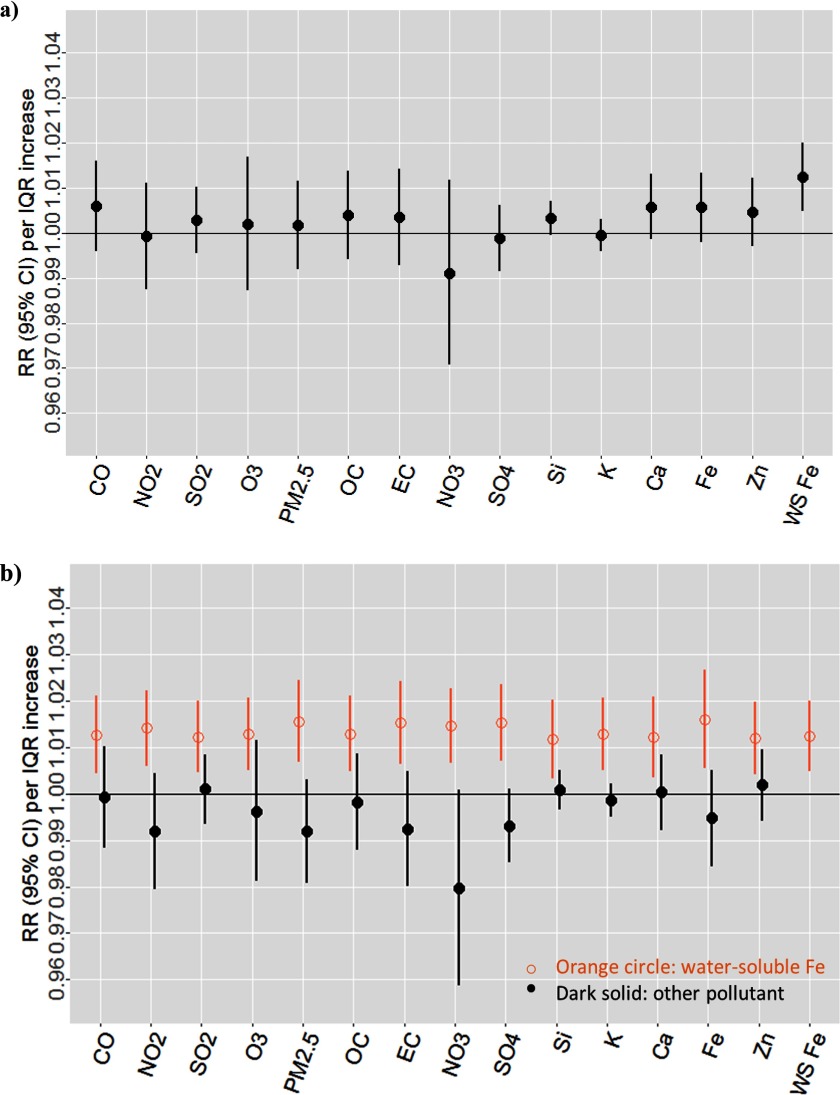
We estimated the associations between cardiovascular ED visits and pollutants available during the full time period using single-pollutant models. The estimated RRs were positive for a number of pollutants, including criteria gases, mass, and components (OC, EC, , Si, Ca, Fe, Zn, water-soluble Fe) (Figure 1). Among them, the estimated RR per IQR increase in water-soluble Fe was the highest [ (95% CI: 1.005, 1.019)].
Figure 1.
Estimated associations between cardiovascular emergency department visits and pollutants available during 1998–2013, year-round analysis (3,303 d), Atlanta, Georgia. Results from single-pollutant models (a); results from two-pollutant models: water-soluble Fe controlling for each of the other pollutants (b). Note: Ca, calcium; CO, carbon monoxide; EC, elemental carbon; Fe, iron; IQR, interquartile range; K, potassium; , nitrogen dioxide; , nitrate; , ozone; OC, organic carbon; , particulate matter with aerodynamic diameter ; RR, rate ratio; Si, silicon; , sulfur dioxide; , sulfate; WS, water soluble; Zn, zinc.
To assess whether the association for water-soluble Fe was confounded by other pollutants, we estimated the associations between cardiovascular ED visits and water-soluble Fe controlling for each of the other measured pollutants in two-pollutant models. The associations for water-soluble Fe changed little when controlling for any of the pollutants. In contrast, the associations for mass and components (OC, EC, , Si, Ca, Fe, Zn) were weaker and consistent with the null when controlling for water-soluble Fe (Figure 1).
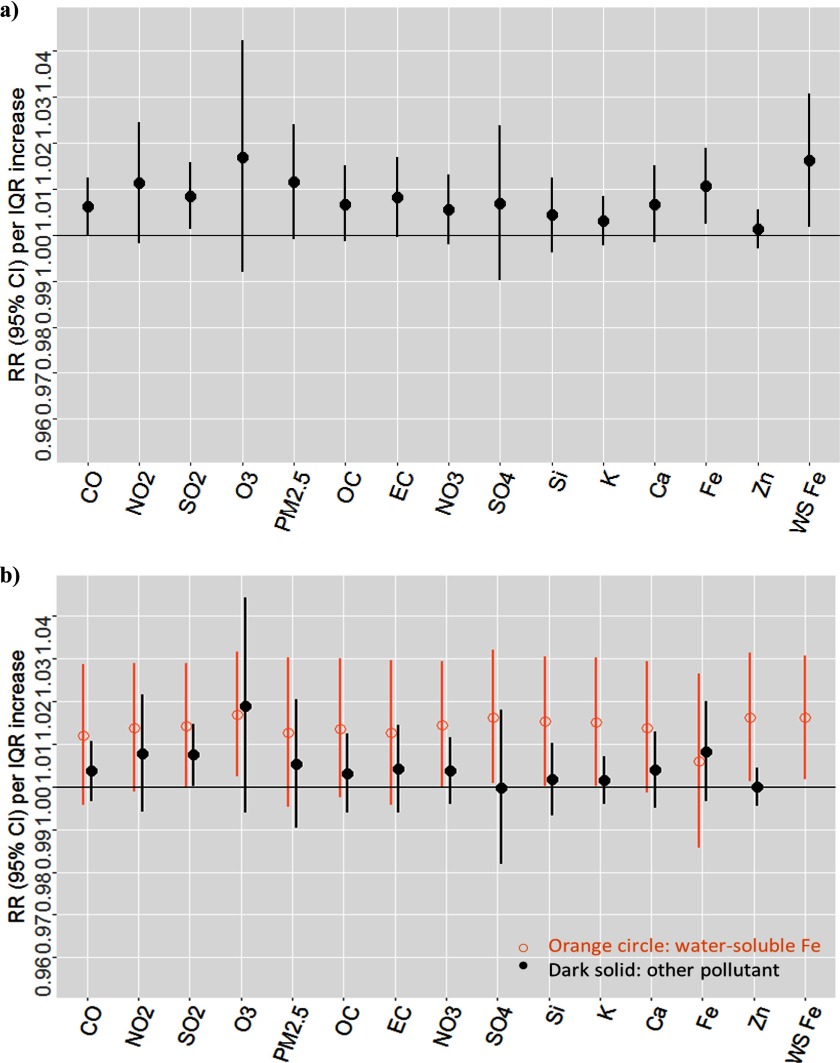
We performed analyses in the warm (May–October) and cold (November–April) seasons separately to see if the patterns of associations were similar. In the warm season, the estimated RR per IQR increase in water-soluble Fe was the highest. The associations for and a number of components (OC, EC, , K) were consistent with the null (Figure 2). Although the estimated RRs for CO, Si, Ca, Fe, and Zn were positive in single-pollutant models, they were lower in two-pollutant models with water-soluble Fe (Figure 2).
Figure 2.
Estimated associations between cardiovascular emergency department visits and pollutants available during 1998–2013, warm-season analysis (1,737 d). Results from single-pollutant models (a); results from two-pollutant models: water-soluble Fe controlling for each of the other pollutants (b). Note: Ca, calcium; CO, carbon monoxide; EC, elemental carbon; Fe, iron; IQR, interquartile range; K, potassium; , nitrogen dioxide; , nitrate; , ozone; OC, organic carbon; , particulate matter with aerodynamic diameter ; RR, rate ratio; Si, silicon; , sulfur dioxide; , sulfate; WS, water soluble; Zn, zinc.
In the cold season, the estimated associations across pollutants were generally higher than those in the warm season (Figures 2 and 3). Among components, the estimated RR for water-soluble Fe was still the highest (Figure 3). The associations for CO, , OC, EC, , , Si, K, and Ca were weaker and consistent with the null when controlling for water-soluble Fe. The association for water-soluble Fe was weaker in two-pollutant models with Fe (Figure 3).
Figure 3.
Estimated associations between cardiovascular emergency department visits and pollutants available during 1998–2013, cold-season analysis (1,566 d). Results from single-pollutant models (a); results from two-pollutant models: water-soluble Fe controlling for each of the other pollutants (b). Note: Ca, calcium; CO, carbon monoxide; EC, elemental carbon; Fe, iron; IQR, interquartile range; K, potassium; , nitrogen dioxide; , nitrate; , ozone; OC, organic carbon; , particulate matter with aerodynamic diameter ; RR, rate ratio; Si, silicon; , sulfur dioxide; , sulfate; WS, water soluble; Zn, zinc.
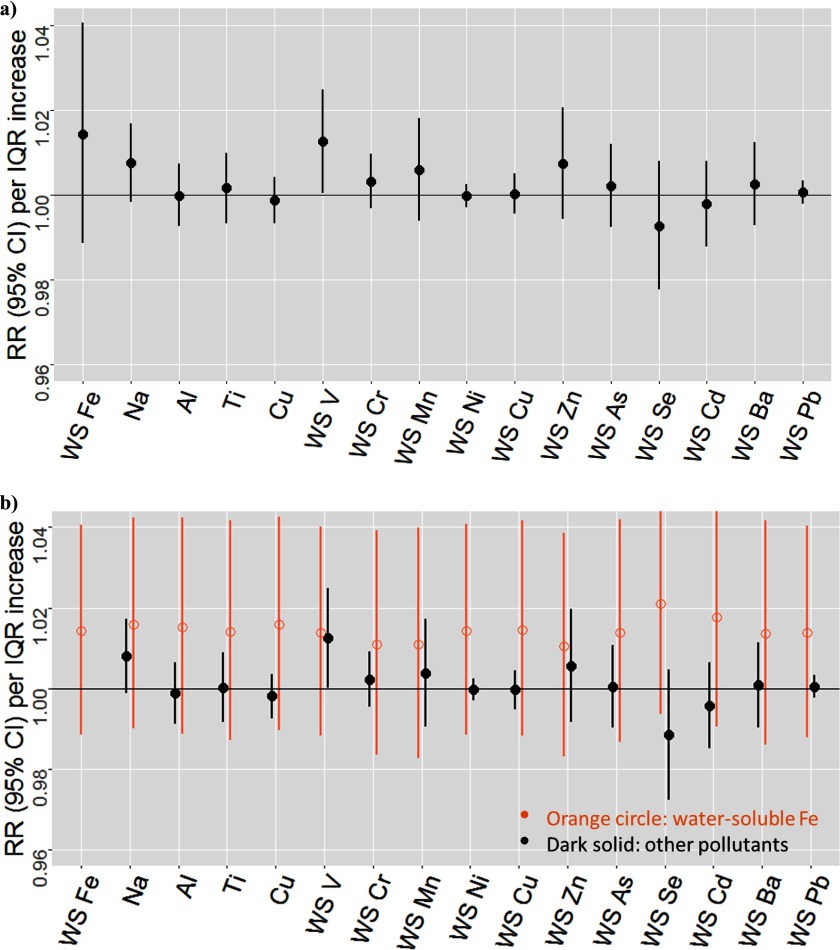
Measurements of an additional 15 metals were only available during the later time period. We estimated their associations with cardiovascular ED visits using single-pollutant models. The estimated RRs were the highest for water-soluble V [ (95% CI: 1.000, 1.025)] and Na [ (95% CI: 0.998, 1.017)]. We also estimated the association for water-soluble Fe in this later time period. The estimated RR (95% CI) for water-soluble Fe was 1.014 (0.988, 1.041) in the single-pollutant model, which was similar to that during the full time period, and the estimated RR had little change when controlling for any of the other metals in two-pollutant models (Figure 4).
Figure 4.
Estimated associations between cardiovascular emergency department visits and pollutants only available during 2008–2013 and water-soluble Fe during 2008–2013, year-round analysis (628 d). Results from single-pollutant models (a); results from two-pollutant models: water-soluble Fe controlling for each of the other pollutants (b). Note: Al, aluminum; Cu, copper; IQR, interquartile range; Na, water-soluble sodium; RR, rate ratio; Ti, titanium; WS, water soluble; WS As, water-soluble arsenic; WS Ba, water-soluble barium; WS Cd, water-soluble cadmium; WS Cr, water-soluble chromium; WS Cu, water-soluble copper; WS Fe, water-soluble iron; WS Mn, water-soluble manganese; WS Pb, water-soluble lead; WS V, water-soluble vanadium; WS Zn, water-soluble zinc.
Sensitivity Analyses
For single-pollutant models in the year-round analysis, we evaluated model misspecification by estimating the associations between tomorrow’s pollutant levels and today’s ED visits, controlling for today’s pollutant and covariate levels. We found associations between cardiovascular ED visits and tomorrow’s levels of WS Ni and WS Mn, suggesting possible model misspecification when estimating these associations (see Figures S1 and S2). All other associations with tomorrow’s pollutant levels were consistent with the null, as expected under a well-specified model.
We restricted the primary analysis to days on which all pollutants were available. However, this led to reduced statistical power. We performed the same set of analyses without this restriction as a sensitivity analysis. We observed patterns of associations similar to those in the primary analysis, except that the association for in the cold season was more positive and the association for was more negative in this sensitivity analysis than in the primary analysis (see Figures S3–S6).
Discussion
In this study, we estimated acute cardiovascular effects of and its components, including a suite of water-soluble metals that are not routinely measured at the ambient level. We performed two-pollutant analysis to account for copollutant confounding, and compared the patterns of associations across pollutants in the warm and cold seasons.
Among the components we examined during the full time period (1998–2013), water-soluble Fe had the strongest estimated effect in both the warm and cold seasons. The associations for and other components were generally weak and consistent with the null when controlling for water-soluble Fe. Among components that were only measured during the later time period (2008–2013), water-soluble V was associated with cardiovascular ED visits.
Oxidative stress has been suggested as a central mechanism by which particulate matter affect health (Ghio et al. 2012). Transition metals can generate reactive oxygen species (ROS) in living systems, leading to oxidative stress (Ghio et al. 2012; Stohs and Bagchi 1995). Redox-active transition metals—such as Fe, Cu, Mn, and V—can act as catalysts of Fenton or Fenton-like reactions, facilitating the conversion of superoxide anion and hydrogen peroxide to hydroxyl radical (Chevion 1988; Stohs and Bagchi 1995). Because particle-bound metals need to dissolve and become metal ions to participate in these reactions, the water-soluble fractions of metals are thought to be more biologically relevant than total metals (Birmili et al. 2006; Urch et al. 2004). Recent studies have used cellular and cell-free assays to measure the oxidative potential of ambient particulate matter and have suggested that water-soluble metals—especially water-soluble Fe, water-soluble Cu, and water-soluble Mn—contribute to the ROS generation of particulate matter (Abrams et al. 2017; Cheung et al. 2012; Fang et al. 2015b; Landreman et al. 2008; Shen and Anastasio 2011; Verma et al. 2010). In our analysis, however, we observed positive associations with water-soluble Fe, but not with water-soluble Cu or water-soluble Mn. One reason could be that these species are less abundant than water-soluble Fe in the ambient air and thus could be more subject to measurement error, resulting in more underestimated health associations.
The observed associations with water-soluble Fe could indicate cardiovascular effects of certain pollution mixtures. Metals are released to the atmosphere from various sources, including natural processes acting on crustal minerals, resuspension of road dust and brake/tire wear abrasion during traffic, combustion of fossil fuels and wood, industrial processes, and waste incineration (Allen et al. 2001; Birmili et al. 2006; Duan et al. 2014; Fang et al. 2015a; Grigoratos and Martini 2015; Ito et al. 2004; Lin et al. 2015; Seinfeld 2006). Crustal species such as silicon, iron, calcium, sodium, aluminum, and potassium are largely found in the resuspension of road dust; meanwhile, copper, barium, manganese, iron, zinc, and chromium are commonly related to brake/tire wear debris; Nickel and vanadium are often attributed to residual oil combustion (Allen et al. 2001; Birmili et al. 2006; Duan et al. 2014; Fang et al. 2015a; Grigoratos and Martini 2015; Ito et al. 2004; Lin et al. 2015; Seinfeld 2006). The water-soluble fractions of these metals are partly from direct emission and partly from secondary processing of the primary insoluble metals by acid dissolution. A recent study in Atlanta investigated source contributions of a suite of water-soluble metals (Fang et al. 2015a). Roadway emissions, such as brake/tire wear debris and the resuspension of road dust followed by secondary processing by acid, were suggested as major contributors of a number of water-soluble metals, including water-soluble Fe, water-soluble Cu, water-soluble Mn, and water-soluble Zn. For water-soluble Fe, source apportionment attributed over 30% to mechanical abrasion of automobile brakes/tires and another 50% to secondary processing of Fe by acid (Fang et al. 2015a). Acid dissolution has been suggested as a major source of water-soluble Fe and other transition-metal ions in recent studies. Size distributions of soluble metals and particle pH have shown that sulfate plays a key role in producing highly acidic fine particles that are capable of dissolving primary transition metals (Fang et al. 2017). Single-particle analysis has shown that the majority of water-soluble Fe in Atlanta is in the form of iron sulfate (Longo et al. 2016; Oakes et al. 2012). We also found in this study that water-soluble Fe is mostly correlated with total Fe () and (). These observations are consistent with the proposed mechanism of metal dissolution by acidic sulfate. The association we observed with water-soluble Fe points to certain aspects of roadway emission, when processed by acidic sulfate, as a mixture harmful for cardiovascular health. In our analysis, however, associations with other roadway-related metal species, such as water-soluble Cu, water-soluble Mn, water-soluble Zn, and water-soluble Ba, were consistent with the null. Again, these species are less abundant than water-soluble Fe in the ambient air and thus could be more subject to measurement error, resulting in more underestimated health associations.
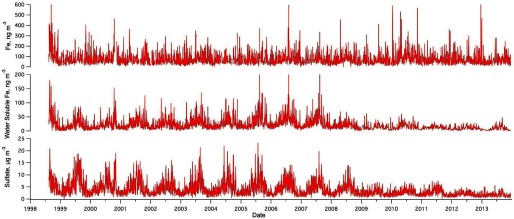
The co-influence of the two sources on the levels of water-soluble Fe—primary roadway emission and secondary processing of the roadway emission by acidic sulfate—is reflected in the temporal trends of total Fe, water-soluble Fe, and sulfate in our study (Figure 5). Fe (i.e., total Fe) has no discernable seasonal or long-term trend. In contrast, the temporal trend of water-soluble Fe follows that of the sulfate: sulfate has a distinct seasonal trend with peaks in summer and a long-term decreasing trend potentially due to controls on coal-fired electrical generating units as well as their replacements (i.e., natural gas-fired units) (de Gouw et al. 2014). These observed trends illustrate how complex interactions between differing pollutant sources could affect the levels of potentially harmful components in and a co-benefit of reduction.
Figure 5.
Daily concentrations of Fe, water-soluble Fe, and sulfate measured at the Atlanta Jefferson Street ambient monitor, 14 August 1998–15 December 2013.
Note: Fe (i.e., total Fe) was analyzed from daily filters using X-ray fluorescence. Water-soluble Fe was analyzed using ICP-OES during 14 August 1998–6 April 2008 and using ICP-MS starting on 7 April 2008. Measurements of water-soluble Fe was daily before 2009 and one-in-three days after 2009. Sulfate was measured daily using filter-based 24-h integrated Federal Reference Methods. Fe, iron; ICP-MS, inductively coupled plasma mass spectrometry; ICP-OES, inductively coupled plasma optical emission spectrometry; , particulate matter with aerodynamic diameter .
Fe (i.e., total Fe) and water-soluble Fe were both included in our analysis over the full time period, and their associations with cardiovascular ED visits were similar in single-pollutant models. In the warm season, the association with total Fe was consistent with the null when controlling for water-soluble Fe, suggesting that the water-soluble fraction was driving the association of Fe. This is expected if iron is a causal agent and its water-soluble fraction is more biologically accessible. However, we did not observe this pattern of associations in the cold season. One reason could be that the concentrations of water-soluble Fe in the cold seasons were much lower than in the warm seasons (see Table S2), and thus could be more subject to measurement error compared with total Fe, whose concentrations were similar in cold and warm seasons.
In fact, in the cold season, other components, such as EC and OC had stronger associations with cardiovascular ED visits than in the warm season. Although the associations for EC and OC were weaker when controlling for water-soluble Fe, the association of water-soluble Fe was also slightly weaker in two-pollutant models with these pollutants. EC and OC are partly from tailpipe emissions, and together with roadway-related species such as total Fe and water-soluble Fe, these pollutants may all contribute to cardiovascular effects of traffic pollution in the cold season.
Epidemiologic evidence on cardiovascular effects of water-soluble metals is sparse. In a time-series study in Edinburgh, Scotland, Heal et al. (2009) estimated the associations between cardiovascular hospital admissions and a number of total and water-soluble metals, including Cu, Fe, Ni, V, and Zn. However, direct measurements of these species were only available for 1 y, during which they did not find significant associations with total or water-soluble metals, nor with mass. Huang et al. (2003) exposed a panel of 38 healthy adults to concentrated ambient particles (CAP) from Chapel Hill, North Carolina, and reported that water-soluble metals in CAP (the V/Cu/Zn factor by principal component analysis) was associated with increased blood fibrinogen levels.
A number of studies have provided general evidence for acute cardiovascular effects of metals, although they only considered total elemental concentrations, not water-soluble fractions of metals (Basagaña et al. 2015; Bell et al. 2014; Bilenko et al. 2015; Huang et al. 2003; Ito et al. 2011; Lippmann et al. 2013; Morishita et al. 2015; Ostro et al. 2007; Suh et al. 2011; Urch et al. 2004; Zhang et al. 2016; Zhou et al. 2011). Suh et al. (2011) combined Cu, Mn, Zn, Ti, and Fe in a transition-metal category and reported positive associations with cardiovascular hospital admissions in a time-series study in Atlanta. In a time-series study in New York City, Ito et al. (2011) reported positive associations between cardiovascular hospital admissions and a number of components (OC, EC, , Ni, V, Zn, Se, Br). Similarly in a time-series study of 64 U.S. counties, Lippmann et al. (2013) found positive associations between cardiovascular hospital admissions and OC, EC, , Fe, V, and Zn. Zhang et al. (2016) reported that short-term exposures to transition metals (Cr, Fe, Cu, Mn, and Ni) in the ambient air were associated with decreased microvascular function in a panel of adults in Los Angeles, California. Morishita et al. (2015) found that a number of metals (As, Ca, Ce, Fe, Mg, Mn, S, Se, Ti) were associated with heart rate in a panel of adults in Dearborn, Michigan.
Some studies reported stronger associations with carbonaceous components than metals (Bell et al. 2014; Sarnat et al. 2015). In a time-series study in the St. Louis, Missouri–Illinois, area, Sarnat et al. (2015) found positive associations between cardiovascular ED visits and carbonaceous constituents (OC, EC, and certain hopanes), but not with metals (Si, K, Ca, Fe, Cu, Zn, and Pb). Bell et al. (2014), in a time-series study in four New England counties, observed positive associations between cardiovascular hospital admissions and black carbon, Ca, Zn, and V, where the association with black carbon was stronger than with the metals and was robust to copollutant adjustment of these metals. The inconsistencies between our study and these previous studies may be due to a number of factors, including the specific components being examined, copollutant confounding, pollutant interactions, nonlinear dose response, differences in population susceptibility, and measurement error. In particular, these studies considered only total elemental, not water-soluble, metals; besides, given that OC is itself a mixture of organic compounds, its health effects also depend on its composition, which likely varies by study location. In addition, previous studies have suggested synergism between organic compounds and metals in generating reactive oxygen species (Ghio et al. 2012; Li et al. 2009). Health associations of organic pollutants could depend on the levels of metals, and vice versa, which further complicates the comparison of health effects across PM components.
There are several limitations to our study. Our results are subject to spatial misalignment and instrument measurement error, and the degree of these sources of error likely differs by pollutant. Compared with pollutants dominated by secondary origins (e.g., , , , , water-soluble metals), primary pollutants (e.g., EC, Fe, Cu, Zn) are likely more subject to spatial misalignment due to their greater spatiotemporal heterogeneity, and thus their estimated associations may be more biased towards the null. Additionally, pollutants with a lower ambient concentration may be more subject to instrument measurement error, leading to an underestimation of effects.
Conclusions
Our study suggests cardiovascular effects of certain water-soluble metals, particularly water-soluble Fe, which has not been well studied previously. Our findings further elucidate the link between traffic emissions, atmospheric secondary processing, and cardiovascular health, and contribute to the ongoing effort to identify causal mixtures in air pollution. The co-influence of two sources on the levels of water-soluble metals, roadway emission and secondary processing of the roadway emission by acidic sulfate, has implications for pollution control strategies.
Supplemental Material
Acknowledgments
The authors acknowledge the contributions of members of the Southeastern Center for Air Pollution and Epidemiology (SCAPE) research group. This publication is based in part upon information obtained from the Georgia Hospital Association and individual hospitals; we are grateful for the support of all participating hospitals.
Research reported in this publication was supported by funding from the Electric Power Research Institute (EPRI, 10002467). This publication was also made possible by a Clean Air Research Center grant to Emory University and the Georgia Institute of Technology from the U.S. Environmental Protection Agency (EPA, RD834799), as well as by grants to Emory University from the U.S. EPA (R82921301), the National Institute of Environmental Health Sciences (R01ES11294), and the EPRI (EP-P27723/C13172 and EP-P4353/C2124).
The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the U.S. EPA. Further, the U.S. EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
References
- Abrams JY, Weber RJ, Klein M, Sarnat SE, Chang HH, Strickland MJ, et al. 2017. Associations between ambient fine particulate oxidative potential and cardiorespiratory emergency department visits. Environ Health Perspect 125(10):107008, PMID: 29084634, 10.1289/EHP1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AG, Nemitz E, Shi JP, Harrison RM, Greenwood JC. 2001. Size distributions of trace metals in atmospheric aerosols in the United Kingdom. Atmos Environ 35(27):4581–4591, 10.1016/S1352-2310(01)00190-X. [DOI] [Google Scholar]
- Atkinson RW, Mills IC, Walton HA, Anderson HR. 2015. Fine particle components and health—a systematic review and meta-analysis of epidemiological time series studies of daily mortality and hospital admissions. J Expo Sci Environ Epidemiol 25(2):208–214, PMID: 25227730, 10.1038/jes.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basagaña X, Jacquemin B, Karanasiou A, Ostro B, Querol X, Agis D, et al. 2015. Short-term effects of particulate matter constituents on daily hospitalizations and mortality in five South-European cities: results from the MED-PARTICLES project. Environ Int 75:151–158, PMID: 25461424, 10.1016/j.envint.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Leaderer BP, Gent JF, Lee HJ, Koutrakis P, et al. 2014. Associations of PM2.5 constituents and sources with hospital admissions: analysis of four counties in Connecticut and Massachusetts (USA) for persons ≥65 years of age. Environ Health Perspect 122(2):138–144, PMID: 24213019, 10.1289/ehp.1306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Peng RD, Samet JM, Dominici F. 2009. Hospital admissions and chemical composition of fine particle air pollution. Am J Respir Crit Care Med 179(12):1115–1120, PMID: 19299499, 10.1164/rccm.200808-1240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilenko N, Brunekreef B, Beelen R, Eeftens M, de Hoogh K, Hoek G, et al. 2015. Associations between particulate matter composition and childhood blood pressure—the PIAMA study. Environ Int 84:1–6, PMID: 26186643, 10.1016/j.envint.2015.07.010. [DOI] [PubMed] [Google Scholar]
- Birmili W, Allen AG, Bary F, Harrison RM. 2006. Trace metal concentrations and water solubility in size-fractionated atmospheric particles and influence of road traffic. Environ Sci Technol 40(4):1144–1153, PMID: 16572768, 10.1021/es0486925. [DOI] [PubMed] [Google Scholar]
- Brook RD. 2008. Cardiovascular effects of air pollution. Clin Sci 115(6):175–187, PMID: 18691154, 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- Cheung K, Shafer MM, Schauer JJ, Sioutas C. 2012. Diurnal trends in oxidative potential of coarse particulate matter in the Los Angeles Basin and their relation to sources and chemical composition. Environ Sci Technol 46(7):3779–3787, PMID: 22380575, 10.1021/es204211v. [DOI] [PubMed] [Google Scholar]
- Chevion M. 1988. A site-specific mechanism for free-radical induced biological damage—the essential role of redox-active transition-metals. Free Radical Bio Med 5(1):27–37, PMID: 2060841, 10.1016/0891-5849(88)90059-7. [DOI] [PubMed] [Google Scholar]
- de Gouw JA, Parrish DD, Frost GJ, Trainer M. 2014. Reduced emissions of CO2, NOx, and SO2 from U.S. power plants owing to switch from coal to natural gas with combined cycle technology. Earths Future 2(2):75–82, 10.1002/2013EF000196. [DOI] [Google Scholar]
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. 2006. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 295(10):1127–1134, PMID: 16522832, 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan JC, Tan JH, Hao JM, Chai FH. 2014. Size distribution, characteristics and sources of heavy metals in haze episode in Beijing. J Environ Sci (China) 26(1):189–196, PMID: 24649706. [DOI] [PubMed] [Google Scholar]
- Fang T, Guo H, Verma V, Peltier RE, Weber RJ. 2015a. PM2.5 water-soluble elements in the southeastern United States: automated analytical method development, spatiotemporal distributions, source apportionment, and implications for heath studies. Atmos Chem Phys 15(12):11667–11682, 10.5194/acp-15-11667-2015. [DOI] [Google Scholar]
- Fang T, Guo HY, Zeng LH, Verma V, Nenes A, Weber RJ. 2017. Highly acidic ambient particles, soluble metals, and oxidative potential: a link between sulfate and aerosol toxicity. Environ Sci Technol 51(5):2611–2620, PMID: 28141928, 10.1021/acs.est.6b06151. [DOI] [PubMed] [Google Scholar]
- Fang T, Verma V, Bates JT, Abrams J, Klein M, Strickland MJ, et al. 2015b. Oxidative potential of ambient water-soluble PM2.5 measured by dithiothreitol (DTT) and ascorbic acid (AA) assays in the southeastern United States: contrasts in sources and health associations. Atmos Chem Phys Discuss 15(21):30609–30644, 10.5194/acpd-15-30609-2015. [DOI] [Google Scholar]
- Flanders WD, Klein M, Darrow LA, Strickland MJ, Sarnat SE, Sarnat JA, et al. 2011. A method for detection of residual confounding in time-series and other observational studies. Epidemiology 22(1):59–67, PMID: 21068669, 10.1097/EDE.0b013e3181fdcabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin M, Koutrakis P, Schwartz P. 2008. The role of particle composition on the association between PM2.5 and mortality. Epidemiology 19(5):680–689, PMID: 18714438, 10.1097/EDE.0b013e3181812bb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio AJ, Carraway MS, Madden MC. 2012. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J Toxicol Environ Health B Crit Rev 15(1):1–21, PMID: 22202227, 10.1080/10937404.2012.632359. [DOI] [PubMed] [Google Scholar]
- Grigoratos T, Martini G. 2015. Brake wear particle emissions: a review. Environ Sci Pollut Res Int 22(4):2491–2504, PMID: 25318420, 10.1007/s11356-014-3696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DA, Edgerton E, Hartsell B, Jansen J, Burge H, Koutrakis P, et al. 2006. Air quality measurements for the Aerosol Research and Inhalation Epidemiology Study. J Air Waste Manag Assoc 56(10):1445–1458, PMID: 17063867, 10.1080/10473289.2006.10464549. [DOI] [PubMed] [Google Scholar]
- Heal MR, Elton RA, Hibbs LR, Agius RM, Beverland IJ. 2009. A time-series study of the health effects of water-soluble and total-extractable metal content of airborne particulate matter. Occup Environ Med 66(9):636–638, PMID: 19451142, 10.1136/oem.2008.045310. [DOI] [PubMed] [Google Scholar]
- Heal MR, Hibbs LR, Agius RM, Beverland IJ. 2005. Total and water-soluble trace metal content of urban background PM10, PM2.5 and black smoke in Edinburgh, UK. Atmos Environ 39(8):1417–1430, 10.1016/j.atmosenv.2004.11.026. [DOI] [Google Scholar]
- Huang YC, Ghio AJ, Stonehuerner J, McGee J, Carter JD, Grambow SC, et al. 2003. The role of soluble components in ambient fine particles-induced changes in human lungs and blood. Inhal Toxicol 15(4):327–342, PMID: 12635002, 10.1080/08958370304460. [DOI] [PubMed] [Google Scholar]
- Ito K, Mathes R, Ross Z, Nádas A, Thurston G, Matte T. 2011. Fine particulate matter constituents associated with cardiovascular hospitalizations and mortality in New York City. Environ Health Perspect 119(4):467–473, PMID: 21463978, 10.1289/ehp.1002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Xue N, Thurston G. 2004. Spatial variation of PM2.5 chemical species and source-apportioned mass concentrations in New York City. Atmos Environ 38(31):5269–5282, 10.1016/j.atmosenv.2004.02.063. [DOI] [Google Scholar]
- Kelly FJ, Fussell JC. 2012. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos Environ 60:504–526, 10.1016/j.atmosenv.2012.06.039. [DOI] [Google Scholar]
- Landreman AP, Shafer MM, Hemming JC, Hannigan MP, Schauer JJ. 2008. A macrophage-based method for the assessment of the reactive oxygen species (ROS) activity of atmospheric particulate matter (PM) and application to routine (daily-24 h) aerosol monitoring studies. Aerosol Sci Tech 42(11):946–957, 10.1080/02786820802363819. [DOI] [Google Scholar]
- Levy JI, Diez D, Dou Y, Barr CD, Dominici F. 2012. A meta-analysis and multisite time-series analysis of the differential toxicity of major fine particulate matter constituents. Am J Epidemiol 175(11):1091–1099, PMID: 22510275, 10.1093/aje/kwr457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QF, Wyatt A, Kamens RM. 2009. Oxidant generation and toxicity enhancement of aged-diesel exhaust. Atmos Environ 43(5):1037–1042, 10.1016/j.atmosenv.2008.11.018. [DOI] [Google Scholar]
- Lin YC, Tsai CJ, Wu YC, Zhang R, Chi KH, Huang YT, et al. 2015. Characteristics of trace metals in traffic-derived particles in Hsuehshan Tunnel, Taiwan: size distribution, potential source, and fingerprinting metal ratio. Atmos Chem Phys 15(8):4117–4130, 10.5194/acp-15-4117-2015. [DOI] [Google Scholar]
- Lippmann M. 2014. Toxicological and epidemiological studies of cardiovascular effects of ambient air fine particulate matter (PM2.5) and its chemical components: coherence and public health implications. Crit Rev Toxicol 44(4):299–347, PMID: 24494826, 10.3109/10408444.2013.861796. [DOI] [PubMed] [Google Scholar]
- Lippmann M, Chen LC, Gordon T, Ito K, Thurston GD. 2013. National Particle Component Toxicity (NPACT) Initiative: Integrated Epidemiologic and Toxicologic Studies of the Health Effects of Particulate Matter Components. Research report. Boston, MA:Health Effects Institute, 5–13. [PubMed] [Google Scholar]
- Longo AF, Vine DJ, King LE, Oakes M, Weber RJ, Huey LG, et al. 2016. Composition and oxidation state of sulfur in atmospheric particulate matter. Atmos Chem Phys 16(21):13389–13398, 10.5194/acp-16-13389-2016. [DOI] [Google Scholar]
- Lough GC, Schauer JJ, Park JS, Shafer MM, Deminter JT, Weinstein JP. 2005. Emissions of metals associated with motor vehicle roadways. Environ Sci Technol 39(3):826–836, PMID: 15757346, 10.1021/es048715f. [DOI] [PubMed] [Google Scholar]
- Metzger KB, Tolbert PE, Klein M, Peel JL, Flanders WD, Todd K, et al. 2004. Ambient air pollution and cardiovascular emergency department visits. Epidemiology 15(1):46–56, PMID: 14712146, 10.1097/01.EDE.0000101748.28283.97. [DOI] [PubMed] [Google Scholar]
- Morishita M, Bard RL, Kaciroti N, Fitzner CA, Dvonch T, Harkema JR, et al. 2015. Exploration of the composition and sources of urban fine particulate matter associated with same-day cardiovascular health effects in Dearborn, Michigan. J Expo Sci Environ Epidemiol 25(2):145–152, PMID: 24866265, 10.1038/jes.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes M, Ingall ED, Lai B, Shafer MM, Hays MD, Liu ZG, et al. 2012. Iron solubility related to particle sulfur content in source emission and ambient fine particles. Environ Sci Technol 46(12):6637–6644, PMID: 22621615, 10.1021/es300701c. [DOI] [PubMed] [Google Scholar]
- Ostro B, Broadwin R, Green S, Feng WY, Lipsett M. 2006. Fine particulate air pollution and mortality in nine California counties: results from CALFINE. Environ Health Perspect 114(1):29–33, PMID: 16393654, 10.1289/ehp.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro B, Feng WY, Broadwin R, Green S, Lipsett M. 2007. The effects of components of fine particulate air pollution on mortality in California: results from CALFINE. Environ Health Perspect 115(1):13–19, PMID: 17366813, 10.1289/ehp.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng RD, Bell ML, Geyh AS, McDermott A, Zeger SL, Samet JM, et al. 2009. Emergency admissions for cardiovascular and respiratory diseases and the chemical composition of fine particle air pollution. Environ Health Perspect 117(6):957–963, PMID: 19590690, 10.1289/ehp.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA III, Dockery DW. 2006. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manage 56(6):709–742, 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- Rohr AC, Wyzga RE. 2012. Attributing health effects to individual particulate matter constituents. Atmos Environ 62:130–152, 10.1016/j.atmosenv.2012.07.036. [DOI] [Google Scholar]
- Sarnat JA, Marmur A, Klein M, Kim E, Russell AG, Sarnat SE, et al. 2008. Fine particle sources and cardiorespiratory morbidity: an application of chemical mass balance and factor analytical source-apportionment methods. Environ Health Perspect 116(4):459–466, PMID: 18414627, 10.1289/ehp.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnat SE, Sarnat JA, Mulholland J, Isakov V, Özkaynak H, Chang HH, et al. 2013. Application of alternative spatiotemporal metrics of ambient air pollution exposure in a time-series epidemiological study in Atlanta. J Expos Sci Environ Epidemiol 23(6):593–605, 10.1038/jes.2013.41. [DOI] [PubMed] [Google Scholar]
- Sarnat SE, Winquist A, Schauer JJ, Turner JR, Sarnat JA. 2015. Fine particulate matter components and emergency department visits for cardiovascular and respiratory diseases in the St. Louis, Missouri–Illinois, metropolitan area. Environ Health Perspect 123(5):437–444, PMID: 25575028, 10.1289/ehp.1307776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seinfeld JPS. 2006. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change. Hoboken, NJ:Wiley-Interscience. [Google Scholar]
- Shen H, Anastasio C. 2011. Formation of hydroxyl radical from San Joaquin Valley particles extracted in a cell-free surrogate lung fluid. Atmos Chem Phys 11(18):9671–9682, PMID: 22121357, 10.5194/acp-11-9671-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafoggia M, Samoli E, Alessandrini E, Cadum E, Ostro B, Berti G, et al. 2013. Short-term associations between fine and coarse particulate matter and hospitalizations in Southern Europe: results from the MED-PARTICLES project. Environ Health Perspect 121(9):1026–1033, PMID: 23777832, 10.1289/ehp.1206151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohs SJ, Bagchi D. 1995. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18(2):321–336, PMID: 7744317, 10.1016/0891-5849(94)00159-H. [DOI] [PubMed] [Google Scholar]
- Suh HH, Zanobetti A, Schwartz J, Coull BA. 2011. Chemical properties of air pollutants and cause-specific hospital admissions among the elderly in Atlanta, Georgia. Environ Health Perspect 119(10):1421–1428, PMID: 21708510, 10.1289/ehp.1002646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert PE, Klein M, Metzger KB, Peel J, Flanders WD, Todd K, et al. 2000. Interim results of the study of particulates and health in Atlanta (SOPHIA). J Expo Anal Environ Epidemiol 10(5):446–460, PMID: 11051535, 10.1038/sj.jea.7500106. [DOI] [PubMed] [Google Scholar]
- Urch B, Brook JR, Wasserstein D, Brook RD, Rajagopalan S, Corey P, et al. 2004. Relative contributions of PM2.5 chemical constituents to acute arterial vasoconstriction in humans. Inhal Toxicol 16(6–7):345–352, PMID: 15204750, 10.1080/08958370490439489. [DOI] [PubMed] [Google Scholar]
- Verma V, Shafer MM, Schauer JJ, Sioutas C. 2010. Contribution of transition metals in the reactive oxygen species activity of PM emissions from retrofitted heavy-duty vehicles. Atmos Environ 44(39):5165–5173, 10.1016/j.atmosenv.2010.08.052. [DOI] [Google Scholar]
- Winquist A, Grundstein A, Chang HH, Hess J, Sarnat SE. 2016. Warm season temperatures and emergency department visits in Atlanta, Georgia. Environ Res 147:314–323, PMID: 26922412, 10.1016/j.envres.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye D, Klein M, Chang HH, Sarnat JA, Mulholland JA, Edgerton ES, et al. 2017. Estimating acute cardiorespiratory effects of ambient volatile organic compounds. Epidemiology 28(2):197–206, PMID: 27984424, 10.1097/EDE.0000000000000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, Franklin M, Koutrakis P, Schwartz J. 2009. Fine particulate air pollution and its components in association with cause-specific emergency admissions. Environ Health 8:58, PMID: 20025755, 10.1186/1476-069X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Staimer N, Tjoa T, Gillen DL, Schauer JJ, Shafer MM, et al. 2016. Associations between microvascular function and short-term exposure to traffic-related air pollution and particulate matter oxidative potential. Environ Health 15(1):81, PMID: 27460097, 10.1186/s12940-016-0157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Ito K, Lall R, Lippmann M, Thurston G. 2011. Time-series analysis of mortality effects of fine particulate matter components in Detroit and Seattle. Environ Health Perspect 119(4):461–466, PMID: 21193387, 10.1289/ehp.1002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.