Abstract
Background:
Electronic cigarettes (e-cigarettes) generate an aerosol by heating a solution (e-liquid) with a metallic coil. Whether metals are transferred from the coil to the aerosol is unknown.
Objective:
Our goal was to investigate the transfer of metals from the heating coil to the e-liquid in the e-cigarette tank and the generated aerosol.
Methods:
We sampled 56 e-cigarette devices from daily e-cigarette users and obtained samples from the refilling dispenser, aerosol, and remaining e-liquid in the tank. Aerosol liquid was collected via deposition of aerosol droplets in a series of conical pipette tips. Metals were reported as mass fractions () in liquids and converted to mass concentrations () for aerosols.
Results:
Median metal concentrations () were higher in samples from the aerosol and tank vs. the dispenser (all ): 16.3 and 31.2 vs. 10.9 for Al; 8.38 and 55.4 vs. for Cr; 68.4 and 233 vs. 2.03 for Ni; 14.8 and 40.2 vs. 0.476 for Pb; and 515 and 426 vs. 13.1 for Zn. Mn, Fe, Cu, Sb, and Sn were detectable in most samples. Cd was detected in 0.0, 30.4, and 55.1% of the dispenser, aerosol, and tank samples respectively. Arsenic was detected in 10.7% of dispenser samples (median ) and these concentrations were similar in aerosol and tank samples. Aerosol mass concentrations () for the detected metals spanned several orders of magnitude and exceeded current health-based limits in close to 50% or more of the samples for Cr, Mn, Ni, and Pb.
Conclusions:
Our findings indicate that e-cigarettes are a potential source of exposure to toxic metals (Cr, Ni, and Pb), and to metals that are toxic when inhaled (Mn and Zn). Markedly higher concentrations in the aerosol and tank samples versus the dispenser demonstrate that coil contact induced e-liquid contamination. https://doi.org/10.1289/EHP2175
Introduction
The use of electronic cigarettes (e-cigarettes) is increasing despite uncertainties about their toxicity and health effects (Giovenco et al. 2015; McCarthy 2015; Schoenborn and Gindi 2015; McQueen et al. 2015; Orr and Asal 2014; Ambrose et al. 2014). e-Cigarettes generate nicotine and non-nicotine containing aerosols by resistance heating a solution (e-liquid) through a metallic coil (Williams et al. 2013; Fuoco et al. 2014). Commonly used coils include Kanthal, made of iron, chromium, and aluminum, and Nichrome, made of nickel and chromium (Farsalinos et al. 2015). Other metals such as tin are used in the joints (Williams et al. 2015). A few studies have detected toxic metals such as chromium, nickel, and lead in e-liquid and in the aerosol produced by e-cigarettes (Williams et al. 2013; Saffari et al. 2014; Goniewicz et al. 2014; Hess et al., 2017). Concern for metal exposure is derived from the serious health effects of metals, including neurotoxicity (Garza et al. 2006) and cardiovascular disease (Navas-Acien et al. 2007) for lead, and respiratory disease and lung cancer for chromium (chromium VI) and nickel (IARC 2012a, 2012b; Jaishankar et al. 2014).
Studies on metals in e-cigarettes have focused on cigalikes (Hess et al., 2017; Mikheev et al. 2016; Williams et al. 2013), which are first generation devices with the shape of conventional tobacco cigarettes. These cigalikes contain a disposable cartomizer that contains the coil and comes preloaded with e-liquid. Daily e-cigarette users, however, often utilize reusable modified devices, known as mods or tank-style devices, which come with a box or cylindrical-shaped battery and a mouthpiece with a tank to refill the e-liquid from a bottle dispenser (Cooper et al. 2016). Tank-style devices are highly diverse in voltage and coil composition, as they can be assembled and manipulated by the user. Direct sampling from e-cigarette consumers rather than purchasing e-cigarettes from a store or company is thus needed to assess typically used devices. Previous research is also lacking in comparisons between metal concentrations in e-liquid from the refilling dispenser (before contact with the device and the heating coil), e-liquid in the device itself (in contact with the heating coil), and the generated aerosol (inhaled by the user).
The goal of this study was to evaluate the potential contribution of the heating coil to metal exposure in e-cigarette users by analyzing a 15-metal panel in samples from different types of tank-style e-cigarettes collected from daily e-cigarette consumers from Maryland. The samples included e-liquid from the refilling dispenser, the tank (after the device was used), and the generated aerosol. We hypothesized higher metal concentrations in samples that have been in contact with the heating coil (aerosol and tank) compared with samples that have never been in contact with the coil (refilling dispenser). We also compared metal concentrations by the type of coil, device voltage, and frequency of coil change, as reported by the user.
Methods
Study Population and Data Collection
We sampled tank-style devices from daily e-cigarette users who were recruited as part of a study to evaluate e-cigarette use in Maryland (Aherrera et al. 2017). The study recruited 58 participants using tank-style devices through vaping conventions and flyers posted in e-cigarette shops. Participants were instructed to bring their regular e-cigarette device and refilling dispenser on the day of the interview. One participant not bringing the e-cigarette device and another not bringing the refilling dispenser were excluded from the analyses, leaving 56 participants for this study. The study was approved by the institutional review board of the Johns Hopkins Bloomberg School of Public Health. All participants provided informed consent.
Trained field workers administered a standardized questionnaire recording information on e-cigarette brand, voltage used (estimated in volts), type of coil (self-reported by the participants and categorized as Kanthal, other/combination, or unknown), and frequency of coil change (self-reported by the participant and categorized as and times per month). For each participant, we collected three types of samples from their device and dispenser. First, we pipetted a minimum of of the refilling e-cigarette liquid (no contact with the coil) directly from the dispenser into a centrifuge tube. Second, we collected of the aerosol generated by the e-cigarette device using the methodology described in Olmedo et al. (2016). Briefly, a peristaltic pump placed inside a fume hood puffed the e-cigarette and the generated aerosol was collected in a centrifuge tube via deposition in a series of conical pipette tips and plastic tubing (, 4 s per puff and 30-s interpuff time). Based on these parameters, the mean puff volume of e-cigarettes in our study was . The collected aerosol sample was then ready for analysis using methods similar to refilling liquid from the dispenser, allowing a direct comparison between both samples. Third, a minimum of of the e-liquid remaining in the mouthpiece tank after puffing the e-cigarette with the peristaltic pump was pipetted into a third centrifuge tube. We could not obtain a sample from the tanks of seven devices, leaving 49 samples for those analyses. All samples were stored at room temperature.
Metal Analyses
All e-liquid samples were shipped to the Institute of Chemistry, University of Graz (Graz, Austria) for metal analyses. External calibrations in the range of were prepared in ultrapure water (; Milli-Q, Merck Millipore; Merck KGaA, Darmstadt, Germany) from aluminum (Al), antimony (Sb), arsenic (As), cadmium (Cd), chromium (Cr), copper (Cu), iron (Fe), lead (Pb), manganese (Mn), nickel (Ni), tin (Sn), titanium (Ti), tungsten (W), uranium (U), and zinc (Zn) single-element standards [CertiPUR® single-element standard solutions for inductively coupled plasma–mass spectrometry (ICP-MS); Merck KGaA, Darmstadt, Germany]. An aliquot of each sample (typically depending on the available total amount) was diluted with ultrapure water. A solution of propylene glycol (High purity grade, Amresco; Solon, OH) and glycerol (Ultra pure; ICN Biochemicals, Aurora, OH) (70% propylene glycol, 30% glycerol) was analyzed () as blank e-liquid to study possible matrix effects. Three blank e-liquid samples were also passed through the conical pipette tips and plastic tubing using the peristaltic pump in the lab to account for potential background air contamination as well as contamination within the sampling device (aerosol blanks). Metal levels in e-liquid and aerosol blanks were in general under or close to the limits of detection (LODs), and the median concentrations are shown in Table S1. The median of the three aerosol blanks was used to correct aerosol samples, whereas the median of the six e-liquid blanks was used to correct the dispenser and tank samples.
The multielement measurements were performed on an Agilent 8800 triple quadrupole ICP-MS (ICPQQQMS) (Agilent Technologies, Santa Clara, CA). The instrument was equipped with a micro-mist nebulizer (Glass Expansion, Melbourne, Australia), a Scott double pass spray chamber, a internal diameter quartz torch, a sampler cone made from copper with a nickel tip and a skimmer cone made from nickel. The instrument was tuned for suitable sensitivity and robustness with cerium (Ce) oxide ratios () and doubly charged ions () in no-gas mode. Oxide ratios and doubly charged ratios were lower in collision mode respectively. Different tune modes were used for the quantification of the different elements. Both in no-gas mode and in helium (He) mode ( He), the ICPQQQMS was operated in single-quadrupole mode.
Quality Assurance.
To ensure accuracy of the results, we used an internal standard and a reference standard. The multielement internal standard consisted of a solution containing of each of the following: beryllium (Be), germanium (Ge), indium (In), and lutetium (Lu) and was added online to the samples prior to the nebulizer of the ICP-MS via a T-piece to compensate for instrumental instabilities and possible matrix effects. The solutions were prepared either in or polypropylene (PP) flasks (Cellstar®; Greiner Bio-One GmbH, Kremsmünster, Austria). In addition to the use of an internal standard, we reanalyzed a reference standard [Reference Material SRM 1640a; NIST SRM® 1640a—Trace Elements in Natural Water; National Institute of Standards and Technology (NIST), Gaithersburg, MD] and two blanks after every 30 samples. All elements of the reference standard were found within 5% of the NIST-certified concentrations. Altogether we analyzed the standard 12 times, with a mean recovery of 98% standard deviation, suggesting a very stable measurement. There was not enough sample volume left for replicate analysis; nevertheless, our quality assurance procedures insured accuracy of the results based on the NIST results. In a previous study (Hess et al., 2017), we conducted an interlaboratory comparison of metal concentrations in e-liquid samples between the laboratory in Austria and the Trace Metal Laboratory at Johns Hopkins University and found high comparability between laboratories (intraclass correlation coefficient for all metals of 0.99 or higher).
We reported metal concentrations in a weight/weight basis [micrograms per kilogram ()] due to the difficulty of measuring volumes of thick and sticky e-liquid samples. LODs in were 5.0 for Al, 1.0 for As, 0.1 for Cd, 0.5 for Cr, 1.0 for Cu, 5.0 for Fe, 1.0 for Mn, 1.0 for Ni, 0.2 for Pb, 0.1 for Sb, 0.1 for Sn, 5.0 for Ti, 0.1 for U, 0.1 for W, and 1.0 for Zn. Concentrations under the LOD were replaced with the LOD divided by the square root of 2 for analysis.
For comparison with aerosol standards and health-based exposure limits, the collected aerosol was assumed to be equivalent to daily consumption, and metal concentrations assumed to represent daily values. Concentrations were converted from the mass fraction () of metal i in the collected liquid as reported by the lab into an air concentration () using Equation 1.
| [1] |
where is the total weight of the sample collected (mg), and is the volume of air required to obtain each sample (). is calculated by multiplying the puffing flow rate Q () times the puffing duration t (4 s/puff) and the number of puffs required to collect the desired volume of aerosol (between 30 and 50 puffs). This number of puffs is an underestimation of a daily average based on our own self-reported data, and others (Aherrera et al. 2017; Robinson et al. 2015). This topography was used to derive a conversion factor of to convert from to mg/puff.
We report air concentrations for Ni, Cr, Pb, Mn, and As because these metals have at least one inhalation health-based limit. We compared our Cr air concentrations to more than one health-based limit because limits depend on the form of the compound, which was not determined in our samples, and thus we cannot be sure which applies. We have used the most protective limits found for each metal. Arsenic is not included in our tables because it was found in only 10/56 aerosol samples. Because of the toxicity of As and the fact that there is no clear source or reason for it to be present in e-liquid, we have reported the most relevant As data in the manuscript text. We estimated by weighing the final remaining sample after analyses, adding the mass used for analysis, and subtracting the mean weight of the vial. Maximum propagation of error () was calculated as 30% using Equation 2:
| [2] |
Statistical Analyses
Medians and interquartile ranges (IQRs) were calculated for each sample type. We graphically described metal concentrations using box plots stratified by sample type. We also described the correlation among metals within and between each sample type using Spearman correlation coefficients. To test whether metal concentrations were higher in samples in contact with the heating coil, mean differences of log-transformed metal concentrations in the aerosol and tank samples were compared to that of the corresponding dispenser sample. This was carried out for each metal by using paired t-test and by estimating geometric mean ratios (95% confidence interval), where the mean difference (equivalent to the coefficient) and corresponding 95% CI are both exponentiated. We further compared metal concentrations by device voltage tertiles, coil materials, and coil change frequency using the test of Kruskal-Wallis. We could not compare metal levels by device brand because a total of 20 different brands were reported by the participants, ranging from 1 up to 9 (median 1) participants per brand. We used R (version 3.3.0; R Core Team) to perform the statistical and graphical analysis of the data. The significance level was set at 0.05 and all tests were two-sided.
Results
Metal Detection
Of the 15 elements analyzed, with results included in Table 1, four (As, Ti, U, and W) were excluded from further analyses shown in Tables 2–8 due to low detection in a majority of the samples. As, Ti, and U were detected in less than 20% of all sample types and W was detected in less than 20% of dispenser and aerosol samples. For the other 11 metals, the percentages of samples with detectable metal concentrations ranged from 0.0% for Cd to 92.9% for Zn in the dispenser samples; from 30.4% for Cd to 100% for Sn in the aerosol samples; and from 55.1% for Cd to 100% for Cr, Cu, Fe, Ni, Pb, Sn, and Zn in the tank samples.
Table 1.
Number (percentage) of e-cigarette samples with detectable metal concentrations in each sample type.
| Metal | LOD () | Dispenser () | Aerosol () | Tank () |
|---|---|---|---|---|
| Al | 5.0 | 45 (80.4) | 55 (98.2) | 48 (98.0) |
| As | 1.0 | 6 (10.7) | 10 (17.9) | 6 (12.2) |
| Cd | 0.1 | 0 (0.0) | 17 (30.4) | 27 (55.1) |
| Cr | 0.5 | 26 (46.4) | 36 (64.3) | 49 (100) |
| Cu | 1.0 | 32 (57.1) | 46 (82.1) | 49 (100) |
| Fe | 5.0 | 44 (78.6) | 33 (58.9) | 49 (100) |
| Mn | 1.0 | 30 (53.6) | 36 (64.3) | 48 (98.0) |
| Ni | 1.0 | 31 (55.4) | 48 (85.7) | 49 (100) |
| Pb | 0.2 | 45 (80.4) | 53 (94.6) | 49 (100) |
| Sb | 0.1 | 17 (30.4) | 34 (60.7) | 35 (71.4) |
| Sn | 0.1 | 49 (87.5) | 56 (100) | 49 (100) |
| Ti | 5.0 | 1 (1.8) | 1 (1.8) | 4 (8.2) |
| U | 0.1 | 3 (5.4) | 0 (0.0) | 3 (6.1) |
| W | 0.1 | 4 (7.1) | 8 (14.3) | 21 (42.9) |
| Zn | 1.0 | 52 (92.9) | 53 (94.6) | 49 (100) |
Note: Al, aluminum; As, arsenic; Cd, cadmium; Cr, chromium; Cu, copper; Fe, iron; LOD, limit of detection; Mn, manganese; Ni, nickel; Pb, lead; Sb, antimony; Sn, tin; Ti, titanium; U, uranium; W, tungsten; Zn zinc.
Table 2.
Median (interquartile range) and limit of detection of metal concentrations () in e-cigarette samples from the dispenser (no previous contact with the device), the aerosol, and the tank (in contact with the device).
| Metal | Dispenser () | Aerosol () | Tank () |
|---|---|---|---|
| Al | 10.9 (7.22–20.2) | 16.3 (12.2–22.2) | 31.2 (17.5–128) |
| Cd | (, ) | (, 0.134) | 0.126 (, 0.267) |
| Cr | () | 8.38 () | 55.4 (17.4–217) |
| Cu | 5.14 () | 15.1 (5.70–51.0) | 148 (42.0–543) |
| Fe | 26.9 (9.14–91.3) | 21.7 () | 382 (127–1,360) |
| Mn | 1.09 () | 2.42 () | 31.9 (13.0–93.9) |
| Ni | 2.03 () | 68.4 (6.19–289) | 233 (69.5–675) |
| Pb | 0.476 (0.243–1.05) | 14.8 (3.10–37.1) | 40.2 (13.6–189) |
| Sb | () | 0.553 () | 0.563 () |
| Sn | 1.33 (0.489–3.55) | 5.65 (2.38–19.4) | 20.3 (9.10–72.2) |
| Zn | 13.1 (6.74–23.0) | 515 (228–809) | 426 (152–1,540) |
Note: Metals with detection in at least one sample type. The number next to the symbol < corresponds to the limit of detection for each specific metal. For some samples the median, the 25th percentile and/or the 75th percentile were below the limit of detection. Al, aluminum; Cd, cadmium; Cr, chromium; Cu, copper; Fe, iron; Mn, manganese; Ni, nickel; Pb, lead; Sb, antimony; Sn, tin; Zn zinc.
Table 8.
Median (range) of daily metal concentrations () in collected aerosol samples with regulatory and health-based limits for Ni, Cr, Pb, and Mn.
| Value | Ni | Cr | Pb | Mn |
|---|---|---|---|---|
| Median | ||||
| Range | ||||
| Regulatory or health-based limitsa (Percent exceeding limit [%]) |
b (57) |
c (68) |
d (48) |
e (14) |
|
f (46) |
g (11) |
h (75) |
Note: To convert results in to mg/puff, multiply by . ATSDR, Agency for Toxic Substances and Disease Registry; Cr, chromium; Mn, manganese; MRL, minimum risk level; NAAQS, National Ambient Air Quality Standard; Ni, nickel; Pb, lead; RfC, cancer reference concentration.
aU.S. EPA NAAQS are regulatory, all other limits are health based.
bATSDR MRL for Ni (ATSDR 2005a; U.S. EPA 2000a).
cMRL for Cr(VI) in mists (ATSDR 2012a). MRLs are daily averages.
dU.S. EPA NAAQS (rolling 3-month average) (U.S. EPA 2016).
eMRL for Mn (ATSDR 2012b). MRLs are daily averages.
fMRL for soluble Cr(III) (ATSDR 2012a). MRLs are daily averages.
gU.S. EPA NAAQS for non-attainment areas (U.S. EPA 2016).
hU.S. EPA RfC, daily values (U.S. EPA 2012).
Metal Concentrations
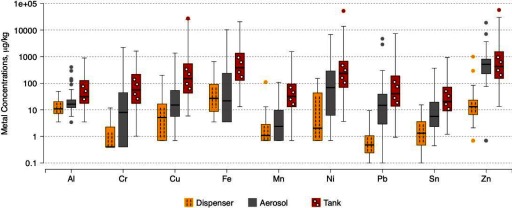
Compared with e-liquid from the dispenser, metal concentrations were higher in aerosol samples, and markedly higher in tank samples for most metals (Figure 1). For Al, Cr, and Ni, metals known to be part of the coil alloys, median concentrations increased from the dispenser sample to the aerosol and tank samples from 10.9 to 16.3, and respectively for Al, from to 8.38, and respectively for Cr, and from 2.03 to 68.4, and respectively for Ni (Table 2). Metals for which the median (interquartile range) concentration increased between the dispenser and aerosol, but was similar between aerosol and tank samples, included Pb [from 0.476 (0.243, 1.05) to 14.8 (3.10, 37.1) and 40.2 (13.6, 189) , respectively] and Zn [from 13.1 (6.74, 23.0) to 515 (228, 809) and 426 (152, 1,540) , respectively]. In contrast, Cu, Mn, Sb, and Sn showed moderate increases in the aerosol samples, but much larger increases in the tank samples compared with dispenser samples. Cd was below the LOD in all dispenser samples and in 70% of aerosol samples, but was detected in 55% of tank samples, with a median value of (, 0.267) . The median (IQR) concentrations among 22 samples with detectable arsenic were 26.7 (12.0–45.6) for the dispenser (), 12.9 (9.33–55.2) for the aerosol (), and 28.5 (12.6–47.6) for the tank samples () (data not shown).
Figure 1.
Boxplots of metal concentrations in e-cigarette dispenser, aerosol, and tank samples. The dispenser sample has not had any contact with the e-cigarette device. The horizontal lines within boxes indicate medians; boxes, interquartile ranges; whiskers, values within 1.5 times the interquartile range from boxes; solid circles outside the boxes, outlier data values. Table 2 lists the raw data for all metals represented in this figure. All metals in Table 2 are represented in this figure except Cd and Sb, as their concentrations were below for most samples. Note: For samples with of the samples below the limit of detection, the minimum and the percentile 25th values are the same and therefore the lower whisker is missing.
In paired sample analyses within devices, the increases in metal concentrations in the aerosol and tank samples compared with the original e-liquid from the dispenser were all statistically significant (all ), except for Fe in the aerosol (Table 3). The highest increases were for Zn (ratio 29.5), Pb (ratio 25.4), Ni (ratio 8.43), and Cr (6.78) in the aerosol, and for Pb (ratio 116), Cr (ratio 70.7), Ni (ratio 64.6), Cu (51.4), and Zn (36.7) in the tank. Only Cd (ratio 2.30), Al (ratio 3.79), and Sb (ratio 4.65) displayed ratios below 10 in tank compared with dispenser samples.
Table 3.
Ratio (95% confidence interval) of metal concentrations in e-cigarette aerosol and tank samples compared with dispenser sample.
| Metal | Aerosol vs. Dispenser () | Tank vs. Dispenser () | ||
|---|---|---|---|---|
| Ratio (95% CI) | p-Value | Ratio (95% CI) | p-Value | |
| Al | 1.73 (1.27, 2.36) | 3.79 (2.62, 5.50) | ||
| Cd | 1.60 (1.26, 2.04) | 2.30 (1.68, 3.15) | ||
| Cr | 6.78 (3.46, 13.3) | 70.7 (41.4, 121) | ||
| Cu | 3.30 (1.54, 7.07) | 0.003 | 51.4 (24.8, 106) | |
| Fe | 1.29 (0.69, 2.40) | 0.41 | 17.6 (9.71, 31.9) | |
| Mn | 1.93 (1.20, 3.09) | 0.007 | 19.6 (12.1, 32.0) | |
| Ni | 8.43 (3.17, 22.4) | 64.6 (27.2, 153) | ||
| Pb | 25.4 (14.0, 45.9) | 116 (64.0, 211) | ||
| Sb | 3.58 (2.26, 5.69) | 4.65 (2.81, 7.71) | ||
| Sn | 6.59 (4.16, 10.4) | 24.2 (14.3, 40.7) | ||
| Zn | 29.5 (17.4, 50.2) | 36.7 (21.4, 62.7) | ||
Note: The ratio of the geometric mean of metal concentrations in e-cigarette aerosol and tank samples compared with the dispenser was obtained by exponentiating the corresponding mean difference (95% confidence interval) in log-transformed metal concentrations. The p-values were obtained with a paired t-test. All tests were two-sided. Al, aluminum; Cd, cadmium; CI, confidence interval; Cr, chromium; Cu, copper; Fe, iron; Mn, manganese; Ni, nickel; Pb, lead; Sb, antimony; Sn, tin; Zn zinc.
Metal Correlations
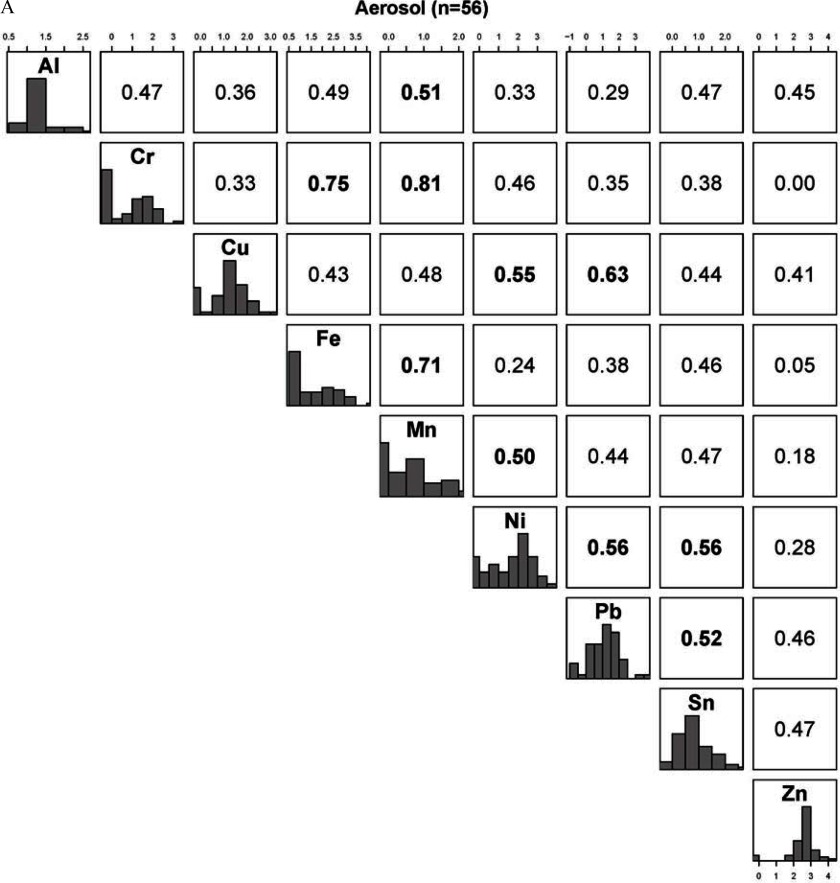
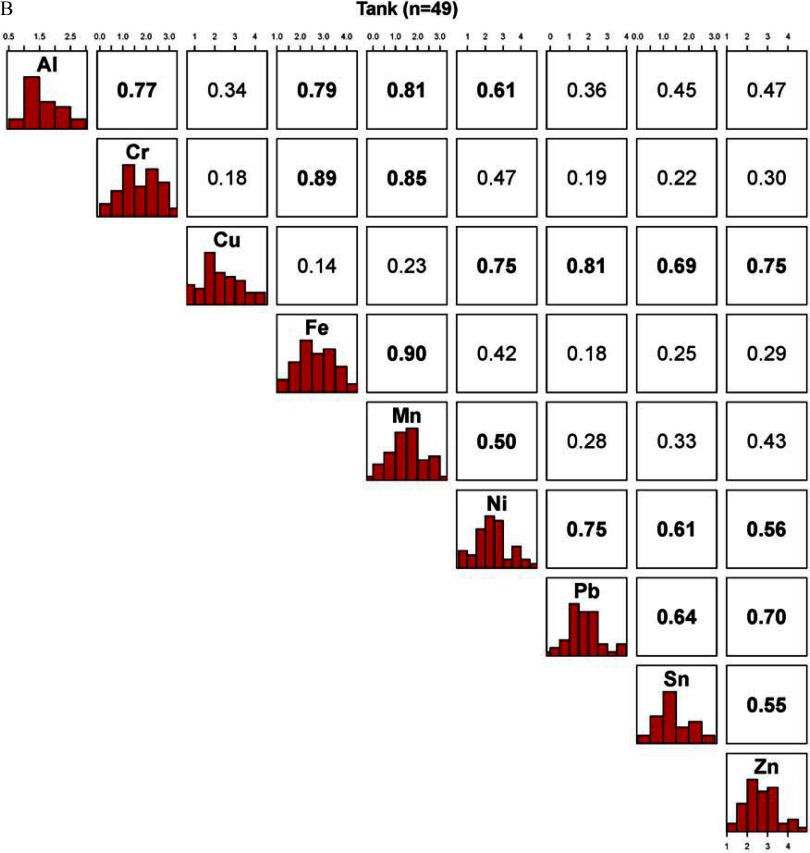
Across metals, Spearman correlations in e-liquid from the dispenser were generally low (well below 0.40) except for Al and Mn (), Fe and Mn (), Sn and Zn (), Mn and Zn (), and Ni and Cu () (see Figure S1); they were higher in aerosol samples, with three correlations being above 0.70 (Cr and Fe, Cr and Mn, and Fe and Mn) and 24 above 0.40 (Figure 2A); and they were markedly higher in tank samples with 23 correlations above 0.40 and 5 above 0.80 (Figure 2B). Within-metal correlations between the dispenser and aerosol samples were statistically significant for Fe, Mn, Sb, and Sn (ranging from 0.28 for Fe to 0.42 for Sb) (Table 4); between the dispenser and tank samples, they were statistically significant for Al, Mn, and Sb (ranging between 0.29 for Al and 0.39 for Mn); and between the aerosol and tank samples, they were all statistically significant, except for Cd and Cu, and ranged between 0.37 for Mn and 0.52 for Al. For As, among the detectable samples, the within-metal correlation was 0.84, 0.97, and 0.81 between the dispenser and aerosol, dispenser and tank, and aerosol and tank samples, respectively (data not shown).
Figure 2.
Correlations between metals in samples from e-cigarette devices: (A) aerosol samples, and (B) tank samples. All metals shown in Figure 1 are shown here. The diagonal panel shows the histograms of the -transformed distribution of each metal. The upper part of the panel represents the Spearman pairwise correlation coefficients between metals. The axes indicate the metal concentrations values that are represented in the histograms. Correlations are bolded.
Table 4.
Within-metal Spearman correlations in e-cigarette samples.
| Metal | Dispenser vs. Aerosol () | Dispenser vs. Tank () | Aerosol vs. Tank () | |||
|---|---|---|---|---|---|---|
| Correlation | p-Value | Correlation | p-Value | Correlation | p-Value | |
| Al | 0.13 | 0.33 | 0.29 | 0.046 | 0.52 | |
| Cda | — | — | — | — | 0.17 | 0.26 |
| Cr | 0.16 | 0.22 | 0.27 | 0.064 | 0.48 | |
| Cu | 0.32 | 0.20 | 0.16 | 0.19 | 0.19 | |
| Fe | 0.28 | 0.038 | 0.16 | 0.28 | 0.42 | 0.003 |
| Mn | 0.30 | 0.025 | 0.39 | 0.006 | 0.37 | 0.009 |
| Ni | 0.11 | 0.04 | 0.79 | 0.43 | 0.002 | |
| Pb | 0.23 | 0.095 | 0.23 | 0.11 | 0.43 | 0.002 |
| Sb | 0.42 | 0.001 | 0.34 | 0.016 | 0.44 | 0.002 |
| Sn | 0.38 | 0.004 | 0.25 | 0.081 | 0.46 | 0.001 |
| Zn | 0.25 | 0.064 | 0.18 | 0.22 | 0.45 | 0.001 |
Note: The p-values were obtained from the Spearman correlation coefficient test. —, no data; Al, aluminum; Cd, cadmium; Cr, chromium; Cu, copper; Fe, iron; Mn, manganese; Ni, nickel; Pb, lead; Sb, antimony; Sn, tin; Zn zinc.
aCd was not detected in any of the dispenser samples; therefore, Dispenser vs. Aerosol and Dispenser vs. Tank correlations were not calculated.
Metal Concentrations by Voltage, Type of Coil, and Frequency of Coil Change
All metals in Table 2 are shown in these analyses except Cd and Sb, because their concentrations were below for most samples. Metal concentrations in dispenser and aerosol samples were not statistically different by voltage (Table 5). In tank samples we found statistically significant differences by voltage tertiles for Al, Fe, and Mn, with the intermediate tertile presenting the highest metal concentrations. For Ni, the difference by voltage was borderline significant () with concentrations also higher at the intermediate tertile (4.00–4.40 V). When analyzed by type of coil, metal concentrations in dispenser samples were similar (Table 6). In aerosol samples, Cr, Fe, Mn, Ni, Pb, and Sn concentrations were higher in those from devices with a Kanthal coil compared with other coils. In tank samples, those from devices for which the user did not know the type of coil showed the highest concentrations for all metals. These differences of metal concentrations by type of coil were not significant (except for Cu in tank samples). There were no statistically significant differences in metal concentrations by frequency of coil change for dispenser and tank samples (Table 7). In aerosol samples, all metals were more concentrated in the aerosol from users who change the coils more than twice per month, with significant differences for Al, Cr, and Mn (Table 7). In tank samples, Al, Cr, Fe, Mn, Ni, and Sn concentrations were also higher for samples from devices for which the participants reported coil change more than twice per month.
Table 5.
Median (interquartile range) metal concentrations () in samples from the dispenser, aerosol, and tank, by voltage tertile.
| Sample | Voltage tertile (V) | a | Al | Cr | Cu | Fe | Mn | Ni | Pb | Sn | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dispenser () | 18 | 8.80 (4.32–16.6) | 1.13 () | 2.04 () | 46.1 (14.4–64.5) | () | () | 0.507 (0.238–1.16) | 0.718 (0.460–3.09) | 13.2 (8.49–24.1) | |
| 4.02–4.42 | 18 | 11.8 (7.37–25.2) | () | 7.20 (4.48–34.0) | 30.1 (9.90–115) | 2.41 () | 9.86 () | 0.513 (0.345–1.11) | 2.29 (0.852–5.13) | 11.4 (5.62–26.1) | |
| 18 | 13.3 (9.12–16.3) | 0.509 () | 2.87 () | 22.8 (11.6–73.2) | 1.21 () | 1.83 () | 0.462 (0.134–0.754) | 1.16 (0.394–2.99) | 12.8 (6.82–14.9) | ||
| p-Value | 0.50 | 0.21 | 0.19 | 0.71 | 0.19 | 0.18 | 0.60 | 0.36 | 0.79 | ||
| Aerosol () | 18 | 15.5 (12.4–17.1) | () | 15.1 (9.29–51.0) | () | 0.869 () | 12.5 (6.24–270) | 14.0 (2.49–38.5) | 4.86 (2.15–20.9) | 549 (234–1,090) | |
| 4.02–4.42 | 18 | 18.5 (13.3–26.5) | 19.7 (4.18–36.9) | 13.8 (1.41–28.1) | 59.3 () | 2.71 () | 101 (8.88–284) | 13.0 (3.41–28.3) | 7.22 (2.20–19.7) | 483 (384–681) | |
| 18 | 16.4 (11.4–28.2) | 24.2 (0.972–58.0) | 38.1 (4.87–75.3) | 58.5 (14.5–213) | 5.92 () | 109 (12.5–299) | 26.6 (4.97–72.2) | 5.49 (3.85–16.3) | 443 (206–791) | ||
| p-Value | 0.61 | 0.18 | 0.30 | 0.23 | 0.37 | 0.94 | 0.41 | 0.96 | 0.75 | ||
| Tank () | 14 | 25.7 (17.5–38.5) | 23.4 (8.43–149) | 168 (57.7–3,375) | 230 (53.8–1,030) | 20.1 (5.86–41.3) | 297 (56.5–664) | 49.2 (20.5–251) | 14.5 (12.1–49.8) | 679 (264–1,560) | |
| 4.00–4.40 | 16 | 62.7 (32.9–136) | 165 (51.9–377) | 128 (55.7–412) | 1,080 (333–2,970) | 61.0 (32.3–262) | 448 (116–4,250) | 42.8 (31.6–192) | 24.3 (18.7–122) | 413 (166–1,190) | |
| 17 | 19.2 (13.6–32.9) | 21.7 (9.73–152) | 67.9 (30.9–479) | 218 (70.4–1,400) | 23.2 (7.73–41.1) | 105 (49.6–302) | 17.7 (11.7–77.8) | 10.2 (7.45–27.6) | 201 (102–537) | ||
| p-Value | 0.04 | 0.07 | 0.46 | 0.04 | 0.03 | 0.05 | 0.20 | 0.08 | 0.37 | ||
Note: The p-values were obtained from Kruskal-Wallis tests. All tests were two-sided. Al, aluminum; Cr, chromium; Cu, copper; Fe, iron; Mn, manganese; Ni, nickel; Pb, lead; Sn, tin; Zn zinc.
aTwo participants did not report the voltage of their devices and were not included in this analysis.
Table 6.
Median (interquartile range) metal concentrations () in samples from the dispenser, aerosol, and tank, by coil material.
| Sample | Coil Category | Al | Cr | Cu | Fe | Mn | Ni | Pb | Sn | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dispenser () | Kanthal | 29 | 10.0 () | () | 6.63 () | 33.5 (14.4–79.7) | 1.28 () | 1.99 () | 0.481 (0.245–0.978) | 2.30 (0.705–3.79) | 13.7 (8.57–26.9) |
| Other/Combination | 13 | 13.4 (12.2–26.3) | () | () | 25.3 (7.93–96.4) | 1.14 () | () | 0.319 () | 0.555 (0.277–2.16) | 8.24 (4.68–14.0) | |
| Unknown | 14 | 9.25 (8.08–15.0) | () | 6.30 () | 13.4 () | () | 9.34 () | 0.462 (0.352–0.996) | 1.33 (0.427–3.40) | 13.1 (12.2–20.7) | |
| p-Value | 0.33 | 0.97 | 0.29 | 0.77 | 0.97 | 0.27 | 0.92 | 0.24 | 0.30 | ||
| Aerosol () | Kanthal | 29 | 16.2 (12.4–20.3) | 15.3 (0.520–46.4) | 15.2 (8.63–58.4) | 38.6 () | 3.43 () | 122 (7.72–268) | 20.4 (7.38–34.8) | 6.29 (4.01–19.1) | 564 (355–723) |
| Other/Combination | 13 | 17.2 (11.4–20.8) | 3.74 () | 21.2 (3.15–164) | 28.9 () | 1.89 () | 57.5 (6.18–411) | 3.86 (2.37–218) | 5.63 (2.75–23.3) | 422 (125–668) | |
| Unknown | 14 | 16.1 (12.1–25.8) | () | 12.7 (6.60–24.0) | 8.60 () | 1.35 () | 36.7 (6.11–148) | 6.89 (4.89–18.2) | 3.18 (1.97–12.3) | 652 (269–848) | |
| p-Value | 0.99 | 0.30 | 0.65 | 0.66 | 0.45 | 0.57 | 0.59 | 0.35 | 0.49 | ||
| Tank () | Kanthal | 25 | 29.6 (17.5–52.8) | 60.3 (17.4–217) | 107 (42.0–298) | 333 (174–1,360) | 31.9 (8.27–86.1) | 147 (39.7–467) | 33.3 (13.2–77.8) | 19.4 (10.2–24.7) | 279 (126–449) |
| Other/Combination | 11 | 27.7 (14.8–157) | 21.5 (12.1–469) | 61.5 (28.7–494) | 251 (108–2,110) | 26.6 (11.2–196) | 302 (98.0–877) | 23.4 (12.7–188) | 9.10 (7.20–40.0) | 416 (127–1,470) | |
| Unknown | 13 | 35.1 (19.4–168) | 69.5 (28.4–177) | 1,410 (80.3–5,150) | 707 (102–1,350) | 41.1 (14.0–93.9) | 397 (158–638) | 189 (40.2–355) | 63.0 (26.7–104) | 1,550 (537–4,080) | |
| p-Value | 0.80 | 0.72 | 0.023 | 0.91 | 0.82 | 0.32 | 0.13 | 0.071 | 0.083 | ||
Note: The p-values were obtained from Kruskal-Wallis tests. All tests were two-sided. Al, aluminum; Cr, chromium; Cu, copper; Fe, iron; Mn, manganese; Ni, nickel; Pb, lead; Sn, tin; Zn zinc.
Table 7.
Median (interquartile range) metal concentrations () in samples from the dispenser, aerosol, and tank, by coil change frequency.
| Sample | Coil change | a | Al | Cr | Cu | Fe | Mn | Ni | Pb | Sn | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dispenser () | times per month | 32 | 11.9 (6.90–19.2) | 0.705 () | 6.83 () | 29.4 () | 1.16 () | 5.04 () | 0.422 (0.202–0.897) | 1.12 (0.489–3.84) | 14.0 (8.91–36.9) |
| times per month | 23 | 10.2 (7.29–21.9) | () | () | 26.6 (10.1–91.9) | 1.05 () | 1.06 () | 0.482 (0.258–1.03) | 1.37 (0.485–2.55) | 10.4 (5.39–13.8) | |
| p-Value | 0.86 | 0.15 | 0.16 | 0.97 | 0.99 | 0.54 | 0.52 | 0.73 | 0.088 | ||
| Aerosol () | times per month | 32 | 15.4 (11.6–17.2) | 0.949 () | 14.6 (5.70–53.1) | 15.2 () | 1.27 () | 68.4 (6.32–252) | 14.4 (3.06–46.4) | 4.99 (2.22–14.8) | 470 (227–809) |
| times per month | 23 | 20.3 (15.0–33.8) | 21.5 (2.13–84.4) | 21.2 (8.79–48.1) | 136 () | 6.02 (2.40–21.2) | 138 (9.27–376) | 16.5 (3.23–32.3) | 6.70 (3.87–24.0) | 591 (292–831) | |
| p-Value | 0.009 | 0.038 | 0.90 | 0.30 | 0.015 | 0.40 | 0.95 | 0.28 | 0.63 | ||
| Tank () | times per month | 30 | 28.6 (14.0–49.8) | 46.6 (17.8–154) | 185 (39.0–1.210) | 303 (125–1,330) | 26.5 (8.55–92.0) | 186 (44.2–636) | 40.8 (15.5–204) | 20.1 (7.52–81.6) | 493 (176–1,640) |
| times per month | 19 | 35.1 (23.5–148) | 132 (18.6–386) | 107 (58.3–430) | 565 (204–2,600) | 33.5 (16.6–160) | 329 (114–877) | 40.2 (13.0–170) | 20.3 (14.3–33.6) | 302 (93.5–1,360) | |
| p-Value | 0.081 | 0.29 | 0.84 | 0.33 | 0.38 | 0.26 | 0.84 | 0.59 | 0.26 | ||
Note: The p-values were obtained from Kruskal-Wallis tests. All tests were two-sided. Al, aluminum; Cr, chromium; Cu, copper; Fe, iron; Mn, manganese; Ni, nickel; Pb, lead; Sn, tin; Zn zinc.
aOne participant did not report the coil change frequency and, also, the tank sample could not be obtained from his/her device.
Aerosol Metal Concentrations
Concentrations for each of the detected metals are estimated to be daily averages, and span several orders of magnitude (Table 8). We focus on Ni, Cr, Pb, Mn, and As because, due to their toxicity when found in aerosols, these compounds have health-based limit concentrations. Ni concentrations ranged from to (median , and 57% of e-cigarette aerosol samples exceeded the Agency for Toxic Substances Disease Registry (ATSDR 2016) daily chronic minimum risk level (MRL) for Ni of (ATSDR 2005a; U.S. EPA 2000a). Cr concentrations ranged from (median . Because we did not determine the valence state of Cr in our samples, we do not know what proportion was Cr(VI) (hexavalent) and which was trivalent. If Cr in our samples were Cr(VI), 68% of the samples would exceed the daily MRL for Cr(VI) in mist (), and 46% of the samples would exceed daily MRL for soluble Cr(III) () if Cr in our samples were Cr(III) (ATSDR 2012a). Pb concentrations ranged from (median , with 48% of aerosol samples exceeding the U.S. EPA National Ambient Air Quality Standard (NAAQS) (U.S. EPA 2016) of and 11% exceeding the standard in nonattainment areas of . Mn concentrations ranged from (median ; 14% of samples exceeded the daily Mn MRL of (ATSDR 2012b) and 75% exceeded the U.S. EPA daily cancer reference concentration (RfC) of (U.S. EPA 2012). Arsenic concentrations, calculated only among the 10 aerosol samples (17.9%) with detectable arsenic (data not shown) ranged from (median . All other metals investigated were also found in concentrations spanning three to four orders of magnitude (Figure 1) in the condensed aerosol, which would translate to several orders of magnitude in the air using Equation 1.
Discussion
In this assessment of metal concentrations in samples collected from tank-style devices of daily e-cigarette users in Maryland, we found that, for most metals, concentrations were markedly higher in samples collected from the tank and the aerosol compared with those collected from the refilling dispenser. Dramatic increases were observed in tank samples for Cr, Cu, Ni, Pb, and Zn concentrations (more than 35 times higher than in the dispenser samples) as well as in aerosol samples for Pb and Zn (more than 25 times higher than in the dispenser samples) and for Cr, Ni, and Sn (more than 6 times higher than in the dispenser samples). For Mn, the concentrations in tank and aerosol samples were 19.6 and 1.93 times higher than the dispenser samples respectively. For Al, Cd, and Sb, the concentrations were between 2.30 and 4.65 times higher in the tank and between 1.60 and 3.58 times higher in the aerosol compared with the dispenser samples. The finding of Pb in e-cigarette aerosol samples, a metal not listed among the components of heating coils but that can be present in metal alloys, is of major concern both directly for the consumer as well as for those involuntarily exposed to e-cigarette aerosol, especially children. For As, 10.7% of the dispenser samples had As detected. The similar concentrations found in the dispenser, aerosol and tank samples, and the high correlation between detected As levels in the dispenser and those found in the aerosol and tank samples supports that when As is present in the dispenser e-liquid it gets transferred to the aerosol. It is concerning that there are e-liquid brands on the market that contain As and Pb in the dispenser. More research is necessary to confirm these findings and to determine how often As and Pb are present in e-liquids, and whether they are related to specific brands or manufacturers.
Higher correlations across metals in the aerosol and tank samples than in the dispenser suggest that several metals are being transferred from the device to the e-liquid in the tank as well as to the aerosol that is inhaled by the user. The most likely source of metals in the device is the heating coil, composed of complex metal alloys in most devices, although we cannot rule out that other parts of the device also contribute.
In our estimations of daily mass concentrations in the aerosol, 57% of e-cigarette aerosol samples exceeded the ATSDR (2016) daily chronic MRL for Ni of (ATSDR 2005a; U.S. EPA 2000a). Sixty eight percent of the samples exceeded the daily MRL for Cr(VI) in mist () if Cr in our samples were Cr(VI), and 46% of the samples would exceed daily MRL for soluble Cr(III) (), if Cr in our samples were Cr(III) (ATSDR 2012a). For Pb, 48% of aerosol samples exceeded the U.S. EPA NAAQS of (U.S. EPA 2016). For Mn, 14% of samples exceeded the daily MRL of (ATSDR 2012b) and 75% exceeded the U.S. EPA daily RfC of (U.S. EPA 2012). Aerosol mass concentrations are likely underestimated, as in our formula we assumed that daily exposure is equivalent to 50 puffs, whereas recent research indicates the average is closer to 200 daily puffs (Aherrera et al. 2017; Robinson et al. 2015). We also assumed that we collected the total weight of the emitted aerosol, although we know that around 20% remains in the tubing and around 10% of the aerosol is lost through the venting groove of the collection device.
Only a few studies have addressed exposure to metals through e-cigarette aerosol. Most of them evaluated only one or two products and none of them formally compared the concentrations of metals in the aerosol to the concentrations in the original e-liquid before being in contact with the heating coil. These studies, however, provide useful information on which metals are detected in e-cigarette emissions and which ones are in higher concentrations compared with others. In a study of secondhand exposure from indoor usage of a single brand tank-style European device, aerosol-laden air was collected on quartz filters and analyzed for metals (Saffari et al. 2014). Indoor air concentrations of the metals with health-based limits (in ) were: for Cr, for Mn, for Ni, and for Pb, whereas we estimated mainstream aerosol concentrations () of for Cr, for Mn, for Ni, and for Pb (Table 8). A reason for why our values are at least an order of magnitude higher is that mainstream aerosol has not undergone mixing in indoor air like secondhand aerosol, which is what was measured in the study by Saffari et al. (2014). Also, the sampling of particles in their study (using quartz filters) could miss metals in vapor phase. In a study of metals in aerosol from 12 electronic cigarettes (with cartridges or cartomizers), collected using gas washing bottles with methanol, immersed in an acetone and dry-ice bath, Cd [range, non-detectable ( puffs], Ni (range, puffs), and Pb (range, puffs) were detected in almost all the devices tested (Goniewicz et al. 2014). Based on a puff, as reported by Goniewicz et al. their results in would be (ranges)—Cd (), Ni (), and Pb ()—which are similar to the ranges that we obtained for Ni () and Pb () (Table 8).
Another study determined metal concentrations in the aerosol of several cigalike devices and a tank-style device (Mikheev et al. 2016) by collecting total particulate matter (TPM) on quartz filters. Of the metals that we report, based on the vaping topography that Mikheev et al. described, and following their assumption that the average mass of TPM/puff was , we estimated the following concentration ranges: for As (), Cr (), Ni (), and Zn () (Mikheev et al. 2016). These results need to be compared with caution because Mikheev et al. (2016) analyzed mostly cigalike devices and, in their own words, they provide only a rough assessment of metal content. Nevertheless, it is interesting to note that even a rough assessment provides mass fractions and variability similar to our results.
In a study of 22 cigalike cartomizers, aerosol was characterized by size, and found that particles contained Sn, Ag, Fe, Ni, and Al, while nanoparticles contained Sn, Cr, and Ni (Williams et al. 2013). Pb was also detected in the aerosol using ICP–optical emission spectrometry ( puffs). In a more recent study by the same investigators, 35 of 36 screened elements were detected in the aerosols of disposable e-cigarettes and electronic hookahs, whereas only 15 were detected in conventional tobacco smoke (Williams et al., 2017). Metals such as Pb, Cu, Ni, or Sn were present at significantly higher concentrations in the aerosols compared with cigarette smoke (Williams et al., 2017). In a study of e-liquid in the cartomizers of five cigalike brands purchased in Maryland, Cd (mean concentration ranged from ), Cr (), Pb (), Mn (), and Ni () were found in the e-liquids analyzed that were in contact with the unused cartomizer coil, indicating the transfer of metals from the coil to the e-liquid in cigalike devices (Hess et al. 2017). A French study analyzing 15 trace elements in e-liquids from refilling dispenser have also shown low concentrations (with the majority of the samples under the lower limits of quantification) of most metals analyzed, except for Al, As, Co, Cr, and Sb (average concentrations 12.9, 1.57, 0.262, 7.16, and , respectively) (Beauval et al. 2016). This is similar to what we found in our study as many of the metals were under the LOD in most of the dispenser e-liquid samples, and those metals detectable in over 50% of the e-liquid samples (Al, Cu, Fe, Mn, Ni, Pb, Sn, and Zn) in general presented low median metal concentrations.
In our study, metal concentrations tended to be lower in aerosol than in tank samples. Correlations between concentrations of different metals were lower in the aerosol than in the tank. We do not have a definite explanation for these differences, but metal concentrations in the tank e-liquid cannot be expected to be equal to those in the aerosol for the following reasons: a) Mass transfer of metal compounds into the aerosol can be expected to be metal specific. b) Some of the metals have been shown to exist as solid beads within the aerosol droplets, and it is hypothesized that the beads originate from metallic e-cigarette components such as the heating coil (Williams et al. 2013). Transfer of these beads from the tank to the aerosol can be expected to be element- and size-specific where size in turn is likely element specific. c) Metals may continue to leach from the coil to the tank even after the generation of the aerosol has stopped. d) The efficiency of our aerosol collection device can be expected to depend on aerosol droplet size (Tien and Ramarao 2007, Long and Hilpert 2009), and it cannot be assumed that different metals are equally distributed in different size fractions. At the beginning of our collection process, (within the first puffs), when drops are starting to be formed inside the tubing, more droplets in the range will escape from the collection device than larger and smaller droplets, which are more efficiently collected on the device walls due to the processes of impaction and diffusion, respectively. After the first liquid drop forms, completely filling the inside diameter of the tubing, all particle sizes are collected with equal efficiency through interception. The liquid formed is pushed towards the collection tube with the incoming aerosol.
Furthermore, we do not know at this point if our collection method can efficiently capture metals in the gas phase of the aerosol, such as those from potentially volatile compounds of Pb and Zn. However, we found similar concentrations of Pb and Zn in aerosol and tank samples compared with other metals, suggesting that the significant loss of these potentially volatile compounds did not occur. More research is needed to investigate the distribution of metals generated in e-cigarettes within particulate and gas phases. In a biomonitoring study conducted with the users of the e-cigarettes analyzed in the present study, concentrations of Ni and Cr in the urine and saliva of these e-cigarette users were more strongly associated with the corresponding metal concentrations measured in the aerosol than with metal concentrations in the tank, supporting that our aerosol sample reflects what an e-cigarette user is inhaling (Aherrera et al. 2017).
Our findings suggest that using e-cigarettes instead of conventional cigarettes may result in less exposure to Cd but not to other hazardous metals found in tobacco. In mainstream smoke from conventional tobacco cigarettes available in the United States (Pappas et al. 2014), the highest concentrations were found for Cd (ranging from to per cigarette), followed by Pb (ranging from to per cigarette). The rest of the element analyzed (As, Co, Cr, Mn and Ni) were below . For Ni and Cr, specifically, most samples were below the lower detection limits. In the Surgeon General Report (CDC 2010), the range of metal concentrations in mainstream smoke were the following for As (), Ni (), Cr (hexavalent) (), Cd (), Co (), and Pb (inorganic) (). Directly comparing smoking a cigarette to vaping behavior is difficult and was not the purpose of our study. However, if we assume that 15 puffs is equivalent to one cigarette (St Helen et al. 2016), and based on a mean puff volume of e-cigarettes in our study of , the range (median) of metal concentration (in nanograms per 15 puffs) in our study would be 0.004–110 (0.444) for Ni, 0.001–30.0 (0.085) for Cr, 0.002–27.0 (0.106) for Pb, 0.001–1.40 (0.020) for Mn, 0.002–66.1 (4.49) for Zn, and 0.008–1.00 (0.151) for As. Saffari et al. (2014) compared the emission rates of different metals in an e-cigarette to a conventional combustible tobacco cigarette and found the emission rates were higher in e-cigarettes for elements like Ti, Cr, Ni, and Ag, and lower for elements like Cu, Cd, Zn, and Pb. Our findings are consistent for Cr, Ni, and Cd; however, for Pb and Zn we found concentrations that were similar to those found in cigarette smoking in some samples. Additional research, including biomarker studies, are needed to compare cigarette smoking and e-cigarette use as sources of metal exposure.
The metals detected in e-cigarettes have been associated with multiple adverse health effects under chronic conditions of exposure. Pb is a major neurotoxicant both for children and aging populations and is also associated with increased risk of cardiovascular disease and kidney disease (Navas-Acien et al. 2007; Fadrowski et al. 2010), diseases that are a major motivation for smokers to quit. Pb is especially of concern because it cannot be easily excreted from the body and because the health effects have been observed at low levels of exposure with no evidence of a threshold (Lin et al. 2006). Any unnecessary Pb exposure should be avoided. In addition, Cr and Ni are established inhalation carcinogens (IARC 2012a, 2012b). The U.S. EPA has stated that the classification of Cr(VI) as a known human carcinogen raises a concern for the carcinogenic potential of Cr(III) because of the possible oxidation of Cr(III) to Cr(VI) within the oxygen-rich environment of the lungs (U.S. EPA 2000b). Therefore, even though we did not speciate our samples for the Cr oxidation state, these results can be of concern.
Other metals that are essential nutrients through the ingestion route can have serious negative effects when inhaled. For example, Fe can produce respiratory irritation, metal fume fever, siderosis, and fibrosis (Johnson et al., 1985); Mn can induce lung irritation, coughing, bronchitis and pneumonitis, reduced lung function, pneumonia, manganism (a Parkinson-like disease), and other neurological outcomes (ATSDR 2012b; O’Neal and Zheng 2015). Cu can produce respiratory irritation, coughing, sneezing, chest pain, and runny nose (ATSDR 2004); and Zn can cause metal fume fever, reduced lung function, chest pain, coughing, dyspnea, and shortness of breath (ATSDR 2005b). The health effects for inhalation of Fe, Mn, Cu, and Zn have been detected mostly in occupational settings during both acute and chronic exposures at relatively high levels. These effects might not translate into chronic e-cigarette exposure. Arsenic, detected in 17.9% of our aerosol samples, also represents a potential concern due to its high toxicity in numerous organs and body systems; for example, cancer and cardiovascular disease have both been associated with inorganic As exposure (Saint-Jacques et al. 2014; Moon et al. 2012). Arsenic speciation, however, was not conducted. Additional research is needed to identify which As species are present in e-cigarette aerosol.
In addition to the device composition, other factors could play a role in e-cigarette metal exposure. We found some suggestion for a role of voltage; among metals that are associated with commonly used coils, Al, Fe, Cr, and Ni concentrations were higher in the middle voltage tertile for tank samples but not for aerosol samples. However, tank concentrations tended to be lower in the upper tertile than in the medium one, whereas aerosol concentrations tended to be higher. These voltage-dependent concentrations need to be interpreted carefully because they are based on self-reported data but they could be related to the rates of mass transfer of the metals and their compounds among the solid alloy of the coil, the tank’s e-liquid surrounding the coil, and the vapor as well as on the chemical equilibria between these different thermodynamic phases. For instance, the higher aerosol concentrations in the upper voltage tertile can at least be partially attributed to a saturated vapor pressure, which increases with temperature and hence voltage. The increased vapor pressure should increase transfer of dissolved metal compounds into the vapor phase, from which the aerosol is formed. This would be consistent with an e-cigarette study that examined parameters affecting the release of aldehydes (Sleiman et al. 2016). They observed that increasing the voltage applied to a single-coil device from 3.3 to 4.8 V caused the mass of e-liquid consumed to double and the total aldehyde emission rates to triple. Age of the device, temperature, and vaping regime could contribute to the degradation of the coil and other metallic parts of the device and increase exposure to metals, although we lacked information on those factors in this study. However, leaching of metals from the coil into the e-liquid could potentially be enhanced by corrosion as has also been observed for Pb in drinking-water pipes (Edwards and Dudi 2004).
Despite some limitations, our findings can inform strategies aimed at reducing the risk of metal exposure in e-cigarette users, including testing for metals as part of the regulation of e-cigarette products. Strengths of our study include the collection of an aerosol sample that has not been filtered or diluted during the collection process and that likely reflects what the consumer is inhaling. Although our sampling method has not been validated against other methods that evaluate metals in aerosol samples through the use of filters, the collection of the aerosol in liquid form allowed the direct comparison with the original e-liquid from the dispenser, as well as liquid from the tank. Another strength is the sampling of a highly diverse number of e-cigarette devices used by daily e-cigarette users in Maryland. Additional research is needed to better understand the metal compounds in e-cigarette emissions, their absorption through the respiratory tract, and the potential health effects of e-cigarette metal related exposures.
Conclusions
Our results add to the existing evidence that e-cigarettes are a relevant source of exposure to a wide variety of toxic metals including Cr, Ni, and Pb as well as to essential metals that are potentially toxic through inhalation such as Mn and Zn. Metal concentrations in the e-liquid from the original dispenser increased markedly in the same e-liquid after it was added to the device and was brought into contact with the heating coil, both in the generated aerosol and in the liquid that remained in the tank. These findings support the hypothesis that metals are transferred from the device (most likely the coil) to the e-liquid and from the e-liquid to the aerosol that is inhaled by the user. Due to potential toxicity resulting from chronic exposure to metals in e-cigarette aerosols, additional research is needed to more precisely quantify metal exposures resulting from e-cigarette use and their implications for human health, and to support regulatory standards to protect public health.
Supplemental Material
Acknowledgments
This study is supported by the Cigarette Restitution Fund (State of Maryland; grant PHPA-G2034). P.O. was supported by the Alfonso Martín Escudero Foundation (postdoctoral fellowship 2014). A.A. was supported by the American Heart Association Tobacco Regulation and Addiction Center (grant 1P50HL120163). A.N.A., M.H., and P.O. are supported by the National Institute of Environmental Health Sciences/National Institutes of Health (grant 5P30ES009089).
References
- Aherrera A, Olmedo P, Grau-Perez M, Tanda S, Goessler W, Jarmul S, et al. 2017. The association of e-cigarette use with exposure to nickel and chromium: a preliminary study of non-invasive biomarkers. Environ Res 159:313–320, PMID: 28837903, 10.1016/j.envres.2017.08.014. [DOI] [PubMed] [Google Scholar]
- Ambrose BK, Rostron BL, Johnson SE, Portnoy DB, Apelberg BJ, Kaufman AR, et al. 2014. Perceptions of the relative harm of cigarettes and e-cigarettes among U.S. youth. Am J Prev Med 47(2 suppl 1):S53–S60, PMID: 25044196, 10.1016/j.amepre.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). 2004. Toxicological profile for copper. Atlanta, GA:U.S. Department of Health and Human Services, Public Health Service; http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=206&tid=37 [accessed 1 November 2016]. [Google Scholar]
- ATSDR. 2005a. Toxicological profile for nickel. Atlanta, GA:U.S. Department of Health and Human Services, Public Health Service; https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=245&tid=44 [accessed 1 November 2016]. [Google Scholar]
- ATSDR. 2005b. Toxicological profile for zinc. Atlanta, GA:U.S. Department of Health and Human Services, Public Health Service; http://www.atsdr.cdc.gov/ToxProfiles/TP.asp?id=302&tid=54 [accessed 1 November 2016]. [Google Scholar]
- ATSDR. 2012a. Toxicological profile for chromium. Atlanta, GA:U.S. Department of Health and Human Services, Public Health Service; https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=62&tid=17 [accessed 14 December 2016]. [Google Scholar]
- ATSDR. 2012b. Toxicological profile for manganese. Atlanta, GA:U.S. Department of Health and Human Services, Public Health Service; http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=102&tid=23 [accessed 1 November 2016]. [Google Scholar]
- ATSDR. 2016. Minimal Risk Levels (MRLs) List. http://www.atsdr.cdc.gov/mrls/mrllist.asp [accessed 21 December 2016].
- Beauval N, Howsam M, Antherieu S, Allorge D, Soyez M, Garçon G, et al. 2016. Trace elements in e-liquids-development and validation of an ICP-MS method for the analysis of electronic cigarette refills. Regul Toxicol Pharmacol 79:144–148, PMID: 27058761, 10.1016/j.yrtph.2016.03.024. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2010. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease. A Report of the Surgeon General. https://www.ncbi.nlm.nih.gov/books/NBK53017/ [accessed 14 October 2017]. [PubMed]
- Cooper M, Harrell MB, Perry CL. 2016. A qualitative approach to understanding real-world electronic cigarette use: implications for measurement and regulation. Prev Chronic Dis 13:E07, PMID: 26766848, 10.5888/pcd13.150502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M, Dudi A. 2004. Role of chlorine and chloramine in corrosion of lead-bearing plumbing materials. J Am Water Works Assoc 96(10):69–81. [Google Scholar]
- Fadrowski JJ, Navas-Acien A, Tellez-Plaza M, Guallar E, Weaver VM, Furth SL. 2010. Blood lead level and kidney function in US adolescents: the Third National Health and Nutrition Examination Survey. Arch Intern Med 170(1):75–82, PMID: 20065202, 10.1001/archinternmed.2009.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Voudris V, Poulas K. 2015. Are metals emitted from electronic cigarettes a reason for health concern? A risk-assessment analysis of currently available literature. Int J Environ Res Public Health 12(5):5215–5232, PMID: 25988311, 10.3390/ijerph120505215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuoco FC, Buonanno G, Stabile L, Vigo P. 2014. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ Pollut 184:523–529, PMID: 24172659, 10.1016/j.envpol.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Garza A, Vega R, Soto E. 2006. Cellular mechanisms of lead neurotoxicity. Med Sci Monit 12(3):RA57–RA65, PMID: 16501435. [PubMed] [Google Scholar]
- Giovenco DP, Hammond D, Corey CG, Ambrose BK, Delnevo CD. 2015. E-cigarette market trends in traditional U.S. retail channels, 2012–2013. Nicotine Tob Res 17(10):1279–1283, PMID: 25542918, 10.1093/ntr/ntu282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, et al. 2014. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 23(2):133–139, PMID: 23467656, 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess CA, Olmedo P, Navas-Acien A, Goessler W, Cohen JE, Rule AM. 2017. E-cigarettes as a source of toxic and potentially carcinogenic metals. Environ Res 152:221–225, PMID: 27810679, 10.1016/j.envres.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer). 2012a. Chromium (VI) compounds. IARC Monographs 100C:147–167. [Google Scholar]
- IARC 2012b. Nickel and nickel compounds. IARC Monographs 100C:169–218. [Google Scholar]
- Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. 2014. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7(2):60–72, PMID: 26109881, 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Moira CY, MacLean L, Atkins E, Dybuncio A, Cheng F, et al. 1985. Respiratory abnormalities among workers in an iron and steel foundry. Br J Ind Med 42(2):94–100, PMID: 2982392, 10.1136/oem.42.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JL, Lin-Tan DT, Li YJ, Chen KH, Huang YL. 2006. Low-level environmental exposure to lead and progressive chronic kidney diseases. Am J Med 119(8):707.e1–707.e9, PMID: 16887418, 10.1016/j.amjmed.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Long W, Hilpert M. 2009. A correlation for the collector efficiency of Brownian particles in clean-bed filtration in sphere packings by a lattice-Boltzmann method. Environ Sci Technol 43(12):4419–4424, PMID: 19603656, 10.1021/es8024275. [DOI] [PubMed] [Google Scholar]
- McCarthy M. 2015. “Alarming” rise in popularity of e-cigarettes is seen among US teenagers as use triples in a year. BMJ 350:h2083, PMID: 25896797, 10.1136/bmj.h2083. [DOI] [PubMed] [Google Scholar]
- McQueen N, Partington EJ, Harrington KF, Rosenthal EL, Carroll WR, Schmalbach CE. 2015. Smoking cessation and electronic cigarette use among head and neck cancer patients. Otolaryngol Head Neck Surg 154(1):73–79, PMID: 26519457, 10.1177/0194599815613279. [DOI] [PubMed] [Google Scholar]
- Mikheev VB, Brinkman MC, Granville CA, Gordon SM, Clark PI. 2016. Real-time measurement of electronic cigarette aerosol size distribution and metals content analysis. Nicotine Tob Res 18(9):1895–1902, PMID: 27146638, 10.1093/ntr/ntw128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon K, Guallar E, Navas-Acien A. 2012. Arsenic exposure and cardiovascular disease: an updated systematic review. Curr Atheroscler Rep 14(6):542–555, PMID: 22968315, 10.1007/s11883-012-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. 2007. Lead exposure and cardiovascular disease–a systematic review. Environ Health Perspect 115(3):472–482, PMID: 17431501, 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmedo P, Navas-Acien A, Hess C, Jarmul S, Rule A. 2016. A direct method for e-cigarette aerosol sample collection. Environ Res 149:151–156, PMID: 27200479, 10.1016/j.envres.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neal SL, Zheng W. 2015. Manganese toxicity upon overexposure: a decade in review. Curr Environ Health Rep 2(3):315–328, PMID: 26231508, 10.1007/s40572-015-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr KK, Asal NJ. 2014. Efficacy of electronic cigarettes for smoking cessation. Ann Pharmacother 48(11):1502–1506, PMID: 25136064, 10.1177/1060028014547076. [DOI] [PubMed] [Google Scholar]
- Pappas RS, Fresquez MR, Martone N, Watson CH. 2014. Toxic metal concentrations in mainstream smoke from cigarettes available in the USA. J Anal Toxicol 38(4):204–211, PMID: 24535337, 10.1093/jat/bku013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RJ, Hensel EC, Morabito PN, Roundtree KA. 2015. Electronic cigarette topography in the natural environment. PLoS One 10(6):e0129296, PMID: 26053075, 10.1371/journal.pone.0129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffari A, Daher N, Ruprecht A, De Marco C, Pozzi P, Boffi R, et al. 2014. Particulate metals and organic compounds from electronic and tobacco-containing cigarettes: comparison of emission rates and secondhand exposure. Environ Sci Process Impacts 16(10):2259–2267, PMID: 25180481, 10.1039/c4em00415a. [DOI] [PubMed] [Google Scholar]
- Saint-Jacques N, Parker L, Brown P, Dummer TJ. 2014. Arsenic in drinking water and urinary tract cancers: a systematic review of years of epidemiological evidence. Environ Health 13:44, PMID: 24889821, 10.1186/1476-069X-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenborn CA, Gindi RM. 2015. Electronic cigarette use among adults: United States, 2014. NCHS Data Brief 217:1–8, PMID: 26555932. [PubMed] [Google Scholar]
- Sleiman M, Logue JM, Montesinos VN, Russell ML, Litter MI, Gundel LA, et al. 2016. Emissions from electronic cigarettes: key parameters affecting the release of harmful chemicals. Environ Sci Technol 50(17):9644–9651, PMID: 27461870, 10.1021/acs.est.6b01741. [DOI] [PubMed] [Google Scholar]
- St Helen G, Havel C, Dempsey DA, Jacob P III, Benowitz NL. 2016. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction 111(3):535–544, PMID: 26430813, 10.1111/add.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien C, Ramarao BV. 2007. Granular Filtration of Aerosols and Hydrosols. 2nd Edition. Amsterdam, Netherlands:Elsevier Science. [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2000a. Nickel Compounds. https://www.epa.gov/sites/production/files/2016-09/documents/nickle-compounds.pdf [accessed 19 December 2017].
- U.S. EPA. 2000b. Chromium Compounds. https://www.epa.gov/sites/production/files/2016-09/documents/chromium-compounds.pdf [accessed 5 October 2016].
- U.S. EPA. 2012. Inhalation Health Effect Reference Values for Manganese and Compounds. nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P100KJKC.TXT [accessed 14 December 2017].
- U.S. EPA. 2016. NAAQS Table. https://www.epa.gov/criteria-air-pollutants/naaqs-table [accessed 5 October 2016].
- Williams M, Bozhilov K, Ghai S, Talbot P. 2017. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS One 12(4):e0175430, PMID: 28414730, 10.1371/journal.pone.0175430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, To A, Bozhilov K, Talbot P. 2015. Strategies to reduce tin and other metals in electronic cigarette aerosol. PLoS One 10(9):e0138933, PMID: 26406602, 10.1371/journal.pone.0138933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P. 2013. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One 8(3):e57987, PMID: 23526962, 10.1371/journal.pone.0057987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.