Summary
Acquired immunodeficiency syndrome (AIDS) is principally a disease of lymphoid tissues, due to the fact that the main target cell of human immunodeficiency virus (HIV) is the CD4+ T lymphocyte that primarily resides within organs of the immune system. The impact of human immunodeficiency virus (HIV) infection on secondary lymphoid tissues, in particular lymph nodes, is critical to delineate, as these immune organs are the principal sites for initiating and facilitating immune responses and are critical for lymphocyte homeostatic maintenance and survival. The underlying structural elements of lymphoid tissues, fibroblastic reticular cell (FRC) network, not only form the architectural framework for these organs, but also play in integral role in the production and storage of cytokines needed for T-cell survival. There is an interdependent relationship between the FRC stromal network and CD4+ T lymphocytes for their survival and maintenance that is progressively disrupted during HIV disease. HIV infection results in profound pathological changes to lymphoid tissues induced by persistent chronic immune activation and inflammation that leads to progressive collagen deposition and fibrosis disrupting and damaging the important FRC network. In this review, I focus on the process, mechanisms, and the implications of pathological damage to important secondary lymphoid tissues, combining what we have learned from HIV-infected individuals as well as the invaluable knowledge gained from studies in non-human primate simian immunodeficiency virus infection models.
Keywords: T cells, AIDS, lymph nodes, inflammation, fibrosis
Introduction
Individuals infected with the human immunodeficiency virus (HIV), the causative agent of the acquired immunodeficiency syndrome (AIDS), eventually succumb to opportunistic infections and malignancies following progressive CD4+ T-cell depletion if they do not receive antiretroviral therapy (ART). The World Health Organization estimates over 25 million people have already died from AIDS since it was first recognized over 3 decades ago, and approximately 34 million people were currently living with HIV infection in 2011 (1).
The hallmark of HIV is its highly focused targeting of cells expressing the CD4 receptor, particularly CD4+ T cells, resulting in the selective and progressive depletion of these critically important lymphocytes. The loss of CD4+ T cells severely weakens the immune system, leading to progressive generalized immunodeficiency restricting the ability of infected individuals to adequately defend against infections and some types of cancer (1). While multiple mechanisms have been implicated in contributing to the loss of CD4+ T cells in HIV-infected individuals, progressive damage to secondary lymphoid tissues (LTs) has been demonstrated to play a significant role in the progressive loss of CD4+ T cells. HIV establishes a disseminated productive infection affecting presumably every LT within the body. Although the infection triggers potent host inflammatory and adaptive immune responses, the virus utilizes multiple mechanisms to evade these responses, maintaining levels of viral replication that establishes and sustains a state of chronic persistent immune activation that continues throughout the course of infection in the absence of ART. Collateral tissue damage typically results from unabated chronic immune activation, regardless of the underlying cause, and the chronic immune activation associated with HIV is no exception. This review focuses on our current understanding of the immunopathology of secondary lymphoid tissues [focusing on lymph nodes (LNs)] in lentiviral infections, combining what we have learned from HIV-infected individuals as well as the invaluable knowledge gained from studies in non-human primate (NHP) models, including progressive pathogenic experimental simian immunodeficiency virus (SIV) infection in macaques and non-progressive infections in natural hosts of SIV such as sooty mangabeys and African green monkeys.
Lymph node anatomy and function
LNs are important organs of the lymphatic system critical for both lymph fluid filtration and multiple key aspects of immune system function, properties that are dependent on maintenance of the intricate structure of these secondary LTs. Lymphocytes and many other leukocytes continually enter and migrate through LNs, which are well organized, roughly oval-shaped glandular organs distributed widely throughout the body and linked together by lymphatic vessels. LNs and other secondary LTs, such as the spleen and gut-associated lymphoid tissue (GALT), are densely packed but highly structured tissues, where the vast majority of mature B and T lymphocytes reside in the body (2). Initial studies estimated that about 2% of total lymphocytes in the body circulate in the blood (3); however, a more recent study using a SPECT imaging approach to quantify CD4+ T cells in macaques has estimated that the peripheral blood only contains between 0.3% to 0.5% of the total CD4+ T cells in the body, with the remaining lymphocytes residing in LNs and other LTs (2). Thus, while the peripheral blood is a convenient compartment for monitoring CD4+ T cells in HIV disease, this compartment represents only a small fraction of the body’s total T-cell population.
The primary function of secondary LTs, in particular the LNs, is to provide the anatomic framework to facilitate induction of adaptive immune responses by antigen-specific B and T cells and to maintain immunologic memory. The anatomic structures of these organs are exquisitely suited to support these functions. LNs are classically described as having three main anatomic compartments: the cortex, paracortex, and medulla. Extravascular lymphocytes and antigen-presenting cells (APCs) can enter the LN through afferent lymphatic vessels, after which they then migrate across the floor of the subcapsular sinus into the cortex or paracortex. In contrast, intravascular lymphocytes (in particular naive lymphocytes), monocytes, and leukocytes enter the LN through specialized endothelial cells that facilitate binding and transmigration of circulating leukocytes, referred to as high endothelial venules (HEVs) (4) (Fig. 1). After entering LNs, most B and T cells migrate into anatomically separate compartments, guided by chemotactic gradients, where they interact with APCs, receive survival signals, and initiate antigen specific immune responses (5) (Fig. 1). The compartmentalization of the LN allows for T and B cells to each interact with their respective specific type of APC needed to undergo clonal expansion. Importantly, the chemotactic migration of lymphocytes occurs along the fibers of specialized stromal cells called fibroblast reticular cells (FRCs), which collectively provide the three-dimensional network of LNs (6–10).
Figure 1. Lymph node anatomy and function.
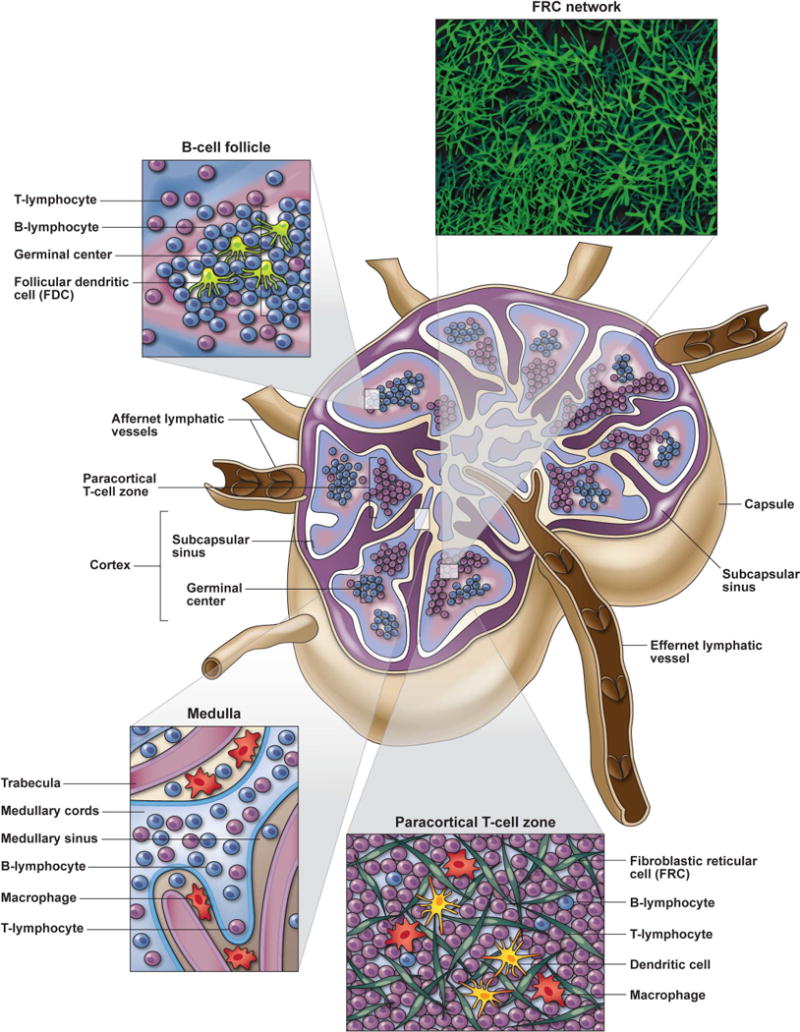
LNs are highly organized lymphoid tissues that facilitate immune responses through the orchestrated movement and interactions of lymphocytes with their respective antigen presenting cell (APC) populations (i.e. DCs for T cells and FDCs for B cells). Lymphocytes and APCs enter the LN either through the afferent lymphatic vessels or HEVs and migrate along the FRC network in response to chemotactic cues. Activated B cells primarily migrate to the superficial cortical region of LNs where B cell follicles are abundantly located. B cells entering follicles rapidly proliferate to form germinal centers and interact with FDCs and TFH to mount humoral immune responses. Most T lymphocytes (except specialized TFH that migrate to B cell follicles) transvers to the paracortical T cell zone (TZ) located intermediate to the superficial cortex and deep medullary cords. Within the TZ, T cells continually migrate along the FRC network interacting with numerous DCs interspersed throughout this 3-deminsional meshwork, sampling MHC-peptide complexes until their TCR binds to their cognate MHC-peptide match at which time they stop migrating, receive activation signals and undergo clonal expansion. FRCs are an important source of homeostatic cytokines, especially IL-7, that are needed for T cell survival during their transition through LTs. Lymphocytes that do not bind to their cognate antigen, egress from LNs through the medullary cords and efferent lymphatic vessel. Lymph fluid contains abundant particulate antigens that need to be removed before it can be recirculated through the blood. LNs serve as an important filtration system for bodily fluids, and this lymph filtration primarily occurs through the medullary
B cells migrate into the superficial cortex, which lies beneath the subcapsular sinus and above and adjacent to the deeper paracortical T-cell zone (TZ). B cells are guided to the edge and eventually into primary follicles by the chemokines CXCL12 and CXCL13 respectively (11–13), where they survey extracellularly retained antigens (in the form of immune complexes) on follicular dendritic cells (FDCs) (5, 7, 8). Upon recognizing their cognate antigen, stimulated B cells migrate and proliferate within follicles forming distinctive germinal centers (GCs) referred to as secondary follicles. Activated B cells receive T-cell help from T-follicular helper (Tfh) cells, undergo somatic hypermutation and interact with FDCs that support the development of effective memory B cells and high affinity antibody responses (14–20).
T lymphocytes that enter the LN through the afferent lymphatics or HEVs enter the paracortical TZ that stretches from the base of (and between) B-cell follicles to the medullary cords (Fig. 1). APCs are positioned throughout the TZ to encounter and activate the rare T cells reactive with any specific presented antigen. Specific chemokines direct T-cell trafficking within the TZ, with the chemokine CCL21 playing a particularly important role in homeostatic trafficking of naive T lymphocytes. T cells continually migrate through LNs in a random walk-like pattern ‘sampling’ the antigens presented by APCs (21). Both naive and memory T cells retained and circulating through LNs require critical signals for survival and homeostatic proliferation provided by certain cytokines and growth factors, such as interleukin-7 (IL-7), produced and retained on the FRC network (6, 22, 23). T cells, particularly naive T cells, migrating through LNs survive by periodic contact of their T-cell receptor (TCR) with self-peptide–major histocompatibility complex (MHC) class I or II complexes plus exposure to survival factors such as IL-7, which sustain expression of antiapoptotic molecules allowing cells to survive during transition from circulation into LTs (24, 25). T lymphocytes that interact with DCs within the TZ presenting antigen on MHC class I and II and recognize their cognate antigen via their TCR proliferate within the TZ. The interactions of DCs with antigen-specific T cells is a bidirectional cross-talk, resulting in timed expression of needed costimulatory molecules as well as the production and secretion of cytokines, which collectively results in the maturation and expansion of antigen-specific lymphocyte populations (26). These interactions of T cells and DCs are accomplished by T cells migrating along the FRC network sampling DCs that are themselves in direct or close contact with FRCs. Thus, induction of adaptive immune responses involves multicellular interactions highly orchestrated in space and time that require cell migration to important LN compartments, cellular coupling, with the release of appropriate cytokines that promote orderly maturation of antigen specific lymphocytes.
LNs have an intricate structural framework that consists of extracellular matrix proteins, such as collagen fibers, surrounded by FRCs that forms an elaborate ‘sponge-like’ meshwork. This FRC meshwork provides structural support, the framework for cell migration, retention (and in some cases production) of needed cytokines necessary for survival and lymphocyte homeostatic proliferation, and anatomic organizations that facilitate interactions between lymphocytes and APCs (7, 8). This organized FRC network facilitates cellular movement along preformed cable-like pathways (often referred to as LN highways), while the basement membrane-like extracellular structure forms a conduit system for fluid transport and delivery of low molecular weight molecules within the LN (9, 10, 23). Intravital microscopy studies have demonstrated that lymphocytes can spend 10 to 100 minutes interacting and moving along the FRC network in each LN, where they can be continually exposed to inflammatory mediators, cytokines and chemokines, and interact with hundreds of DCs (7, 27). Thus, the FRC network and conduit system of LNs distributes chemokines, survival signals (i.e. homeostatic cytokines, growth factors, etc.), and inflammatory mediators within secondary lymphoid organs. These mediators facilitate lymphocyte transmigration across HEVs and cellular migration, localization and compartmentalization of lymphocyte populations after entering the LN parenchyma that is critical for the efficient development of productive adaptive immune responses. Given the importance of the intricate structure of LNs in supporting key aspects of immune system function, it is not hard to envision how processes that disrupt this structure could have adverse consequences for the host.
Historical perspective
While the FRC network and basement membrane provide the framework that supports and gives structure to LNs, these tissues retain remarkable plasticity, as during infections lymphocytes induced to proliferate to fight a particular pathogen increase in number, causing the LN to expand and become enlarged. This clinical manifestation of enlarged LNs (i.e. lymphadenopathy) was one of the first recognized symptoms in patients at the inception of the AIDS epidemic (28–30).
From the establishment of the HIV-1 epidemic, before AIDS was even known as AIDS, it was associated with a particular pattern of immunopathology that included generalized lymphadenopathy, lymphopenia, and a decreased ratio of CD4+ to CD8+ T cells (previously referred to as helper to suppressor T cells ratios) (28, 30, 31). While AIDS was initially described in the US among young homosexual men, it was also found in hemophiliacs and intravenous drug users. Many of these patients presented with rare neoplasms (such as Kaposi’s sarcoma and B-cell lymphoma) and serious opportunistic infections (28, 32, 33). Currently the global epidemiology of the disease is such that the vast majority of newly acquired infections are heterosexually transmitted, with young women in sub-Saharan Africa experiencing a disproportionately higher burden of new infections (34).
Initial studies of HIV disease recognized the importance the secondary LT system played in this disease and focused on understanding the histological and pathological features reflecting HIV infection of these important immune tissues. These studies coalesced on some common histopathological features of the affected LTs. In early studies of patients with AIDS, the histopathological lesions within secondary LTs segregated into three common but distinct patterns, with most patients presenting with an explosive follicular hyperplasia with focal coalescence of follicles seen as large irregularly shaped follicles (29, 35). The second pattern consisted of follicular involution and atrophy with only small hypocellular, sometimes hyalinized or fibrotic, GCs and paracortical hyperplasia with the medullary cords densely packed with sheets of plasma cells (29, 36). All patients with follicular involution had severe T-cell dysfunction as demonstrated by inversion of the CD4+-to-CD8+ T-cell ratio, depressed response to mitogens, and deficient natural killer cell activity, with many patients with this type of histopathology demonstrating opportunistic infections, such as lymphoma and Kaposi’s sarcoma that we now delineate as AIDS defining clinical end points (29). Immunohistology showed a temporal and structural relation between follicular involution, disappearance of FDCs, and follicular invasion by T cells. These observations suggested elimination of FDCs as part of a pathogenic mechanism in follicular involution (36). The third pattern of histopathology consisted of follicular hyperplasia in parts of the node and follicular involution with paracortical expansion in other areas, and it was soon realized that this mixed pattern really represented a transition phase from explosive follicular hyperplasia to follicular involution and atrophy (29, 36). Subsequent studies refined the scheme to four distinct classifications that were demonstrated to have prognostic value in the evaluation of the progression of HIV disease based upon the degree of damage to follicular structures: (i) follicular hyperplasia, (i) follicular lysis, (iii) follicular atrophy with or without fibrosis, and (iv) follicular and lymphocytic depletion (37, 38). These early studies demonstrated a progressive pattern of secondary LT destruction culminating in end-stage AIDS, with destruction of the architecture of the LTs compounding the immune dysfunction (39–41).
Further studies that looked extensively at the histopathology of LNs from patients with AIDS demonstrated additional abnormal features that included focal hemorrhages, extensive cellular destruction, accumulation of neutrophils, phagocytosis of nuclear debris, proliferation of blood vessels, immunoblasts, and peculiar aggregates of clear cells that were classified as acute lymphadenitis. Importantly, these presentations suggested that the systemic, persistent lymphadenitis of AIDS patients was induced by a microorganism, likely a virus with lymphotropism that resulted in the destruction of a CD4+ T lymphocytes leading to the induction of AIDS (30, 42, 43).
Following the identification of HIV as the causative virus for AIDS (44, 45), HIV antigens and viral particles were found within LNs, in particular GCs associated with FDCs, from patients at all phases of disease (46–52). These data further highlighted the integral role of secondary LTs in this disease. In fact, of the >1010 virions estimated to be generated per day (53), the vast majority were demonstrated to be produced and stored in massive quantities in secondary LTs as immune complexes bound to FDCs (53).
Soon after these findings in humans, SIV-infected macaques were demonstrated to have a disease presentation and progression to AIDS remarkably similar to humans, and analysis of LTs from infected macaques revealed strikingly parallel histologic features (54, 55). These studies highlighted the usefulness of SIV-induced disease in macaques as a model for the study of AIDS in humans (54, 55). Since these pioneering early studies, SIV macaque models have emerged as arguably the most robust set of animal models ever used in infectious disease research, recapitulating nearly every aspect of HIV disease in humans. While these NHP models have been utilized for all aspects of HIV disease research, from transmission studies to preclinical evaluations of ART, vaccines, passive antibody administration and microbicides, they have also proven to be absolutely invaluable to pathogenesis studies aimed at understanding the complex mechanisms driving the progressive immunopathology of LTs in HIV infection (56, 57).
The role of pathological damage of lymph node architecture in HIV/SIV pathogenesis
At the heart of HIV disease is the progressive loss of CD4+ T cells. Since the discovery that CD4+ T lymphocytes were the principal target cells for HIV, numerous groups have been focused on dissecting the complex multifactorial mechanisms contributing to destruction of CD4+ T cells. Because most T cells reside in and are dependent on tophic interactions occurring in secondary LTs for their survival, it became evident over time that the HIV-infection associated immunopathology of these important immune organs was likely linked to CD4+ T-cell loss. Several longitudinal studies of individuals with progressive HIV infection identified progressive changes in secondary LT structure, from the gross presentation of small and fibrotic LNs, to microscopic changes characterized by diffuse augmentation of collagen III, elastic fibers, laminin, fibronectin and proteoglycans. Strikingly, the patients with the most severe LN damage had the most marked CD4+ T-cell depletion (39, 58). More recently, LN fibrosis, resulting from extensive collagen deposition initially within the TZ, was demonstrated to be a major component of the pathological damage in these important immune tissues in HIV-infected individuals. The link between CD4+ T cells and LT damage was further made by the observation that the extent of the fibrosis was inversely correlated with the size of the CD4+ T-cell population within the TZ compartment of LNs in HIV-infected patients (59–61).
How does LN fibrosis impact CD4+ T-cell loss? Fibrosis, in general, is a condition marked by increased extracellular matrix constituents (primarily collagen I and III and fibronectin), produced by resident tissue fibroblasts, and is a characteristic component in the pathogenesis of a wide spectrum of diseases (i.e. chronic infections, cancer, inflammatory disorders) (62). As fibrosis develops, normal tissue is replaced by fine scar-like structures, which create obstructions that limit lymph drainage, resulting in further accumulation of extracellular matrix proteins that exacerbates the fibrotic process (63). The functional component of the tissue is gradually replaced by the accumulating extracellular matrix proteins, disrupting normal tissue structure and function. Thus, fibrosis within the parenchyma of secondary LTs could have a profound impact on immunological function and lymphocyte population dynamics.
As discussed above, secondary LTs provide a critical infrastructure for key immune functions, and this capability is dependent on maintenance of anatomic microenvironmental niches. Thus, progressive damage to these tissues could have deleterious effects on lymphocyte populations and function. Growing evidence in both HIV patient cohorts and SIV NHP models demonstrates that AIDS virus infection associated chronic immune activation and persistent inflammation induces profound damage to secondary LTs that limits restoration of CD4+ T-cell populations (63–68). Chronic immune activation and inflammation-driven damage to secondary LTs is manifested by progressive deposition of fibrotic collagen, first within the TZ compartment, which then extends into all LT compartments as disease progresses (59, 68) (Fig. 2). The fibrotic collagen accumulation in LNs continues in a quantifiable and progressive stage-dependent process that ultimately results in LNs in end-stage AIDS that are profoundly disorganized and that can have over 30% of the parenchyma replaced by fibrotic collagen (69).
Figure 2. Progressive pathological lymph node fibrosis in HIV/SIV infection.

Cartoon schematic (top panel) showing the effect of progressive fibrosis on CD4+ T cell and FRC loss during different stages of HIV/SIV infection. NHP SIV-infection models have provided important insight into the pathological damage of LTs at different stages of disease, with particular insight into the acute phase. Progressive collagen (middle panel; brown IHC staining) and fibronectin (bottom panel; red immunefluorescent staining) deposition occurs early during the acute phase and continues through the chronic phase of SIV infection as a consequence of persistent chronic immune activation and inflammation.
Among infectious pathogens, HIV is unique in its ability to establish a persistent infection within presumably every LT within the body, causing chronic pathological systemic immune activation. Even other pathogens that establish chronic infections, such as hepatitis C virus (HCV) and hepatitis B virus (HBV), with persistent viral replication in the liver producing sustained systemic viral loads up to two to three logs greater than seen in chronic HIV infection (70), do not result in systemic immune activation and secondary LT damage like that are seen in HIV disease. Because HIV/SIV is able to directly establish infection in ostensibly every LT, this infection has a profound effect on organ systems that are integrally linked to secondary LTs and immune function, such as the gastrointestinal tract. As such, HIV/SIV infection results in epithelial barrier damage and associated microbial translocation, providing yet another persistent source of immune stimulation (71–74). The response to this chronic inflammatory stimulation includes pathways that result in progressive fibrotic damage to secondary LTs. Thus, it is not simply the structural and functional breakdown of one or even a series of LNs but the cooperative collective damage to effectively all the host’s secondary immune organs systemically that leads to a vicious cycle that contributes to the deterioration of the immune system that manifests clinically as AIDS.
ART, LN pathology, and CD4+ T-cell recovery
Most patients respond to ART with significant reductions in plasma virus loads and with decreases in FDC-associated HIV-1 and productively infected cells in the LNs (75). However, while virological responses following ART occur in most patients, a large body of accumulated clinical experience has identified a subpopulation of up to a quarter of treated patients, designated ‘immunologic non-responders’, who experience little or no increase in CD4+ T-cell counts despite achieving apparently effective viral suppression (76, 77). The best CD4+ T-cell recovery outcomes are achieved in patients that receive ART early after becoming infected with higher baseline CD4+ T-cell counts (CD4>350 cells/μL), compared to patients that start ART later in the chronic phase of the disease (CD4<350 cells/μL) (78, 79). The more variable and generally poorer immunological recovery seen in chronically infected HIV patients receiving ART can be put in perspective when one recognizes that most of the T cells in the body reside within LTs. While many HIV-infected patients have some level of normalization of immune parameters on ART, such as decreased immune activation, partial repopulation of LN lymphocyte populations, and reformation of active secondary follicles (75), virtually all treated HIV-infected patients have evidence of persisting LT abnormalities (i.e. follicular absence, regression, and hyperplasia, abnormal CD4+ T-cell population size, and damaged LT architecture) (80). Importantly, even with significant recovery of peripheral blood CD4+ T cells, CD4+ T-cell populations within secondary LTs remain markedly reduced (63, 80).
While the connection between clinical measures of CD4+ T-cell counts and LN immunopathology have been previously suggested (81), the relationship between progressive fibrotic damage in LNs and CD4+ T-cell recovery was first demonstrated by Schacker et al. (59, 82), who showed that the amount of collagen deposited in the TZ of LNs at the time of initiation of suppressive ART predicts the magnitude of recovery of the peripheral CD4+ T-cell pool with treatment. The contribution of secondary LT fibrosis to CD4+ T-cell loss and limited recovery has been referred to as the ‘damaged niche hypothesis’, since it is the physical accumulation of fibrotic scar tissue that leads to the functional loss of the normal parenchyma of the LT that is so critical for T-cell survival (60, 61, 63–68, 83).
In addition to effects on CD4+ T-cell populations, the progressive damage to the architecture of secondary LTs, particularly within the paracortical TZ, may have implications on other important physiological systems and processes, including the autonomic nervous system. The finding of reduced catecholaminergic neural fibers in SIV-infected LNs suggests that lentiviral pathology may alter autonomic innervation in ways that could potentially affect neuroimmune interactions (84). In addition, alterations and damage to the LT architecture may also help explain the functional changes in APC populations that fail to fully mature and adopt tolerogenic properties (85), demonstrating the far-reaching effects damage to these important immune organs can have.
Collectively, the progressive structural and functional damage to important lymphoid organs leads to progressive quantitative and qualitative immunodeficiency that is ultimately manifested clinically in AIDS defining opportunistic infections or neoplasms. Recently it has been suggested that there is a key inflection point in the progression of HIV/SIV infection at which the ability of the remaining CD4+ TCM compartment to maintain adequate ongoing replenishment of the CD4+ TEM cells that are the preferred target cells destroyed by the virus drops below a threshold, leading to a clinically significant immunocompromised state (86, 87). Since as adults age peripheral T-cell expansion plays an increasingly important role in maintaining T-cell populations compared to the thymus (88), progressive damage to secondary LT microenvironments may be an important process that limits CD4+ effector T-cell generation over time. The cumulative loss of CD4+ effector T cells may be the consequence of significant decreases of particular immune populations, such as the decline of CD4+ TCM cells below a key threshold, but other mechanisms may contribute, including immune ‘organizational failures’ of several partially depleted and excessively stimulated cell populations to function in a coordinated way (86, 87). These ‘organizational failures’ may be a function of progressive LT fibrotic damage, which may have a particularly harmful impact on the induction of productive T-cell responses, because it has been shown that collagen is a potent negative regulator of immunological synapse formation (89). Thus, collagen deposition in LTs in HIV infection may not only play a role in quantitative CD4+ T-cell depletion but also in qualitative immune dysfunction. Progressive inflammation related fibrosis of secondary LT likely contributes to deterioration of immune function and the process of disease progression to AIDS mediated by multiple potential mechanisms.
Role of Inflammation and Chronic Immune Activation on HIV/SIV Lymph Node Pathology
Early immunopathological studies of LNs in HIV-1 infected patients suggested that LT changes represent different stages of a dynamic process progressing from hyperplasia to atrophy and lymphocytic depletion (90) and highlight that HIV-1 infection is primarily an infection of the lymphoid system.
What are the underlying mechanisms driving LN pathology in HIV-1 infection? While much of what we know comes from studies from HIV-infected patients, NHP models of HIV pathogenesis have provided key insights into the mechanistic pathways leading to pathologic damage to these important secondary lymphoid organs.
Early studies in HIV-infected patients and SIV-infected macaques showed evidence for type I interferon (IFN) responses during progressive disease (91). Although, the importance and implications of these findings were not fully realized at first we now know that these responses are a persistent manifestation of chronic inflammation within these tissues. Several groups since have shown that the type 1 and 2 IFN-stimulated genes and pathways, as a whole, are significantly elevated in all stages of disease from the early acute to late end-stage in progressive HIV-1 and SIV infections (75, 92).
Although definitions of immune activation vary depending on the study, it is clear that there is broad hyperactivation of immune cell populations and heightened levels of a variety of immune cytokines in chronic HIV-1 infection (26). Several studies aimed at understanding the underlying causes for the LT immunopathology have provided compelling evidence for persistently elevated immune activation characterized by polyclonal B cell activation (93), increased T-cell turnover (94), increased frequencies of T cells with an activated phenotype (95), and increased levels of pro-inflammatory molecules in both peripheral blood and secondary LT s (96). In addition, visualizing SIV-infected RMs using positron emission tomography (PET) allowing whole body imaging revealed a pattern of widespread and dramatic LT activation consistent with these earlier studies (97). This persistent inflammatory response and chronic immune activation we now recognize as a fundamental hallmark of disease progression in pathogenic HIV/SIV primate lentiviral infections and has been demonstrated to be a stronger predictor of disease progression than either CD4+ T-cell count or plasma viral load (98).
The importance of immune activation and inflammation to disease progression in HIV/SIV infections is highlighted by the low levels of immune activation measured during the chronic phase of SIV infection in non-progressive natural hosts such as African green monkeys (AGMs) and Sooty mangabeys (SMs), which abide high-levels of viral replication and viremia, but rarely experience significant CD4+ T cell decline and do not typically progress to AIDS (99). While these natural hosts of SIV have been demonstrated to mount robust levels of immune activation and inflammatory responses during the acute phase of disease, they rapidly resolve these responses during the transition from acute-to-chronic disease in the absence of viral clearance, which is in stark contrast to progressive SIV infections in macaques that demonstrate unabated inflammatory responses (92, 100–102). The resolution of immune activation and inflammation in natural SIV hosts likely is critical for preserving immune integrity and function, as these natural hosts do not display any abnormalities in LN morphology, histology, and lack any evidence of LT fibrosis (99, 100).
Mechanism of Pathologic Collagen Deposition in Lymph Nodes in HIV/SIV Infection
As with other fibrotic diseases, the progressive pathological fibrosis in LNs during HIV/SIV infection is intimately linked to the chronic immune activation and inflammation within the affected tissues. However, what are the molecular signal(s) that stimulate the excessive accumulation of collagen and other ECM components in HIV/SIV disease? In studies utilizing a progressive SIV infection NHP model, the pathological production and deposition of fibrotic collagen was linked to the early induction of an immunoregulatory response in secondary LTs that consisted of T regulatory cells (Tregs), as well as other cell types that expressed transforming growth factor (TGF)β (68, 103). The rapid induction of a heterogeneous immunosuppressive response consisting of multiple Treg populations shortly after SIV infection in RMs recapitulates the reported increases and accumulation of Treg cells and TGFβ expression in secondary LTs of HIV-infected individuals (104–107).
Observations from many animal models of fibrotic diseases implicating TGFβ as an important mediator of pathologic fibrosis (108–111), suggested that this cytokine might be an important profibrotic signal in HIV/SIV disease. Utilizing a RM progressive SIV infection model, in which the timing of collagen deposition could be studied and its spatial and temporal association with TGFβ expressing cells (including Tregs) examined, we showed that the initiation of fibrotic damage in the paracortical TZ was both rapid and progressive with collagen deposition quantitatively apparent by 7 days post-infection (>8-fold increase) and dramatically augmented by 28 days post-infection (>20-fold increase) compared to LN levels of collagen before infection (68). Collagen deposition continued unabated throughout all stages of disease in the absence of intervention until AIDS defining illness and death ensued. The extent of collagen deposition was significantly related to depletion of CD4+ T cells, albeit in a complex nonlinear manner (68).
The extent and timing of collagen deposition in this model paralleled increases in immune activation (Ki67+ cells) and TGFβ+ cells, with TGFβ-expressing cells found spatially localized within regions of high collagen deposition during acute infection and their frequency correlated with the magnitude of collagen increase (68). These findings suggested that the increase in TGFβ as one arm of an immunosuppressive regulatory response intended to counter potential harmful effects of acute inflammation and immune activation following SIV infection may also have resulted in collateral fibrotic tissue damage. This in vivo process is nicely modeled in an ex vivo experimental system in which TGFβ-expressing Tregs were co-cultured with primary autologous or allogeneic fibroblasts. In this system, TGFβ+ Tregs, but not conventional activated T cells, were directly linked to collagen expression and deposition by fibroblasts (68).
Subsequent work has shown that up-regulation of TGFβ by Tregs and the TGFβ receptors on fibroblasts in HIV infected patients leads to activation of the TGFβ signaling pathway (66). Increased TGFβ mediated signaling in LNs of HIV infected patients (as measured by phosphorylated STAT2/3 expression) resulted in progressively increased production of procollagen and expression of chitinase 3-like-1 (CHI3L1), an enzyme that enhances maturation of procollagen into the collagen fibrils that are the major component of fibrotic scar tissue (66). The importance of immune activation driving the induction of TGFβ expressing Tregs and the prominence of collagen deposition in the pathologic damage to secondary LTs in vivo was further highlighted by the absence of elevated TGFβ expression or LN fibrosis in non-progressive SIV infection of Sooty mangabeys, a natural host of the virus in which infection is typically not pathogeneic (68). Thus, the rapid induction of TGFβ+ cells, and other anti-inflammatory responses, shortly after SIV infection may not only adversely dampen adaptive antiviral immune responses during a time that these responses are critically needed (103), but can also cause harmful effects on CD4+ T cell populations by inducing the progressive, pathological process of fibrotic collagen deposition in secondary LTs (68).
Further support for the model of immune activation driving TGFβ expression and LT fibrosis in HIV/SIV disease came from a recent NHP study aimed to better understand the contribution of acute inflammation to lentiviral pathogenesis. In this study, RMs newly infected with SIV were treated with an anti-TNF blocking antibody (adalimumab; Humira) (112). While anti-TNF antibody treatment did attenuate the expression of many proinflammatory genes, this treatment did not affect plasma SIV vRNA levels or classical measures of immune activation (CD38 or Ki67 expression) in peripheral blood or LN T cells (112). However, compared with untreated RMs, anti-TNF antibody treated animals showed attenuated expression of anti-inflammatory regulatory responses to SIV infection including reduced TGFβ expression, that was associated with reduced LT fibrosis and better preservation of CD4+ T cells (112). Thus, similar to non-progressive SIV infection in natural hosts, dampening LN inflammatory responses and attenuating TGFβ expression can limit pathologic LN fibrosis even in the setting of ongoing viral replication, observations that point to the potential therapeutic benefit of anti-inflammatory and anti-fibrotic therapy in conjunction with ART to modulate LT pathology and improve immunological recovery.
While the production and deposition of collagen (and other ECM proteins) induced by TGFβ is a major pathophysiological feature of fibrosis, TGFβ can influence the expression of other factors that contribute to the progression of fibrosis such as the imbalance between matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) (113). TGFβ has been demonstrated to directly induce TIMP-1 expression in myofibroblasts (114), implicating TGFβ in multiple aspects of the fibrosis pathway. Recently, it was demonstrated that HIV-infected patients that have significantly increased collagen deposition in secondary LTs not only had fewer CD4+ T cells, but also decreased MMP-9/TIMP-1 and MMP-2/TIMP-2 ratios compared with HIV-negative control patients (61). Collectively, the host’s ardent immunosuppressive regulatory response mounted to dampen HIV/SIV driven inflammation and immune activation ultimately results in the sustained high expression levels of the profibrotic cytokine TGFβ within secondary LTs culminating in the production and deposition of collagen and other fibrotic ECM proteins and resulting in the disruption of normal LN architecture and function.
The Reciprocal Dependency of the Stromal FRC Network and CD4+ T Cells
Although the recognized profibrotic signals (namely TGFβ) that result in LN fibrosis in HIV/SIV infections and the link between pathological secondary LT fibrosis and CD4+ T cell population size has been realized now for several years (59, 60, 64, 82, 83), the mechanisms by which fibrosis of LT directly impacts CD4+ T cells and impairs immune reconstitution in lentiviral infections have not been well defined until recently (69). These mechanistic studies were only possible by utilizing SIV-infected NHP and small animal models, which allowed for the detailed analysis of LTs obtained in longitudinal, followed by confirmation in cross-sectional, studies.
First, using an SIV NHP model in which LNs were collected prior to SIV infection and during the acute and chronic phase of infection, it was demonstrated that FRCs are the major source of IL-7, the primary survival factor for naïve T cells, and that LT fibrosis limits lymphocyte access to FRC-derived IL-7, resulting in depletion of T cells, particularly naïve CD4+ T cells, through apoptosis (69). T cell loss in turn diminishes the availability of T cell–derived and expressed lymphotoxin-β (LTβ), on which the FRC network depends for its survival (115). The decreased availability of LTβ-producing cells, along with collagen-restricted access to FRCs, results in loss of both the FRC network and IL-7 production. This further impairs the survival of naïve T cells, perpetuating a vicious cycle of continuous and cumulative loss of both T cells and the FRC network with progressive fibrosis (69).
The direct reciprocal relationship and dependency between the FRC network and T cell populations were further shown in a small animal model in which mice were administered either LTβR-Ig, an anti-CD3 depleting antibody, or control antibody. Mice with depleted FRC network (after LTβR-Ig treatment) demonstrated reduced T cell populations and mice depleted of T cells (after anti-CD3 treatment) demonstrated a reciprocal loss in the FRC network (69). Further analysis of NHP LNs demonstrated that T cells were a major source of LTβ (~80% of the LTβ+ cells were T cells) in the TZ for which FRCs are dependent, and the loss of LTβ+ T cells followed a parallel time course with the loss of the FRC network in SIV infection (69). This reciprocal relationship between T cells and the FRC network was confirmed in additional NHP models that used antibody-mediated depletion of CD4+ or CD8+ T cells in both RMs and sooty mangabeys (116). These studies demonstrated that CD4+ T cell depletion, but not depletion of CD8+ T cells, in both NHP species, resulted in FRC network loss and that this loss was associated with decreased lymphotoxin-β expression, which was mainly produced by the CD4+ T cells (116).
Further support of this reciprocal relationship between T cell populations and the FRC network was provided in human studies that showed that collagen deposition and progressive loss of the FRC network in LNs prior to suppressive ART restricted both T cell access to and a major source of the survival factor IL-7 (67). Furthermore, the extent the FRC network loss and magnitude of the pathological collagen deposition predicted the extent of restoration of the naïve T cell populations after 6 months of ART (67). Importantly, the extent of restoration of the FRC network after initiation of ART was correlated with the stage of disease at which the therapy was initiated. Restoration of the FRC network and reconstitution of naïve T cell populations were only optimal when therapy was initiated in the early/acute stage of infection (67, 116). This relationship between CD4+ T cells and the FRC network extends beyond lentiviral infections, as CD4+ T cell depletion in cancer patients induced by chemotherapy and irradiation was associated with FRC network loss in secondary LTs (67). Collectively, the emerging evidence suggests that chronic immune activation during HIV infection indirectly impairs the survival of naïve T cells via induction of pathological damage to secondary LTs structures and that CD4+ T cells play a central role in the maintenance of LN architecture necessary for their own homeostasis and survival.
Summary.
AIDS is fundamentally a disease of lymphoid tissues, but although HIV replication is necessary for disease, it is not sufficient, and pathogenesis is substantially determined by the host response to infection. This is particularly true of the host responses in secondary lymphoid tissues, the primary site of viral replication in HIV infection. Inflammatory responses triggered by viral replication lead to countervailing immunoregulatory responses that lead to fibrosis of these tissues, resulting in a vicious cycle of progressive reciprocal destruction of the interdependent FRC network and T cell populations. This resultant structural and functional damage to lymphoid tissues can limit immune reconstitution, even when viral replication is suppressed by antiretroviral drug treatment. It is becoming increasing clear that in addition to controlling viral replication through potent ART, additional adjunctive therapeutic strategies are needed to restore immunological function in some HIV infected patients. Potentially promising strategies would target pathways that further attenuate the elevated immune activation and inflammation that drives pathological changes within secondary LTs, and anti-fibrotic approaches that are aimed at restoring the structural integrity and function of secondary immune organs.
Acknowledgments
I would like to thank Allen Kane with SPGM at the Frederick National Laboratory for Cancer Research for generation of the figures and Jeff Lifson for critically reading the manuscript and thoughtful input. This project has been funded with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.WHO. http://www.who.int/mediacentre/factsheets/fs360/en/index.html.2012.
- 2.Di Mascio M, et al. Noninvasive in vivo imaging of CD4 cells in simian-human immunodeficiency virus (SHIV)-infected nonhuman primates. Blood. 2009;114:328–337. doi: 10.1182/blood-2008-12-192203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trepel F. Number and distribution of lymphocytes in man. A critical analysis. Klin Wochenschr. 1974;52:511–515. doi: 10.1007/BF01468720. [DOI] [PubMed] [Google Scholar]
- 4.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 5.Willard-Mack CL. Normal structure, function, and histology of lymph nodes. Toxicol Pathol. 2006;34:409–424. doi: 10.1080/01926230600867727. [DOI] [PubMed] [Google Scholar]
- 6.Bajenoff M, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gretz JE, Anderson AO, Shaw S. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol Rev. 1997;156:11–24. doi: 10.1111/j.1600-065x.1997.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 8.Gretz JE, Kaldjian EP, Anderson AO, Shaw S. Sophisticated strategies for information encounter in the lymph node: the reticular network as a conduit of soluble information and a highway for cell traffic. J Immunol. 1996;157:495–499. [PubMed] [Google Scholar]
- 9.Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med. 2000;192:1425–1440. doi: 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaldjian EP, Gretz JE, Anderson AO, Shi Y, Shaw S. Spatial and molecular organization of lymph node T cell cortex: a labyrinthine cavity bounded by an epithelium-like monolayer of fibroblastic reticular cells anchored to basement membrane-like extracellular matrix. Int Immunol. 2001;13:1243–1253. doi: 10.1093/intimm/13.10.1243. [DOI] [PubMed] [Google Scholar]
- 11.Bleul CC, Schultze JL, Springer TA. B lymphocyte chemotaxis regulated in association with microanatomic localization, differentiation state, and B cell receptor engagement. J Exp Med. 1998;187:753–762. doi: 10.1084/jem.187.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansel KM, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 13.Casamayor-Palleja M, Mondiere P, Verschelde C, Bella C, Defrance T. BCR ligation reprograms B cells for migration to the T zone and B-cell follicle sequentially. Blood. 2002;99:1913–1921. doi: 10.1182/blood.v99.6.1913. [DOI] [PubMed] [Google Scholar]
- 14.Deenick EK, Ma CS. The regulation and role of T follicular helper cells in immunity. Immunology. 2011;134:361–367. doi: 10.1111/j.1365-2567.2011.03487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 16.Burton GF, Conrad DH, Szakal AK, Tew JG. Follicular dendritic cells and B cell costimulation. J Immunol. 1993;150:31–38. [PubMed] [Google Scholar]
- 17.Kapasi ZF, et al. Follicular dendritic cell (FDC) precursors in primary lymphoid tissues. J Immunol. 1998;160:1078–1084. [PubMed] [Google Scholar]
- 18.Liu YJ, Johnson GD, Gordon J, MacLennan IC. Germinal centres in T-cell-dependent antibody responses. Immunol Today. 1992;13:17–21. doi: 10.1016/0167-5699(92)90199-H. [DOI] [PubMed] [Google Scholar]
- 19.MacLennan IC, Germinal centers Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 20.Tew JG, Wu J, Qin D, Helm S, Burton GF, Szakal AK. Follicular dendritic cells and presentation of antigen and costimulatory signals to B cells. Immunol Rev. 1997;156:39–52. doi: 10.1111/j.1600-065x.1997.tb00957.x. [DOI] [PubMed] [Google Scholar]
- 21.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 22.Link A, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 23.Sixt M, et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol. 2009;9:823–832. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- 25.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Lederman MM, Margolis L. The lymph node in HIV pathogenesis. Semin Immunol. 2008;20:187–195. doi: 10.1016/j.smim.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 28.Strand OA. Acquired immunodeficiency syndrome (AIDS) in homosexual men–a new public health concern. NIPH Ann. 1982;5:41–49. [PubMed] [Google Scholar]
- 29.Fernandez R, Mouradian J, Metroka C, Davis J. The prognostic value of histopathology in persistent generalized lymphadenopathy in homosexual men. N Engl J Med. 1983;309:185–186. doi: 10.1056/NEJM198307213090314. [DOI] [PubMed] [Google Scholar]
- 30.Ioachim HL, Lerner CW, Tapper ML. The lymphoid lesions associated with the acquired immunodeficiency syndrome. Am J Surg Pathol. 1983;7:543–553. doi: 10.1097/00000478-198309000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Mildvan D, et al. Opportunistic infections and immune deficiency in homosexual men. Ann Intern Med. 1982;96:700–704. doi: 10.7326/0003-4819-96-6-700. [DOI] [PubMed] [Google Scholar]
- 32.Gill JC, Menitove JE, Wheeler D, Aster RH, Montgomery RR. Generalized lymphadenopathy and T cell abnormalities in hemophilia A. J Pediatr. 1983;103:18–22. doi: 10.1016/s0022-3476(83)80768-9. [DOI] [PubMed] [Google Scholar]
- 33.Khan FA, Wollschlager CM. Acquired immune deficiency syndrome. A deadly new disease. Postgrad Med. 1983;74:180–185. 188–191. doi: 10.1080/00325481.1983.11698389. [DOI] [PubMed] [Google Scholar]
- 34.WHO. http://www.afro.who.int/en/clusters-a-programmes/dpc/acquired-immune-deficiency-syndrome/overview.html.2012.
- 35.Metroka CE, et al. Generalized lymphadenopathy in homosexual men. Ann Intern Med. 1983;99:585–591. doi: 10.7326/0003-4819-99-5-585. [DOI] [PubMed] [Google Scholar]
- 36.Biberfeld P, Porwit-Ksiazek A, Bottiger B, Morfeldt-Mansson L, Biberfeld G. Immunohistopathology of lymph nodes in HTLV-III infected homosexuals with persistent adenopathy or AIDS. Cancer Res. 1985;45:4665s–4670s. [PubMed] [Google Scholar]
- 37.Vago L, et al. Morphogenesis, evolution and prognostic significance of lymphatic tissue lesions in HIV infection. Appl Pathol. 1989;7:298–309. [PubMed] [Google Scholar]
- 38.Baroni CD, Uccini S. Lymph nodes in HIV-positive drug abusers with persistent generalized lymphadenopathy: histology, immunohistochemistry, and pathogenetic correlations. Prog AIDS Pathol. 1990;2:33–50. [PubMed] [Google Scholar]
- 39.Biberfeld P, et al. Histopathology and immunohistology of HTLV-III/LAV related lymphadenopathy and AIDS. Acta Pathol Microbiol Immunol Scand A. 1987;95:47–65. doi: 10.1111/j.1699-0463.1987.tb00009_95a.x. [DOI] [PubMed] [Google Scholar]
- 40.Ioachim HL, Cronin W, Roy M, Maya M. Persistent lymphadenopathies in people at high risk for HIV infection. Clinicopathologic correlations and long-term follow-up in 79 cases. Am J Clin Pathol. 1990;93:208–218. doi: 10.1093/ajcp/93.2.208. [DOI] [PubMed] [Google Scholar]
- 41.Pantaleo G, et al. Role of lymphoid organs in the pathogenesis of human immunodeficiency virus (HIV) infection. Immunol Rev. 1994;140:105–130. doi: 10.1111/j.1600-065x.1994.tb00867.x. [DOI] [PubMed] [Google Scholar]
- 42.Ioachim HL, Lerner CW, Tapper ML. Lymphadenopathies in homosexual men. Relationships with the acquired immune deficiency syndrome. JAMA. 1983;250:1306–1309. doi: 10.1001/jama.250.10.1306. [DOI] [PubMed] [Google Scholar]
- 43.Domingo J, Chin NW. Lymphadenopathy in a heterogeneous population at risk for the acquired immunodeficiency syndrome (AIDS)–a morphologic study. Am J Clin Pathol. 1983;80:649–654. doi: 10.1093/ajcp/80.5.649. [DOI] [PubMed] [Google Scholar]
- 44.Barre-Sinoussi F, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 45.Gallo RC, et al. Isolation of human T-cell leukemia virus in acquired immune deficiency syndrome (AIDS) Science. 1983;220:865–867. doi: 10.1126/science.6601823. [DOI] [PubMed] [Google Scholar]
- 46.Le Tourneau A, Audouin J, Diebold J, Marche C, Tricottet V, Reynes M. LAV-like viral particles in lymph node germinal centers in patients with the persistent lymphadenopathy syndrome and the acquired immunodeficiency syndrome-related complex: an ultrastructural study of 30 cases. Hum Pathol. 1986;17:1047–1053. doi: 10.1016/s0046-8177(86)80089-2. [DOI] [PubMed] [Google Scholar]
- 47.Pekovic DD, et al. Pathogenicity of HIV in lymphatic organs of patients with AIDS. J Pathol. 1987;152:31–35. doi: 10.1002/path.1711520105. [DOI] [PubMed] [Google Scholar]
- 48.Ward JM, et al. Immunohistochemical localization of human and simian immunodeficiency viral antigens in fixed tissue sections. Am J Pathol. 1987;127:199–205. [PMC free article] [PubMed] [Google Scholar]
- 49.Cameron PU, Dawkins RL, Armstrong JA, Bonifacio E. Western blot profiles, lymph node ultrastructure and viral expression in HIV-infected patients: a correlative study. Clin Exp Immunol. 1987;68:465–478. [PMC free article] [PubMed] [Google Scholar]
- 50.Piris MA, Rivas C, Morente M, Rubio C, Martin C, Olivia H. Persistent and generalized lymphadenopathy: a lesion of follicular dendritic cells? An immunohistologic and ultrastructural study. Am J Clin Pathol. 1987;87:716–724. doi: 10.1093/ajcp/87.6.716. [DOI] [PubMed] [Google Scholar]
- 51.Schuurman HJ, Krone WJ, Broekhuizen R, Goudsmit J. Expression of RNA and antigens of human immunodeficiency virus type-1 (HIV-1) in lymph nodes from HIV-1 infected individuals. Am J Pathol. 1988;133:516–524. [PMC free article] [PubMed] [Google Scholar]
- 52.Baroni CD, et al. Expression of HIV in lymph node cells of LAS patients. Immunohistology, in situ hybridization, and identification of target cells. Am J Pathol. 1988;133:498–506. [PMC free article] [PubMed] [Google Scholar]
- 53.Haase AT. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu Rev Immunol. 1999;17:625–656. doi: 10.1146/annurev.immunol.17.1.625. [DOI] [PubMed] [Google Scholar]
- 54.Chalifoux LV, et al. Lymphadenopathy in macaques experimentally infected with the simian immunodeficiency virus (SIV) Am J Pathol. 1987;128:104–110. [PMC free article] [PubMed] [Google Scholar]
- 55.McClure HM, Anderson DC, Fultz PN, Ansari AA, Lockwood E, Brodie A. Spectrum of disease in macaque monkeys chronically infected with SIV/SMM. Vet Immunol Immunopathol. 1989;21:13–24. doi: 10.1016/0165-2427(89)90126-8. [DOI] [PubMed] [Google Scholar]
- 56.Picker LJ, Hansen SG, Lifson JD. New paradigms for HIV/AIDS vaccine development. Annu Rev Med. 2012;63:95–111. doi: 10.1146/annurev-med-042010-085643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lifson JD, Haigwood NL. Lessons in nonhuman primate models for AIDS vaccine research: from minefields to milestones. Cold Spring Harb Perspect Med. 2012;2:a007310. doi: 10.1101/cshperspect.a007310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paiva DD, Morais JC, Pilotto J, Veloso V, Duarte F, Lenzi HL. Spectrum of morphologic changes of lymph nodes in HIV infection. Mem Inst Oswaldo Cruz. 1996;91:371–379. doi: 10.1590/s0074-02761996000300023. [DOI] [PubMed] [Google Scholar]
- 59.Schacker TW, et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Invest. 2002;110:1133–1139. doi: 10.1172/JCI16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diaz A, et al. Factors associated with collagen deposition in lymphoid tissue in long-term treated HIV-infected patients. AIDS. 2010;24:2029–2039. doi: 10.1097/QAD.0b013e32833c3268. [DOI] [PubMed] [Google Scholar]
- 61.Diaz A, et al. Lymphoid tissue collagen deposition in HIV-infected patients correlates with the imbalance between matrix metalloproteinases and their inhibitors. J Infect Dis. 2011;203:810–813. doi: 10.1093/infdis/jiq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wight TN, Potter-Perigo S. The extracellular matrix: an active or passive player in fibrosis? Am J Physiol Gastrointest Liver Physiol. 2011;301:G950–955. doi: 10.1152/ajpgi.00132.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Estes JD. Role of collagen deposition in lymphatic tissues and immune reconstruction during HIV-1 and SIV infections. Curr HIV/AIDS Rep. 2009;6:29–35. doi: 10.1007/s11904-009-0005-0. [DOI] [PubMed] [Google Scholar]
- 64.Schacker TW, et al. Lymphatic tissue fibrosis is associated with reduced numbers of naive CD4+ T cells in human immunodeficiency virus type 1 infection. Clin Vaccine Immunol. 2006;13:556–560. doi: 10.1128/CVI.13.5.556-560.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nies-Kraske E, et al. Evaluation of the pathogenesis of decreasing CD4(+) T cell counts in human immunodeficiency virus type 1-infected patients receiving successfully suppressive antiretroviral therapy. J Infect Dis. 2009;199:1648–1656. doi: 10.1086/598980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zeng M, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zeng M, et al. Lymphoid tissue damage in HIV-1 infection depletes naive T cells and limits T cell reconstitution after antiretroviral therapy. PLoS Pathog. 2012;8:e1002437. doi: 10.1371/journal.ppat.1002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Estes JD, et al. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J Infect Dis. 2007;195:551–561. doi: 10.1086/510852. [DOI] [PubMed] [Google Scholar]
- 69.Zeng M, Haase AT, Schacker TW. Lymphoid tissue structure and HIV-1 infection: life or death for T cells. Trends Immunol. 2012;33:306–314. doi: 10.1016/j.it.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 70.Stacey AR, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 72.Brenchley JM, Douek DC. The mucosal barrier and immune activation in HIV pathogenesis. Curr Opin HIV AIDS. 2008;3:356–361. doi: 10.1097/COH.0b013e3282f9ae9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Estes JD, et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 2010;6:e1001052. doi: 10.1371/journal.ppat.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brenchley JM, Douek DC. Microbial translocation across the GI tract. Annu Rev Immunol. 2012;30:149–173. doi: 10.1146/annurev-immunol-020711-075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Q, Schacker T, Carlis J, Beilman G, Nguyen P, Haase AT. Functional genomic analysis of the response of HIV-1-infected lymphatic tissue to antiretroviral therapy. J Infect Dis. 2004;189:572–582. doi: 10.1086/381396. [DOI] [PubMed] [Google Scholar]
- 76.Valdez H, et al. Limited immune restoration after 3 years’ suppression of HIV-1 replication in patients with moderately advanced disease. AIDS. 2002;16:1859–1866. doi: 10.1097/00002030-200209270-00002. [DOI] [PubMed] [Google Scholar]
- 77.Robbins GK, et al. Incomplete reconstitution of T cell subsets on combination antiretroviral therapy in the AIDS Clinical Trials Group protocol 384. Clin Infect Dis. 2009;48:350–361. doi: 10.1086/595888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007;44:441–446. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 79.Sterne JA, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schacker TW, et al. Persistent abnormalities in lymphoid tissues of human immunodeficiency virus-infected patients successfully treated with highly active antiretroviral therapy. J Infect Dis. 2002;186:1092–1097. doi: 10.1086/343802. [DOI] [PubMed] [Google Scholar]
- 81.Fauci AS, Pantaleo G, Stanley S, Weissman D. Immunopathogenic mechanisms of HIV infection. Ann Intern Med. 1996;124:654–663. doi: 10.7326/0003-4819-124-7-199604010-00006. [DOI] [PubMed] [Google Scholar]
- 82.Schacker TW, et al. Amount of lymphatic tissue fibrosis in HIV infection predicts magnitude of HAART-associated change in peripheral CD4 cell count. AIDS. 2005;19:2169–2171. doi: 10.1097/01.aids.0000194801.51422.03. [DOI] [PubMed] [Google Scholar]
- 83.Estes JD, Haase AT, Schacker TW. The role of collagen deposition in depleting CD4+ T cells and limiting reconstitution in HIV-1 and SIV infections through damage to the secondary lymphoid organ niche. Semin Immunol. 2008;20:181–186. doi: 10.1016/j.smim.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sloan EK, Tarara RP, Capitanio JP, Cole SW. Enhanced replication of simian immunodeficiency virus adjacent to catecholaminergic varicosities in primate lymph nodes. J Virol. 2006;80:4326–4335. doi: 10.1128/JVI.80.9.4326-4335.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krathwohl MD, Schacker TW, Anderson JL. Abnormal presence of semimature dendritic cells that induce regulatory T cells in HIV-infected subjects. J Infect Dis. 2006;193:494–504. doi: 10.1086/499597. [DOI] [PubMed] [Google Scholar]
- 86.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat Med. 2006;12:289–295. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 87.Okoye A, et al. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med. 2007;204:2171–2185. doi: 10.1084/jem.20070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol. 2000;18:529–560. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 89.Dustin ML, Allen PM, Shaw AS. Environmental control of immunological synapse formation and duration. Trends Immunol. 2001;22:192–194. doi: 10.1016/s1471-4906(01)01872-5. [DOI] [PubMed] [Google Scholar]
- 90.Racz P, et al. Lymphatic tissue changes in AIDS and other retrovirus infections: tools and insights. Lymphology. 1990;23:85–91. [PubMed] [Google Scholar]
- 91.Khatissian E, et al. The relationship between the interferon alpha response and viral burden in primary SIV infection. AIDS Res Hum Retroviruses. 1996;12:1273–1278. doi: 10.1089/aid.1996.12.1273. [DOI] [PubMed] [Google Scholar]
- 92.Bosinger SE, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lane HC. Polyclonal activation of B cells in homosexual men. New Engl J Med. 1984;31:536–537. doi: 10.1056/NEJM198408233110814. [DOI] [PubMed] [Google Scholar]
- 94.Hellerstein M, et al. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat Med. 1999;5:83–89. doi: 10.1038/4772. [DOI] [PubMed] [Google Scholar]
- 95.Hazenberg MD, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. Aids. 2003;17:1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 96.Valdez H, Lederman MM. Cytokines and cytokine therapies in HIV infection. AIDS Clin Rev. 1997:187–228. [PubMed] [Google Scholar]
- 97.Scharko AM, Perlman SB, Hinds PWn, Hanson JM, Uno H, Pauza CD. Whole body positron emission tomography imaging of simian immunodeficiency virus-infected rhesus macaques. Proc Natl Acad Sci U S A. 1996;93:6425–6430. doi: 10.1073/pnas.93.13.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Giorgi JV, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 99.Silvestri G, et al. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18:441–452. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 100.Estes JD, et al. Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques. J Immunol. 2008;180:6798–6807. doi: 10.4049/jimmunol.180.10.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harris LD, et al. Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. J Virol. 2010;84:7886–7891. doi: 10.1128/JVI.02612-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jacquelin B, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119:3544–3555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Estes JD, et al. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J Infect Dis. 2006;193:703–712. doi: 10.1086/500368. [DOI] [PubMed] [Google Scholar]
- 104.Nilsson J, et al. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108:3808–3817. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Airoldi I, et al. Cytokine gene expression and T-cell proliferative responses in lymph node mononuclear cells from children with early stage human immunodeficiency virus infection. Haematologica. 2000;85:1237–1247. [PubMed] [Google Scholar]
- 106.Pal S, Schnapp LM. HIV-infected lymphocytes regulate fibronectin synthesis by TGF beta 1 secretion. J Immunol. 2004;172:3189–3195. doi: 10.4049/jimmunol.172.5.3189. [DOI] [PubMed] [Google Scholar]
- 107.Andersson J, et al. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J Immunol. 2005;174:3143–3147. doi: 10.4049/jimmunol.174.6.3143. [DOI] [PubMed] [Google Scholar]
- 108.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 109.Border WA, Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest. 1992;90:1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mauviel A. Transforming growth factor-beta: a key mediator of fibrosis. Methods Mol Med. 2005;117:69–80. doi: 10.1385/1-59259-940-0:069. [DOI] [PubMed] [Google Scholar]
- 111.Verrecchia F, Mauviel A. Transforming growth factor-beta and fibrosis. World J Gastroenterol. 2007;13:3056–3062. doi: 10.3748/wjg.v13.i22.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tabb B, et al. Reduced inflammation and lymphoid tissue immunopathology in rhesus macaques receiving anti-tumor necrosis factor treatment during primary simian immunodeficiency virus infection. J Infect Dis. 2013;207:880–892. doi: 10.1093/infdis/jis643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G245–249. doi: 10.1152/ajpgi.2000.279.2.G245. [DOI] [PubMed] [Google Scholar]
- 114.Medina C, et al. Transforming growth factor-beta type 1 receptor (ALK5) and Smad proteins mediate TIMP-1 and collagen synthesis in experimental intestinal fibrosis. J Pathol. 2011;224:461–472. doi: 10.1002/path.2870. [DOI] [PubMed] [Google Scholar]
- 115.Katakai T, Hara T, Sugai M, Gonda H, Shimizu A. Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J Exp Med. 2004;200:783–795. doi: 10.1084/jem.20040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zeng M, et al. Critical role of CD4 T cells in maintaining lymphoid tissue structure for immune cell homeostasis and reconstitution. Blood. 2012;120:1856–1867. doi: 10.1182/blood-2012-03-418624. [DOI] [PMC free article] [PubMed] [Google Scholar]


